Abstract
Infant rats learn to prefer stimuli paired with pain, presumably due to the importance of learning to prefer the caregiver to receive protection and food. With maturity, a more ‘adult-like’ learning system emerges that includes the amygdala and avoidance/fear learning. The attachment and ’adult-like’ systems appear to co-exist in older pups with maternal presence engaging the attachment system by lowering corticosterone (CORT). Specifically, odor-shock conditioning (11 odor-0.5mA shock trials) in 12-day old pups results in an odor aversion, although an odor preference is learned if the mother is present during conditioning. Here, we propose a mechanism to explain pups ability to ‘switch’ between the dual learning systems by exploring the effect of maternal presence on hypothalamic paraventricular nucleus (PVN) neural activity, norepinephrine (NE) levels and learning. Maternal presence attenuates both PVN neural activity and PVN NE levels during odor-shock conditioning. Intra-PVN NE receptor antagonist infusion blocked the odor aversion learning with maternal absence, while intra-PVN NE receptor agonist infusion permitted odor aversion learning with maternal presence. These data suggest maternal control over pup learning acts through attenuation of PVN NE to reduce the CORT required for pup odor aversion learning. Moreover, these data also represent pups’ continued maternal dependence for nursing, while enabling aversion learning outside the nest to prepare for pups future independent living.
Keywords: corticosterone, attachment, fear conditioning, amygdala, infant, microdialysis, paraventricular nucleus of hypothalamus, norepinephrine
INTRODUCTION
Infant rats rapidly learn an odor preference following odor-pain (0.5mA shock or tailpinch) conditioning during the first 10 days of life (Camp and Rudy, 1988; Haroutunian and Campbell, 1979; Moriceau and Sullivan, 2004b; Roth and Sullivan, 2005; Sullivan et al., 1986a,b, 2000a). This failure to learn to avoid odors paired with pain is not due to pups’ inability to detect pain (Barr, 1995; Collier and Bolles, 1980; Emerich et al., 1985; Fitzgerald, 2005; Sullivan et al., 2000a; Stehouwer and Campbell, 1978) and is related to pups’ low corticosterone (CORT) levels during the infant stress hyporesponsive period (SHRP) when some stressors fail to induce a CORT increase (Grino et al., 1994; Levine, 2001; Rosenfeld et al., 1992). Indeed, a systemic injection of CORT or direct infusion of CORT into the amygdala permits odor-0.5mAshock conditioning to prematurely enable pups to learn to avoid odor (Moriceau and Sullivan, 2004b; Moriceau et al., 2006), as well as inhibitory conditioning (Collier et al., 1979). Additionally, in older pups (passed SHRP), simply lowering CORT levels (adrenalectomy) or blocking CORT receptors, reinstates the early life odor preference learning induced by odor-0.5mA shock conditioning (Moriceau et al., 2006).
Once the SHRP declines, maternal presence appears to reinstate the SHRP by decreasing the levels of CORT (Moriceau and Sullivan, 2006; Richardson et al., 1989; Stanton and Levine, 1990; Wiedenmayer et al., 2003). Due to the important role of CORT in pup odor aversion learning, attenuating pups’ CORT naturalistically though maternal presence could switch pup odor aversion learning to odor preference learning following odor-shock fear conditioning (Moriceau and Sullivan, 2006). Furthermore, this early learning modifies the neural circuitry supporting overall pup learning, with activation of the amygdala during learning only occurring if the mother is absent and pups learn an odor aversion (Moriceau and Sullivan, 2006). Indeed, direct infusion of CORT into the amygdala permits young pups in the SHRP to learn an odor aversion (Moriceau and Sullivan, 2004b; Moriceau et al., 2006).
Here we assess one potential mechanism for maternal modulation of pup CORT levels and odor learning by exploring the influence of maternal presence on the neural and noradrenergic activity of the paraventricular nucleus of the hypothalamus (PVN). The PVN is a major area controlling CORT levels, receiving projections from different parts of the brain to activate the hypothalamus pituitary adrenal (HPA) axis and, more particularly, permit the release of CORT. Specifically, among these projections, various stressful stimuli increase norepinephrine (NE) release in the PVN (Pacak et al., 1992, 1993), including shock used during the fear conditioning (Otagiri et al., 2000). The PVN’s major source of noradrenergic fibers originate from A1, A2 and A6 (locus coeruleus) cell groups of the brainstem (McKellar and Loewy, 1981; Petrov et al., 1993), which regulate the PVN (Mezey and Palkovits, 1991; Pacak et al., 1992, 1993; Plotsky et al., 1989; Szafarczyk et al., 1987, 1990). Upon stimulation by NE, the PVN will indirectly release CORT through a cascade of events (Leibowitz et al., 1989; Pacak et al., 1995).
METHODS
Animals
We used postnatal day (PN) 12–13 male and female Long-Evans rat pups born and bred in our animal vivarium (originally from Harlan Lab Animals). Mothers were housed in polypropylene cages (34 × 29 × 17 cm) with an abundant amount of aspen wood shavings for nest building, and kept in a 20°C environment with a 12:12 light cycle. Food and water were available ad libitum. The day of parturition was considered PN0 and litters were culled to 12 on PN0-1. The University of Oklahoma Institutional Animal Care and Use Committee, which follows guidelines from the National Institutes of Health, approved all animal care and experimental procedures.
Pup Odor- 0.5mAShock Conditioning
At PN12-13, pups were assigned to one of the following 45min classical conditioning groups: 1) Paired odor-0.5mA shock, 2) Unpaired odor-0.5mA shock, and 3) Odor-only. Pups were trained in individual 600 ml plastic beakers and were given a 10 min adaptation period to recover from experimental handling. During a 45 min training session, pups received 11 presentations of a 30-sec peppermint odor (CS; McCormick Pure Peppermint, Hunt Valley, Maryland) and a 1sec 0.5mA tail shock (US; Lafayette), with an intertrial interval of 4min. Peppermint odor was delivered by a flow dilution olfactometer (2 L/min flow rate) at a concentration of 1:10 peppermint vapor. Paired odor-shock pups received 11 pairings of the 30sec odor with shock overlapping during the last 1sec of the odor presentation. Unpaired odor-shock pups received the shock 2min after each odor presentation. Odor-only pups received only the peppermint odor presentations (Moriceau and Sullivan, 2004b, 2006; Moriceau et al., 2006; Roth and Sullivan, 2001, 2003, 2005; Sullivan et al., 1986a,b, 2000a, 2000b).
Maternal Presence
The mother, which was the same postpartum age as pups’ biological mother, was injected with the anesthetic Urethane to prevent movement and the milk ejection reflex. The mother was placed on her side to permit pups access to her nipples. Before the first shock, some pups occasionally nursed, although no pups nursed after the first shock.
CORT RIA
Duplicate plasma samples were analyzed for CORT using the Rat corticosterone Coat-a-Countkit (Radioassay Systems Labs, In., Carson, CA). The sensitivity of the assay was 5ng/ml. The intraassay coefficient of variation was 1–9%. Heart blood samples were taken from PN12 pups, centrifuged at 14,000cpm for 6min, the plasma aliquoted and stored at −70°C for later analysis.
Pup Behavioral Testing
The day following conditioning, a Y-maze was used to assess pups’ expression of an odor preference or aversion. The Y-maze required pups to choose between two arms of a Plexiglas Y-maze (start box: 8.5 cm width, 10cm length, 8 cm height; choice arms: 8.5 × 24 × 8 cm), one containing the peppermint odor (20μl of peppermint odor on a KimWipe placed at the end of the alley) and the other a familiar odor (the same wood shavings used as nest bedding but clean; 20ml of clean shavings in a petri dish placed at the end of the alley). Pups were placed in the start box for 5sec, the alley doors opened and pups were given 60sec to choose an arm (pup entire body passed the alley entrance). Pups received 5 sequential trials and between trials, the pup was placed in a holding cage for 10sec and the floor wiped clean with water and dried. Observations during testing were made blind to the training condition.
PVN cannula/probe implantation
On PN10, pups were anesthetized by inhalation (isoflurane) and placed in an adult stereotaxic apparatus adapted for use with infants. Stainless steel cannulae (30-gauge tubing) or microdialysis guide cannulae (8mm long, 500μm diameter acrylic resin; EICOM corp, Kyoto, Japan) were implanted bilaterally or unilaterally, respectively, in the PVN through holes drilled in the overlying skull. Stereotaxic coordinates derived from the atlas of Paxinos et al (1991) were used as a reference and adapted through pilot work for implanting cannulae into the PVN (caudal −0.9 mm; lateral ±0.60 mm from lambda). The cannulae were lowered 7.0mm from the surface of the skull, which placed the tip near the PVN. The cannula assembly was fixed to the skull with dental cement and anchor wires. To ensure patency of the cannulae, guide wires were placed in the lumen of the tubing until training. Following recovery from surgery (generally within 30min), pups were returned to the litter and dam until training two days later. On PN12, pups were placed in training chambers and bilateral cannulae were connected via PE10 tubing to a Harvard syringe pump driving two Hamilton microliter syringes. The cannulae were filled (14sec for PVN cannulae at 0.5μl/min) with either drug (described below) or vehicle. Pups were odor-shock conditioned as described above. During the first 20-min training period, pups received drug or control solution infused at 0.1μl/min, for a total infusion volume of 2.0μl as previously described (Moriceau and Sullivan, 2004a; Sullivan et al., 1992, 2000b). Following training, pups were disconnected from the syringe pump and returned to the nest until testing, the following day.
Infusions with maternal presence
Pups received either phenylephrine (NE α1 noradrenergic receptor agonist; 0.25 or 0.50mM) or vehicle (saline).
Infusions without maternal presence
Pups received prazoxin (NE α1 noradrenergic receptor antagonist; 125 or 250nM) or saline.
PVN neural activity
Pups were injected with 14C 2-deoxyglucose (2-DG; 20 μCi/100g, sc) 5min prior to the 45min odor-shock conditioning. Immediately after training, brains were removed, quickly frozen in 2-methylbutane at −45°C and stored in a −70°C freezer. Brains were sectioned (20μm) in a −20°C cryostat and every other section was placed on a cover slip and exposed for 5 days along with 14C standards (14C standards 10×.02 mCi, American Radiolabeled Chemicals, Inc., St. Louis, MO) to x-ray film (Coopersmith and Leon, 1986; DiRocco and Hall, 1988; Nudo and Masterton, 2004; Sulivan and Wilson, 1995). The autoradiographs were analyzed using National Institutes of Health image software that allows pseudocolor imaging and quantitative optical densitometry. The PVN was identified by counterstaining sections with cresyl violet and making a template of that brain area, which was then placed over the enlarged autoradiograph image to guide optical density measurements. Corpus callosum optical density, which did not vary between conditioning groups, was used to form a ratio of amygdala optical density to control for differences in section thickness and exposure (Sullivan et al., 2000a). All measurements were done blind to the experimental conditions. An increase in autoradiographic density indicates increased neural activity but does not discriminate between inhibitory and excitatory neural activity.
Microdialysis procedure
On the day of the experiment, pups were placed in a 27cm diameter acrylic circular cage (EICOM corp., Kyoto, Japan) and were able to move freely and kept at 27°C. The microdialysis probe (A-I-8-02, 8mm length, 2mm membrane, 220μm diameter; EICOM corp., Kyoto, Japan) was inserted into the guide cannula. The probes were perfused with artificial cerebrospinal fluid (ACSF; 147mM NaCl, 2.7mM KCl, 1.2mM CaCl2, 0.85mM MgCl2) at a flow rate of 1.5μl/min.
Dialysate was collected automatically every 10 minutes in a refrigerated (4°C) microfraction collector (EICOM corp., Kyoto, Japan; EFC-82) in which every vial contained 2μl of 12.5mM perchloric acid/250μM EDTA. After completion of the experiment, dialysate samples were immediately stored at −80°C until HPLC analysis. Norepinephrine (NE) and its metabolite (3-MT, 3-methoxytyramine) were assessed by high-pressure liquid chromatography with electrochemical detection (HPLC-EC). HPLC-EC consisted of a 150 × 2.1mm SC-5ODS, 5μm particle column (EICOM corp., Kyoto Japan). Mobile phase (0.1M citric acid, 0.25mM octyl sulfate sodium salt, 0.5mM EDTA, 0.085 tryethylamine, and 6% acetonitrile, pH 2.4) was delivered at 0.23ml/min by a EICOM EP-300 pump. Neurotransmitters were detected with a graphite carbon detector electrode maintained at +0.75V relative to an Ag/AgCl reference electrode. Neurochemical concentrations were estimated using chromatographic peak areas and calibration curves obtained with standard mixtures of known monoamine compounds. During the course of dialysate autoinjection fractions, a standard mixture was injected every fifth sample to monitor and correct calibration curves.
Histological verification of cannula/probe placement
Following microdialysis experiments or after testing following PVN infusion, pups’ brains were removed, frozen and sectioned at 20μm using a −20°C cryostat. Sections were stained with cresyl violet for identification of the microdialysis probes and cannula placement in relation to the PVN using a neonatal atlas (Paxinos et al., 1991). Additional pups were implanted to characterize the extent of drug diffusion within and outside of the PVN. These pups were infused with 2μl of a saline solution of [3H]NE (56.9 mCi/μM, 0.37 μCi/μl; NEN Research Products) during conditioning simulation, followed by brains removal, brain freezing in 2-methylbutane at −45°C, and sliced in 20 μm coronal sections in a cryostat. The slides were apposed to a tritium storage phosphor screen during 14 days (Amersham Biosciences, USA). Then, the screen was scanned at a pixel density of 50 μm (5000 dots per cm2) with a STORM 820 Phosphor Imager (Molecular Dynamics, Sunnyvale, CA). Phosphorimaging of the slides results in a TIFF image file for analysis of 3H diffusion (Moriceau and Sullivan, 2004a, 2006; Tucker et al., 2002).
Statistical analysis
For all experiments, comparisons were made between groups using analysis of variance (ANOVA) followed by post hoc Fisher tests (Winkler and Hays, 1975).
RESULTS
Maternal presence effect on learning
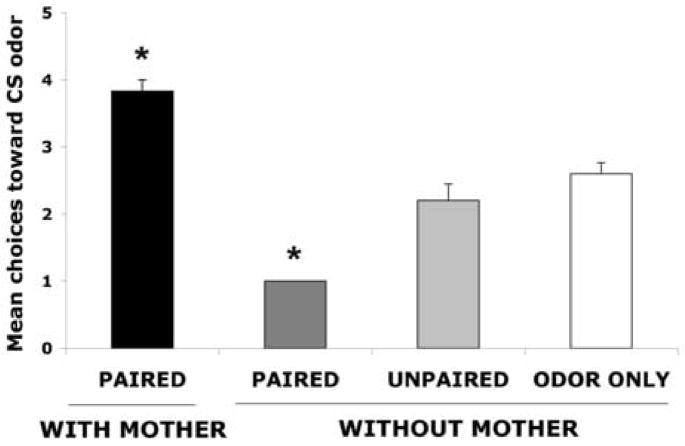
As illustrated in Figure 1, at PN12-13, following odor- 0.5mA shock conditioning without maternal presence, pups learn an odor aversion, whereas maternal presence during odor-0.5mA shock conditioning permits the odor preference learning. ANOVA analysis revealed a significant effect of training condition (F(3,17) = 44.810, p < 0.0001); post hoc Fisher tests revealed that both paired groups with and without maternal presence differed significantly from each of the control groups at p < 0.05 level. These results replicate our previous results showing maternal presence causes Paired odor-shock conditioning to produce an odor preference, although controls (Unpaired and Odor-only) were conditioned in maternal presence and also did not show learning (Moriceau and Sullivan, 2006).
Figure 1.
Mean (± sem) number of choices toward the conditioned stimulus (CS) odor during the Y-maze test (total of 5 trials) for postsensitive period PN12-15 with or without maternal presence. Asterisk represents a significant difference from each of the other groups (p<0.05). n=5–6.
Effect of maternal presence on CORT levels
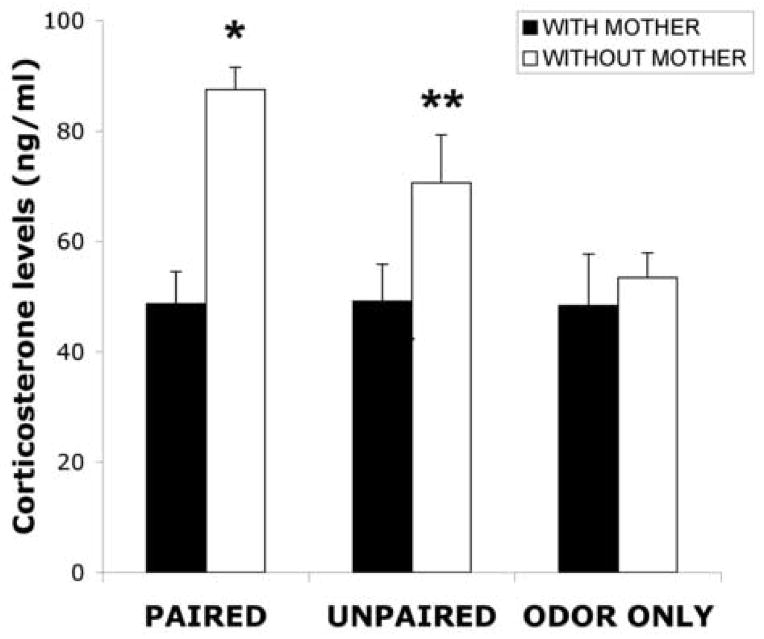
As illustrated by Figure 2, paired and unpaired odor-shock pups without maternal presence had increased levels of CORT compared to paired and unpaired odor-shock pups with maternal presence and odor only pups with/without maternal presence. ANOVA analysis for the CORT levels revealed a significant main effect of maternal presence (F(2,32) =14.599, p < 0.001); post hoc Fisher tests revealed that the paired and unpaired odor-shock conditioning without maternal presence groups were significantly different from all other groups, although the paired and unpaired groups were also significantly different from one another at the p < 0.05 level.
Figure 2.
Mean (± sem) of corticosterone (CORT) levels showed in ng/ml for post-sensitive period PN12 pups comparing animals with maternal presence with animals without maternal presence during odor-shock conditioning. Asterisk represents a significant difference from each of the other groups (p<0.05). n=5–8.
Effect of maternal presence on parvocellular PVN neural activity
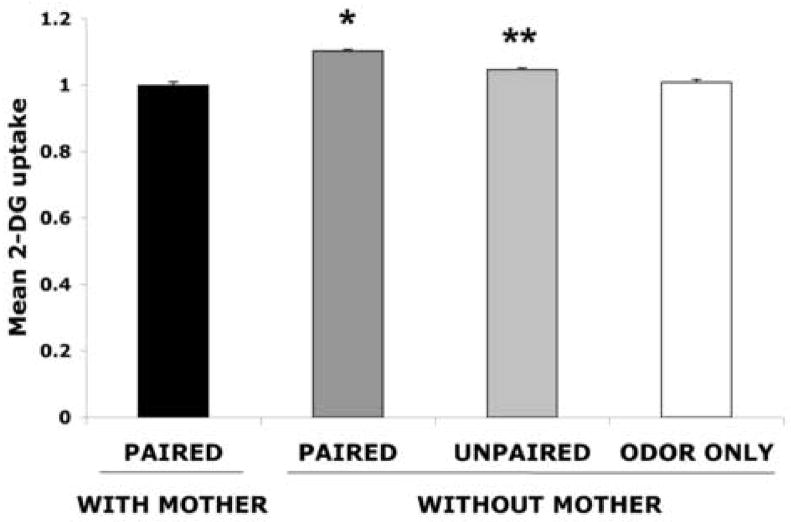
As illustrated by Figure 3, paired and unpaired odor-shock pups without maternal presence had enhancement parvocellular PVN activity (ANOVA, F(3,20) =41.124, p < 0.0001); post hoc Fisher tests revealed that the odor-shock conditioning without maternal presence group was significantly different from the paired group with maternal presence and each of the control groups at the p < 0.0001 level. Additional post hoc Fisher tests revealed that the paired and unpaired odor-shock conditioning without maternal presence groups are significantly different from each other at the p < 0.05 level.
Figure 3.
Effect of maternal presence on the 2-DG relative uptake into the parvocellular PVN of postsensitive period pups (PN12-15) during odor-shock conditioning. Bars represent the Mean (± sem) level of 2-DG uptake in the PVN. Asterisk represents a significant difference from all other groups (p<0.05). n=5–7.
Effect of maternal presence on NE release in PVN
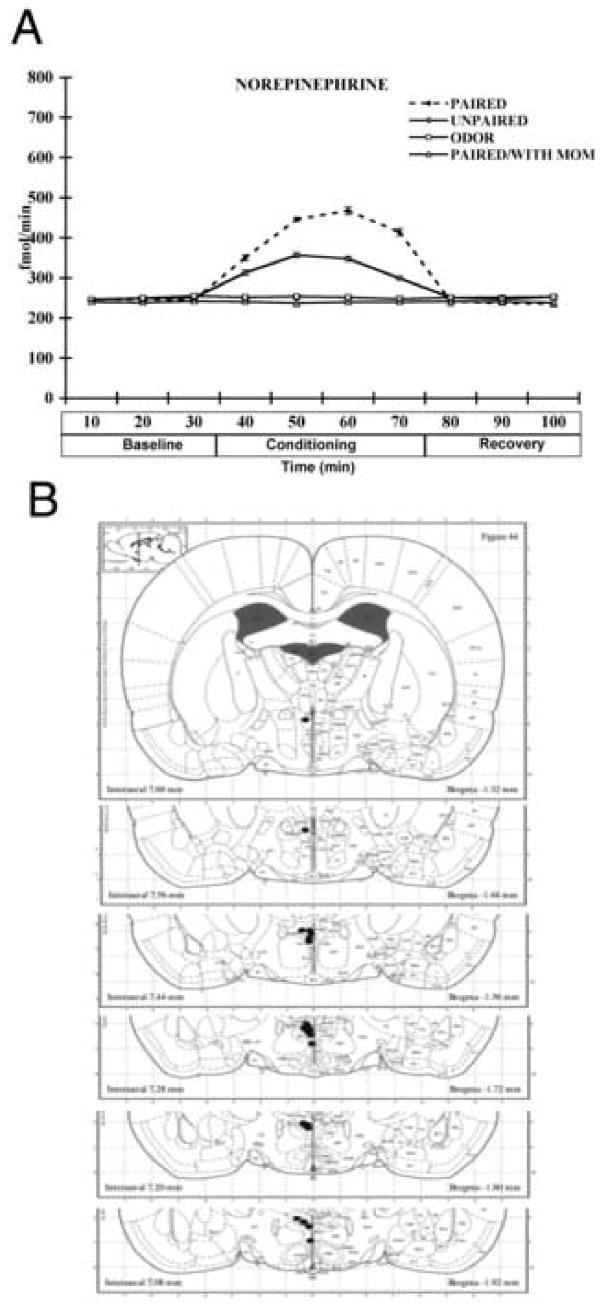
Figure 4A showed that paired and unpaired odor-shock conditioning without maternal presence showed an increase of NE in the PVN while maternal presence attenuated this NE increase. ANOVA analysis for NE levels revealed a significant main effect of training condition (F(3,153) = 553.754, p <0.0001), a main effect of time (F(9,153) = 1036.79, p < 0.0001), and a significant interaction between training condition and time (F(27,153) = 452.986, p < 0.0001). Post hoc Fisher tests revealed that the paired odor-shock conditioning without maternal presence groups was significantly different from all other groups at 50, 60 and 70 minutes at the p < 0.05 level. Also, unpaired odor-shock conditioning without maternal presence group was significantly different from the paired with and without maternal presence and odor only groups at 50, 60 and 70 minutes at the p < 0.05 level. No significant differences in NE metabolite (3-methoxytyramine) were found, also no difference in basal levels of NE was found between groups. Microdialysis probe placements were distributed within 1 mm of the PVN (Figure 4B).
Figure 4.
(A) Effect of maternal presence on the level of NE into the PVN of postsensitive period pups (PN12-15) during odor-shock conditioning. (B) location of microdialysis probe tips (solid circles) in rats used for NE measurement in the PVN. Asterisk represents a significant difference from all other groups (p<0.05). n=6.
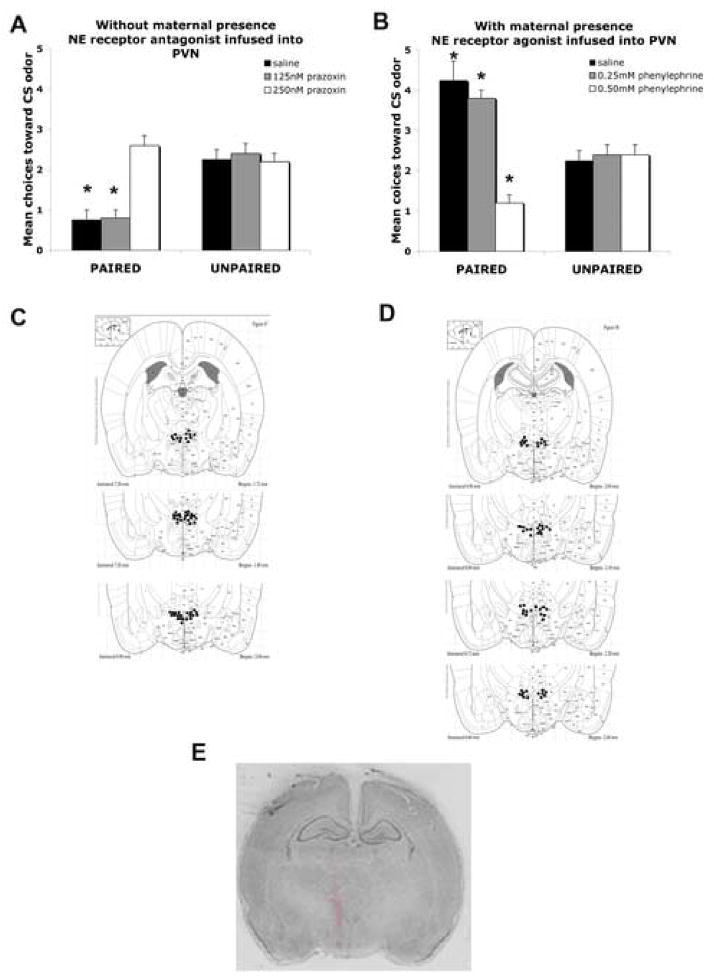
Intra-PVN infusion of NE α1 antagonist blocks the aversion learning in absence of maternal presence
As shown in Figure 5A, odor-shock pups with vehicle infused into the PVN with no mother present showed odor aversion learning. However, learning was blocked in paired odor-shock pups given PVN infusion of 250 nM of α1 noradrenergic receptor antagonist (prazoxin). The PVN infusion of a lower dose (125nM) of α1 noradrenergic receptor antagonist (prazoxin) had no effect on the learned odor aversion. ANOVA analysis for the PVN infusion revealed a significant main effect of training condition (F(1,22) = 22.431, p < 0.0001), a main effect of drug treatment (F(2,22) = 9.257, p < 0.005), and a significant interaction between training condition and drug treatment (F(2,22) = 12.314, p < 0.005); post-hoc Fisher tests revealed that pups infused with saline or low dose (125nM) of α1 noradrenergic receptor antagonist into the PVN are significantly different than each of the other groups (p< 0.05). PVN cannulae tip placements were verified as within or no greater than 1mm from the PVN (Figure 5C). The analysis of 3H NE diffusion showed infused volumes largely limited to the PVN and immediate PVN region (Figure 5E).
Figure 5.
(A) Mean (± SEM) number of choices toward the conditioned stimulus (CS) odor during the Y-Maze test following odor-shock conditioning without maternal presence during NE α1antagonist (prazoxin) infusion directly into the PVN. Asterisks represent significant differences from the control groups (p<0.001) n=5, (B) Mean (± SEM) number of choices toward the conditioned stimulus (CS) odor during the Y-Maze test following odor-shock conditioning with maternal presence during NE α1 agonist (phenylephrine) infusion directly into the PVN. Asterisks represent significant differences from the control groups (p<0.001) n=5, (C) locations of cannula tips (solid circles) in rats used for NE infusion without maternal presence into the PVN, (D) locations of cannula tips (solid circles) in rats used for NE infusion during maternal presence into the PVN, (E) section from a PN12 pup counterstained with cresyl violet and characterizing the extent of H3 NE drug diffusion within the PVN.
Intra-PVN infusion of NE α1 agonist permits an odor aversion learning during maternal presence
As shown in Figure 5B, pups with vehicle infused into the PVN during odor-shock conditioning with maternal presence showed odor preference learning, although PVN infusion of 0.5mM of α1 noradrenergic receptor agonist (phenylephrine) permitted odor aversion learning. ANOVA analysis for PVN infusion revealed a significant main effect of training condition (F(1,22) = 10.921, p < 0.005), a main effect of drug treatment (F(2,22) = 17.757, p < 0.0001), and a significant interaction between training condition and drug treatment (F(2,22) = 19.957, p < 0.0001); post-hoc Fisher tests revealed that pups infused with saline and 0.25 or 0.5mM of α1 noradrenergic receptor agonist into the PVN are significantly different than each of the other groups, although the 0.5mM group is significantly different from the saline and 0.25mM α1 noradrenergic groups (p < 0.05). Cannulae tip placements at the PVN were verified and all tip placements were within 1mm of the targeted PVN (Figure 5D).
DISCUSSION
Our data suggest that the attachment and ’adult-like’ learning systems co-exist in older rat pups with maternal presence engaging the attachment system by lowering CORT. Here we assessed and manipulated a central area controlling CORT release, the PVN and its NE content, during pup odor preference/aversion learning from odor 0.5mA shock conditioning. Specifically, we found that parvocellular PVN neural activity, PVN NE content and blood CORT level increased when pups received paired, and to a lesser extent unpaired shock, although these increases were attenuated by maternal presence. A causal relationship between the presumed control of CORT via PVN NE and learning was established by manipulation of NE α1 noradrenergic receptor agonist and antagonist, which was sufficient to control odor preference/aversion learning. Since increased CORT levels are critical in permitting pups to learn aversions to odors paired with 0.5mA shock and amygdala plasticity, maternal presence appears to lower PVN NE, which ensures pups will be prevented from learning to avoid maternal odor.
Maternal presence effect on pup learning
Pups learn an odor preference from odor-0.5mA shock conditioning when CORT is low, either during the SHRP or after provided the mother is present to attenuate the stress-induced CORT release (Camp and Rudy, 1988; Haroutunian and Campbell, 1979; Moriceau and Sullivan, 2004b, 2006; Roth and Sullivan, 2001, 2005; Sullivan et al., 1986a,b, 2000a). This replicates previous behavioral work from our lab, although, in that study, control groups were conditioned in maternal presence and also did not show learning (Moriceau and Sullivan, 2006). In either of these conditions, increasing CORT either systemically or within the amygdala permits pups to learn an odor aversion from this conditioning (Moriceau and Sullivan, 2004b; Moriceau et al., 2006). The critical role of the amygdala, CORT and pup odor-0.5mA shock fear conditioning was verified by temporary lesions of the amygdala with muscimol (Moriceau and Sullivan, 2006).
CORT is also important in other types of pups learning. In effect, low CORT levels during the sensitive period prevent pups’ from learning conditioned inhibition and passive avoidance (Bialik et al., 1984; Collier et al., 1979). Furthermore, adrenalectomy prevent the normal emergence of inhibitory conditioning and the developmental emergence of fear to natural odors in PN10 pups, while injecting a PN8 pup with CORT causes the premature expression of fear to natural odors (Bialik et al., 1984; Moriceau et al., 2004; Takahashi and Rubin, 1993; Takahashi, 1994). In early life, CORT has a unique role of switching odor-0.5mA shock conditioning from preference to aversion learning (Moriceau et al., 2006; Moriceau et al., 2006). However, in adult, CORT has a more modulatory role that attenuates or enhances fear conditioning or inhibitory conditioning. Specifically, systemic CORT injection increases freezing during fear conditiong although blocking CORT reduces fear and memory retrieval but never lead to a preference learning (Conrad et al., 2004; Cordero et al., 2003; Corodimas et al., 1994; Hui et al., 2004; Macri and Wurbel, 2006; Pugh et al., 1997; Roozendaal et al., 1996, 2006; Roozendaal, 2002; Thompson et al., 2004; Seckl and Meaney, 2004; Shore, 2006).
Maternal presence effect on pup CORT and PVN activity
During the neonatal SHRP, sensory stimuli from the mother (i.e. milk, tactile stimulation) maintains infant rats’ low CORT levels, although simple maternal presence also prevents stress-induced CORT release even in the older pups used in the experiments presented here (Huot et al., 2002; Kent et al., 1997; Levine et al., 1998; Moriceau and Sullivan, 2006; Rosenfeld et al., 1992; Stanton and Levine, 1990; Wiedenmayer et al., 2003). An essential brain area controlling the CORT stress response is the hypothalamic parvocellular PVN. Under stressful conditions, the PVN expresses corticotropin-releasing hormone (CRH) that controls release of the pituitary hormone adrenocorticotropic (ACTH), leading to release of adrenal gland CORT release. The present study demonstrated that the PVN is activated during odor-shock conditioning without maternal presence, which confirms previous work using CRH hnRNA, CRH mRNA and c-fos expression in stressed pups (Dent et al., 2000a,b; Makino et al., 1999; Mamalaki et al., 1992; Smith et al., 1997; Vazquez et al., 2006; Wiedenmayer et al., 2005). The importance of maternal stimulation in controlling PVN activity has been previously demonstrated through the induction of PVN activity by removal of maternal stimulation (maternal deprivation) and subsequent decrease in PVN activity through tactile stimulation (stroking) to mimic maternal grooming (Van Oers et al., 1998b). In the present study, maternal presence (anesthetized mother) is sufficient to block neural activity increase in the PVN. While the mother was not actively stimulating pups, pups remained close to the mother and continued to probe and nuzzle the mother resumably enabling the mother to passively stimulate pups.
Maternal presence effects on CORT appears to be mediated by PVN NE
A major neurotransmitter controlling PVN activity and ultimate CORT release is NE, which is stimulated during stress. Electrophysiological and neurochemical studies have determined that the brain’s noradrenergic system is stimulated by a vast diversity of stressful stimuli, such as immobilization, noise, shock, cold and others (Abercrombie and Jacobs, 1987; Glavin, 1985; Morilak et al., 1987a, b; Pacak et al., 1993; Pardon et al., 2003; Valentino et al., 1998). Considerable evidence also suggests NE increases in the PVN during stress is responsible for activation of the hypothalamic-pituitary axis (HPA) since NE microinjection directly into the PVN increase CRF gene transcription and release of CORT from the adrenal gland (Chen et al., 2004; Cole and Sawchenko, 2002; Feldman et al., 1988; Helmreich et al., 2001; Ishizuka et al., 2000; Itoi et al., 1994; Leibowitz et al., 1989; Pacak et al., 1992, 1995; Palkovits et al., 1999; Szafarczyk et al., 1988; Tjurmina et al., 1999), although other pathways also exist (Cole and Sawchenko, 2002; De Kloet, 2004; Habib et al., 2001; Herman et al., 2002). The source of PVN NE is predominantly from medullary catecholamine neurons (A1 and A2) since the locus coeruleus provides only a minimal direct projection to the PVN (Cunnigham and Sawchenko, 1988; Pacak et al., 1993; Sawchenko and Swanson, 1981, 1982; Tanaka et al., 1982; Ziegler et al., 1999).
Direct manipulation of PVN α1-adrenoceptor receptors support a direct link between PVN NE levels and maternal manipulation of pup’s aversion learning dependence on CORT. Specifically, based on the existing literature, which suggests PVN α1-adrenoceptor receptors activate the stress response (Feldman and Weidenfeld, 2004; Ma and Morilak, 2005), we used PVN α1-adrenoceptor agonists and antogonists to mimick maternal presence or absence PVN NE effects. To override the maternal presence suppression of PVN NE, we used bilateral PVN microinjections of α1-adrenoceptor receptor agonists PVN during learning, which permited the paired presentations of odor-shock to produce a learned odor aversion. Similarly, we mimicked the maternal suppression of PVN NE in pups conditioned without the mother with PVN α1-adrenoceptor receptor antagonist, which blocked shock-induced odor aversion learning. The adult literature suggests α1-adrenoceptors within the PVN augmentate glutamatergic tone and attenuation of GABAergic inputs to activate the HPA axis (Chen et al., 2006).
Parallels to the parturient females’ SHRP
The newly parturient mother exhibits a SHRP, which also appears dependent upon reduced PVN NE (Deschamps et al., 2003; Shanks et al., 1999; Toufexis and Walker, 1996; Toufexis et al., 1998; Walker et al., 2004). However, in early lactating females, the presence of pups eliminates this blunted HPA response to a threatening stressor (Deschamps et al., 2003). Indeed, lactating females are more responsive to pups, display larger CORT increases to stress, show increased CRH mRNA expression into the central amygdala and the PVN during stressful stimuli, and increased aggression toward male intruders or predator odor (Deschamps et al., 2003; Erskine et al., 1978; Smotherman et al., 1977). Removal of pups from the lactating female blocks these effects. Thus, pups have a crucial role in modulating the maternal response to stressors threatening their survival.
Summary
These data provide insight into one unique characteristic of the infant’s stress response and its impact on the infant learning the maternal odor used for attachment. In effect, PVN NE levels seem important for deciding between the preference learning used for attachment or the more ‘adult-like’ amygdala-dependent aversion learning through CORT modulation. A similar phenomenon of pup modulation of the mother’s CORT response has been identified suggesting a reciprocal regulation of mother-infant behavior (Erskine et al., 1978; Leuner and Shors, 2006; Stanton and Levine, 1990; Suchecki et al., 1993; Toufexis et al., 1998; Walker et al., 2004; Wiedenmayer et al., 2003;).
Acknowledgments
This work was funded by grants NICHD-HD33402, NSF IOB-0544406 & Oklahoma Center for Science and Technology OCAST to RMS.
Grants: This work was funded by grants NICHD-HD33402, NSF IOB-0544406 & Oklahoma Center for Science and Technology OCAST to RMS and Riken Institute Research funds to KS.
Footnotes
Disclosure Statement: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats I Acutely presented stressful and nonstressful stimuli. J Neurosci. 1987;7:2837–2843. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA. Ontogeny of nociception and antinociception. NIDA Res Monograph. 1995;158:172–201. [PubMed] [Google Scholar]
- Bialik RJ, Pappas BA, Roberts DC. Neonatal 6-hydroxydopamine prevents adaptation to chemical disruption of the pituitary-adrenal system in the rat. Horm Behav. 1984;18:12–21. doi: 10.1016/0018-506x(84)90046-1. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Chen XQ, Du JZ, Wang YS. Regulation of hypoxia-induced release of corticotropin-releasing factor in the rat hypothalamus by norepinephrine. Regul Pept. 2004;113:221–228. doi: 10.1016/j.regpep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Chen Q, Li DP, Pan HL. Presynaptic alpha1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmacol Exp Ther. 2006;316:733–742. doi: 10.1124/jpet.105.094797. [DOI] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosc. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Bolles RC. The ontogenesis of defensive reactions to shock in preweanling rats. Dev Psychobiol. 1980;13:141–150. doi: 10.1002/dev.420130206. [DOI] [PubMed] [Google Scholar]
- Collier AC, Mast J, Meyer DR, Jacobs CE. Approach-avoidance conflict in preweanling rats: A developmental study. Ani Learn Behav. 1979;7:514–520. [Google Scholar]
- Conrad CD, MacMillan DD, Tsekhanov S, Wright RL, Baran SE, Fuchs RA. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiol Learn Mem. 2004;81:185–199. doi: 10.1016/j.nlm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Res. 1986;371:400–403. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of conditioned fear in rats. Ann NY Acad Sci. 1994;746:392–393. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Hormones and the Stressed Brain. Ann NY Acad Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Dent GW, Okimoto DK, Smith MA, Levine S. Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinol. 2000a;71:333–342. doi: 10.1159/000054554. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinol. 2000b;141:1593–1598. doi: 10.1210/endo.141.5.7455. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J Neuroendocrinol. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- DiRocco RJ, Hall WG. Metabolic neural mapping in neonatal rats. J Neurosci Res. 1981;6:13–19. doi: 10.1002/jnr.490060103. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Scalzo FM, Enters EK, Spear N, Spear L. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Dev Psychobiol. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav Biol. 1978;23:206–218. doi: 10.1016/s0091-6773(78)91814-x. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Melamed E. Hypothalamic norepinephrine mediates limbic effects on adrenocortical secretion. Brain Res Bull. 1988;21:587–590. doi: 10.1016/0361-9230(88)90197-9. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. Involvement of endogeneous glutamate in the stimulatory effect of norepinephrine and serotonin on the hypothalamo-pituitary-adrenocortical axis. Endocrinol. 2004;79:43–53. doi: 10.1159/000076044. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–20. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Glavin GB. Stress and brain noradrenaline: a review. Neurosci Biobehav Rev. 1985;9:233–243. doi: 10.1016/0149-7634(85)90048-x. [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha 2-adrenoreceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinol. 1994;135:2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Itoi K, Lopez-Figueroa MO, Akil H, Watson SJ. Norepinephrine-induced CRH and AVP gene transcription within the hypothalamus: differential regulation by corticosterone. Brain Res Mol Brain Res. 2001;88:62–73. doi: 10.1016/s0169-328x(01)00018-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of paraventricular microenvironment in stress regulation. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Ishizuka Y, Ishida Y, Jin Q, Kato K, Kunitake T, Mitsuyama Y, Kannan H. Differential profiles of nitric oxide and norepinephrine releases in the paraventricular nucleus region in response to mild footshock in rats. Brain Res. 2000;862:17–25. doi: 10.1016/s0006-8993(00)02061-8. [DOI] [PubMed] [Google Scholar]
- Itoi K, Suda T, Tozawa F, Dobashi I, Ohmori N, Sakai Y, Abe K, Demura H. Microinjection of norepinephrine into the paraventricular nucleus of the hypothalamus stimulates corticotropin-releasing factor gene expression in conscious rats. Endocrinol. 1994;135:2177–2182. doi: 10.1210/endo.135.5.7956940. [DOI] [PubMed] [Google Scholar]
- Kent S, Tom C, Levine S. Pituitary adrenal, feeding, and immune responses to interleukin 1-β in the neonate rat: interaction with maternal deprivation. Stress. 1997;1:213–231. doi: 10.3109/10253899709013742. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Diaz S, Tempel D. Norepinephrine in the paraventricular nucleus stimulates corticosterone release. Brain Res. 1989;496:219–27. doi: 10.1016/0006-8993(89)91069-x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Learning during motherhood: a resistance to stress. Horm Behav. 2006;50:38–51. doi: 10.1016/j.yhbeh.2006.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Stanton ME, Gutierrez YR. Maternal modulation of pituitary-adrenal activity during ontogeny. Adv Exp Med Biol. 1988;245:295–310. doi: 10.1007/978-1-4899-2064-5_24. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic – pituitary – adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Lookingland KJ, Ireland LM, Gunnet JW, Manzanares J, Tian Y, Moore KE. 3-Methoxy-4-hydroxyphenylethyleneglycol concentrations in discrete hypothalamic nuclei reflect the activity of noradrenergic neurons. Brain Res. 1991;559:82–88. doi: 10.1016/0006-8993(91)90289-8. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Macri S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, Gold PW, Hashimoto K. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999;850:136–143. doi: 10.1016/s0006-8993(99)02114-9. [DOI] [PubMed] [Google Scholar]
- Mamalaki E, Kvetnansky R, Brady LS, Gold PW. Repeated immobilization stress alters tyrosine hydroxylase, corticotropin-releasing hormone and corticosteroid receptor messenger ribonucleic acid levels in rat brain. J Neuroendocrinol. 1992;4:689–699. doi: 10.1111/j.1365-2826.1992.tb00220.x. [DOI] [PubMed] [Google Scholar]
- McKellar S, Loewy AD. Organization of some brain stem afferents to the paraventricular nucleus of the hypothalamus in the rat. Brain Res Rev. 1981;217:351–357. doi: 10.1016/0006-8993(81)90010-x. [DOI] [PubMed] [Google Scholar]
- Mezey E, Palkovits M. CRF-containing neurons in the hypothalamic paraventricular nucleus: Regulation, especially by catecholamines. Front Neuroendocrinol. 1991;12:23–37. [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004a;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive period learning. Behav Neurosci. 2004b;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22:415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence during infant odor-shock conditioning produces a paradoxical odor preference and attenuated amygdala function. Nature Neurosci. 2006;9:1004–1006. [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Corticosterone serves as a switch between love and hate in infancy: Dual circuitry for odor-shock conditioning during development. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Fornal CA, Jacobs BL. Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. II Cardiovascular challenge. Brain Res. 1987a;422:24–31. doi: 10.1016/0006-8993(87)90536-1. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Fornal CA, Jacobs BL. Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. I Thermoregulatory challenge. Brain Res. 1987b;422:17–23. doi: 10.1016/0006-8993(87)90535-x. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol. 2004;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- Otagiri A, Wakabayashi I, Shibasaki T. Selective corticotropin-releasing factor type 1 receptor antagonist blocks conditioned fear-induced release of noradrenaline in the hypothalamic paraventricular nucleus of rats. J Neuroendocrinol. 2000;12:1022–1026. doi: 10.1046/j.1365-2826.2000.00563.x. [DOI] [PubMed] [Google Scholar]
- Pacak K, Armando I, Fukuhara K, Kvetnansky R, Palkovits M, Kopin IJ, Goldstein DS. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: an in vivo microdialysis study. Brain Res. 1992;589:91–6. doi: 10.1016/0006-8993(92)91165-b. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kvetnansky R, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the paraventricular nucleus of rats with brainstem hemisections: a microdialysis study. Neuroendocrinol. 1993;58:196–201. doi: 10.1159/000126533. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kvetnansky R, Yadid G, Kopin IJ, Goldstein DS. Effects of various stressors on in vivo norepinephrine release in the hypothalamic paraventricular nucleus and on the pituitary-adrenocortical axis. Ann N Y Acad Sci. 1995;771:115–130. doi: 10.1111/j.1749-6632.1995.tb44675.x. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Baffi JS, Pacak K. The role of ascending neuronal pathways in stress-induced release of noradrenaline in the hypothalamic paraventricular nucleus of rats. J Neuroendocrinol. 1999;11:529–539. doi: 10.1046/j.1365-2826.1999.00365.x. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971:55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Tork I, Tecott LH, Valentino KL. Atlas of the developing rat brain. San Diego: Academic; 1991. [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. Branching projections of catecholaminergic brainstem neurons to the paraventricular hypothalamic nucleus and the central nucleus of the amygdala in the rat. Brain Res. 1993;609:81–92. doi: 10.1016/0006-8993(93)90858-k. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET, Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocrinol Rev. 1989;10:437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual fear conditioning. Behav Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- Richardson R, Siegel MA, Campbell BA. Effect of maternal presence on the cardiac and behavioral responses to shock in rats as a function of age. Dev Psychobiol. 1989;22:567–583. doi: 10.1002/dev.420220604. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: Effects on emotion and memory. Psychoneuroendocrinol. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem. 2006;86:249–55. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Guttierrez YA, Martin AM, Mallet HA, Alleva E, Levine S. Maternal regulation of the adrenocortical response in preweanling rats. Physiol Behav. 1991;50:661–671. doi: 10.1016/0031-9384(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci Biobehav Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Dev Psychobiol. 2001;39:188–198. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiol Behav. 2003;78:135–142. doi: 10.1016/s0031-9384(02)00961-7. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psy. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res Rev. 1982;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid Programming. Ann NY Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Shore TJ. Stressful experience and learning across the lifespan. Ann Rev Psych. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks P, Wood S, Ingram CD, Lightman SL. The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J Neuroendocrinol. 1999;11:587–586. doi: 10.1046/j.1365-2826.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Kim SY, Van Oers HJ, Levine S. Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinol. 1997;138:4622–4628. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Mendoza SP, Levine S. Ontogenic changes in pup-elicited maternal pituitary-adrenal activity: pup age and stage of lactation effects. Dev Psychobiol. 1977;10:365–371. doi: 10.1002/dev.420100412. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ, Campbell BA. Habituation of the forelimb-withdrawal response in neonatal rats. J Exp Psychol Ani Behav Processes. 1978;4:104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinol. 1993;57:204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake SC, Hofer MA, Williams CL. Huddling and independent feeding of neonatal rats can be facilitated by a conditioned change in behavioral state. Dev Psychobiol. 1986a;19:625–35. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986b;19:615–23. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Dev Brain Res. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Dissociation of behavioral and neural correlates of early associative learning. Dev Psychobiol. 1995;28:213–219. doi: 10.1002/dev.420280403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy: Ontogeny of conditioned fear and the amygdala. Nature. 2000a;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci. 2000b;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafarczyk A, Guillame V, Conte-Devolx B, Alonso G, Malaval F, Pares-Herbute N, Oliver C, Assenmacher I. Central catecholaminergic system stimulates secretion of CRH at different sites. Am J Physiol. 1988;255:E463–E468. doi: 10.1152/ajpendo.1988.255.4.E463. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, Gaillet S, Barbanel G, Malaval F, Assenmacher I. Implication of alpha-2 adrenergic post-synaptic receptors in the central catecholaminergic stimulation of the corticotropic axis in rats. Acad Sci. 1990;31:81–88. [PubMed] [Google Scholar]
- Szafarczyk A, Malaval F, Laurent A, Gibaud R, Assenmacher I. Further evidence for a central stimulatory action of catecholamines on adrenocorticotropin release in the rat. Endocrinol. 1987;121:883–892. doi: 10.1210/endo-121-3-883. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Dev Brain Res. 1994;81:121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Rubin WW. Corticosteroid induction of threat induced behavioral inhibition in preweanling rats. Behav Neurosc. 1993;107:860–866. doi: 10.1037//0735-7044.107.5.860. [DOI] [PubMed] [Google Scholar]
- Tanaka MY, Kohno Y, Nakagawa R, Ida Y, Takeda S, Nagasaki N. Time-related differences in noradrenaline turnover in rat brain regions by stress. Pharmacol Biochem Behav. 1982;16:315–319. doi: 10.1016/0091-3057(82)90166-6. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRHmRNA expression in the amygdala. Behav Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Goldstein DS, Palkovits M, Kopin IJ. Alpha2-adrenoceptor-mediated restraint of norepinephrine synthesis, release, and turnover during immobilization in rats. Brain Res. 1999;826:243–252. doi: 10.1016/s0006-8993(99)01281-0. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Walker CD. Noradrenergic facilitation of the adrenocorticotropin response to stress is absent during lactation in the rat. Brain Res. 1996;737:71–77. doi: 10.1016/0006-8993(96)00627-0. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Thrivikraman Plotsky PM, Morilak DA, Huang N, Walker CD. Reduced noradrenergic tone to the hypothalamic paraventricular nucleus contributes to the stress hyporesponsiveness of lactation. J Neuroendocrinol. 1998;10:417–427. doi: 10.1046/j.1365-2826.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Tucker DL, Tucker N, Conway T. Gene expression profiling of the pH response in E. coli. J Bacteriol. 2002;183:6551–6558. doi: 10.1128/JB.184.23.6551-6558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation. Adv Pharmacol. 1998;42:781–784. doi: 10.1016/s1054-3589(08)60863-7. [DOI] [PubMed] [Google Scholar]
- Van Oers HJ, De Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998b;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, Lopez JF, Levine S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121:83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Deschamps S, Proulx K, Tu M, Salzman C, Woodside B, Lupien S, Gallo-Payet N, Richard D. Mother to infant or infant to mother? Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. J Psychiatry Neurosci. 2004;29:364–382. [PMC free article] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Ann NY Acad Sci. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Age-specific threats induce CRF expression in the paraventricular nucleus of the hypothalamus and hippocampus of young rats. Horm Behav. 2005;47:139–150. doi: 10.1016/j.yhbeh.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Winkler RL, Hays WL. Sampling theory, experimental design, and analysis of variance. Statistics probability, interference and decision. 1975:P782–783. [Google Scholar]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]