Summary
ADP-ribosylation factors (ARFs) are small (21 kDa), monomeric GTPases that are important regulators of membrane traffic. When membrane bound, they recruit soluble adaptors to membranes and trigger the assembly of coating complexes involved in cargo selection and vesicular budding. N-myristoylation is a conserved feature of all ARF proteins that is required for its biological functions, though the mechanism(s) by which the myristate acts in ARF functions is not fully understood. Here, we present the first structure of a myristoylated ARF1 protein, determined by solution NMR methods, and an assessment of the influence of myristoylation on association of ARF1·GDP and ARF1·GTP with lipid bilayers. A model in which myristoylation contributes to both the regulation of guanine nucleotide exchange and stable membrane association is supported.
Introduction
ARFs are ∼20 kDa GTPases that have been very highly conserved throughout eukaryotic evolution, are ubiquitously expressed in eukaryotic organisms, and play essential roles in the regulation of membrane traffic (Gillingham and Munro, 2007; Nie et al., 2003; Donaldson et al., 2005). ARFs interact with a number of proteins at membrane surfaces, including guanine nucleotide exchange factors (GEFs) that facilitate GDP for GTP exchange (Peyroche et al., 1996; Chardin et al., 1996), GTPase activating proteins (GAPs) that impart GTPase activity (Cukieman et al., 1995; Inoue and Randazzo, 2007), enzymes involved in lipid metabolism (Brown et al., 1993; Godi et al., 2004; Godi et al., 1999), and the protein adaptors that aid in the recruitment of specific cargos (Serafini, et al., 1991; Bonifacino, 2004; Styers and Faundez, 2003; Hill et al., 2003). A central aspect of ARF-dependent cell regulation is their cycling between the predominantly cytosolic GDP-bound and the membrane-associated GTP-bound forms. Alterations in the exposure of both an N-terminal myristoyl group and an N-terminal amphipathic helix upon nucleotide exchange have been postulated to play roles in membrane association and dissociation. However, confirmation of these changes through structural investigations has been difficult because recombinant protein preparations available in the required quantities lack the N-terminal myristoyl group, and in some cases, the critical N-terminal amino acids as well. Because the presence of activated, membrane-associated ARF is viewed as the critical initiator of vesicle biogenesis, the molecular details of the processes of membrane association and activation are essential to the generation of models of membrane traffic.
Early models of ARF-membrane interaction postulated that the exchange of GTP for GDP caused exposure of the myristoyl group and that myristoyl-lipid interaction subsequently recruited ARF to the membrane (Helms et al., 1993; Tanigawa et al., 1993). This is similar to the calcium-switch mechanism postulated for recoverin (Zozulaya et al., 1992). However, other data cast doubts on this simple model. First, non-myristoylated ARF was shown to be capable of stable membrane interaction through the N-terminus, raising questions regarding the function of the highly conserved myristoyl group (Franco et al., 1993). Second, a high concentration of phospholipids is normally required for the guanine nucleotide exchange, as catalyzed by ARF GEFs (Franco et al., 1996). This suggests that the membrane surface may actually provide a platform essential for the exchange reaction. This suggestion is consistent with the observations that the rate of dissociation of the tightly bound GDP is increased in the presence of lipids, detergents, or membranes (Weiss et al., 1989). Together, these observations open the possibility that myristoyl insertion into the membrane is not a simple consequence of guanine nucleotide exchange but that it may play earlier roles in the overall processes of membrane association, ARF activation, and recruitment of GEFs, GAPs, and effectors.
Crystal structures exist for GDP-bound forms of full-length human ARFs lacking myristate and GTP-loaded forms of ARFs lacking the N-terminal 17 residues (ARF1Δ17) as well as myristate (Amor et al., 1994; Goldberg, 1998). ARF1Δ17 has improved solubility and is able to undergo efficient guanine nucleotide exchange in the absence of a GEF without the requirements for phospholipids or detergents (Kahn et al., 1992). These features facilitated the later structural study, which together with the earlier one, provided significant insight into the mechanisms of guanine nucleotide exchange and hydrolysis. However, truncation of the N-terminus introduces ambiguity because structures thus obtained may carry artifacts resulting from the N-terminal deletion (Seidel III et al., 2004). Previously we have shown that substantial structural discrepancies exist between ARF1·GDP and ARF1Δ17·GDP, and thus it is necessary to deconvolute such differences from other sources of structural changes, such as those from nucleotide exchange (Seidel III et al., 2004). Moreover, the lack of the N-terminal residues and the myristoyl group precludes investigation of the role of myristoylation and the N-terminal amphiphilic region in events such as membrane association/dissociation, nucleotide exchange and GTP hydrolysis. Clearly, extending studies to a myristoylated form of ARF in a relatively native lipid-containing environment promises to yield novel and significant understanding of the biological activities of ARF.
Changes in solubility and crystallization properties upon myristoylation have posed difficulties for structural determination by both X-ray crystallography and NMR. Multidimensional heteronuclear NMR, and more recent experimental developments such as Transverse Relaxation Optimized Spectroscopy (TROSY) (Pervushin et al., 1997), Residual Dipolar Coupling (RDC) measurement (Tolman et al., 1995; Tjandra and Bax, 1997), and methyl-protonation with background perdeuteration of samples (Rosen et al., 1996), have greatly improved the prospects for structural and dynamic studies on large proteins and membrane-proteins by NMR. NMR has, in fact, been used to study myristoylated proteins, but only in a few cases (Tanaka et al., 1995; Tang et al., 2004). Prerequisite to such studies is actual production of a myristoylated protein. In this study yeast Saccharomyces cerevisiae ARF1 (yARF1) was adopted due to its high myristoylation efficiency when co-expressed in bacteria with an N-myristoyltransferase (Randazzo and Kahn, 1995). Human and yeast ARF1 share >74% identity, and the fact that human protein can complement the deletion of yARF1 is indicative of close functional and structural conservation despite the evolutionary distance between these organisms (Kahn et al., 1991; Logsdon and Kahn, 2004). Here these favorable properties have been used to determine a solution structure of the myristoylated form of yARF1·GDP along with data on the interaction of both the GDP-bound and GTP-bound forms with membrane mimetics. The structure clearly places the myristoyl chain in a hydrophobic groove between a C-terminal helix and a loop connecting β-strands 3 and 4. The positioning suggests a clash with this loop would occur as it exists in the GTP-bound form, a fact that may require membrane association to accommodate an expelled myristoyl chain before GDP exchange can occur. The data on interaction with membrane mimetics show that myristoylation enhances membrane interactions of both GDP and GTP forms of yARF1. The interactions of the GDP form are weaker, but they also display an interesting preference for interaction with more extended membrane structures that may be of functional significance.
Results and Discussion
Structure determination
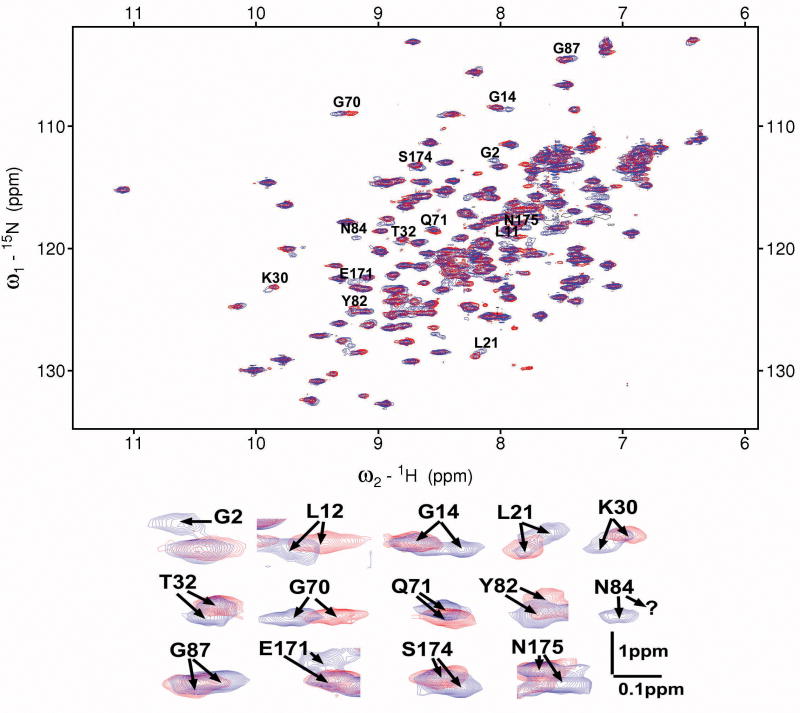
Yeast ARF1 was expressed in bacteria either alone (myr-) or co-expressed with the yeast N-myristoyltransferase NMT1, to generate the acylated (myr+) protein. The extensive similarity between the HSQC spectra of myr(+) and myr(−)-yARF1·GDP indicates that myristoylation does not extensively alter the global fold of yARF1 (Figure 1). However, specific changes in positions or intensities of resonances from sites throughout the protein suggest that effects of myristoylation on structure do occur and these may have biological consequences. Some of the affected residues are near the N-terminus where anticipated, e.g., G2 is invisible in myr(−)-yARF1 but shows up in myr(+)-yARF1 due to the quenched amine proton exchange rate upon myristoyl conjugation. Other perturbed N-terminal residues include L12 and G14. Perturbed residues more C-terminal in the sequence include those traditionally associated with functionally important parts of the protein, including switch II (G70, Q71, and Y82), the nucleotide binding site (K30 and T32), and the C-terminal helix (E171, S174, and N175).
Figure 1.
15N-1H HSQC spectra of myr(+)- and myr(−) – yARF1·GDP (blue and red respectively). Blown-up views of chemical shift perturbed residues are displayed on the bottom.
Numerous perturbed resonances also display some level of heterogeneity. These include G14, L21, K30, T32, E171, S174, and N175, where two sets of resonances are observed upon acylation. One of the members of the pairs is usually minimally perturbed from that of myr(−) and the other is shifted substantially (Figure 1). This could result from partial N-myristoylation of the yARF1 in bacteria. Closer examination by electro spray ionization mass spectrometry and fatty acid analysis revealed that greater than 90% of the purified protein is acylated, but only ∼50% is myristoylated while the remaining ∼40-50% is lauroylated. The presence of covalently attached laurate on yARF1 is presumably caused by the broader specificities for fatty acids (Kishore et al., 1993) of yeast or human NMT1 when expressed in bacteria and differences in the levels of acyl-coA substrates among organisms. Incorporation of acyl chains other than myristate into ARF proteins co-expressed in bacteria with NMT has been reported previously (Randazzo and Kahn, 1995), though in that report the diversity of acyl chain lengths was much greater. Because lauroyl-ARF1 differs from the physiologically relevant myristoyl-ARF1 by only two carbons, and the acyl chain is relatively unstructured (see below) we believe it is unlikely the heterogeneity in acyl group compromises conclusions drawn. Nevertheless, in an effort to resolve the effects of the different acyl groups an isotope labeling strategy was employed in which myristate and laurate were isotope-labeled differently and X-filtered NMR experiments were applied to help suppress the N-lauroyl-yARF1 signals (see “EXPERIMENTAL PROCEDSURES”).
Several types of structural data were acquired (Table I) including distance restraints between (i) amide protons, (ii) amide and a number of side-chain methylene/methyl protons, (iii) sets of methyl protons, (iv) amide and myristoyl protons, and (v) methyl and myristoyl protons. Residual Dipolar Couplings (RDCs) were collected from a compressed charged gel and a stretched neutral gel which yielded two uncorrelated alignment conditions and therefore two sets of complementary RDC data. The presence of bound GDP is confirmed from 6 assigned NOEs between the protein amides and the labile HN1 proton of the guanine base (other GDP protons are invisible due to perdeuteration or fast solvent exchange). Given the consistency of these NOEs with what is expected from the crystal structures of ARF·GDP, conserved hydrogen bonds between ARF and GDP observed in the crystal structures were utilized to help restrain the nucleotide during structure calculations. The structure was solved applying these restraints in a simulated annealing protocol run under the program CNS (Brünger et al., 1998).
Table I.
Statistics of structure calculations based on 15 accepted structures
| Total NOEs observed | 1398 |
| Total nonredudent NOEs | 830 |
| intraresidue (i=j) | 182 |
| sequential (|i-j|=1) | 272 |
| medium-range (1<|i-j|<5) | 146 |
| long-range (|i-j|>4) | 173 |
| ambiguous | 57 |
| ARF-myristoyl NOEs | 14 |
| ARF-GDP NOEs | 6 |
| Dihedral angle restraints | |
| Φ | 101 |
| ψ | 100 |
| Residual Dipolar Couplings * | |
| HN (alignment 1 + alignment 2) | 108 + 99 |
| NC′ (alignment 1) | 58 |
| HNC′ (alignment 1) | 57 |
| Hydrogen bond restraints | 180 |
| RMSD of distance violations (maximum) (Å) | 0.0136 ± 0.0005 (0.129 ± 0.017) |
| RMSD of dihedral angle violations (maximum) (deg) | 0.371 ± 0.026 (2.05 ± 0.58) |
| RMSD of RDC violations (Hz) / Maximum of RDC (Hz) | |
| HN (alignment 1) | 1.49 ± 0.06 / 22.45 ± 0.13 |
| HN (alignment 2) | 1.82 ± 0.03 / 20.31 ± 0.14 |
| NC′ (alignment 1) | 0.26 ± 0.02 / 2.72 ± 0.02 |
| HNC′ (alignment 1) | 0.77 ± 0.02 / 7.12 ± 0.04 |
| RMSD of covalent geometry | |
| bond lengths (Å) | 0.0017 ± 0.00003 |
| bond angles (deg) | 0.3537 ± 0.0035 |
| impropers (deg) | 0.2872 ± 0.0104 |
| Ramachandran plot statistics (%) | Residues (18-71, 77-178) |
| Most favored | 91.4 |
| Additionally allowed | 5.7 |
| Generously allowed | 0 |
| Disallowed | 2.9 |
| RMSD for NMR ensemble (Å) | Residues (18-71, 77-178) |
| Backbone | 0.762 |
| Heavy atoms | 1.391 |
HN RDCs were collected for two highly uncorrelated alignments. Alignment 1 is by negatively charged compressed gel and alignment 2 by neutral stretched gel. See “Methods” section for details.
Structure of myr(+)-yARF1·GDP
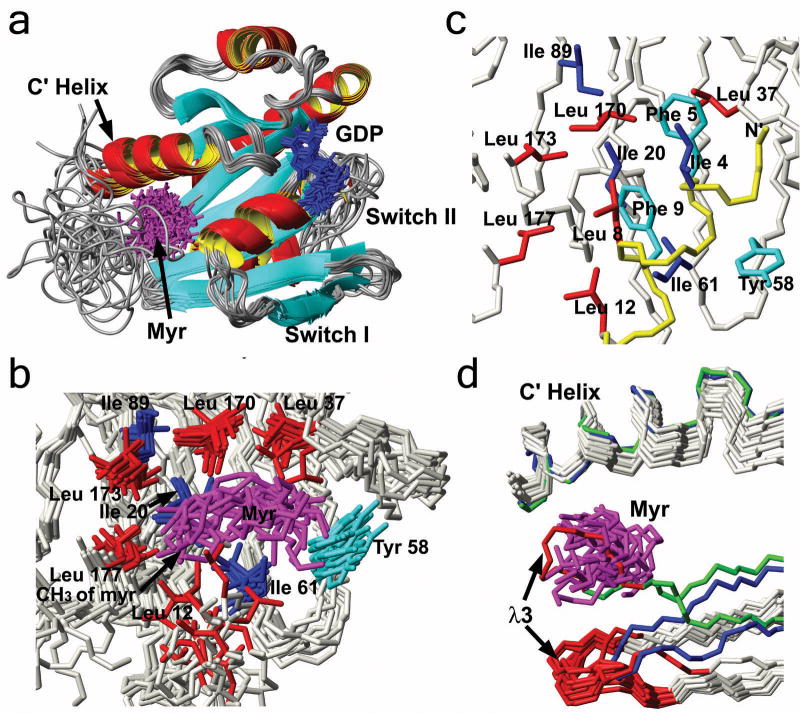
Myristoylated yARF1·GDP in solution adopts an overall fold that is very similar to the non-myristoylated ARF proteins whose crystal structures have previously been reported (Amor et al., 1994; Amor et al., 2001). A superposition of 15 of the lowest energy structures is depicted in Figure 2A (a stereo view of the superimposed structures is given as Supplemental Data). The N-terminus (G2-N15) and part of Switch II (D72-S76) are not well defined because a majority of residues in these regions lack detectable NMR signals, presumably due to the presence of multiple conformational states interconverting on the μs-ms time scale.
Figure 2.
Structure of myristoylated yARF1 and comparisons to related structures. a. Overlap of 15 accepted myr(+)-yARF1·GDP structures out of 100 trials. GDP is shown in blue and myristoyl in purple. b. The myristoyl binding pocket with leucines shown in red, isoleucines in blue, tyrosine in cyan and myristoyl in purple. c. The myristoyl binding pocket lies under the N-terminal amphiphilic helix in the myr(−)-hARF·GDP structure (PDB:1hur). d. The inter-strand loop λ3 clashes with the myristoyl chain in the GTP-bound conformation but not in the GDP-bound conformation. Backbone of myr(+)-yARF1·GDP is shown in ivory, myr(−)-hARF1·GDP in blue, hARF1Δ17·GTP in green. λ3 of all molecules is shown in red, and myristoyl is shown in purple.
Myristoyl chain representations (Fig. 2, purple) are also not particularly closely clustered, but clearly sequestered in an extended hydrophobic groove lined by multiple non-sequential residues, including L12*, I20*, L37, Y58, I61, I89*, L170*, L173*, and L177 (NOEs to the myristoyl group were detected for residues marked with an asterisk). The distribution of the myristoyl group structures may reflect a certain level of mobility, or the absence of sufficient NOE constraints along the central region of the myristoyl chain. The hydrophobic pocket buries roughly half the myristoyl chain while the other half is kept from the solvent by an overlay of the N-terminus. In crystal structures of full-length myr(−)-ARF proteins and NMR structures of the isolated myr(+)-N-terminus, the N-terminal residues generally form an amphipathic helix, the length of which varies among different ARF proteins (Amor et al., 1994; Amor et al., 2001; Losonczi and Prestegard, 1998; Gizachew and Oswald, 2006). A high-resolution picture of the N-terminus is unavailable in our structure due to the broadened or missing resonances of this region, but it is tempting to speculate that the hydrophobic face of the amphiphilic helix faces the myristoyl chain with the hydrophilic face solvent-exposed. The severe line broadening and absence of resonances is consistent with interchange of multiple N-terminal conformations of comparable free energy. A similar statement can be made for missing residues in Switch II. The high structural plasticity of these regions may be related to the need for dramatic conformational change during guanine nucleotide exchange and myristoyl chain exposure. Interestingly, the myristoyl group is not proximal to Switch II or the GDP binding site, although these regions display perturbed chemical shifts upon myristoylation (Figure 1). Instead, the acyl chain may have an allosteric effect on nucleotide exchange properties, consistent with the myristate playing a role as clamp to slow GDP dissociation and prevent spontaneous nucleotide exchange in aqueous solution, as seen earlier in nucleotide binding and exchange assays (Randazzo et al, 1995).
The myristoyl binding pocket can be recognized in the crystal structure of myr(−)-yARF2·GDP (Amor et al., 2001) but it is partly shielded from water by the hydrophobic face of the N-terminal helix (Figure 2C). In the GTP-bound conformation as shown in the crystal structure of the hARF1Δ17 (Goldberg, 1998), the displacement of the β2-β3 strands shifts the inter-switch loop λ3 (E57-I61) into the myristoyl binding pocket; this is predicted to lead to steric clashes preventing myristoyl sequestration (Figure 2D). Structural comparison of myr(−)-ARF·GDP and ARFΔ17·GTP had previously suggested that intrusion of λ3 is responsible for myristoyl release during guanine nucleotide exchange by sterically expelling the N-terminal helix (Goldberg, 1998; Renault et al., 2003). Here it is clear that the positional change of λ3 leads directly to interference with myristoyl binding, rather than through an indirect effect on the N-terminal helix.
myr(+)-yARF1 GDP weakly interacts with lipids
To assess membrane interactions, a number of experiments designed to examine changes in motional correlation times on exposure to model membranes were employed. The effective rotational correlation time (τc), measured from NMR relaxation experiments or reflected in NMR spectral linewidths, is closely related to the tumbling rate of a molecule in solution. Therefore, assuming other factors, such as temperature and viscosity, remain constant, it reports on the apparent molecular size. Association of ARF with membrane-like fragments should clearly lead to an increased effective size and an increase in correlation times. To extract correlation times (τc), a previously published NMR technique (Liu and Prestegard, 2008) was applied to yARF1 in the presence and absence of lipid bicelles. Initially, a small bicelle system was utilized as a membrane mimic; it consists of dimyristoyl-phosphatidylcholine (DMPC) and dihexanoyl-phosphatidylcholine (DHPC) at 1:4 molar ratio (q = [DMPC]:[DHPC] = 0.25). This ratio yields bicelle disks with a radius of ∼ 24Å and thickness of ∼ 40 Å (Glover et al., 2001; Luchette et al., 2001). Complete association of ARF with this fragment should roughly double the effective correlation time, but still provide favorable high-resolution NMR spectra.
The 15N spin relaxation experiments show relatively little variation in τc determinations with residue position, particularly in the C-terminal half of the protein (Figure 3A, B). These invariant regions report most directly on overall correlation time. Both myr(+) and myr(−)-yARF1·GDP displayed modestly elevated τcs for residues in these regions (40% & 34% respectively) in the presence of 10% (w/v) lipids (Figure 3A, B). This can be interpreted as weak lipid interaction, but it may also be an effect of increased solution viscosity. Estimations based on a modified Stokes-Einstein-Debye equation predict a 20% increase in rotational correlation time due to viscosity changes upon the addition of small bicelles (Ross and Minton, 1977; Lavalette et al., 2006). Given the approximations in this equation, the observed change in correlation times could largely result from the change in viscosity. It is notable that the changes are approximately the same for both myr and non-myr forms. The lack of an observable enhanced interaction for the GDP loaded, myristoylated, form could be a consequence of the stability of the myristoyl chain in the well shielded binding pocket relative to its stability in the small bicelle structure. Small levels of interaction may also be difficult to detect given the modest increase in effective molecular size on interaction with small bicelles.
Figure 3.
a & b, The residue-specific effective rotational correlation times of myr(-) and myr(+)-yARF1·GDP in the absence or presence of 10% DMPC/DHPC small bicelles. c. One dimensional 15N-1H HSQC scans of myr(−) and myr(+)-yARF1·GDP mixed with DMPC/DHPC bicelles of varying sizes. d. Effective rotational correlation times of myr(−) and myr(+)-yARF1·GTP in the presence of 10% DMPC/DHPC small bicelles.
It is possible to further distinguish viscosity effects from effective size effects, as well as improve sensitivity to association, using an experiment in which the lipid concentration is fixed at 8% (in order to minimize the viscosity changes) while varying the bicelle size by adjusting the q value. The q values of 0.75, 1.5, 2.25, 3.0, and 3.5, correspond to bicelle disk radii of 57.5, 105.6, 153.1, 200.5, and 232 Å (Vold & Prosser, 1996). The bicelle thickness is independent of q and stays at ∼ 40 Å in this simplified bicelle model (Vold & Prosser). This progressive increase in bicelle size with q value increases sensitivity to weak protein interactions as it produces increasingly larger changes in correlation time upon bicelle interaction.
For the experiments with larger bicelles a 1D 15N-1H HSQC experiment was used to monitor τc changes as reflected in signal intensity changes. Due to the exponential decay of transverse magnetization during coherence transfer between nuclei (INEPT and reverse-INEPT steps) in HSQC experiments, and the direct dependence of this decay on τc for most resonances, peak intensity in 1D HSQC experiments is a sensitive reporter of changes in τc. While myr(−)-yARF1·GDP has minimal dependence on bicelle size within the examined range (Figure 3C, upper panel), myr(+)-yARF1·GDP showed a marked decrease in intensity at q=3.0 and above (Figure 3C, lower panel). Because the viscosity is identical for myr(+) and myr(−) samples at the same q value, the differential dependence of intensity on the q ratio can be directly correlated to the differential lipid interaction tendencies of the two forms of the GDP loaded protein. Note that this is not necessarily inconsistent with the τc data reported in Figure 3B, which report only data collected at relatively low q value (0.25). One interpretation of the data in Figure 3A-C is that myr(+)-yARF1·GDP does interact more strongly with bicelles than does the myr(−)-yARF1·GDP, although this interaction is still relatively weak and undergoes fast exchange. As a result, this difference between myr(+) and myr(−) proteins is best seen at higher q values where lipid association has more exaggerating effects on the rotational correlation time. However, an alternate interpretation of these data is that there may be preferential interaction of myr(+)-yARF1·GDP with bicelles of higher q values because they display a more planar membrane character, or a surface region that is simply more compatible with myristoyl chain insertion (DMPC acyl chains are well matched to the myr(+) chain length). In the ideal bicelle model, the short-chained DHPC molecules form the rim of the disc while the long-chained DMPC molecules are segregated in the center. However, near the interface of DHPC and DMPC, neither could be ideally packed due to the mis-match in chain length. Thus at low q values, a significant percentage of DMPC could be in this loosely packed region which does not represent an ideal binding surface for ARF. As the q value increases, more planar regions with compatible chain lengths would form in the center of the disc, and this might lead to a more stable interaction with ARF. Recognition of a planar surface is particularly attractive possibility because it could reflect an important extension of the current model that some ARF GAPs are activated by increases in membrane curvature (Bigay et al., 2003; Bigay et al., 2005). Thus, the cycle of ARF activation/deactivation would be coupled at both ends if myr(+)ARF·GDP is first attracted to planar membranes, where it interacts with an ARF GEF to generate activated myr(+)ARF·GTP, recruit adaptors required for specific carrier biogenesis and membrane deformation, leading to ARF GAP activation, GTP hydrolysis, and release of the resulting myr(+)ARF·GDP to the cytosol.
Once associated with a membrane surface, it is interesting to consider what changes in protein structure would be required for, or result from, insertion of myristate into the bilayer. A direct insertion of the myristoyl chain would necessarily involve displacement of the N-terminus, as it keeps the myristoyl chain hidden from the solvent in the absence of lipids. Previous studies have shown that myr(+)-ARF·GDP requires both GEF and high concentrations of phospholipids for efficient guanine nucleotide exchange at physiological Mg2+ concentrations (Franco et al., 1996), while ARFΔ17-GDP only requires GEF (Goldberg, 1998). This suggests that displacement of the N-terminus, whether by actual deletion from the protein sequence, or by a lipid environment favoring exposure of the myristoyl chain, can facilitate nucleotide exchange. Based on the crystal structure of hARF1Δ17 complexed with a Sec7 domain (Goldberg, 1998), a GEF promotes guanine nucleotide exchange by inducing a nucleotide-free form of ARF that structurally resembles the GTP-bound form, a conformation also promoted by N-terminal deletion (Seidel III et al., 2004). According to the structure presented here, the myristoyl group would have to move to prevent a steric clash with λ3 (Figure 2 D) in a GTP-bound structure similar to hARF1Δ17·GTP. Myristoyl insertion in lipids could allow a GTP-like structure, and promote nucleotide exchange, by eliminating this potential clash. Thus, N-myristoylation would play an important regulatory role during guanine nucleotide exchange by ensuring that efficient exchange occurs only on the membrane surface.
The myristoyl group contributes to yARF1·GTP membrane interaction
While GTP-loaded forms of yARF1 are not sufficiently soluble in the absence of lipids to conduct NMR experiments, it was possible to conduct experiments on both myr(+) and myr(-)-yARF1·GTP in the presence of small bicelles. The results are presented in Figure 3D. In comparison to correlation times extracted for both GDP-bound forms in the absence of bicelles (Figure 3A, B) there is a substantial increase (80-100%) in correlation times for the GTP bound forms. Given partial contribution from changes in viscosity, this may not be as large as expected for a rigid integration with the bicelle structure, but it likely indicates a high degree of bicelle-protein interaction. This is consistent with previous data suggesting that ARF·GTP is capable of stable membrane association both in the presence and absence of a myristoyl chain (Franco et al., 1993).
There is actually a small difference in correlation times for the myr(+) and myr(-) forms, with the myr(+) form being more immobilized. This can be seen in the averaged τc values, but also in a residue by residue comparison. While resonances for the GTP forms were not assigned, chemical shift changes between myr(+) and myr(−)-yARF1·GTP are very small, allowing residue by residue comparisons of τc. A systematic increase in τc is seen for the myr(+) form here as well. A difference in τc would not be expected if association is complete (as expected from the lack of solubility in the absence of lipids) and the structural properties of the protein-bicelle complexes are similar. A possible explanation is that the motion of the two proteins at the bicelle surface is different. Membrane/membrane-associated proteins generally have ns-μs time scale rotational or wobbling motions on the membrane surface. When this motion is on a timescale that is shorter or similar to that of the global tumbling motion of the protein-bicelle complex, it has significant effects on the effective rotational correlation time. The increase in τc of myr(+) over myr(−)-yARF1·GTP could be due to decreased local motions of ARF when association is enhanced by lipid-myristoyl interaction. A decrease in local motion could entropically favor further associations with other accessory proteins (e.g., adaptor proteins) at the membrane surface. This may suggest an additional role for ARF myristoylation.
In summary, the data presented support a model in which the myristoyl group plays an important role during guanine nucleotide exchange (Franco et al., 1993; Franco et al., 1996). Structural comparisons of myr(+)-yARF1·GDP with ARFΔ17·GTP (Goldberg, 1998) and ARFΔ17·GTP/Sec7 (Goldberg, 1998; Renault et al., 2003) provide a structural basis for a possible regulatory function of the myristoyl group. Basically, its presence inhibits movement of protein elements necessary for nucleotide exchange, unless a membrane is available to accommodate the myristoyl chain. Hence, exchange occurs only after association with a membrane surface. Comparison of the tendencies of various yARF1 forms to associate with large and small bicelles also provides insight into the role of myristoylation. Both ARF·GTP forms associate strongly with even small bicelles. However, the myristoyl group appears to further stabilize the interaction, which may be important for the interaction of ARF·GTP with other proteins on the membrane surface. In comparison, neither myr(−)-yARF1·GDP nor myr(+)-yARF1·GDP could be demonstrated to associate with small bicelles. However, using a larger bicelle, with a more extended bilayer surface, myr(+)-yARF1·GDP was found to have a substantial tendency to associate with a membrane surface, and to do so more strongly than myr(−)-yARF1·GDP. While the inability to demonstrate this with small bicelles could simply be a reflection of weak binding, it is worth noting that an increased specificity for planar membranes would be consistent with the role of ARF as the initiator of membrane traffic carrier biogenesis.
Experimental Procedures
Protein Expression and Purification
Full length yARF1 was cloned into a pET20(b) (Novagen, Inc) vector using NdeI and XhoI sites. A thrombin cleavage site was introduced for removal of the C-terminal H6 tag included in the vector. For production of myr(+)-yARF1, yARF1-pET20(b) was co-transformed with the pBB131 vector containing the human N-myristoyl transferase 1 (hNMT1) open reading frame into the BL-21(DE3) cell-line. Cells were grown in M9 medium containing 1g/L NH4Cl, 2g/L glucose, 100mg/L ampicillin and 50mg/L kanamycin at 37°C until OD600nm reached 0.5 at which point sodium myristate was added to 25μM. IPTG (0.4mM) was added when OD600nm reached 1.0∼1.1 and cells were grown at 28°C overnight. For production of perdeuterated ARF, freshly transformed cells were inoculated into 40mL LB medium in H2O. When OD600nm reached 0.5∼0.6, cells were spun down and re-suspended in 200mL M9 medium in H2O. Growth continued until OD600nm reached 0.5∼0.6 at which point cells were spun down and re-suspended in 1L M9 medium in D2O with 2g/L 2D7-13C6-glucose and 1g/L 15NH4Cl. Sodium myristate was added to 25μM at OD600nm of ∼0.8 and IPTG was added to 0.4mM at an OD600nm of 1.0∼1.2. After induction, cells were held at 37°C for 6 hours or at 28°C overnight.
Production of methyl-protonated protein in a partially perdeuterated background followed the aforementioned procedure except that 3,3-2D2-1,2,3,4-13C4-2-keto-butyrate (100mg/L) and 3-2D1-1,2,3,4-13C4-2-keto-isovalerate (100mg/L) were added together with sodium myristate (25μM) ∼1 hour before induction (OD600nm = ∼0.8). In addition, protonated 13C6-glucose (2g/L) was used here as the background carbon source. This allows a sufficient level of protonation on the methyl groups of Ala and Met for additional NOE measurements while preserving the spectral simplicity and favorable relaxation behavior of perdeuterated samples. The myristoylation efficiency was usually ∼ 50% with the other half dominated by lauroylated forms as suggested by ESI Mass Spectrometry and fatty acid analysis (data not shown). No steps were taken to isolate the myristoylated from the other acylated forms. Signals from the myristoylated ARF were selectively investigated through an isotope labeling strategy in which the myristoyl and the other fatty acids were differentially labeled.
For production of myr(−)-yARF1, yARF1-pET20(b) but not hNMT1-pBB131 was transformed, kanamycin was not included in the growth medium, and sodium myristate was not added; Other growth conditions remain identical. Purification of both myr(+) & myr(−)-yARF1·GDP followed the same protocol. Proteins were first purified by affinity chromatography on a HisTrap column and the H6 tag removed with thrombin. Proteins were further subjected to ion-exchange chromatography on a Q-Sepharose column. A final yield of 20∼30mg of protein per liter of culture was usually achieved.
Preparation of Lipid Containing Samples
For τc measurements, a 10 % (w/v) small bicelle solution containing DMPC and DHPC at a 1:4 molar ratio was employed. For interactions with bicelles of varying sizes, the total lipid concentration was fixed at 8% with the relative amount of DMPC and DHPC adjusted as specified in the main text. Protein was maintained at a concentration of 0.5mM in NMR buffer (10mM K2HPO4-KH2PO4 (pH 7.0), 50mM NaCl, 10mM K2SO4, 2mM MgCl2, and 5mM dithiothreitol) throughout these assays.
Guanine Nucleotide Exchange in Lipid Containing Solution
myr(+) or (−)-yARF1·GDP was first mixed with DMPC and DHPC to a final lipid concentration of 10% and protein concentration of 0.5mM in NMR buffer. GTP and EDTA were then added to 10mM and 2mM, respectively. The sample was incubated at room temperature overnight and MgCl2 (to 2mM) was added before transferring the sample to an NMR tube. The exchange resulted in a preparation containing mostly the GTP form (>3:2), as determined by the appearance of new chemical shift distinct HSQC cross-peaks allowing relaxation time measurements specifically for this form.
NMR Spectroscopy
Spectra were acquired on 600, 800, and 900 MHz spectrometers (Varian, Inc) on samples containing 0.5∼0.8mM protein in NMR buffer. All experiments were conducted at 25°C. Backbone resonances were assigned using data from HNCACB, HN(CO)CA, HN(COCA)CB, and CBCA(CO)NH experiments on a fully protonated sample. Methyl groups in selectively protonated samples were assigned using a doubly-enhanced, 13C excited CCmHm-TOCSY experiment modified from previously published pulse sequences (Permi et al., 2004; Yang et al., 2004). NOESY-15N-HSQC on the same sample proved helpful in solving assignment ambiguities by correlating the methyl protons to the amides. A number of Hα and Hβ protons were assigned based on HNHA, 1H-1H-TOCSY-15N-HSQC, and NOESY-15N-HSQC experiments collected on a fully protonated 15N sample. The myristoyl protons were assigned using 2-dimensional water-gated NOESY on a myristoyl protonated, but otherwise highly perdeuterated sample.
An attempt was made to selectively retrieve data on the myristoylated form of ARF in the presence of other acylated ARF species (mostly lauroylated) through the following approach. In preparations of fully perdeuterated protein, 1H27-12C14-sodium myristate was supplemented in a growth medium containing D2O and 2D7-glucose, thus the endogenous lauroyl group was expected to be perdeuterated while the majority of the myristoyl would come from the supplemented material and be protonated. A NOESY-15N-HSQC experiment revealed cross-peaks to amide protons from the myristate but not from the other acyl-groups. In the preparation of methyl-protonated, background-deuterated protein, 1H27-12C14-sodium myristate was applied to a medium containing D2O and protonated 13C6-glucose. The endogenous lauroyl was thus expected to be 13C labeled while most of the myristoyl adduct would be protonated and 12C labeled. Thus, signals from the acyl-groups can be eliminated by 13C-filtered experiments. NOE's between myristoyl protons and methyl protons were revealed and assigned using 13C-filtered-NOESY-13C-edited-HSQC experiments. Sample panels from this experiment showing connections from myristoyl methyl and methylene groups to side chains of specific residues in the protein are given as Supplemental Data. The approach presumes minimal metabolic conversion of 1H27-12C14-sodium myristate to a 1H27-12C14-laurate species. We were unable to quantify this possible conversion and thus must acknowledge possible contamination of myristoyl with lauroyl data.
A negatively charged (50% 2-acrylamido-2-methyl-1-propanesulfonic acid + 50 % acrylamide) compressed gel (Cierpicki and Bushweller, 2004) and a neutral (100% acrylamide) stretched gel (Sass et al., 2000) were employed to obtain two highly uncorrelated alignments for the measurement of residual dipolar couplings. These were measured from an interleaved TROSY-HSQC pair of acquisitions (Ottiger and Bax, 1998). The correlation coefficient between the two alignments is 0.02. Spin relaxation data for the extraction of correlation times were acquired with an experiment designed to directly detect chemical shift anisotropy – dipole-dipole cross correlated relaxation times (Liu and Prestegard, 2008).
Structural Determination
Structural calculation was conducted using a simulated annealing protocol provided in the CNS software (Brünger et al., 1998). Distance restraints were classified into 3 categories: strong (1.8-2.7Å), medium (1.8-3.7Å) and weak (1.8-5.5Å), based on the volumes of NOESY cross peaks. Backbone dihedral angles were derived from N, HN, Cα, Cβ, and C′ chemical shifts using the TALOS program (Cornilescu et al., 1999). Errors for NH RDCs were estimated from duplicate experiments. The force constants during simulated annealing were adjusted so that the final deviations between experimental and back-calculated RDCs were comparable to experimental errors (see Table I). Force constants for DNH, DNC′, and DHNC′ were ramped from 0.01 to 0.6, 0.3 and 0.3 kcal.mol-1.Hz-2, respectively. The resulting 15 lowest energy structures have been deposited in the PDB (2k5u). NMR assignments and constraint data have been deposited in the BMRB (15809).
Supplementary Material
Supplemental data include a stereo view of Figure 2A and examples of panels from the 13C-filtered-13C-edited NOESY experiment used to identify myristoyl-protein contacts. This material can be found at http://www.structure.org.
Acknowledgments
This work was supported by a grant from the National Institutes of Health, GM61268.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amor JC, Harrison DH, Kahn RA, Ringe D. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature. 1994;372:704–708. doi: 10.1038/372704a0. [DOI] [PubMed] [Google Scholar]
- Amor JC, Horton JR, Zhu X, Wang Y, Sullards C, Ringe D, Cheng X, Kahn RA. Structures of yeast ARF2 and ARL1: distinct roles for the N terminus in the structure and function of ARF family GTPases. J Biol Chem. 2001;276:42477–42484. doi: 10.1074/jbc.M106660200. [DOI] [PubMed] [Google Scholar]
- Bigay J, Casella J, Drin G, Mesmin B, Antonny B. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 2005;24:2244–2253. doi: 10.1038/sj.emboj.7600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J, Gounon P, Robineau S, Antonny B. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature. 2003;426:563–566. doi: 10.1038/nature02108. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS. The GGA proteins: Adaptors on the move. Nature Reviews Molecular Cell Biology. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–44. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Brünger AT, Adams PD, Clore GM, Delano WL, Gros P, Crosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Chardin P, Paris S, Antonny B, Robineau S, Béraud-Dufour S, Jackson CL, Chabre M. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- Cierpicki T, Bushweller JH. Charged gels as orienting media for measurement of residual dipolar couplings in soluble and integral membrane proteins. J Am Chem Soc. 2004;126:16259–16266. doi: 10.1021/ja046054g. [DOI] [PubMed] [Google Scholar]
- Cornilescu G, Delaglio F, Bax A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR. 1999;13:289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Huber I, Rotman M, Cassel D. The ARF1 GTPase-activating protein - zinc-finger motif and Golgi-complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Honda A, Weigert R. Multiple activities for Arf1 at the Golgi complex. Biochim Biophys Acta. 2005;1744:364–373. doi: 10.1016/j.bbamcr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Franco M, Chardin P, Chabre M, Paris S. Myristoylation is not required for GTP-dependent binding of ADP-ribosylation factor ARF1 to phospholipids. J Biol Chem. 1993;268:24531–24534. [PubMed] [Google Scholar]
- Franco M, Chardin P, Chabre M, Paris S. Myristoylation-facilitated binding of the G protein ARF1GDP to membrane phospholipids is required for its activation by a soluble nucleotide exchange factor. J Biol Chem. 1996;271:1573–1578. doi: 10.1074/jbc.271.3.1573. [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. 2007. [DOI] [PubMed] [Google Scholar]
- Gizachew D, Oswald R. NMR structural studies of the myristoylated N-terminus of ADP ribosylation factor 6 (Arf6) FEBS Lett. 2006;580:4296–4301. doi: 10.1016/j.febslet.2006.06.086. [DOI] [PubMed] [Google Scholar]
- Glover KJ, Whiles JA, Wu G, Yu N, Deems R, Struppe JO, Stark RE, Komives EA, Vold RR. Structural evaluation of phospholipid bicelles for solution-state studies of membrane-associated biomolecules. Biophys J. 2001;81:2163–2171. doi: 10.1016/s0006-3495(01)75864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Goldberg J. Structural basis for activating of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–248. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- Helms JB, Palmer DJ, Rothman JE. Two Distinct Populations of ARF Bound to Golgi Membranes. J Cell Biol. 1993;121:751–760. doi: 10.1083/jcb.121.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Li YW, Bennett M, McKay M, Zhu XJ, Shern J, Torre E, Lah JJ, Levey AI, Kahn RA. Munc18 interacting proteins - ADP-ribosylation factor-dependent coat proteins that regulate the traffic of beta-Alzheimer's precursor protein. Journal of Biological Chemistry. 2003;278:36032–36040. doi: 10.1074/jbc.M301632200. [DOI] [PubMed] [Google Scholar]
- Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic. 2007;8:1465–1475. doi: 10.1111/j.1600-0854.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- Kahn RA, Kern FG, Clark J, Gelmann EP, Rulka C. Human ADP-ribosylation factors. A functionally conserved family of GTP-binding proteins. J Biol Chem. 1991;266:2606–2614. [PubMed] [Google Scholar]
- Kahn RA, Randazzo P, Serafini T, Weiss O, Rulka C, Clark J, Amherdt M, Roller P, Orci L, Rothman JE. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J Biol Chem. 1992;267:13039–13046. [PubMed] [Google Scholar]
- Kishore NS, Wood DC, Mehta PP, Wade AC, Lu T, Gokel GW, Gordon JI. Comparison of the acyl chain specificities of human myristoyl-CoA synthetase and human myristoyl-CoA:protein N-myristoyltransferase. J Biol Chem. 1993;268:4889–4902. [PubMed] [Google Scholar]
- Lavalette D, Hink MA, Tourbez M, Tétreau C, Visser AJ. Proteins as micro viscosimeters: Brownian motion revisited. Eur Biophys J. 2006;35:517–522. doi: 10.1007/s00249-006-0060-z. [DOI] [PubMed] [Google Scholar]
- Liu Y, Prestegard JH. Direct measurement of dipole-dipole/CSA cross-correlated relaxation by a constant-time experiment. J Magn Reson. 2008;193:23–31. doi: 10.1016/j.jmr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon JM, Kahn RA. The ARF family GTPases. Kluwer Academic Publishers; 2004. pp. 1–22. [Google Scholar]
- Losonczi JA, Prestegard JH. Nuclear magnetic resonance characterization of the myristoylated, N-terminal fragment of ADP-ribosylation factor 1 in a magnetically oriented membrane array. Biochemistry. 1998;37:706–716. doi: 10.1021/bi9717791. [DOI] [PubMed] [Google Scholar]
- Luchette PA, Vetman TN, Procsser RS, Hancock RE, Nieh MP, Glinkda CJ, Krueger S, Katsaras J. Morphology of fast-tumbling bicelles: a small angle neutron scattering and NMR study. Biochim Biophys Acta. 2001;1513:83–94. doi: 10.1016/s0005-2736(01)00358-3. [DOI] [PubMed] [Google Scholar]
- Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. doi: 10.1016/s0955-0674(03)00071-1. 2003. [DOI] [PubMed] [Google Scholar]
- Ottiger M, Bax A. Determination of relative N-HN, N-C′, Cα-C′, and Cα-Hα effective bond lengths in a protein by NMR in a dilute liquid crystalline phase. J Am Chem Soc. 1998;120:12334–12341. [Google Scholar]
- Permi P, Tossavainen H, Hellman M. Efficient assignment of methyl resonances: enhanced sensitivity by gradient selection in a DE-MQ-(H)CCmHtm-TOCSY experiment. J Biomol NMR. 2004;30:275–282. doi: 10.1007/s10858-004-3222-2. [DOI] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wüthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyroche A, Paris S, Jackson CL. Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature. 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Kahn RA. Myristoylation and ADP-ribosylation factor function. Methods Enzymol. 1995;257:128–135. doi: 10.1016/0076-6879(95)50087-1. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Terui T, Sturch S, Fales HM, Ferrige AG, Kahn RA. The myristoylated amino-terminus of ADP-ribosylation factor-1 is a phospholipid-sensitive and GTP-sensitive switch. J Biol Chem. 1995;270:14809–14815. doi: 10.1074/jbc.270.24.14809. [DOI] [PubMed] [Google Scholar]
- Renault L, Guibert B, Cherfils J. Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature. 2003;426:525–530. doi: 10.1038/nature02197. [DOI] [PubMed] [Google Scholar]
- Rosen MK, Gardner KH, Willis RC, Parris WE, Pawson T, Kay LE. Selective methyl group protonation of perdeuterated proteins. J Mol Biol. 1996;263:627–636. doi: 10.1006/jmbi.1996.0603. [DOI] [PubMed] [Google Scholar]
- Ross PD, Minton AP. Hard quasispherical model for the viscosity of hemoglobin solutions. Biochem Biophys Res Comm. 1977;76:971–976. doi: 10.1016/0006-291x(77)90950-0. [DOI] [PubMed] [Google Scholar]
- Sass HJ, Musco G, Stahl SJ, Wingfield PT, Grzesiek S. Solution NMR of proteins within polyacrylamide gels: diffusional properties and residual alignment by mechanical stress or embedding of oriented purple membranes. J Biomol NMR. 2000;18:303–309. doi: 10.1023/a:1026703605147. [DOI] [PubMed] [Google Scholar]
- Seidel RD, III, Amor JC, Kahn RA, Prestegard JH. Conformational changes in human Arf1 on nucleotide exchange and deletion of membrane-binding elements. J Biol Chem. 2004;279:48307–48318. doi: 10.1074/jbc.M402109200. [DOI] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles - a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Styers M, Faundez V. In: ARF Family GTPases. Kahn RA, editor. Springer; NY: 2003. pp. 259–281. [Google Scholar]
- Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- Tang C, Loeliger E, Luncsford P, Kinde I, Beckett D, Summers MF. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc Natl Acad Sci USA. 2004;101:517–522. doi: 10.1073/pnas.0305665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandra N, Bax A. Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science. 1997;278:1111–1114. doi: 10.1126/science.278.5340.1111. [DOI] [PubMed] [Google Scholar]
- Tolman JR, Flanagan JM, Kennedy MA, Prestegard JH. Nulclear magnetic dipole interactions in field-oriented proteins: information for structure determination in solution. Proc Natl Acad Sci USA. 1995;92:9279–9283. doi: 10.1073/pnas.92.20.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold RR, Prosser RS. Magnetically oriented phospholipid bilayered micelles for structural studies of polypeptides. Does the ideal bicelle exist? J Magn Reson B. 1996;113:267–271. [Google Scholar]
- Weiss O, Holden J, Rulka C, Kahn RA. Nucleotide binding and cofactor activities of purified bovine brain and bacterially expressed ADP-ribosylation factor. J Biol Chem. 1989;264:21066–21072. [PubMed] [Google Scholar]
- Yang D, Zheng Y, Liu D, Wyss DF. Sequence-specific assignments of methyl groups in high-molecular weight proteins. J Am Chem Soc. 2004;126:3710–3711. doi: 10.1021/ja039102q. [DOI] [PubMed] [Google Scholar]
- Zozulya S, Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci USA. 1992;89:11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data include a stereo view of Figure 2A and examples of panels from the 13C-filtered-13C-edited NOESY experiment used to identify myristoyl-protein contacts. This material can be found at http://www.structure.org.