Potent marine neurotoxins known as brevetoxins are produced by the ‘red tide’ dinoflagellate Karenia brevis. They kill large numbers of fish and cause illness in humans who ingest toxic filter-feeding shellfish or inhale toxic aerosols1. The toxins are also suspected of having been involved in events in which many manatees and dolphins died, but this has usually not been verified owing to limited confirmation of toxin exposure, unexplained intoxication mechanisms and complicating pathologies2–4. Here we show that fish and seagrass can accumulate high concentrations of brevetoxins and that these have acted as toxin vectors during recent deaths of dolphins and manatees, respectively. Our results challenge claims that the deleterious effects of a brevetoxin on fish (ichthyotoxicity) preclude its accumulation in live fish, and they reveal a new vector mechanism for brevetoxin spread through food webs that poses a threat to upper trophic levels.
In the spring of 2002, 34 endangered Florida manatees (Trichechus manatus latirostris) died in southwest Florida, and 107 bottlenose dolphins (Tursiops truncatus) died in waters off the Florida panhandle in the spring of 2004. In both of these unusual mortality events, extensive water surveys revealed that only low concentrations of K. brevis were present.
We tested for the presence of brevetoxin in the fluids and tissues of 63 of these animals (27 manatees, 36 dolphins) and found very high concentrations in the tissues of all of them (see supplementary information), confirming that the animals must have been exposed to brevetoxin. In a previous event, in which 149 manatees died, lung pathology indicated that brevetoxins had been inhaled5. In our examples, the absence of similar pathology excluded the possibility of poisoning through aerosol exposure, and the high toxin concentrations measured in the stomach contents indicated that the toxin was from a dietary source.
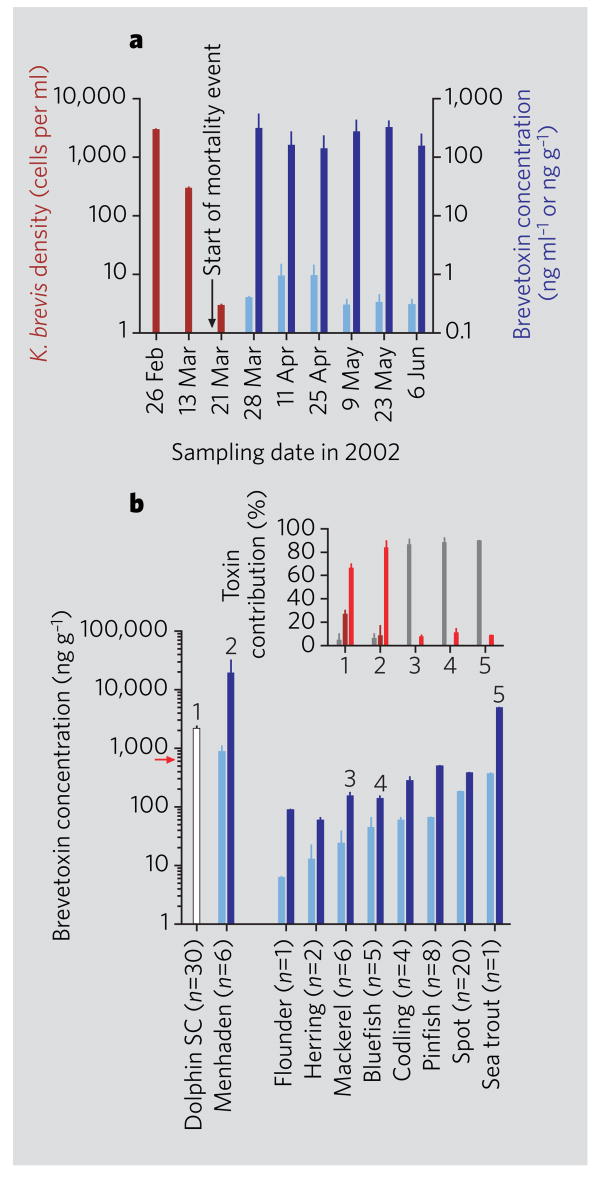
Manatee stomach contents were composed exclusively of seagrass; filter-feeding tunicates, which were suspected vectors in a 1982 mortality event3, were notably absent. Analysis of seagrass (Thalassia testudinum) collected at several locations in the area of death revealed high concentrations of brevetoxins (Fig. 1a), mainly in the epiphytic fraction (epiphytes, 83% of total brevetoxins; blades, 7%; rhizomes, 10%). The accumulation mechanism could involve active uptake or passive adsorption of the toxin. As the red tide that previously affected the area had almost dissipated by the start of the mortality event (Fig. 1a), the comparable toxin concentrations in manatee stomach contents and in the seagrass beds (up to 1,136 and 1,263 ng brevetoxin per g, respectively) indicated that seagrass was the primary source of brevetoxin for the manatees.
Figure 1. Brevetoxin concentrations in seagrass and fish during mass-mortality events.
a, Density of red-tide algae Karenia brevis (red bars) and brevetoxin concentrations in seagrass (Thalassia testudinum) (dark blue bars; ng g−1) and in sea water (light blue bars; ng ml−1), collected during and after the 2002 manatee mortality event in Charlotte Harbor, Florida. Error bars, standard deviation between samples collected from four sites. b, Brevetoxin concentrations in dolphin stomach contents (SC), in undigested menhaden, and in fish collected live (light blue, in muscle; dark blue, in viscera) (flounder, Paralichthys lethostigma; herring, Opisthonema oglinum; mackerel, Scomberomorus maculatus; bluefish, Pomatomus saltatrix; codling, Urophycis floridana; pinfish, Lagodon rhomboides; spot, Leiostomus xanthurus; and sea trout, Cynoscion nebulosus) from St Joseph Bay, Florida, in spring 2004. Red arrow, regulation limit for brevetoxin in shellfish. Error bars, standard deviation between individual fish, except for pinfish and spot (pooled). Inset, toxins identified by liquid chromatography and mass spectroscopy in selected samples, as numbered in the main bar chart; those in dolphin stomach contents and in menhaden (1, 2) differed from the profile found in fish collected live two weeks after the onset of the mortality (3–5). Bars: dark red, brevetoxin-2; light red, brevetoxin-3; grey, brevetoxin-2 disulphide metabolite. For methods, see supplementary information.
An extensive pathological, pathogenic and environmental investigation conducted in response to the dolphin mortalities failed to identify any consistent mortality factor other than brevetoxin6. Although no K. brevis was evident at the time, the contents of the dolphins' stomachs were acutely toxic. Stomachs were full and menhaden (Brevoortia spp.), a type of plankton-eating fish, were identified as the dominant prey in 50% of the 28 animals examined. Surprisingly, there was a high level of brevetoxin contamination in all undigested menhaden tested and, to a lesser extent, in all fish that were collected live two weeks after the onset of the dolphin deaths (Fig. 1b).
Until now, it was uncertain whether live fish could accumulate and transfer brevetoxins to upper trophic levels, as brevetoxins kill fish even at low concentrations7 and typically result in high fish mortalities during red tides1. To determine how brevetoxins might accumulate in fish, we exposed omnivorous and planktivorous fish to toxic shellfish (which retain brevetoxins after blooms have dissipated1) and to bloom concentrations of healthy K. brevis cultures with low extracellular toxin concentrations (as sometimes observed during red tides8), respectively. We found that brevetoxin accumulates in both types of feeder (results not shown). Because brevetoxins are sequestered in their food (shellfish and K. brevis cells), the fish remained healthy while brevetoxin concentrations increased in their tissues (up to 2,675 ng g−1 in the viscera and 1,540 ng g−1 in the muscle of omnivorous fish exposed for two weeks to toxin-containing clams).
Brevetoxin poisoning in humans has so far been restricted to the consumption of contaminated shellfish (neurotoxic shellfish poisoning). Although the accumulation of brevetoxins in live fish to the levels measured in the menhaden is probably short-lived and unusual, this finding, together with the dolphin deaths (given that dolphins are a sentinel species9), raises concerns that humans could also be poisoned by contaminated fish.
These findings show not only that brevetoxin-contaminated food webs pose a threat to marine mammals, but also that toxin vectors can result in delayed or remote animal exposure. Biological toxins should therefore be considered as possible culprits when investigating unusual marine animal mortalities, even in the absence of toxin-producing algae.
Florida manatees (3 metres long, on average) are susceptible to toxins from the red tide alga Karenia brevis (inset; cell diameter, 30-35 mm).

Footnotes
Supplementary information accompanies this communication on Nature's website.
Competing financial interests: declared none.
References
- 1.Steidinger KA. In: Algal Toxins in Seafood and Drinking Water. Falconer I, editor. Academic; London: 1993. pp. 1–28. [Google Scholar]
- 2.Geraci JR. Report to the National Marine Fisheries Service and US Navy, Office of Naval Research and Marine Mammal Commission. Ontario Veterinary College; Guelph, Ontario: 1989. Clinical Investigation of the 1987-1988 Mass Mortality of Bottlenose Dolphins along the US Central and South Atlantic Coast. [Google Scholar]
- 3.O'Shea TJ, Bonde RK, Buergelt CD, Odell DK. Mar Mamm Sci. 1991;7:165–179. [Google Scholar]
- 4.Van Dolah FM, Doucette GJ, Gulland FMD, Rowles TL, Bossart GD. In: Toxicology of Marine Mammals. Vos JG, Bossart GD, Fournier M, O'Shea TJ, editors. Taylor & Francis; New York: 2003. pp. 247–269. [Google Scholar]
- 5.Bossart GD, Baden DG, Ewing RY, Roberts B, Wright SD. Toxicol Pathol. 1998;26:276–282. doi: 10.1177/019262339802600214. [DOI] [PubMed] [Google Scholar]
- 6.Working Group on Marine Mammal Unusual Mortality Events. The Bottlenose Dolphin (Tursiops truncatus): Unusual Mortality Event along the Panhandle of Florida March-April 2004. National Marine Fisheries Service; 2004. [Google Scholar]
- 7.Baden DG, Mende TJ. Toxicon. 1982;20:457–461. doi: 10.1016/0041-0101(82)90009-5. [DOI] [PubMed] [Google Scholar]
- 8.Pierce RH, Henry MS, Blum P, Payne S. In: Harmful Algal Blooms 2000. Hallegraeff GM, et al., editors. Intergovernmental Oceanic Commission; Paris: 2001. pp. 421–424. [Google Scholar]
- 9.Ross PS. Hum Ecol Risk Ass. 2000;6:29–46. [Google Scholar]