Abstract
Chronic Chagasic patient immunoglobulins (CChP-IgGs) recognize an acidic amino acid cluster at the second extracellular loop (el2) of cardiac M2-muscarinic acetylcholine receptors (M2AChRs). These residues correspond to a common binding site for various allosteric agents. We characterized the nature of the M2AChR/CChP-IgG interaction in functional and radioligand binding experiments applying the same mainstream strategies previously used for the characterization of other allosteric agents. Dose-response curves of acetylcholine effect on heart rate were constructed with data from isolated heart experiments in the presence of CChP or normal blood donor (NBD) sera. In these experiments, CChP sera but not NBD sera increased the efficacy of agonist action by augmenting the onset of bradyarrhythmias and inducing a Hill slope of 2.5. This effect was blocked by gallamine, an M2AChR allosteric antagonist. Correspondingly, CChP-IgGs increased acetylcholine affinity twofold and showed negative cooperativity for [3H]-N-methyl scopolamine ([3H]-NMS) in allosterism binding assays. A peptide corresponding to the M2AChR-el2 blocked this effect. Furthermore, dissociation assays showed that the effect of gallamine on the [3H]-NMS off-rate was reverted by CChP-IgGs. Finally, concentration-effect curves for the allosteric delay of W84 on [3H]-NMS dissociation right shifted from an IC50 of 33 nmol/L to 78 nmol/L, 992 nmol/L, and 1670 nmol/L in the presence of 6.7 × 10−8, 1.33 × 10−7, and 2.0 × 10−7 mol/L of anti-el2 affinity-purified CChP-IgGs. Taken together, these findings confirmed a competitive interplay of these ligands at the common allosteric site and revealed the novel allosteric nature of the interaction of CChP-IgGs at the M2AChRs as a positive cooperativity effect on acetylcholine action.
Keywords: Chagas’ disease, M2 muscarinic acetylcholine receptor, Autoantibody, Allosteric binding site, N-methyl scopolamine, Acetylcholine, Gallamine, W84
INTRODUCTION
Chagasic cardiopathy is the most frequent complication of chronic Trypanosoma cruzi infection. As many as 30% of T. cruzi-infected patients develop the disease, which can lead to heart failure and sudden death (1). The presence of anticardiac G protein-coupled receptor (GPCR) autoantibodies has been described in the sera of patients with idiopathic dilated cardiomyopathy and chagasic cardiopathy (2,3). Chiale and coworkers (4) reported a strong correlation between the presence of antibodies against the cardiac M2 muscarinic acetylcholine receptors (M2AChRs) and the occurrence of sinus node dysfunction, whereas the presence of anti-β1 adrenergic receptor (β1AR) antibodies was correlated to the occurrence of ventricular arrhythmias. Moreover, extensive evidence shows that this antibody-receptor interaction elicits an agonist-like functional response (5,6) that may contribute to the abnormalities observed in these disorders (7).
We recently demonstrated, by acting in an agonist-like manner, that antibodies of the immunoglobulin G class present in the serum of chronic chagasic patients (CChP-IgG) were able to impair L-type Ca2+ currents in rabbit cardiomyocytes by binding and activating cardiac M2AChRs in the absence of other agonists (8). In addition, the agonist-like nature of these antibodies was preserved when specific fractions of CChP-IgGs were purified against the second extracellular loop (el2) of the M2AChRs. In fact, at least for the chagasic cardiopathy, the acidic amino acids in the el2 of the M2AChRs seemed to be involved in the anticardiac autoimmune response observed in this disease (6,9,10).
Coincidently, the most thoroughly studied allosteric site on a GPCR is located on the M2AChR el2. This site was mapped to the acidic amino acid cluster contained in the EDGE sequence (Glu172-Asp173-Gly174-Glu175) of the M2AChR el2 (11,12). Most of the muscarinic allosteric agents, such as gallamine, alcuronium, brucine, tubocurarine, obidoxime, WDuo3, strychnine, and W84, were shown to act by binding to this “common allosteric site.” Consequently, any of these drugs is able to modify the affinity of orthosteric binding-site muscarinic ligands, such as acetylcholine (ACh), N-methyl scopolamine (NMS), and quinuclidinyl benzilate (13,14).
Although the pharmacological allosteric modulation of the affinity of several muscarinic receptor agonists and antagonists has been broadly characterized (11–14), data demonstrating an allosteric modulation of the M2AChR binding properties and function through the action of endogenous autoantibodies is novel. Therefore, the main scope of this study was to characterize the interaction of autoantibodies present in the serum of chronic chagasic patients with the allosteric binding site at the M2AChR el2 and their capacity to exert cooperative effects on ACh (endogenous agonist) binding and signal transduction.
In the first part of this study, the effect of CChP-IgG on the functional response to ACh was studied under near-physiological conditions using isolated rabbit hearts. In these experiments, the effect of varying dilutions of CChP sera on the profile of the ACh dose-response curves was characterized. In some of these experiments, gallamine; an M2AChR allosteric agent (12), was used as an autoantibody competitor to show the functional interaction of CChP-IgGs with the el2 “common allosteric site.” In the second part of the study, the allosteric effect of CChP-IgG on the binding of [3H]-NMS and ACh to cardiac M2AChRs from porcine atrium membranes or heterologously expressed in CHO-K1 cells was characterized. We opted for NMS as the tracer ligand in binding assays because this drug has been widely used to describe the potency of various allosteric modulators that interact with the NMS-occupied receptors (13,14). Finally, following the same rationale used for the functional assays, gallamine and W84 were used to test the specificity of the CChP-IgG interaction with the “common allosteric site.”
The results of this study show for the first time the allosteric nature of this interaction at the M2AChR el2 and the positive cooperativity effect exerted by the CChP-IgGs on ACh binding to the M2AChRs and its functional response.
MATERIALS AND METHODS
Patient Selection, Sera Collection, Enzyme-Linked Immunosorbent Assays (ELISA) and Western Blots
The present study used sera from 10 CChP (hereby randomly designated as CChP-01 through CChP-10): 4 from group II (CChP-01, CChP-03, CChP-05, and CChP-10) and 6 from group III (CChP-02, CChP-04, CChP-06, CChP-07, CChP-08, and CChP-09) according to the Los Andes classification (15). Sera from five normal blood donors (NBD) were used as controls (designated as NBD-01 through NBD-05). Broader descriptions of the clinical staging of the subjects used in this study have been previously reported (16,17). NBDs were individuals submitted to orthopedic surgery who were otherwise healthy. Written consent was obtained from each patient (or NBD), and World Health Organization and Helsinki Treaty regulations were followed (18). In addition, approval for the study was obtained from the Institutional Committee on Research using Human subjects of the Hospital Universitário Clementino Fraga Filho (Rio de Janeiro, Brazil).
The relative titers of anti-M2AChRs-el2 in the sera collected from the CChP or NBD were determined by enzyme-linked immunosorbent assays (ELISA). These were performed by coating microtitre plates (high binding; Corning Inc, Corning, NY, USA) with 1 μg/well of a synthetic peptide (Sigma-Genosys, The Woodlands, TX, USA) corresponding to the el2 of the human M2AChR (168V-R-T-V-E-D-G-E-C-Y-I-Q-F-F-S-N-A-A-V-T-F-G-T-A-I192) in carbonate-bicarbonate buffer (50 mmol/l, pH = 9.6). The plates were incubated overnight at 4°C. Afterward, wells were saturated with PBS + 5% v/v fetal bovine serum + 0.1% v/v Tween 20 (PBS-FBS-T) solution for 120 min at room temperature. Sample sera were serially diluted in PBS-FBS-T solution. Serial dilutions of a monoclonal antibody raised against the N-terminus of the M2AChRs-el2 (residues V-R-T-V-E) (a gift from Dr. Johan Hoebeke, Institute for Molecular and Cellular Biology, Strasbourg, France) were used as a positive control. Primary antibodies were incubated for 120 min at 37°C. Afterward, goat biotinylated anti-mouse and anti-human IgG (H+L) antibodies (1:5000 in PBS-FBS-T) were allowed to react for 60 min at 37°C. The bound antibodies were detected with a streptavidin-horseradish peroxidase conjugate (1 μg/mL) solution in PBS-T. TMB (3,3′,5,5;-tetramethyl benzidine) was used as substrate for the peroxidase. Color development was measured as absorbance at 450 nm.
The Western blot analysis of the reactivity of affinity-purified CChP- or NBD-IgGs was performed as follows: Proteins from porcine heart membrane preparations or homogenates of CHO-K1 cells heterologously expressing the human M2AChRs (20 μg/lane), were submitted to SDS acrylamide electrophoresis and subjected to electrotransfer to nitrocellulose membranes following standard procedure. Affinity purified anti-M2AChR-el2-peptide CChP or NBD-IgGs (20–25 μg) dissolved in Tris-buffered saline (Tris 10 mol/L, NaCl 140 mol/L, pH = 7.4) supplemented with 0.05% (v/v) Tween 20 and 5% (w/v) skimmed milk, were incubated for 120 min at room temperature with the membranes. The monoclonal antibody against the N-terminus of the M2AChRs-el2 used in the ELISA assays was also used as a positive control. Affinity-purified goat anti-human or anti-mouse IgGs (1:1000 v/v) conjugated to alkaline phosphatase were allowed to react with the antibodies for 60 min at room temperature. Antibody detection was performed on the basis of the BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) procedure.
Antibody Purification
Total IgGs from each CChP (or NBD) were purified by standard affinity chromatography on a Hi-Trap protein A column (Amersham Biosciences, Piscataway, NJ, USA). The antibodies were preequilibrated with Tris-HCl buffer (1 mol/L, pH 8.0) and loaded onto a preequilibrated protein A column at pH 8.0, followed by elution with Glycine buffer (100 mmol/L, pH 3.0). The antibodies were then dialyzed against PBS. Purity was checked by double-immunodiffusion assays using anti-total-human immunoglobulins and anti-human-IgG antibodies (MP Biomedicals, Irvine, CA, USA). Only one line of precipitation was obtained in all purified IgGs used. Protein concentrations in the fractions were determined by the Lowry method (19).
Affinity-purified anti-M2AChR-el2-peptide IgGs were used in some of the experiments of this study. These were obtained by the elution of the purified IgG fractions on a Sepharose 4B-CNBr-activated column (Amersham Biosciences, Piscataway, NJ, USA) covalently linked to the M2AChR-el2 peptide (same peptide used in the ELISA assays). The affinity-purified IgGs were eluted with 3 mol/L potassium thiocyanate (pH 7.4) followed by immediate extensive dialysis against PBS.
Electrocardiogram Recordings of Isolated Rabbit Hearts
Young rabbits (1.5–2.0 kg) were killed by cervical dislocation, and the excised hearts were cannulated via the aorta for the continuous perfusion of the coronary circulation with Tyrode solution (composition in mmol/L: NaCl, 127; KCl, 2.7; NaHCO3, 12; MgCl2, 0.5; Glucose, 10; CaCl2, 2.7; pH 7.2, gassed with an O2/CO2 mixture). The cannulated hearts were immersed in warmed Tyrode solution and a water-jacketed flask to maintain a constant temperature of 35–36°C. Three glass electrodes filled with NaCl (1 mol/L) were used to obtain electrocardiographic recordings. Two of the electrodes were connected to the differential input of a high gain amplifier (model 3A9; Tektronix Inc., Beaverton, OR, USA), and the third wire was grounded. The electrocardiograms were recorded on a chart recorder (model 2200, Gould Inc., Cleveland, OH, USA) for posterior analysis.
The experimental protocol consisted of a control recording for 30 min in Tyrode solution, a 4-min perfusion (longer incubation times were prone to receptor desensitization) with Tyrode solution containing one of the ACh concentrations to be tested (from 1 × 10−8 to 1 × 10−4 mol/L), in the absence or presence of a fixed concentration of CChP serum (1:1000, 1:500, 1:250, or 1:100 v/v dilutions), ending with a 30-min washout period when basal heart rate was regained. Only experiments where the basal heart rate was regained were considered for posterior analysis. The same protocol was used to test the effect of the NBD serum at the 1:100 v/v dilution. In another set of experiments, the ACh and serum effects were recorded in the presence of gallamine 100 μmol/L. Each ACh or serum concentration was assayed in a separate experiment (a different isolated heart preparation) resulting in three to ten replicates for a given data point (sera or ACh dilution).
No effects were observed on ACh efficacy when the cholinesterase inhibitor physostigmine (0.1 μmol/L) was added in separate experiments aimed at evaluating the necessity of this agent. Thus, we concluded that the overall cholinesterase activity in rabbit hearts under the experimental conditions used was not significant, and the use of cholinesterase inhibitors was therefore unnecessary.
The variations of heart rate were measured as a fractional response (E) established from the difference between the concentrations of ACh used in the absence and presence of a fixed concentration of CChP or NBD serum and gallamine. ACh dose-response curves for the different serum dilutions used were plotted and data were analyzed by nonlinear regression. The animals used in these experiments were cared for in accordance with the guiding principles for research involving animals and human beings published by the American Physiological Society and the World Medical Association (20).
Preparation of Porcine Atrium Membranes and Homogenates of CHO-K1 Cells Expressing the Human M2AChRs
Fresh porcine atria were obtained from a local slaughterhouse. Atrial tissue (approximately 160 g) was cut into small pieces and washed in HEPES 10 mmol/L, pH 7.4. Afterward, the tissue was homogenized in buffer A (HEPES buffer 25 mmol/L, pH 7.4; EDTA 1 mmol/L; sodium azide 0.02% w/v; MgCl2 1 mmol/L; PMSF 0.1 mmol/L; pepstatin A 1 μg/mL) with a Waring blender and subsequently, an Ultraturrax homogenizer. To separate coarse particles, the resulting homogenate was centrifuged for 10 min at 600 × g. The supernatant was then centrifuged for 60 min at 70,000 × g. The resulting pellet was resuspended in buffer A and applied to a 28% and 13% (w/v) discontinuous sucrose gradient in buffer A. The samples were centrifuged for 60 min at 113,000 × g. The 13% sucrose layer, including the interface, was collected, partitioned in 1-mL aliquots, and stored at −80°C until use. Total protein content was quantified according to the Lowry method (19) and ranged from 1.0 to 1.7 mg/mL of membrane suspension.
A plasmid DNA construct (pcDNA3-hM2) bearing the human M2AChR sequence was used to express the receptors in CHO-K1 cells (ATCC number CCL-61; American Type Culture Collection, Manassas, VA, USA). This construct was prepared by subcloning a 1950 bp AvrII restriction fragment from the pcD-hM2 plasmid that originally included the M2 muscarinic receptor sequence (from Dr. Edward Hulme, NIMR, London, UK) into the XbaI unique site in the pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA, USA).
The CHO K1 cells were subcultured and grown to a confluence of 50%–60% in Ham’s F12 nutrient medium supplemented with 10% v/v fetal bovine serum in 100-mm culture dishes (Corning Inc, Corning, NY, USA). The cells were transfected with the pcDNA3-hM2 plasmid construct using the Lipofectamine™ transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Forty-eight hours after transfection, the culture dishes were scraped, the cell suspension was homogenized in a Dounce cell homogenizer, and the homogenates were centrifuged twice at 48,000 × g. The final pellet was resuspended in HEPES 25 mmol/L (pH 7.4), EDTA 1 mmol/L, PMSF 0.1 mmol/L, and MgCl2 2 mmol/L and stocked at −80°C until use.
Allosterism Assays
Cardiac atrial membrane preparations or CHO-K1-M2AChR cell homogenates were incubated with [3H]-NMS 400 pmol/L in the absence or presence of several concentrations of CChP- or NBD-IgGs at a total protein concentration of 60 μg/mL for 180 min at 37°C and a final volume of 2 mL of HEPES 25 mmol/L (pH 7.4) and MgCl2 2 mmol/L buffer. The same procedure was repeated in the absence or presence of a fixed concentration of ACh (2 μmol/L). For the assays performed in the presence of ACh, guanosine-5′-triphosphate (GTP) was added at a concentration of 0.2 mmol/L. The specific binding of a high and saturating concentration (1 nmol/L) of [3H]-NMS alone was also measured. The cardiac membranes or the cell homogenates were collected by rapid vacuum filtration through glass fiber GF/B filters by means of a cell harvester (Brandel, Gaithersburg, MD, USA) followed by three rapid washes with 5 mL of ice-cold sodium phosphate buffer (10 mmol/L). The filters were dried, placed in scintillation vials, and soaked with 5 mL of toluene-based scintillation cocktail. Afterward, the membrane-bound radioactivity was determined by liquid scintillation counting (efficiency of 45%; Packard Instruments Co., Meriden, CT, USA). Nonspecific binding was defined as the tracer binding in the presence of atropine 2 μmol/L and was subtracted in all experiments.
To block the CChP-IgG antibody action on agonist binding, a separate set of experiments were performed by preincubating the CChP-IgGs with 90 μg of the M2AChR-el2 peptide per μg of total IgG for 12 hr at 4°C.
The [3H]-NMS saturation binding parameters under control conditions for the porcine atrial membranes were previously determined as 150 ± 26 pmol/L (mean ± SEM; n = 4 independent experiments) for the equilibrium dissociation constant (KD) and 280 ± 16 fmol/mg of protein as total binding sites for the radioligand at a saturating concentration (BMAX). Similar binding parameters were determined for the CHO-K1 cells heterologously expressing the human M2AChRs.
To assess the presence of allosteric interactions and the magnitude of the cooperativity between the autoantibodies and both [3H]-NMS and unlabeled ACh, specific binding data were transformed into affinity ratios (ratios of apparent affinity) of the primary ligands [3H]-NMS or ACh in the presence of several concentrations of CChP- or NBD-IgGs compared with the affinity of these ligands alone. This experimental procedure is described in detail by Lazareno and Birdsall (13,21). The affinity ratio of [3H]-NMS (rNMS) in the presence of a distinct concentration of CChP- or NBD-IgG was estimated by using Eq. (1):
| (1) |
where BLX is the specific binding in the presence of the lower [3H]-NMS concentration (400 pmol/L) and a given concentration of IgG, BL1 is the specific binding in the presence of the higher radioligand concentration (1 nmol/L), BL is the specific binding in the presence of the lower [3H]-NMS concentration alone and q is the ratio between the lower and higher [3H]-NMS concentrations.
The affinity ratio of ACh (rACh) in the presence of a given IgG concentration was estimated using Eq. (2):
| (2) |
where BLAX is the specific binding in the presence of the lower [3H]-NMS concentration, 2 μmol/L ACh, and a distinct concentration of CChP- or NBD-IgG. BLA is the specific binding in the presence of the lower [3H]-NMS concentration and 2 μmol/L ACh.
As a corollary aiding in the interpretation of the generated plots, when the affinity ratio (rNMS or rACh) values are greater than 1, a positive cooperative process applies. If on the other hand, values smaller than 1 are obtained, negative cooperativity is present. Values equal to 1 suggest the absence of cooperativity (21).
Dissociation Assays
Cardiac membranes (60 μg/mL) were incubated with [3H]-NMS 200 pmol/L in 2 mL of HEPES 25 mmol/L buffer (pH 7.4) for 60 min (sufficient time to reach equilibrium as determined by association assays, data not shown) at 4°C to slow down the overall radioligand dissociation rate and simplify analysis. After the formation of the radioligand/receptor complexes, the allosteric ligands (gallamine or W84) and the antibodies (CChP-IgGs) were simultaneously added immediately before the beginning of the dissociation phase. Dissociation of the labeled ligand was then initiated by the addition of 2 μmol/L atropine, in the presence or absence of the indicated concentrations of gallamine or W84 and the indicated concentration of CChP-IgGs. The incubation was allowed to continue at adequate time intervals over a period of at least 100 min. The incubation was terminated by vacuum filtration, which was conducted as described above. Nonspecific binding was determined by the inclusion of atropine 2μmol/L during the prelabeling period.
Materials
All reagents used in the ELISA assays or the immunoblots were acquired from Zymed (San Francisco, CA, USA). l-[N methyl-3H] scopolamine methyl chloride ([3H]-NMS) with a specific activity of 84 Ci/mmol was purchased from Amersham Biosciences (Piscataway, NJ, USA). W84 was purchased from Tocris (Bristol, UK). Atropine sulfate, acetylcholine, gallamine, HEPES, and all other reagents and drugs were of the highest grade available and were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Data Analysis and Statistics
The data from the functional and dissociation binding experiments were analyzed by least square nonlinear regression using the Prism (version 4.03) software (GraphPad, San Diego, CA, USA). The antibody titration curves were fitted with an empiric four-parameter sigmoidal function. The mid inflexion point parameter was considered as the relative titer of anti-M2AChR-el2 specific antibody. In the functional assays, dose-response curves for the effects of CChP and NBD sera on ACh isolated-heart responses were obtained by curve fitting of experimental data to a sigmoid one-site dose-response model with a variable-slope parameter. Hill coefficients (nH) and EC50 values were determined for each experimental condition. Curve fitting to the dissociation data was based on a mono-exponential decay equation. The apparent rate constant Koff for the dissociation of [3H]-NMS was determined for each concentration of allosteric modulator and divided by the true rate constant, determined in the presence of 2 μmol/L atropine (control condition). Thus, the resulting data were expressed as a percentage of Koff,, where values less than 100 indicate a delay in the dissociation rate of [3H]-NMS. Curve fits of the concentration-effect curves for the allosteric delay of the apparent rate constant Koff of the [3H]-NMS dissociation were based on a four-parameter logistic function. The shifts on W84 concentration-effect curves induced by the CChP-IgGs were analyzed according to Lew and Angus (22). The accuracy of the models used and the “best fits” obtained by nonlinear regression analysis were statistically assessed with the partial F test. For this and other statistical analysis, a value of P < 0.05 was taken as the criterion of significance.
RESULTS
Anti-M2AChR Specific Antibody Response in the Sera from Chronic Chagasic Patients and Normal Blood Donors
A synthetic peptide corresponding to the amino acid sequence of the M2AChR el2 was used to detect the antibody response against this domain in the sera of CChP and NBD (Fig. 1). Thus, the relative titers of specific anti-el2 antibodies were determined by a limiting dilutions ELISA assay. The relative titers were established as the estimated sera dilutions that gave the half-maximal response. The results from these assays are depicted in Figure 1A together with the estimated relative titers for the serum from each individual used in this study. The mean relative titer of anti-M2AChR el2 was 1:990 v/v in the CChP sera, whereas this value was determined to be 1:119 v/v for the NBD sera. To normalize the skewed distribution, the log10 transforms of these values were compared by using an unpaired t-test with P < 0.0001. On the other hand, the mean relative titers between group II (1:874 v/v) (Los Andes) and group III Chagasic patients (1:1073 v/v) were equal (P = 0.168, unpaired t-test).
Figure 1.
Anti-M2AChR specific antibody response in the sera from CChPs and NBD. (A) The relative titers of anti-M2AChR el2 IgGs were characterized by serial limiting dilutions of the sera from the CChP and NBD individuals. The data were fitted with an empiric four-parameter sigmoidal function (see Materials and Methods section for further details). The values of the relative titers expressed as dilutions for the CChP and NBD sera are tabulated on the right. The means of the log10 transforms of the relative titers from each group were compared with the unpaired t-test. (B) Recognition of M2AChRs from porcine membrane preparations by specific anti M2AChR-el2 affinity-purified CChP-IgGs. Lane 1: Specific anti M2AChR-el2 monoclonal antibody. Lanes 2 and 4: Specific IgGs from samples CChP-02 and CChP-06, respectively. Lanes 3 and 5: Unbound fractions from the samples for CChP-02 and CChP-06. Lanes 6 and 7: Recovered and unbound fractions from NBD-03, respectively.
Given the presence of specific anti-M2AChR-el2 antibodies in the sera of CChP, the affinity-purification of this IgG fraction using a Sepharose 4B-CNBr-activated column covalently linked to the M2AChR-el2 peptide was possible. The Western blots depicted in Figure 1B show that the affinity-purified IgGs specifically recognize the M2AChRs from porcine atrial membrane preparations (Fig. 1B) or CHO-K1 cell homogenates expressing these receptors (data not shown). This response was comparable in extent to the response of an M2AChR-el2 specific monoclonal antibody (Fig. 1B, lane 1). Lanes 2 and 4 correspond to the eluted specific IgGs from samples CChP-02 and CChP-06, respectively. Lanes 3 and 5 show the corresponding flow-through fractions that did not recognize the M2AChR. An NBD sample submitted to the same procedure did not yield anti-M2AChR-el2 specific IgGs (lanes 6 and 7), confirming the presence of anti-M2AChR el2 antibodies in the CChP sera and not in the NBD sera.
From these results, it can be assumed that the CChP sera samples were homogenous, the specific anti-M2AChR el2 antibody concentration was roughly the same for all the CChP sera used in this study, and these titers were significantly higher than the titers found in NBD individuals. In consequence, the homogenous concentration of specific anti-M2AChR-el2 antibodies in the sera of CChPs or NBDs allowed for the use of these agents as a ligand in the functional and binding assays and the analyses described in the following sections.
Enhancement of Muscarinic Receptor Function in Isolated Rabbit Hearts by Chagasic Antibodies
We have previously documented the agonistic effect of anti-M2AChR el2 antibodies from CChP by the reduction of the cardiac L type Ca2+ channel currents (8). Sera from CChP have also been shown to moderately affect chronotropy in isolated rabbit hearts (16,17). For this reason, and as a starting point, the well-described Langendorff isolated rabbit heart setup was used to investigate the presumed partial-agonistic action of the same CChP antibodies used in the abovementioned reports (8,16,17). For this purpose, the inhibitory effect of ACh on the heart rate of isolated hearts was measured in the presence of various dilutions of CChP or NBD sera.
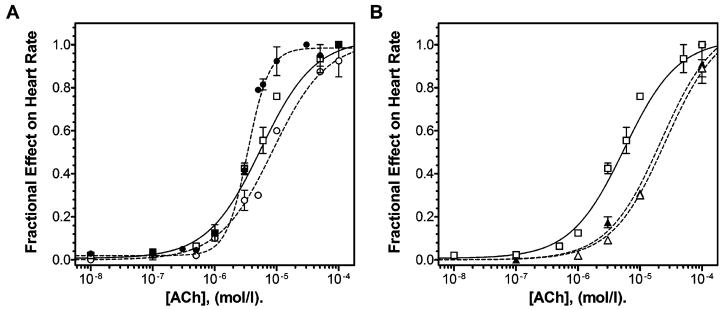
Figure 2 depicts the raw electrocardiographic tracings recorded from isolated rabbit hearts in the presence of various concentrations of ACh (1 × 10−7 to 1 × 10−5 mol/L), CChP sera (dilution of 1:100 v/v) or gallamine (100 μmol/L) plus the same dilution of CChP sera. Each of these traces was obtained in a separate independent experiment. The left column shows the progressive dose-dependent effect of increasing concentrations of ACh on heart rate. In this case, heart rate in the presence of ACh 1 × 10−7 mol/L was 126 bpm and for ACh 1 × 10−5 mol/L, 18 bpm. The enhancement of the negative chronotropic acetylcholine response by CChP sera is clearly visible by comparing the electrocardiographic tracings in the presence of ACh alone (left column) and ACh and CChP serum (central column). When the serum from the CChP was added to the experiment with an ACh concentration of 1 × 10−5 mol/L a sinus arrest was induced. Besides, several types of bradyarrhythmias (such as AV-conduction abnormalities) were also observed as seen in the tracing for 3 × 10−6 mol/L ACh and CChP serum (indicated by arrowheads in Fig. 2). Most importantly, the effect of the CChP sera on the ACh response was reverted by the presence of gallamine as seen by comparing the tracings in the central column (without gallamine) and right-hand column (with gallamine) of Figure 2. Moreover, gallamine seemed to act as an antagonist by right shifting the ACh response.
Figure 2.
Electrocardiographic tracings showing the enhancement of the ACh-induced negative chronotropic effect by CChP sera in isolated rabbit hearts. ACh (1 × 10−7 to 1 × 10−5 mol/L in this figure) produced a dose-dependent reduction of the heart rate in isolated rabbit hearts (left column). The responses to 1 × 10−6 to 1 × 10−5 mol/L ACh were significantly augmented by the presence of 1:100 CChP sera (central column). The arrowheads on the record corresponding to an ACh concentration of 3 × 10−6 mol/L and CChP sera highlight nonconducting P waves characterizing an AV block. The effect of CChP sera on ACh response was completely blocked when the allosteric antagonist gallamine (100 μmol/L) was added (right column). This experiment was performed with the CChP-10 sera and this set of data is included in the graphs in Figure 3. The protocol used in the course of the experiments is shown in the bottom left-hand corner. The horizontal bar represents 20 s.
The raw data from the experiments such as those depicted in Figure 2 were used to construct the ACh dose-response curves shown in Figure 3. Under control conditions (Fig. 3A, open squares and continuous line) ACh reduced the heart rate in a concentration-dependent manner with a pEC50 value of 5.25 ± 0.09 and a Hill coefficient (nH) equal to one. In the presence of CChP sera (individually tested in separate experiments) at a 1:100 v/v dilution (Fig. 3A, filled circles and dashed line) the ACh dose-response curve became steeper, enhancing the ACh agonist action. Accordingly, the nH was increased to 2.5 (partial F test, P < 0.05 when compared to the fit with a sigmoid curve model with a slope equal to 1). CChP sera at this dilution did not affect the basal ormaximal response (EMAX) nor the pEC50 value of the ACh dose-response curves (5.47 ± 0.02). The increase in the ACh dose-response curve slope induced by the CChP sera was retained at a dilution of 1:250 (nH = 2.44) also without significantly affecting the pEC50 value (5.52 ± 0.16, data not shown). The threshold concentration for the enhancement of ACh effectiveness with the CChP sera was therefore found to be close to this dilution (1:250 v/v). This is due to the fact that at higher sera dilutions (1:500 and 1:1000 v/v) the ACh dose-response curves had an nH of one with no significant shifts in the dose-response curves were observed (pEC50 values, 4.86 ± 0.18, 5.17 ± 0.28, respectively, data not shown). In contrast, 1:100 v/v NBD sera (Fig. 3A, open circles and dashes line) did not alter the slope or induce significant shifts in the ACh dose-response curve (pEC50 = 5.07 ± 0.04).
Figure 3.
Enhancement of the negative chronotropic response to ACh by human chagasic antibodies in isolated rabbit hearts. (A) Dose-response curves of varying concentrations of ACh (1 × 10−8 to 1 × 10−4 mol/L) in the absence (control condition: open squares and continuous line) or presence (filled circles and dashed line) of 1:100 v/v of CChP or NBD sera (open circles and dashed line). (B) Dose-response curves of varying concentrations of ACh (1 × 10−8 to 1 × 10−4 mol/L) in the absence (control condition: open squares and continuous line; same set of experiments as in panel A) or presence of gallamine 100 μmol/L (open triangles and dashed line) or the combination of gallamine 100 μmol/L and 1:100 v/v CChP sera (filled triangles and dashed line). Each data point from the curves represents the means ± SEM from three to ten independent experiments with each of the sera from all the CChP or NBD individuals used in the study. The curves were generated from raw data as the shown in Figure 2. pEC50 and Hill slopes were derived from nonlinear regression analysis (see Materials and Methods section for more details).
To determine whether the observed enhancement of the ACh functional response induced by the CChP sera was caused by the antibody action at the M2AChR common allosteric site, ACh dose-response curves were performed with CChP sera and gallamine. This drug was shown to act as an allosteric modulator at cardiac M2AChRs in functional studies (23). Figure 3B shows the effect of 100 μmol/L gallamine (open triangles and dashed line) and the effect of the combination of this modulator with 1:100 v/v CChP sera (filled triangles and dashed line) on the ACh heart rate dose-response curve. In this case, gallamine behaved as a competitive antagonist shifting the ACh dose-response curve for the cardiac M2AChRs to the right (pEC50 = 4.67 ± 0.11, P < 0.01 compared to the control and the CChP sera curves, one-way ANOVA), without a significant reduction in the maximal inhibition of heart rate. When the CChP sera (1:100 v/v) were combined with gallamine (100 μmol/L), they failed to enhance the ACh agonist action and the Hill slope of the curve remained equal to 1. The pEC50 values of the ACh dose-response curve in the presence of CChP sera and gallamine were significantly increased to 4.75 ± 0.20 (P < 0.001 compared to the control and 1:100 v/v CChP sera curves, one-way ANOVA) without affecting the maximal agonist response (Fig. 3B). Taken together, these results suggest that gallamine and the CChP sera (or IgGs) share the same site of action at the cardiac M2AChRs.
Allosteric Interactions Between Human Chagasic Antibodies and Acetylcholine at the Cardiac Muscarinic Receptors
The CChP sera enhancement of ACh action suggests that the interaction at the common allosteric site can modulate the endogenous agonist (ACh) affinity for the M2AChR. Therefore, to determine whether the effect of CChP-IgG on the affinity of ACh for the M2AChR is of relevance at the molecular level, two experimental binding approaches were used to study the interactions between CChP-IgGs, the M2AChRs, and, in some cases, ACh. These interactions were characterized in a low ionic strength buffer to favor the conditions under which cooperative interactions at the M2AChRs could be detected (24).
A thorough characterization of the putative allosteric binding of CChP-IgG was obtained from assays that semiquantitatively measured the effects of an allosteric agent on the binding of [3H]-NMS and ACh. These binding assays were performed following the method described by Lazareno and Birdsall (13,21). Figures 4A and 4B show the affinity ratio plots of the specific binding of [3H]-NMS at the cardiac M2AChRs in the absence (filled circles) and presence (open circles) of ACh 2 μmol/L and various concentrations of CChP-IgG or NBD-IgG. These plots were generated by using the raw data from Figures 4C and 4D, applying Eqs, (1) and (2) (see Materials and Methods section). The estimated affinity ratios of acetylcholine (rACh) and [3H]-NMS (rNMS) in the presence of the highest concentration of CChP- or NBD-IgGs used in these assays are summarized in Table 1. It is evident from Figure 4A and Table 1 that CChP-IgG increased the binding of ACh to cardiac M2AChRs with an affinity ratio of 2.24 ± 0.12 and decreased [3H]-NMS binding (affinity ratio estimates of 0.43 ± 0.12). In contrast, NBD-IgG did not affect the binding of ACh (affinity ratio estimates of 1.00 ± 0.04) to cardiac M2AChRs and decreased [3H]-NMS binding (affinity ratio estimates of 0.52 ± 0.12) in a similar manner to CChP-IgGs for [3H]-NMS binding (Figs. 4A, 4B, and Table 1). To validate and reinforce the data obtained by using porcine atria, these experiments were also performed in membrane homogenates from CHO-K1 cells heterologously expressing the human M2AChRs. These results are also summarized in Table 1, showing comparable values for rACh and rNMS to those obtained with porcine atrial membranes. According to these data, CChP-IgG showed positive cooperativity with ACh and negative cooperativity with [3H]-NMS at the M2AChR while NBD-IgG showed neutral cooperativity with ACh and negative cooperativity for [3H]-NMS.
Figure 4.
Effect of Chagasic antibodies on the ratio of the apparent affinities of [3H]-NMS and ACh at the cardiac M2AChRs. Affinity ratio plots (A and B) were derived from quadruplicate observations of the binding of [3H]-NMS 400 pmol/L to cardiac M2AChRs (raw data presented in panels C and D). These experiments were carried out in the absence (filled circles) or the presence (open circles) of ACh and in the presence of various concentrations of CChP (A and C) and NBD purified IgGs (B and D). (A, inset): A 25-residue peptide corresponding to the M2AChR el2 was preincubated with the CChP-IgGs prior to the semiquantitative allosterism assay. Guanosine-5′-triphosphate (GTP) was added at a concentration of 0.2 mmol/L to assays performed in the presence of ACh. Data represent the means ± SEM of five independent experiments. CChP-02, CChP-03, CChP-06, CChP-07, and CChP-10 IgGs, and all NBD-IgGs were used in the distinct experiments. The estimated affinity ratios of acetylcholine (rACh) and [3H]-NMS (rNMS) in the presence of the highest concentration of CChP- or NBD-IgGs used in these assays were summarized in Table 1. The pKD, log affinities of the antibodies for the free unbound receptors estimated from these assays are mentioned in the text.
Table 1.
Affinity ratios of acetylcholine and [3H]-NMS in the presence of CChP- or NBD-IgGs for M2AChR binding measured in the allosterism assays1
| Porcine M2AChR (n=5)2 |
Human M2AChR (n=2)3 |
|||
|---|---|---|---|---|
| Antibody | rACh | rNMS | rACh | rNMS |
| CChP-IgG | 2.24 ± 0.12 | 0.43 ± 0.12 | 2.57 [1.85; 3.28] | 0.30 [0.31; 0.29] |
| NBD-IgG | 1.00 ± 0.04 | 0.52 ± 0.12 | 0.93 [0.90; 0.95] | 0.58 [0.55; 0.60] |
Affinity ratios of acetylcholine (rACh) and [3H]-NMS (rNMS) for the highest concentration of CChP- or NBD-IgGs used are shown as means ± standard errors when applicable.
M2AChR from porcine cardiac membranes.
M2AChR from CHO-K1 cells transfected with the human receptor coding plasmid (pcDNA3-hM2).
A reversion of the CChP-IgG-positive cooperative action on ACh binding to the M2AChRs was partially achieved when the antibodies were preincubated with a 25-amino acid peptide corresponding to the M2AChR el2, on an equimolar basis. These results are depicted in the inset of Figure 4A. This set of data shows that the positive cooperative effect on ACh binding was abolished at the two lower concentrations of CChP-IgG. This effect was reestablished at the higher antibody concentration used, which could be due to el2 peptide depletion at the higher antibody concentration.
The IC50 values obtained from the affinity ratio plots that correspond in theory to the KD (or pKD) values of the allosteric agent for the unoccupied receptors were estimated to be in the micromolar range for CChP-IgG (pKD = 5.34 ± 0.46). Similar results to those obtained with the porcine atrial preparation were obtained from experiments using the CHO-K1/M2AChRs cells. Correspondingly, CChP-IgG had pKD values for the unoccupied human M2AChRs in the micromolar range (pKD = 6.51 ± 0.14, P > 0.05 compared to porcine pKD values). The pKD values of NBD-IgG were also similar in the unoccupied porcine and human M2AChRs (pKD = 5.99 ± 0.63 for the porcine receptors and 6.22 ± 1.82 for the heterologously expressed receptors).
In addition, the values of pKD and pKA for [3H]-NMS and ACh calculated by means of the affinity ratio assay data were 9.84 ± 1.86 and 9.58 ± 0.86 for [3H]-NMS in porcine cardiac membranes and CHO-K1 cells, respectively, and 6.12 ± 0.29 and 5.75 ± 0.02 for ACh in the receptors from these sources. Thus, the data obtained from the allosterism binding assays show that both the porcine cardiac M2AChRs and the human counterpart expressed in a heterologous system share similar ligand binding parameters and allosteric modulation by the CChP-IgGs.
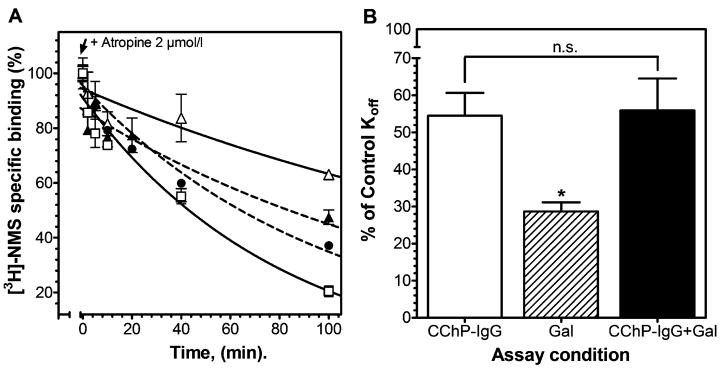
A well-known property of the agents that bind to allosteric sites is their ability to slow down the off-rate of certain agonist or antagonist ligands. As a result, we performed kinetic binding studies to determine the effect of CChP-IgGs on the dissociation of cardiac M2AChR-bound [3H]-NMS. These experiments were carried out in the presence of gallamine that was used to corroborate the CChP-IgG interaction at the common allosteric binding site on the M2AChR el2. The dissociation of [3H]-NMS 200 pmol/l (Fig. 5A), a concentration representing an occupation of nearly 50% of the cardiac NMS-binding sites, was monophasic in the control condition (open squares and continuous line, Koff = 0.01215 ± 0.003 min−1). In agreement with previously reported results (25,26), a low concentration of gallamine (1 μmol/L) markedly decelerated the dissociation of [3H]-NMS (Fig. 5A, open triangles and continuous line). The effect of CChP-IgG 8 × 10−7mol/L (value near the IC50 estimated from competition assays, data not shown) on the Koff of [3H]-NMS was also characterized (filled circles and dashed line). Again in this case CChP-IgG significantly decreased the rate of [3H]-NMS dissociation (Koff = 0.0071 ± 0.0015 min−1), but to a lesser extent than gallamine alone. It is interest that when gallamine was combined with CChP-IgG (filled triangles and dashed line), a significant inhibition of the effect of gallamine on the Koff of [3H]-NMS was found. This latter result implies the existence of a blocking action of CChP-IgG on the allosteric effect of gallamine (Koff gallamine = 0.00348 ± 0.0006 min−1 and Koff gallamine + CChP-IgG = 0.00679 ± 0.0021 min−1). On the other hand, NBD-IgG did not influence the dissociation of the radioligand (Koff = 0.01805 ± 0.005 min−1, data not shown). In Figure 5B, the effects of CChP-IgG and gallamine on the [3H]-NMS dissociation (Fig. 5A) are presented as the percentages of [3H]-NMS Koff in the presence of these agents compared to the control value. In this figure, the competitive interaction of gallamine and CChP-IgGs at the M2AChR allosteric site is clearly observed as an inhibition of the effect of gallamine on the [3H]-NMS Koff by the presence of CChP-IgG.
Figure 5.
Effect of human chagasic antibodies and gallamine on the dissociation rate of [3H]-NMS. (A) The effect of 8 × 10−7 mol/L CChP-IgG on the apparent rate constant Koff of the dissociation of [3H]-NMS 200 pmol/L was determined alone (filled circles and dashed line) and in combination (filled triangles and dashed line) with gallamine 1 μmol/L. The dissociation rate of [3H]-NMS (control condition, open squares, and continuous line) and the allosteric effect of gallamine 1 μmol/L alone (open triangles and continuous line) were also determined. The dissociation curves for the [3H]-NMS specific binding are expressed as percentages of the radioligand specific binding at the equilibrium. (B): The effects of CChP-IgG and gallamine on the [3H]-NMS dissociation from the experiments depicted in (A) are presented as percentages of [3H]-NMS Koff in the presence of these agents as compared to the control value (Koff in the absence of modulators = 0.01215 ± 0.003 min−1). The data correspond to the means ± SEM of a representative experiment derived from four independent dissociation experiments in quadruplicates. The purified IgG fractions from CChP-03, CChP-05, CChP-07, and CChP-09 were used in these experiments. n.s., difference between the means not significant (P > 0.05), Gal, gallamine, *P< 0.05; one way ANOVA and Bonferroni post-test.
The distinct allosteric effects that CChP-IgG exerted on ACh and [3H]-NMS affinities for the M2AChRs indicated that CChP-IgG may by itself differentially recognize free and occupied receptors by specific and distinctive interaction at the allosteric site. Thus, these interactions were further studied by using concentration-effect curves for the inhibition of the [3H]-NMS dissociation rate constant by W84 in the presence of various concentrations of affinity-purified anti-M2AChR el2 CChP-IgGs. Figure 6A shows the right shift induced by the affinity-purified CChP-IgG for the allosteric delay effect of W84 on the [3H]-NMS dissociation. The IC50 of W84 on the Koff of [3H]-NMS was 33 nmol/L, comparable to the results in previous reports under similar experimental conditions using porcine cardiac M2AChRs (14,27). Significantly, values of 78 nmol/L, 992 nmol/L, and 1670 nmol/L for the IC50 of W84 were obtained in the presence of 6.7 × 10−8, 1.33 × 10−7, and 2 × 10−7 mol/L of affinity-purified CChP-IgGs. When these results were analyzed according to Lew and Angus (22) and presented in the form of a Schild plot (Fig. 6B), the rightward shift of the curves induced by the affinity-purified CChP-IgGs was compatible with a simple competitive antagonism for the interaction of the CChP-IgGs and W84. In addition, the pKD value from the Schild analysis corresponds to the KD value of the affinity-purified CChP-IgG at its site of competitive action (el2) against W84 in the [3H]-NMS-occupied receptors. Furthermore, the right-shift of the W84 effect can readily be inferred from the dissociation curves presented in Figure 6C. In this case, W84 3 μmol/L decreased the dissociation rate of [3H]-NMS, and this effect was partially reverted with the addition of CChP-IgGs as also seen for gallamine (Fig. 4A). The inferred affinity of the purified CChP-IgGs for the allosteric site on the M2AChR el2 was approximately 60 nmol/L.
Figure 6.
Competitive behavior of M2AChRs-el2 affinity-purified human chagasic IgGs on the allosteric delay of W84 on [3H]-NMS dissociation rate. (A) Concentration effect curves for the W84 action on the Koff of the dissociation of [3H]-NMS 200 pmol/L in the absence or presence of 6.7 × 10−8, 1.3 × 10−7, and 2.0 × 10−7 mol/l of affinity-purified anti-M2 CChP-IgGs. The Koff values of the [3H]-NMS dissociations in the presence of the different W84 concentrations were expressed as percentages of the Koff of the control assays. Data points represent the means ± SEM of Koff values derived from two to six dissociation curves performed in quadruplicates. Anti-M2 CChP-IgGs purified from CChP 01, CChP-02, CChP-05, CChP-07, CChP-08, and CChP-10 were used in these experiments. (B) Schild plot derived from the data shown in (A) of the competitive effect of the affinity-purified CChP-IgGs on the W84 allosteric delay of [3H]-NMS dissociation. The line was fitted by linear regression giving a slope of 1 and the pKD (affinity of the anti-M2AChR el2 affinity-purified IgGs for the allosteric binding site) was estimated as 7.194. The Koff value under control conditions for this set of experiments was 0.01104 ± 0.003 min−1. (C), Representative dissociation curves of the specific binding of [3H]-NMS 200 pmol/L expressed as a percentage of the radioligand binding at equilibrium in the absence or presence of either affinity-purified anti-M2 CChP-IgG 2 × 10−7 mol/L or W84 3 μmol/L (refer to Materials and Methods section for more details).
DISCUSSION
The core molecular mechanisms underlying the interactions between endogenous antibodies and GPCRs remain for the most part unexplored. Data demonstrating the binding of CChP-IgGs to a specific epitope localized at the M2AChR el2 domain have been provided by several authors (8,9,28–31). These research groups have shown the partial-agonist action of the anti-M2AChR antibodies in spontaneously beating newborn rat cardiomyocyte cultures, hearts isolated by means of the Langendorff technique, and in vitro assays showing the decrease of intracellular cAMP, increase of cGMP, and impairment of L type Ca2+ currents (8,28–30,32). In some of these reports, the partial-agonist effect of the anti-M2AChR chagasic antibodies was inhibited by preincubating the CChP-IgGs with a peptide corresponding in sequence to the M2AChR-el2 (30,32,33). These later findings were in agreement with those of previous reports that mapped the predominant epitope to the region comprising the EDGE sequence of the M2AChR el2 based on immunochemical data in chronic chagasic patients (9,29). In contrast, the majority of reports proposed that the agonist-like effect of the chagasic sera resulted from antibody binding at the same site recognized by agonists and competitive antagonist, according to the law of mass action (28,34). Recent data suggest a departure from this view as the anti-M2AChR antibodies from CChP and idiopathic dilated cardiomyopathy patients seem to bind to a site different to the “primary” or orthosteric binding site (8).
As for the orthosteric binding site; mutagenesis, protein labeling, and spectroscopic studies suggest that similar mechanisms of activation operate in rhodopsin and several other cationic amine receptors, including the muscarinic acetylcholine receptor (35,36). Among the G protein-coupled receptors that bind biogenic amines, a conserved aspartate residue in the third transmembrane helix is critical for orthosteric agonist binding (37–39). Furthermore, the site-directed mutant Y403F of the M2AChR, which is located in the sixth transmembrane helix, reduced absolute agonist affinities for this receptor (40). In addition, detailed alanine scanning mutagenesis studies for the M1AChR have identified other critical residues in the third and sixth transmembrane domains for orthosteric ligand binding (39). Therefore, the “primary” agonist-binding site of rhodopsin-related GPCRs is thought to lie in the transmembrane bundle of amphipathic α-helices (35,36,39), probably inaccessible to the anti-M2AChR IgGs.
Concerning the muscarinic receptor allosteric binding site, a number of observations have suggested that allosteric ligands bind near the extracellular entrance to the ACh-binding pocket of the muscarinic receptor. This location is compatible with the nearly universal property of these ligands of delaying the kinetics of the binding of classical (orthosteric) antagonists (41,42). Leppik et al. (12) sequentially mutated the acidic amino acids of the M2AChR-el2, in which the EDGE sequence of the el2 domain was changed to the neutral LAGQ sequence. The manipulated receptor displayed an eightfold reduction in affinity for gallamine at the allosteric site, in comparison with the wild-type receptor. From a wide perspective, these and other reports show a convergence of the anti M2AChR-el2 CChP-IgG binding site (9,43,44) and the “common” allosteric binding site at the M2AChR el2 domain (11,12,45,46).
Previous data from our group support this assertion. We recently (8) documented a decrease in [3H]-NMS BMAX when saturation binding experiments were performed in the presence of CChP-IgGs purified from the same patients included in the current study. No significant change in the equilibrium dissociation constant (KD) was observed in these assays. Furthermore, CChP-IgGs failed to inhibit completely [3H]-NMS binding in competition curves (8). This equilibrium binding behavior is in agreement with a negative cooperative action of the CChP antibodies on [3H]-NMS occupied receptors (47,48) as we demonstrated in the present study with the semiquantitative allosterism assays. Moreover, the muscarinic inverse-agonist atropine blocked the CChP-IgG agonist action on L type Ca2+ currents only when preincubated with the isolated cardiomyocytes (8) and not when concomitantly added with the antibodies. In addition, results from our group (LE Giménez unpublished results) showed that M2AChR-el2 affinity-purified CChP-IgGs did not influence M2AChR internalization when measured by [3H]-NMS binding in CHO-K1 cells stably expressing these receptors as carbamylcholine does. The presence of these antibodies more likely induces an imbalance in adrenergic and muscarinic receptor input leading to cardiac muscarinic acetylcholine receptor overexpression as previously demonstrated by our group in a murine model of cardiomyopathy (7).
Regarding the effect of CChP-IgG on agonist binding, the partial agonist character of the antibodies described above does not exclude the possible allosteric nature of the CChP-IgG/M2AChR interaction because it is expected that an allosteric modulator can also act as an agonist enhancer (49). To describe the character of this CChP-IgG/M2AChR interaction, the pharmacological strategies that have become mainstream tools in the analysis of allosteric ligand-receptor interactions (11,13,14,21,47) were applied in the context of the chagasic antibodies used in this study.
In our experiments, the first indication of the allosteric nature of the interaction of CChP-IgGs with cardiac M2AChRs came from the isolated rabbit heart functional assays. We examined the effect of several CChP sera on the chronotropic response to ACh. CChP-sera mainly affected ACh action by increasing the slope to values near 2.5 (Fig. 3A), although no effect in ACh EC50 was observed. This would be an unexpected outcome if the positive cooperative effect of the CChP-IgGs on ACh action were limited to the M2AChRs (47). Regarding this, information concerning the functional consequences of the allosteric modulation of agonist action is scarce. Maass et al. (50) explored the enhancement of the NMS antimuscarinic effect by the allosteric modulator alcuronium in dose-response curves of oxotremorine-M on guinea pig atria contraction force. These authors found that in the presence of varying concentrations of alcuronium, the Arunlakshana-Schild analyses for NMS on oxotremorine-M action departed from linearity as seen for the CChP sera. However, this is not necessarily the case for agonist action in the presence of an allosteric enhancer (47). In fact, Birdsall et al. (51) assessed the functional effects (guanosine-5′-O-(3-[35S]thio) triphosphate, GTPase, cAMP, and intra-cellular Ca2+ mobilization assays) of brucine analogs on ACh action in most muscarinic receptor subtypes. These authors reported an increase in ACh potency in the presence of the allosteric enhancers with no reported effect on the slope of the curves. This reveals the complex interactions of the CChP antibodies that emerge in the context of the isolated heart preparations. Hence, a direct experimental methodology should be implemented in future studies such as intracellular cAMP level determinations in response to ACh stimulation in the presence or absence of the CChP antibodies and gallamine to determine the pharmacological behavior of the antibodies at the receptor site of action.
We also found that besides enhancing the ACh chronotropic response (Fig. 2 and Fig. 3A), the CChP sera increased the incidence of arrhythmias (Fig. 2). The allosteric nature of the interaction of CChP-IgGs with its binding site on the cardiac M2AChRs was confirmed when the CChP serum lost its effect when assayed in the presence of the allosteric modulator gallamine (Fig. 2 and Fig. 3B). Analogous results were reported by Lanzafame et al. (46), who demonstrated, by using functional experiments conducted on guinea pig atria, that gallamine, alcuronium and C7/3′-phth (the heptane homologue of W84) competitively shared the same allosteric binding site on the M2AChRs, distinct to the ACh or carbachol binding site. The specific nature of the interaction between CChP-IgG and ACh at the cardiac M2AChRs was confirmed when a lack of effect of NBD sera on ACh action was observed (Fig. 3A). These findings clearly supported the hypothesis that the CChP-IgG-receptor-ACh complexes accounted for the increase in the efficacy of the agonist on effector mechanism activation in the isolated heart.
We were also able to demonstrate the allosteric nature of the CChP-IgG/M2AChR interaction at the molecular level, reinforcing the data obtained from the functional experiments. The degree of cooperativity for the CChP-IgG interaction at the muscarinic receptors was established by using semiquantitative equilibrium assays previously implemented by Lazareno and Birdsall (21). In these assays, separate patterns of cooperative interactions between CChP-IgGs and the [3H]-NMS or the ACh (and [3H]-NMS) occupied receptors were detected (Fig. 4). When CChP-IgGs bound to the ACh occupied receptors, a more than twofold degree of positive cooperativity for the agonist binding was evidenced (Fig. 4A). On the other hand, when CChP-IgGs acted on the [3H]-NMS occupied receptors, negative cooperativity was observed (Fig. 4A). As for the NBD-IgGs, interaction with the receptor proved to be neutral for the agonist (Fig. 4B) and similarly negative cooperative for the antagonist. Thus, the CChP-IgGs acted as allosteric enhancers of ACh binding to the M2AChRs, supporting the existence of positive cooperativity in the same fashion as the brucine analogs previously described by Birdsall et al. and other authors (52,53). The specific nature of this positive cooperative effect of the CChP-IgGs on ACh binding was further demonstrated when it was blocked by preincubating the CChP-IgG with a peptide corresponding to the sequence of the M2AChR-el2 (inset of Fig. 4A).
According to the allosterism assays, the affinity of CChP-IgGs for the NMS-unoccupied receptor was highly compatible with the affinity demonstrated by the same antibodies in the [3H]-NMS equilibrium binding assays previously reported by our group (8). Furthermore, the strong negative cooperativity observed in the [3H]-NMS binding induced by CChP-IgGs when the allosteric binding sites were occupied at the higher antibody concentrations (Figs. 4A and 4B) is in line with the apparent reduction in BMAX observed in the saturation binding assays also previously reported by our group (8). In support of these observations, a strong negative cooperative effect can be indistinguishable from a noncompetitive interaction, and this explains why we previously observed a reduction in BMAX for [3H]-NMS binding despite the high affinity (allosteric) CChP-IgG binding site being occupied instead of the lower affinity orthosteric site (47,48).
Even though NBD-IgG displayed similar values of pKD for the NMS-unoccupied M2AChRs, this interaction seemed to correspond to a nonspecific interaction of the NBD antibodies to the receptor since ACh affinity was unaffected and the degree of inhibition of [3H]-NMS specific binding was smaller than 30%, as previously reported (8).
In accordance with the effect of most allosteric ligands on antagonist dissociation (24–26), we found that the CChP-IgGs decelerated the dissociation rate of [3H]-NMS in the same way as gallamine and other muscarinic allosteric modulators; but to a somewhat lesser degree. To demonstrate this effect for the CChP-IgGs, dissociation assays were performed (Fig. 5), showing that the human chagasic antibodies affected the potency of gallamine for the allosteric delay of [3H]-NMS dissociation. These observations were similar to the findings reported by Ellis and Seidenberg (45) using gallamine and obidoxime to delay the orthosteric antagonist dissociation. Therefore, on the basis of the assays depicted in Figure 5, we postulate that the CChP-IgGs competitively interact on the gallamine binding site at the M2AChR el2 domain.
This assumption was further demonstrated when we applied the experimental approach of Ellis and Seidenberg (45) to evaluate whether the affinity-purified anti-M2 CChP-IgGs antagonize the allosteric delay of the dissociation of [3H]-NMS induced by W84, in a competitive manner. This agent is an allosteric antagonist that binds to the EDGE sequence at the M2AChR el2 where gallamine also binds (14,26). The results obtained from the [3H]-NMS dissociation assays in the presence of W84 and the affinity-purified anti-M2 CChP-IgG confirmed the interaction of CChP-IgG with the allosteric site of the NMS-occupied M2AChRs with an estimated KD value of 60 nmol/L (Fig. 6B). A comparable value of KD for a monoclonal antibody raised against the N-terminal sequence VRTVE of the M2AChRs el2 (KD =15 nmol/L) (44) was previously reported. This value was obtained from the Arunlakshana-Schild analyses of the allosteric delay of W84 on the dissociation of [3H]-NMS in the presence of various concentrations of CChP-IgGs (Figs. 6A and 6B) and represents an estimate of the affinity of the CChP-IgGs for the “common” allosteric binding site at the M2AChR el2. Because of the polyclonal nature of the CChP-IgGs, it is reasonable to assume that lower affinity and nonspecific binding sites might be present in other receptor domains. This possibility accounts for the higher KD values for the CChP-IgGs and the unoccupied receptors (refer to the Results section) derived from the semiquantitative allosterism assays.
The observation of positive cooperativity between the endogenous ligand and antibodies at the cardiac M2AChRs raises the possibility that, in the chronic chagasic cardiopathy, the enhancement of the ACh response by the allosteric interaction of CChP-IgG may account for the major symptoms reported for this illness such as sinusal dysfunction and AV conduction block. In fact, these findings are strengthened by the fact that all CChP included in this study had sinus node dysfunction (17), which suggests that a pharmacological intervention on allosteric deregulation of cardiac muscarinic function by CChP-IgG might be therapeutically effective as a coadjuvant treatment of chagasic disease. In line with this assumption, Matsui et al. (54) showed that the treatment with AF-DX 116, a specific M2 receptor allosteric antagonist, had a protective effect on experimental dilated cardiomyopathy induced in rabbits immunized with an M2AChRs el2 peptide. Taken together, the results presented in this study show for the first time the occurrence of specific allosteric interactions between the chagasic antibodies and the cardiac M2 muscarinic acetylcholine receptors and highlight the functional implications of the antibody allosteric modulation of endogenous agonist action.
Acknowledgments
This work was supported by grants from: Conselho Nacional de Desenvolvimento Científico e Tecnológico (PRONEX-CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), and NIH (HL.073732-01). We thank Dr. Roberto C Pedrosa for supplying the CChP and NBD sera, as well as Mrs. Daisy Avanzi and Mr. Rui Manuel Domingues for technical assistance.
References
- 1.Schmunis GA. Chagas' Disease and the Nervous System Scientific publication. Vol. 547. Washington, DC: Pan- American Health Organization; 1994. American Tripanosomiasis as a public health problem; pp. 3–31. [Google Scholar]
- 2.Calabrese F, Thiene G. Myocarditis and inflammatory cardiomyopathy: Microbiological and molecular biological aspects. Cardiovasc Res. 2003;60:11– 25. doi: 10.1016/s0008-6363(03)00475-9. [DOI] [PubMed] [Google Scholar]
- 3.Mason JW. Myocarditis and dilated cardiomyopathy: An inflammatory link. Cardiovasc Res. 2003;60:5–10. doi: 10.1016/s0008-6363(03)00437-1. [DOI] [PubMed] [Google Scholar]
- 4.Chiale PA, Ferrari I, Mahler E, Vallazza MA, Elizari MV, Rosenbaum MB, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–1771. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- 5.Matsui S, Fu ML. Myocardial injury due to G-protein coupled receptor- autoimmunity. Jpn Heart J. 1998;39:261–274. doi: 10.1536/ihj.39.261. [DOI] [PubMed] [Google Scholar]
- 6.Kierszenbaum F. Views on the autoimmunity hypothesis for Chagas disease pathogenesis. FEMS Immunol Med Microbiol. 2003;37:1–11. doi: 10.1016/S0928-8244(03)00097-X. [DOI] [PubMed] [Google Scholar]
- 7.Gimenez LE, Hernandez CC, Mattos EC, Brandao IT, Olivieri B, Campelo RP, et al. DNA immunizations with M2 muscarinic and β1 adrenergic receptor coding plasmids impair cardiac function in mice. J Mol Cell Cardiol. 2005;38:703–714. doi: 10.1016/j.yjmcc.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez CC, Barcellos LC, Gimenez LE, Cabarcas RA, Garcia S, Pedrosa RC, et al. Human chagasic IgGs bind to cardiac muscarinic receptors and impair L-type Ca2+ currents. Cardiovasc Res. 2003;58:55–65. doi: 10.1016/s0008-6363(02)00811-8. [DOI] [PubMed] [Google Scholar]
- 9.Elies R, Ferrari I, Wallukat G, Lebesgue D, Chiale P, Elizari M, et al. Structural and functional analysis of the B cell epitopes recognized by anti-receptor autoantibodies in patients with Chagas’ disease. J Immunol. 1996;157:4203–4211. [PubMed] [Google Scholar]
- 10.Ferrari I, Levin MJ, Wallukat G, Elies R, Lebesgue D, Chiale P, et al. Molecular mimicry between the immunodominant ribosomal protein P0 of Trypanosoma cruzi and a functional epitope on the human beta 1-adrenergic receptor. J Exp Med. 1995;182:59–65. doi: 10.1084/jem.182.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christopoulos A, Lanzafame A, Mitchelson F. Allosteric interactions at muscarinic cholinoceptors. Clin Exp Pharmacol Physiol. 1998;25:185–194. doi: 10.1111/j.1440-1681.1998.t01-4-.x. [DOI] [PubMed] [Google Scholar]
- 12.Leppik RA, Miller RC, Eck M, Paquet JL. Role of acidic amino acids in the allosteric modulation by gallamine of antagonist binding at the m2 muscarinic acetylcholine receptor. Mol Pharmacol. 1994;45:983–990. [PubMed] [Google Scholar]
- 13.Lazareno S, Birdsall NJ. Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: Interactions of strychnine and acetylcholine at muscarinic receptors. Mol Pharmacol. 1995;48:362–378. [PubMed] [Google Scholar]
- 14.Trankle C, Mohr K. Divergent modes of action among cationic allosteric modulators of muscarinic M2 receptors. Mol Pharmacol. 1997;51:674–682. doi: 10.1124/mol.51.4.674. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa R, Carrasco HA, Belandria F, Fuenmayor AM, Molina C, Gonzalez R, et al. Life expectancy analysis in patients with Chagas’ disease: prognosis after one decade (1973–1983) Int J Cardiol. 1985;8:45–56. doi: 10.1016/0167-5273(85)90262-1. [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira SF, Pedrosa RC, Nascimento JH, Campos de Carvalho AC, Masuda MO. Sera from chronic chagasic patients with complex cardiac arrhythmias depress electrogenesis and conduction in isolated rabbit hearts. Circulation. 1997;96:2031–2037. doi: 10.1161/01.cir.96.6.2031. [DOI] [PubMed] [Google Scholar]
- 17.Costa PC, Fortes FS, Machado AB, Almeida NA, Olivares EL, Cabral PR, et al. Sera from chronic chagasic patients depress cardiac electrogenesis and conduction. Braz J Med Biol Res. 2000;33:439–446. doi: 10.1590/s0100-879x2000000400010. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–374. [PMC free article] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.World Medical Association, American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 21.Lazareno S, Birdsall N. Measurement of competitive and allosteric interactions in radioligand binding studies. In: Haga T, Berstein G, editors. G Protein-Coupled Receptors. Boca Raton: CRC Press; 1999. pp. 1–48. [Google Scholar]
- 22.Lew MJ, Angus JA. Analysis of competitive agonist-antagonist interactions by nonlinear regression. Trends Pharmacol Sci. 1995;16:328–337. doi: 10.1016/s0165-6147(00)89066-5. [DOI] [PubMed] [Google Scholar]
- 23.Clark AL, Mitchelson F. The inhibitory effect of gallamine on muscarinic receptors. Br J Pharmacol. 1976;58:323–331. doi: 10.1111/j.1476-5381.1976.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee NH, el-Fakahany EE. Allosteric antagonists of the muscarinic acetylcholine receptor. Biochem Pharmacol. 1991;42:199–205. doi: 10.1016/0006-2952(91)90703-8. [DOI] [PubMed] [Google Scholar]
- 25.Ellis J, Huyler J, Brann MR. Allosteric regulation of cloned m1-m5 muscarinic receptor subtypes. Biochem Pharmacol. 1991;42:1927–1932. doi: 10.1016/0006-2952(91)90591-r. [DOI] [PubMed] [Google Scholar]
- 26.Trankle C, Kostenis E, Burgmer U, Mohr K. Search for lead structures to develop new allosteric modulators of muscarinic receptors. J Pharmacol Exp Ther. 1996;279:926–933. [PubMed] [Google Scholar]
- 27.Burgmer U, Schulz U, Trankle C, Mohr K. Interaction of Mg2+ with the allosteric site of muscarinic M2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:363–370. doi: 10.1007/pl00005180. [DOI] [PubMed] [Google Scholar]
- 28.Goin JC, Borda E, Leiros CP, Storino R, Sterin-Borda L. Identification of antibodies with muscarinic cholinergic activity in human Chagas’ disease: pathological implications. J Auton Nerv Syst. 1994;47:45–52. doi: 10.1016/0165-1838(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 29.Goin JC, Leiros CP, Borda E, Sterin-Borda L. Interaction of human chagasic IgG with the second extracellular loop of the human heart muscarinic acetylcholine receptor: Functional and pathological implications. FASEB J. 1997;11:77–83. doi: 10.1096/fasebj.11.1.9034169. [DOI] [PubMed] [Google Scholar]
- 30.Masuda MO, Levin M, De Oliveira SF, Dos Santos Costa PC, Bergami PL, Dos Santos Almeida NA, et al. Functionally active cardiac antibodies in chronic Chagas’ disease are specifically blocked by Trypanosoma cruzi antigens. FASEB J. 1998;12:1551–1558. doi: 10.1096/fasebj.12.14.1551. [DOI] [PubMed] [Google Scholar]
- 31.Wallukat G, Nissen E, Morwinski R, Muller J. Autoantibodies against the beta- and muscarinic receptors in cardiomyopathy. Herz. 2000;25:261–266. doi: 10.1007/s000590050017. [DOI] [PubMed] [Google Scholar]
- 32.Goin JC, Borda ES, Auger S, Storino R, Sterin-Borda L. Cardiac M(2) muscarinic cholinoceptor activation by human chagasic autoantibodies: Association with bradycardia. Heart. 1999;82:273–278. doi: 10.1136/hrt.82.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterin-Borda L, Joensen L, Bayo-Hanza C, Esteva M, Borda E. Therapeutic use of muscarinic acetylcholine receptor peptide to prevent mice chagasic cardiac dysfunction. J Mol Cell Cardiol. 2002;34:1645–1654. doi: 10.1006/jmcc.2002.2114. [DOI] [PubMed] [Google Scholar]
- 34.Sterin-Borda L, Gorelik G, Borda ES. Chagasic IgG binding with cardiac muscarinic cholinergic receptors modifies cholinergic-mediated cellular transmembrane signals. Clin Immunol Immunopathol. 1991;61:387–397. doi: 10.1016/s0090-1229(05)80010-8. [DOI] [PubMed] [Google Scholar]
- 35.Gether U. Uncovering molecular mechanisms involved in activation of G protein- coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 36.Mirzadegan T, Benko G, Filipek S, Palczewski K. Sequence analyses of G- protein-coupled receptors: Similarities to rhodopsin. Biochemistry. 2003;42:2759– 2767. doi: 10.1021/bi027224+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser CM, Wang CD, Robinson DA, Gocayne JD, Venter JC. Site-directed mutagenesis of m1 muscarinic acetylcholine receptors: conserved aspartic acids play important roles in receptor function. Mol Pharmacol. 1989;36:840–847. [PubMed] [Google Scholar]
- 38.Page KM, Curtis CA, Jones PG, Hulme EC. The functional role of the binding site aspartate in muscarinic acetylcholine receptors, probed by site-directed mutagenesis. Eur J Pharmacol. 1995;289:429–437. doi: 10.1016/0922-4106(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 39.Hulme EC, Lu ZL, Saldanha JW, Bee MS. Structure and activation of muscarinic acetylcholine receptors. Biochem Soc Trans. 2003;31:29–34. doi: 10.1042/bst0310029. [DOI] [PubMed] [Google Scholar]
- 40.Vogel WK, Sheehan DM, Schimerlik MI. Site-directed mutagenesis on the m2 muscarinic acetylcholine receptor: The significance of Tyr403 in the binding of agonists and functional coupling. Mol Pharmacol. 1997;52:1087–1094. doi: 10.1124/mol.52.6.1087. [DOI] [PubMed] [Google Scholar]
- 41.Stockton JM, Birdsall NJ, Burgen AS, Hulme EC. Modification of the binding properties of muscarinic receptors by gallamine. Mol Pharmacol. 1983;23:551– 557. [PubMed] [Google Scholar]
- 42.Proska J, Tucek S. Mechanisms of steric and cooperative actions of alcuronium on cardiac muscarinic acetylcholine receptors. Mol Pharmacol. 1994;45:709–717. [PubMed] [Google Scholar]
- 43.Krejci A, Tucek S. Changes of cooperativity between N-methylscopolamine and allosteric modulators alcuronium and gallamine induced by mutations of external loops of muscarinic M(3) receptors. Mol Pharmacol. 2001;60:761–767. [PubMed] [Google Scholar]
- 44.Elies R, Fu LX, Eftekhari P, Wallukat G, Schulze W, Granier C, et al. Immunochemical and functional characterization of an agonist-like monoclonal antibody against the M2 acetylcholine receptor. Eur J Biochem. 1998;251:659–666. doi: 10.1046/j.1432-1327.1998.2510659.x. [DOI] [PubMed] [Google Scholar]
- 45.Ellis J, Seidenberg M. Two allosteric modulators interact at a common site on cardiac muscarinic receptors. Mol Pharmacol. 1992;42:638–641. [PubMed] [Google Scholar]
- 46.Lanzafame A, Christopoulos A, Mitchelson F. Three allosteric modulators act at a common site, distinct from that of competitive antagonist, at muscarinic acetylcholine M2 receptors. J Pharmacol Exp Ther. 1997;282:278– 285. [PubMed] [Google Scholar]
- 47.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 48.Birdsall NJ, Lazareno S, Popham A, Saldanha J. Multiple allosteric sites on muscarinic receptors. Life Sci. 2001;68:2517–2524. doi: 10.1016/s0024-3205(01)01047-5. [DOI] [PubMed] [Google Scholar]
- 49.May LT, Christopoulos A. Allosteric modulators of G-protein-coupled receptors. Curr Opin Pharmacol. 2003;3:551–556. doi: 10.1016/s1471-4892(03)00107-3. [DOI] [PubMed] [Google Scholar]
- 50.Maass A, Kostenis E, Mohr K. Potentiation by alcuronium of the antimuscarinic effect of N-methylscopolamine in guinea pig left atria. Eur J Pharmacol. 1995;272:103–106. doi: 10.1016/0014-2999(94)00664-s. [DOI] [PubMed] [Google Scholar]
- 51.Birdsall NJ, Farries T, Gharagozloo P, Kobayashi S, Lazareno S, Sugimoto M. Subtype-selective positive cooperative interactions between brucine analogs and acetylcholine at muscarinic receptors: functional studies. Mol Pharmacol. 1999;55:778–786. [PubMed] [Google Scholar]
- 52.Birdsall NJ, Farries T, Gharagozloo P, Kobayashi S, Kuonen D, Lazareno S, et al. Selective allosteric enhancement of the binding and actions of acetylcholine at muscarinic receptor subtypes. Life Sci. 1997;60:1047–1052. doi: 10.1016/s0024-3205(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 53.Jakubik J, Bacakova L, El-Fakahany EE, Tucek S. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol Pharmacol. 1997;52:172–179. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- 54.Matsui S, Fu ML, Hayase M, Katsuda S, Yamaguchi N, Teraoka K, et al. Beneficial effect of muscarinic-2 antagonist on dilated cardiomyopathy induced by autoimmune mechanism against muscarinic-2 receptor. J Cardiovasc Pharmacol. 2001;38:S43–49. doi: 10.1097/00005344-200110001-00010. [DOI] [PubMed] [Google Scholar]