Abstract
Purpose
This report describes the development and preclinical qualification tests of 2nd generation (gen) anti-carcinoembryonic (CEA) designer T cells for use in human trials.
Experimental Design
The progenitor 1st generation immunoglobulin-T cell receptor (IgTCR) that transmits Signal 1-only effectively mediated chimeric immune receptor (CIR)-directed cytotoxicity, but expressor T cells succumbed to activation-induced cell death (AICD). The 2nd generation CIR (termed “Tandem” for two signals) was designed to transmit TCR Signal 1 and CD28 Signal 2 to render T cells resistant to AICD and provide prolonged anti-tumor effect in vivo.
Results
A CIR was created that combines portions of CD28, TCR zeta and a single chain antibody domain (sFv) specific for CEA into a single molecule (IgCD28TCR). As designed, the gene-modified Tandem T cells exhibit the new property of being resistant to AICD, showing instead an accelerated proliferation on tumor contact. Tandem T cells are more potent than 1st generation in targeting and lysing CEA+ tumor. Tandem T cells secrete high levels of IL2 and IFNγ on tumor contact that 1st generation T cells lacked, but secretion was exhaustible, suggesting need for IL2 supplementation in therapy even for these 2nd generation agents. Finally, 2nd generation T cells were more effective in suppressing tumor in animal models.
Conclusion
An advanced generation anti-CEA designer T cell is described with features that promise a more potent and enduring anti-tumor immune response in vivo. These preclinical data qualify the human use of this agent that is currently undergoing trial in patients with CEA+ cancers under BB-IND 10791.
INTRODUCTION
The use of tumor-infiltrating lymphocytes (TILs) in patients with melanoma or renal cell carcinoma (RCC) possessed a rationale based on a native but weak response of the host to the tumor prior to therapeutic intervention that was augmented by ex vivo manipulation and re-administration with IL2 supplementation [1]. However, this recognition was imperfect, or the tumor would have regressed on its own; and the degree and durability of the responses was infrequently satisfactory, whether due to induced tolerance or antigen modulation.
A rational approach to build on the encouraging results in these two tumor systems (melanoma, RCC) would be to provide patient T cells the power to recognize tumor-associated antigens by design. Such therapies applying chimeric immune receptors (CIR) have been variously termed T-bodies, universal receptors or designer T cells [reviewed in ref.2]. In one configuration, these molecules are fusion products of an antibody binding domain (immunoglobulin, Ig) with the zeta signaling chain of the TCR, to form IgTCR. When expressed by gene therapy techniques in recipient T cells, the resulting designer T cells are redirected by the neo-specificity of the CIR to attack tumors expressing the surface antigen (Ag) recognized by the Ig. This strategy is designed to bypass a major drawback of cancer immunotherapies, which have been hampered by the fact that most “tumor antigens” are normal self-proteins to which the patient is already tolerized.
These protocols have the advantage that one may design therapies to attack other types of tumors than the limited set susceptible to TIL therapies. Any cancer can be targeted that has a known cell surface marker that is expressed in a tumor-restricted fashion. This strategy has the advantage that peripheral blood cells may be employed without resort to tumor sampling and the associated challenges of tumor-induced propagation of T cells. In chimeric receptor approach, the receptors are MHC-independent and the vector to modify autologous T cells is therefore an off-the-shelf reagent available to all patients irrespective of HLA-type. Further, being non-MHC-dependent means that the T cells are not thwarted by mutations affecting antigen processing (TAP proteins) or down-regulation of MHC that can lead to tumor evasion [3,4]. Additionally, transgene modification of CD4+ T cells in bulk PBMCs provides a mechanism for generating CD4 T helper cell activity that is typically missing from classic TIL protocols. Finally, the weak anti-tumor response among TILs in patients that is sampled for later TIL therapy may be improved upon by the stronger, engineered interactions that foster "more effective" T cell responses [e.g. ref. 5] In principle, the antitumor T cells so-designed may respond to the tumor as in an organ rejection, with cytokine secretion, proliferation and cytotoxicity against antigen-expressing targets. These features constitute the rationale for the designer T cell approach for cancer therapy.
In parallel with these developments, an evolution in our understanding of T cell biology was taking place. It had been noted that T cells would gradually die after engagement of the TCR (Signal 1), although killing many target cells in the interim. This death process in T cells was termed activation-induced cell death (AICD). Investigations showed early on that AICD was a form of apoptosis, mediated through the so-called death receptor pathway [6]. AICD was associated with DNA fragmentation, phosphotidylserine inversion (measured by annexin V binding), and an abbreviated survival that was not rescued by any cytokine supplementation. In contrast, when TCR Signal 1 was supplemented with co-stimulatory Signal 2 by CD28 engagement on the T cell, there was a suppression of AICD and of its associated correlate measures with an accompanying proliferation and improved survival [7,8].
We previously created so-called 1st generation Signal 1-only anti-CEA designer T cells [9] and instituted a clinical trial in colorectal and breast cancers. Biologic responses were observed, providing proof-of-principle of anti-tumor immune activity, but the responses were transient in nature [10]. Thereupon, we embarked on laboratory studies that confirmed loss of modified T cells in an antigen-dependent manner over a period of one week in vitro [11], corresponding to the time of expiration of immune activity in vivo. Like normal T cells, 1st generation designer T cells received only Signal 1 on antigen contact, supplied by the grafted TCR zeta, that would be predicted to render the designer T cells susceptible to AICD. In a test of the potential of CD28 Signal 2 to rescue cells from loss after Signal 1, designer T cells were stimulated by tumor cells with B7 artificially co-expressed to engage T cell CD28 co-receptor: as a result, T cell death was blocked and an accelerated T cell proliferation ensued with prolonged tumor cell killing.
These data [11] provided the impetus and justification for a further modification to the original design, to incorporate CD28 signaling into the chimeric receptor. In comparison to the 1st generation IgTCR, the 2nd generation exhibits induced proliferation on contact with tumor and resistance to AICD, superior cytotoxicity and cytokine production, and improved activity in animal tumor models.
Our goal is to create an effective, self-sustaining immune attack on recurrent or refractory CEA expressing cancers, including colorectal, breast, lung and others, in a therapy that combines the advantages of Ab specificity with the homing, tissue penetration and target cell destruction capabilities of T cells. Between 100,000 and 150,000 people die of CEA+ tumors per year in the United States alone who could be helped by this new therapy. The product of this study is the next iteration in the cycle of bench-to-bedside-to-bench-to-bedside towards achieving this goal. This 2nd generation designer T cell product is now (11/07) entering human clinical trials under FDA BB-IND 10791. The following describe the supporting preclinical data for this application.
MATERIALS AND METHODS
See Supplemental Materials online.
RESULTS
Vector construction
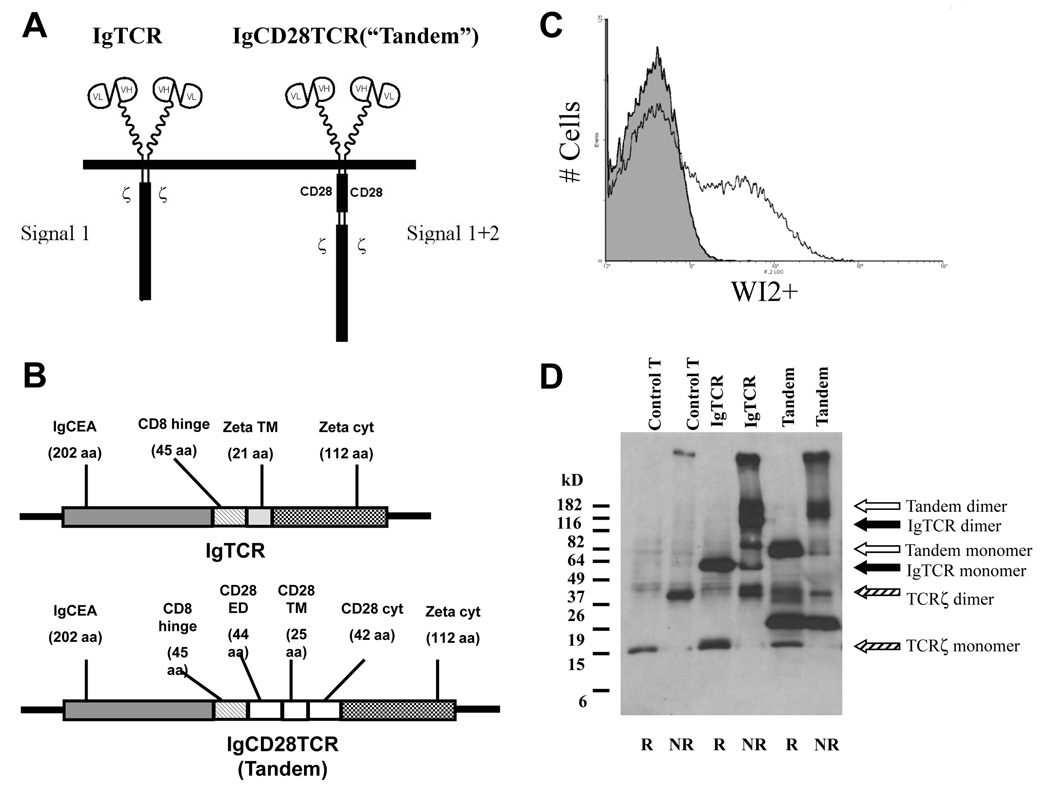
Model studies previously showed that co-stimulation could be engaged in a similar Ag-dependent fashion as the IgTCR, either as IgCD28 or in a colinear structure with TCR signaling molecules [12,13,14]. We examined CIR constructs with IgTCR + IgCD28 in the same retroviral vector, with an intervening internal ribosome entry site (IRES). However, these proved unstable on infecting the vector producer cells, undergoing recombination deletion between the Ig domains to generate an IgCD28 without IgTCR (Q Ma & R Junghans, unpublished results). To maintain the goal of a single viral vector for gene expression, we turned our focus to adopting a co-linear design. We re-engineered our 1st generation IgTCR [9] by inserting the CD28 cytoplasmic domain between the membrane and zeta chain to create an IgCD28TCR (Fig.1A). Previous studies had shown this orientation to be preferred over an orientation with CD28 distal from the membrane [13]. The CIR incorporates the CD28 transmembrane (TM) domain plus a portion of the CD28 extracellular domain (ECD) (Fig.1B) that maintains CD28 dimerization [14]. (The molecular junctions of the chimeric molecule are represented in Fig.S1 of Supplemental Online Materials.) This molecule was termed “Tandem” to signify the two signaling regions (Signals 1+2) in the same molecule. Whereas the IgTCR has a TCRzeta TM domain that directs it to assemble into the native TCR, the CD28 TM domain allows Tandem to express on the T cell independently of the TCR.
Fig. 1.
Anit-CEA IgCD28TCR molecular construction and expression. A. IgCD28TCR versus IgTCR. The IgCD28TCR construct was created from IgTCR by molecular insertion of the complete CD28 transmembrane (TM) and cytoplasmic domains and a portion of the CD28 extracellular domain (ECD). Upper: schematic representation of surface expressed molecules. Lower: molecular design of colinear constructs. Note that both constructs have identical sFv-CD8α hinge and TCR.ζ cytoplasmic domains. B. IgCD28TCR expression. Transduced normal human T cells were stained with WI2 anti-MN14-idiotype Ab and analyzed by FACS. Shaded profile, untransduced control; open profile, transduced cells. Transduced fraction measured as 22%. C. Sizes of CIRs. Western blots of membrane fractions from untransduced or transduced (~40%) human T cells were detected with anti-TCRζ monoclonal antibody. Monomeric and dimeric forms of the endogenous TCRζ, IgTCR and IgCD28TCR are indicated by arrows. Other forms are interpreted as cleavage products.5
Expression
Supernatants from GALV-pseudotyped vector producer cells were used to infect activated normal human T cells that were modified to 20–30% after 2–5 rounds of infection (Fig.1C). Surface expression of the IgCD28TCR transgene per cell was at a level comparable to or higher than the corresponding 1st generation IgTCR (not shown). Western blot confirmed a disulfide-linked dimer under native conditions of apparent MW of 120 kDa (Fig.1D). Under reduced conditions a monomer molecular weight of 64 kDa was observed versus a calculated 56 kDa for the mature protein, with glycosylation accounting for the difference. A size increment of 10 kDa is seen in relation t\o the prior IgTCR version that is 46 kDa by calculation.
Function
Insertion of the chimeric TCR into T cells genetically instructs these cells to respond in an MHC-unrestricted manner to immobilized (but not soluble) CEA with activation of T cell effector functions [9,15]. Three mechanisms are assayed relevant to anti-tumor activity of the 2nd generation versus the 1st generation product.
1. Proliferation/survival of T cells
To examine the role of co-stimulation in T cell proliferation and death, we created a panel of tumor lines that could provide to the T cells either Signal 1, by engaging the TCR, or Signal 2, by engaging the CD28 co-stimulatory molecule. Because epithelial tissues and cancers do not express B7, the natural ligand for CD28, there was no opportunity for the modified T cells to receive Signal 2 on tumor contact. To examine the dependency of modified T cells on co-stimulation, we created tumor targets that artificially expressed the B7.1 gene (Methods). With this panel, tumor cells could be selected to provide to 1st generation IgTCR either No signal (MIP101), Signal 1 (MIPCEA), Signal 2 (MIP101-B7) or Signal 1+2 (MIPCEA-B7). Our earlier studies showed that Signal 1 alone led to death in 1st generation designer T cells in a process compatible with AICD, but that the addition of Signal 2 via MIPCEA-B7 was sufficient to rescue the modified T cells from cell death and induce their super-proliferation [11]. (In the following, we apply the term AICD to mean T cell death that is selectively mediated via antigen stimulation. Prior studies have repeatedly correlated this means of inducing T cell death with diverse molecular markers of apoptosis [6,7,8], including annexin V staining which we reproduce below.)
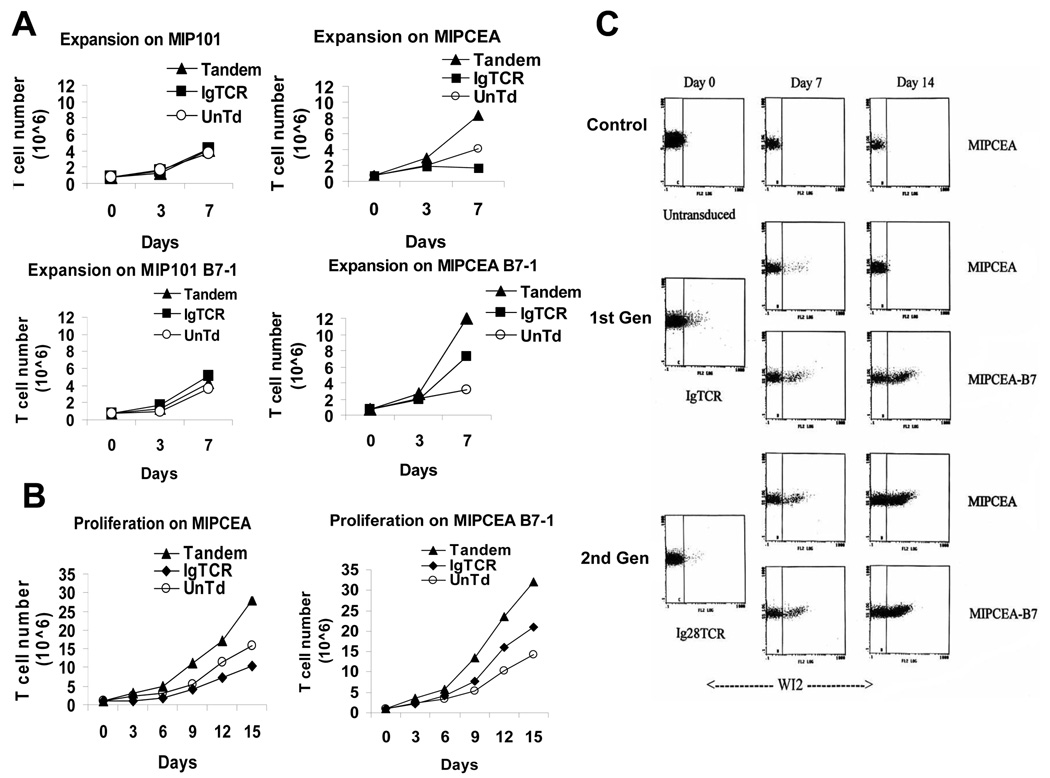
Designer T cells created as a 15–45% fraction of the total T cells were cultured on tumor cells and then the T cells were monitored for growth (Fig.2A). Untransduced (UnTd) T cells grew equally on all tumor cell types, irrespective of CEA antigen or B7 expression, and IgTCR and Tandem T cells maintained basal expansion on CEA-negative MIP101 and MIP101-B7 cells (Fig.2A).1 These results confirm that activation (for AICD or for proliferation) depends upon antigen recognition (Signal 1) and that Signal 2 by itself induces no response. On MIPCEA tumor cells, however, Tandem IgCD28TCR T cells proliferated at an accelerated rate relative to the untransduced (UnTd) T cells, and also lysed all CEA+ tumor targets in the culture. IgTCR T cells also killed all MIPCEA tumor targets, but ultimately died as expected by AICD. The net loss in total T cell number relative to UnTd at 7 days represented the death of the original 45% IgTCR+ fraction, as seen on flow cytometry (below). When the same IgTCR T cells were grown on MIPCEA-B7, in which B7 provided co-stimulation, the AICD was reversed and a super-expansion of the IgTCR+ T cells was achieved, but at a level below that with Tandem T cells. This confirms our prior results with AICD in 1st generation IgTCR T cells [11], and establishes the activity of 2nd generation Tandem T cells to obtain Signal 1+2 and resist AICD from binding CEA on tumor – but without need for B7 co-expression. A more prolonged assay (Fig.2B) with a second restimulation at day 7 confirmed the pattern of selective expansion of Tandem T cells and selective depletion of IgTCR-modified T cells on the same CEA+B7(−) tumor cells over a two-week interval. 2 Interestingly, the close juxtaposition of Signal 1 and Signal 2 in the same molecule (IgCD28TCR) is more efficient than their signaling via separate molecules (IgTCR + B7-CD28). This effect in the co-linear molecule is apparently also at saturation for co-stimulation inasmuch as the addition of B7-CD28 to IgCD28TCR across several experiments produced no reproducible increment in the proliferative response.
Fig. 2.
Proliferation. A. Enhanced proliferation is both CEA-dependent and Signal 2-dependent. On days specified, T cells (untransduced, IgTCR [45% modified], Tandem [15% modified]) were counted, split and mixed with an equal number of irradiated tumor cells: MIP101 (CEA neg) or MIPCEA (CEA+), without or with B7 co-expression. B. Longer term assay. Experiment as in A., except for two weeks duration and only employing CEA+ tumor targets with T cells. A and B are single experiments, repeated on three occasions with comparable results. C. Selective expansion of Tandem T cells. 1st or 2nd generation designer T cells were prepared and adjusted to very low (~2%) starting fractions. (See Methods.) T cells were co-cultured with specified tumor cells, and the fractions of modified T cells were assayed by WI2+ staining on FACS. D. Summary of results from C. Proliferative result (“Prolif”) is relative to basal expansion of activated but un-restimulated T cells.
With a mixed population of unmodified and Ig(CD28)TCR+ T cells, tumor Ag-induced AICD or proliferation predicts depletion or enrichment of the Ig(CD28)TCR+ fraction. An experiment was designed analogous to Fig.2B but using a very small starting proportion of designer T cells and monitoring by FACS for modified cells (Fig.2C). There was a selective loss of 1st generation designer T cells on MIPCEA, with Signal 1 alone, but enrichment/expansion on MIPCEA-B7, with Signal 1+2, confirming our prior results [11]. On the other hand, Tandem T cells show equal enrichment on MIPCEA or MIPCEA-B7 with a 15-fold fractional enrichment of IgCD28TCR+ cells over a 2-week period (Fig.2D). Like the proliferation results (Fig.2A,B), 2nd generation alone regularly exceeded that which was attainable with 1st generation plus B7 costimulation (Fig.2D). To account for this degree of enrichment, calculations (Methods) indicate that the growth rate constant for the Tandem T cells after Ag stimulation was increased to >200% of the basal expansion rate of the unmodified cells. In two experiments with different donors beginning with 5% Tandem fractions, the final fractions after 2 weeks were 39% and 41%.
Finally, the presumed correlation of cell loss with AICD in the 1st generation IgTCR construct was tested by annexin V binding, a procedure that marks the externalization and exposure of phosphatidyl serine on the cell surface, an early event in apoptotic cell death. Flow analysis of annexin V staining after 72 hours on contact with CEA+ targets showed 30% apoptosis of IgTCR+ cells by this assay, but without any effect on IgCD28TCR+ cells in the same test. (See Figure S2 of Supplemental Online Materials.) This confirms susceptibility of Signal 1-only designer T cells to AICD and resistance to the same in Signal 1+2 designer T cells, in accord with expectation (6,7,8). These selective death and survival effects were reflected in decreasing or increasing fractions of CIR-modified T cells in this 3-day assay (Figure S2C), as seen more prominently in longer term assays in Figure 2C.
2. Cytotoxicity
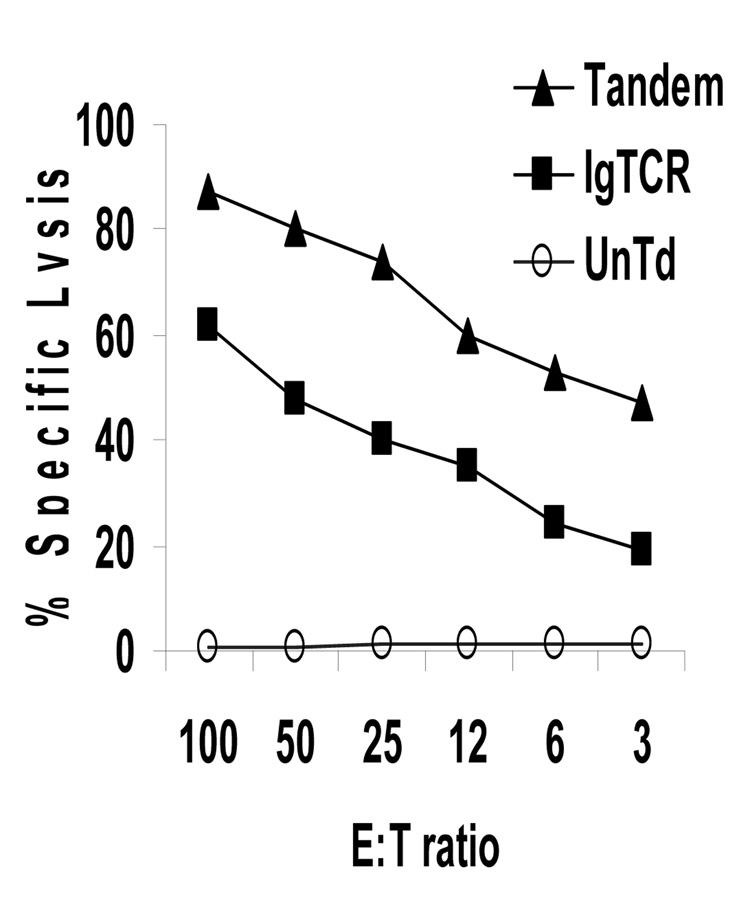
The single most critical feature of designer T cells in anti-tumor therapies is their antigen-directed killing capacity. Binding of antigen on tumor cells by 1st generation CD8+ designer T cells resulted in cytotoxicity against CEA+ tumor cell targets. This feature was preserved in the 2nd generation version. In multiple tests, 2nd generation (Tandem) designer T cells were actually more potent than 1st generation (Fig.3), although the basis for this superiority is presently uncertain. For lower E:T ratios, the 2nd generation T cells killed approximately twice as many targets. When expressed in terms of lytic units per cell, the Tandem T cells were approximately eight-fold more potent on a per cell basis. (Compare activity with E:T = 3 for IgTCR to E:T = 0.38 for Tandem.) Thus, the second goal of preserved cytotoxicity was achieved and surpassed.
Fig. 3.
Specific anti-tumor cytotoxicity of 2nd generation designer T cells. Untransduced, 1st and 2nd generation designer T cells were incubated at specified ratios of designer CTL effectors to MIPCEA targets (E:T) for 4 hrs in a chromium-release assay. CEA(−) MIP101 cells in parallel assays were <5% lysed at the highest E:T ratios (not shown).
3. Cytokine secretion
Cytokine secretion by T cells in support of their own growth and activity is a characteristic of T lymphocyte biology. IL2 is an essential growth factor for T cell survival and secretion of gamma interferon has been correlated with tumor rejection. Other studies showed that IL2 is secreted principally by CD4 helper T cells and that gamma interferon (IFNg) may be secreted by both CD4 and CD8 cells on antigen stimulation [e.g., ref.39]. We assayed for IL2 and IFNg in our bulk unfractionated modified T cells to assess whether these cytokines could be detected after antigen stimulation. The IgTCR 1st generation designer T cells were inert on MIPCEA for cytokine secretion, producing IL2 only if tumor artificially also provided B7 antigen for stimulation of native CD28 on the T cells.3 In contrast, insertion of the IgCD28TCR gene into CD4+ T cells leads to cellular activation and IL2 secretion on MIPCEA (Fig.4A). The 2nd generation designer T cells also secreted high levels of IFNg, marking these T cells as potent Th1/Tc1 effectors [16] (Fig.4B).
Fig. 4.
Cytokine secretion. A, B. High cytokine production with IgCD28TCR. Designer T cells (20% modified) were cultured with tumor cells overnight and supernatants harvested and assayed for (A) IL2 and (B) gamma interferon (IFNg) by ELISA. All cultures were negative for cytokine production on MIP101 and MIP101-B7 (not shown). By the international unit definition (WHO standard), 30 ng/ml of IL2 corresponds to 450 IU/ml. C. Tandem T cells die without IL2. T cells from A during log-phase expansion in IL2 were washed and placed in medium -IL2 without tumor cells. T cells were replaced with fresh medium -IL2 on day 3. D. Limited survival of Tandem T cells when depending solely upon their own stimulated IL2 secretion. At time zero and each time point, T cells as in C. were fed irradiated MIPCEA tumor cells and placed in fresh medium, (D1) +IL2 (100 IU/ml) or (D2) -IL2. Note 5X scale difference, with lower cell numbers for -IL2. (Data in D1 reproduced in part from Fig.2B.) E. Exhaustion of IL2 secretion with restimulation. Tandem T cells were incubated with MIPCEA cells as in D2 with medium change and re-plating of viable T cells with fresh irradiated MIPCEA tumor every 48 hours. IL2 measured at each 48-hour time point by ELISA. IL2 was unmeasurable after the third stimulation (days 4–6).
We then examined the impact of IL2 secretion on T cell survival. Activated prior to transduction and maintained in IL2, all T cells expanded rapidly (e.g., Fig.2A,B). When subjected to IL2 withdrawal in the absence of antigen stimulation, however, all cells died within 7 days (Fig.4C). These data confirm that designer T cells, 1st and 2nd generation alike, remain absolutely dependent upon IL2 for their survival.
For Tandem T cells trafficking to tumor with activation on contact with tumorous CEA, there may be local secretion of IL2, possibly at levels sufficient for the IL2 needs of an anti-tumor immune response. We sought to derive a functional in vitro test of this hypothesis.
In vivo, the IL2 that is secreted diffuses out of the tissue and into the blood where it is cleared by renal filtration with an abbreviated half-life of minutes to hours [17]. In vitro, the culture flask artificially retains the secreted IL2 and sustains activity that would otherwise dissipate in vivo. To model better the in vivo setting, therefore, we periodically exchanged the flasks with fresh medium (-IL2) two times per week, each time also feeding with fresh CEA+ tumor cells. This forces the T cells to depend for their survival on IL2 that was re-secreted with each stimulation.
Under +IL2 conditions (Fig.4D1), normal untransduced and 1st generation T cell cultures maintained viability and basal expansion and Tandem T cells exhibited accelerated proliferation, as previously shown. Under the –IL2 condition (Fig.4D2), control unmodified and 1st generation T cells died quickly, as in Fig.4C. In contrast, Tandem T cells on CEA+ tumor exhibited an initial period of expansion, indicating that the IL2 secreted by the Tandem T cells was sufficient for growth in the first stimulations (Fig.4D2). This expansion is in contrast to death of Tandem T cells over the same period when placed into -IL2 medium without stimulation (Fig.4C). However, with subsequent re-stimulations, this proliferation began to lag behind that observed in the presence of added IL2: by day 9, there were only 1/3rd as many T cells in the -IL2 culture (Fig.4D2) as in the control Tandem culture +IL2 (Fig.4D1) (note scale difference), after which there was a progressive T cell decline.
When testing IL2 production, we confirmed an exhaustion of autogenous IL2 secretion, even with the advantage of the co-stimulation (Fig.4E). Because CD28 co-stimulation is stated to upregulate Bcl family proteins [18] that oppose apoptosis on cytokine withdrawal [19], it was conceivable that the Tandem T cells would be IL2-independent and not require either autologous or supplemented IL2 for their in vivo survival. However, our results of T cell death indicates that any Bcl upregulation after CD28 co-activation [18,19] was insufficient to render the T cells immune to IL2 deprivation. This scenario presents us with the likelihood of a value for IL2 supplementation in human therapies for extended in vivo efficacy of 2nd generation designer T cells.
Animal model
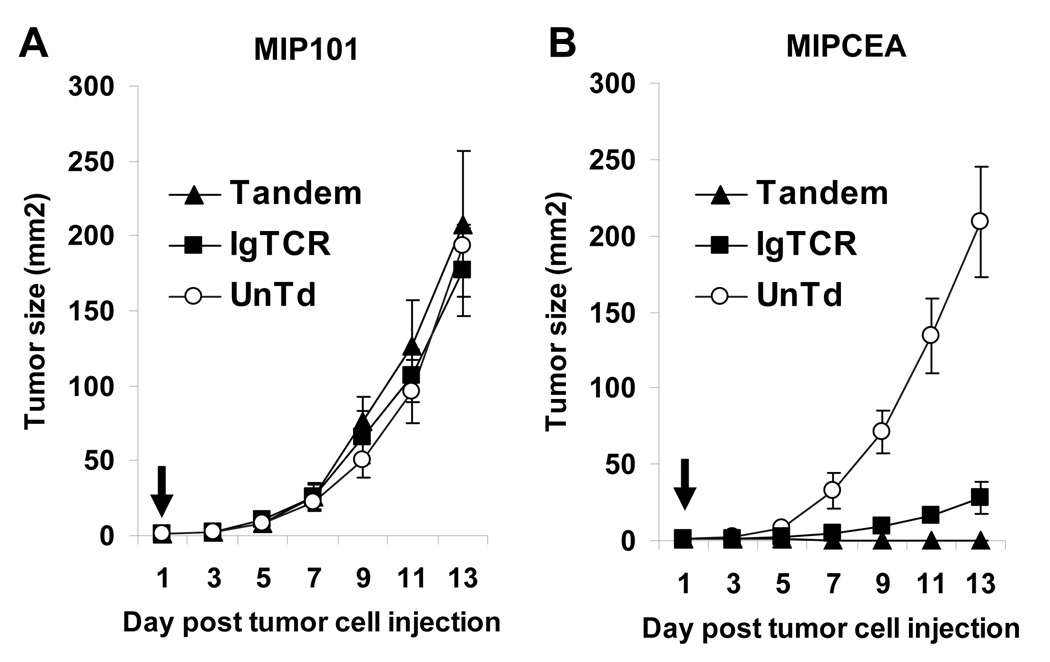
Finally, the effect of the 2nd versus 1st generation designer T cells was examined in a mouse model with human tumor xenografts (Fig.5). 1st generation designer T cells delayed CEA+ tumors relative to CEA(−) control or untransduced T cells, but all tumors grew. In contrast, 2nd generation T cells suppressed tumors in all animals (p<0.02) 4. By 7 days, masses that were initially palpable at 2–3 days were no longer detectable and no tumors were evident at two weeks when animals were sacrificed due to large CEA(−) control tumor size. No toxicity was evident in any animal.
Fig. 5.
Treatment of in vivo tumor model. Nude mice (4 per group) were injected s.c. with 5×106 MIP101 (left panel) and MIPCEA (right panel) tumor cells on opposing flanks. After 1 day, a small nodule was palpable at each site, and 5×107 T cells were injected via tail vein (arrow). The modified T cell fraction was 20–22% of total cells for both IgTCR and Tandem. Tumor size was tracked over two weeks (average +/− SEM), when animals were sacrificed due to large size of control tumors.
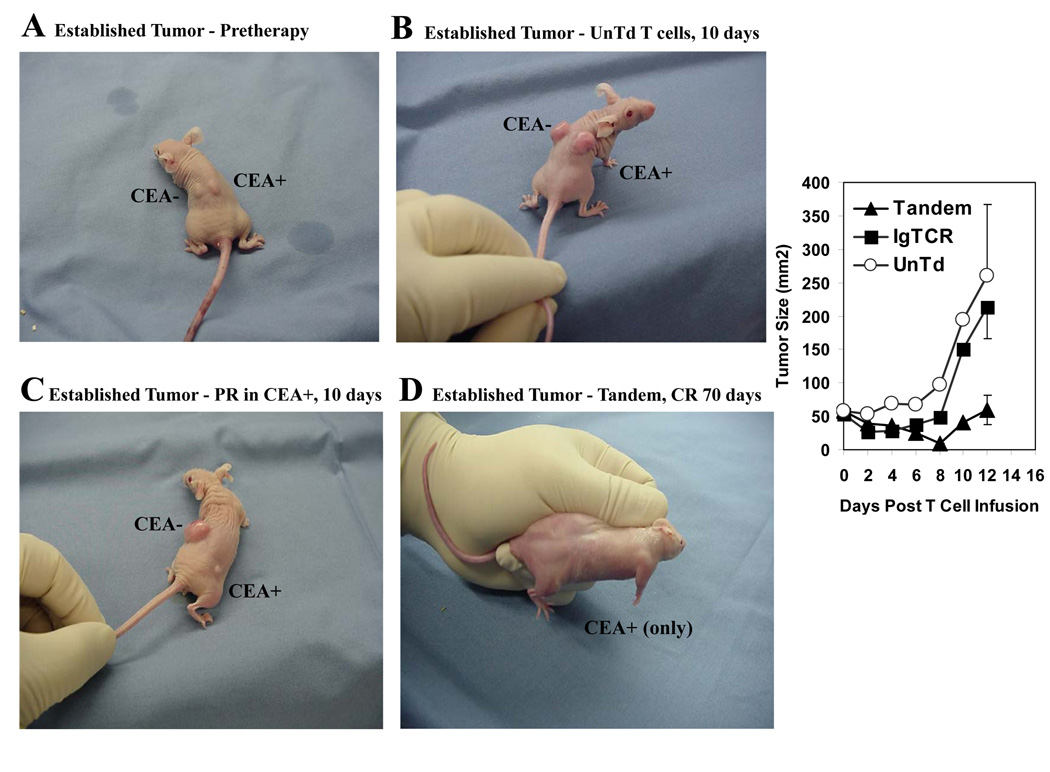
In pilot tests with established tumors (Fig.6A), there was also an immunotherapeutic benefit. Prior to designer T cell injection, these established tumors were allowed to grow six days instead of one day as in Fig.5. Because of an expected longer duration of activity required of the infused T cells for cure in the established tumor setting, we added IL2 in vivo to support the T cells beyond the 3–6 days of their anticipated survival in the absence of IL2 as seen in Fig.4C. Animals treated with activated but unmodified T cells had equivalent growth of CEA− and CEA+ tumors at 10 days (Fig.6B). With designer T cells, CEA+ tumor was reduced and CEA- tumor unaffected (Fig.6C). In a comparison among animals carrying just CEA+ tumors (Fig.6 right), there was an initial delay of tumor growth by activated normal T cells supplemented with IL2, while actual tumor suppressions required the anti-CEA specificity of the modified cells, seen with both IgTCR and Tandem, as also seen in Fig.5. However, the response duration was longer for Tandem than IgTCR to result in smaller mean tumor size at 12 days (50 versus 210 mm2) (p<0.05), despite a lower fraction of modified T cells in the Tandem treatment group (25% versus 40%). (At the same 12-day time point, the difference was non-significant between IgTCR and control unmodified T cells.) Among the Tandem treated group with established tumors, 1/4 animals was without tumor at 12 days (in contrast to 4/4 with small tumors), which was still tumor-free at 70 days, potentially representing cure (Fig.6D).
Fig. 6.
Treatment of established tumors. Nude mice (4 per group) were injected with MIP101 and MIPCEA as in Fig.5 and observed for 6 days until tumors were approximately 7 mm in diameter (~50 mm2). At this time (six days = day 0 on chart), 5×107 T cells were injected by tail vein, either untransduced (UnTd) or IgTCR or Tandem (both 22% modified), and IL2 was initiated at 2.5 × 105 IU s.c. every 12 hours. Tumor size was tracked for 12 days (+/− SEM), at which time animals were sacrificed due to large size of control tumors. A. Tumors at time of T cell injection. B. Tumors at 10 days after treatment with UnTd T cells. C. Tumors at 10 days after treatment with Tandem T cells, showing partial response (PR) of CEA+ tumor. D. Tumor (CEA+ only) with complete response (CR) at 7 days after treatment with Tandem T cells that was maintained at 70 days. Right panel: Average and standard error for CEA+ tumor sizes (mice bearing CEA+ tumors only) at times after T cell injection (N=4 for each group).
DISCUSSION
To date, chimeric immune receptors have been generated to a variety of tumor-associated antigens [2]. The target to which this agent is directed, carcinoembryonic antigen (CEA), is expressed at high levels on tumor cells of many colorectal, breast and lung carcinomas and other cancers [20]. CEA is expressed on normal cells of gastric fovea, colonic epithelium, and elsewhere in the GI tract, but mainly on microvilli on lumenal surfaces that should topologically be sequestered to attack. Furthermore, tumor cells typically express quantitatively much higher levels of CEA (estimated at 35-fold), which enhances discrimination between normal and tumorous expression of the protein [21]. MN14 [22] is a Primus class III antibody, defined as reacting only with CEA in the family of CEA-related proteins. We acquired a humanized version of MN14 for engineering human T cells, with the advantage of reducing or eliminating host responses that rarely target the small retained murine segments in the antibody binding domain [23].
Designer T cells have had a number of clinical tests, all 1st generation [10,24,25,26,27]. One agent induced hepatotoxicity by action against normal expression of antigen on bile duct [27], but others have been well-tolerated, to date also without major responses. Our own 1st generation anti-CEA clinical trial [10] showed adequate patient tolerance and proof-of-principle anti-tumor immune activity, but that was limited by lack of sustained effect. Evolving understandings of the normal role of co-stimulation in T cell biology led to a belief that AICD was at least contributory to the inadequacy of these early products [11,28,29]. Signal 1 through the T cell receptor (TCR) or the chimeric receptor (IgTCR) was adequate for T cell cytotoxicity but leads to little or no IL-2 production and promotes anergy or apoptosis without effector cell expansion. In contrast, Signal 1 in conjunction with CD28 (Signal 2) induces robust IL2 production and up-regulation of anti-apoptosis genes such as Bcl-2, Bcl-xL and cFLIP [18,19,30,31,32]. Accordingly, a number of researchers have generated fusion molecules that confer on the engrafted T cells a functional CD28 signal after encountering specific antigen [13,14,15].
The data in this report validate core premises of our motivation for creating 2nd generation anti-CEA designer T cells. This modification results in improved features of blocked-AICD and tumor-induced T cell proliferation that appeared key in the face of time-limited efficacies in clinical testing of our 1st generation construct. We previously showed that IgTCR Signal 1-only designer T cells die over a period of days while nonetheless killing CEA+ targets. In the present report, we confirm that this death is apoptotic as supposed for AICD and that Tandem T cells were resistant to such death. Further, the incorporation of CD28 in the CIR was confirmed to lead to augmented proliferation on antigen contact, with a doubling of growth rates above the basal expansion of the previously activated T cells. In correlative reconstruction studies, the effect of CD28 recruitment in our Tandem constructs was to induce resistance to both AICD and fas-apoptosis, which are linked via FLIP [33], but not to render T cells IL2-independent, a function ascribed to Bcl family members [19, Gomes et al, in prep.]. Tandem T cells were also more potent in lytic units per cell than the 1st generation construct. Additionally, Tandem T cells exhibited heightened secretion of cytokines IL2 and gIFN on tumor contact. Finally, in an animal model, Tandem T cells with their improved survival, greater cytolytic potency, and heightened cytokine secretion, were superior in in vivo tumor therapy models with cures of small tumors and some established tumors.
Although improved cytokine production is widely reported for Tandem-type designer T cells, our functional data are the first to examine whether effective cytokine production by T cells is sustainable, from which there appears to be a limited capacity on restimulation. This raises significant question of the ability of modified T cells in this format to support their own growth with autogenous IL2 that may require yet other signals for persistent secretion (e.g., LFA1 [28,29], E Gomes & R Junghans, unpublished results). The benefit of this improved, albeit limited, feature of improved cytokine production is less critical because the designer T cells may be supported by infusional IL2 in patients. By contrast, beyond being a marker for Th1/Tc1 cells [16], the therapeutic role of IFNg is less clear. IFNg has been touted as stimulating up-regulation of MHC to increase efficiency of TCR target recognition [34], but this would not assist designer T cells, which are MHC-independent. Even in situations where T cells are dependent upon MHC for cytotoxic targeting, clinical data have shown no correlation of T cell IFNg production with clinical benefit [35]. IFNg has been cited to oppose the action of TGF-beta in tumor microenvironment [36,37]. In mouse models, IFNg was critical to the therapeutic elimination of tumor by 2nd generation designer T cells [38,39]. Incorporation of Signal 2 was separately shown to render designer T cells resistant to regulatory T cell (Treg) suppression [40], and the expression of IFNg that is secreted may plausibly mediate this resistance that correlates with improved anti-tumor activity in model systems. This has not as yet been directly addressed.
Cytotoxicity was improved in our 2nd generation versus 1st generation designer T cells. Calculated on a basis of lytic units per cell, it translated into a 4- to 8-fold improvement. In some prior reports, CD28 inclusion improved killing over 1st generation [41,42], but most comparisons showed no difference in killing potential [38,43,44]. We have also seen this advantage of 2nd generation in our GD3 constructs [45; A Lo and RPJ, in preparation]. The basis for an improvement is not presently understood and was not explored further.
Finally, animal tests corroborated an improvement with Tandem T cells in vivo that was seen in vitro. Animal models have in general shown benefit to adding co-stimulation when compared to 1st generation T cells [38,44], paralleling still earlier studies with bispecific antibodies that recruited CD28 co-stimulation [46].
For small tumors, treatment with 1st generation T cells showed specificity and growth delay in all CEA+ tumors, but no cures, while 2nd generation T cells cured all animals. The tumor ablation was not immediate: tumor grew for 2 days after Tandem infusion before regressing. This demonstrates that the relatively few designer T cells that will traffick to tumor can suppress a much larger number of malignant cells. For larger (~7 mm) tumors, 2nd generation T cells exhibited the same relative benefit over 1st gen, but with a lower rate of cure than in small tumors.
Given that our therapeutic goal with Tandem in suppressing AICD is precisely to secure prolonged T cell persistence in tumors, the differences in immune environments (complement, ligands, adhesion molecules, etc) that limit human T cell persistence will be detrimental to this same goal in mice: if the T cells cannot survive long periods in vivo, the full benefit of these modifications to suppress large tumors will not be appreciated. Further, it has been shown that IFNg is critical to the cure of established tumors in mice, whereas the human cytokine released by the designer T cells does not interact with the murine IFN receptors to recruit cooperating host cellular activities for tumor elimination [38,39]. As suggested by Moeller et al [39], there are “several problems” with testing the efficacy of human T cells in mice. This likely dictates that further product advances for treating solid tumors in humans that depend upon human T cell persistence and expansion will not be demonstrated in this mixed-species in vivo model using human T cells, but that purely murine systems will continue to be applied for proof-of-principle demonstrations. It is our hypothesis, therefore, that responses with this agent will be much better in the autologous setting in humans than was observable with human T cells in our mice – where we already proved that systemically administered 2nd generation designer T cells can cure established tumors. The cure of established (e.g., stage IV) tumor in any patient with CEA-positive colon, breast or lung cancer will be a revolutionary outcome. Hence, for this reason especially, clinical trials of these advanced agents are awaited with anticipation for the display of their full potential.
With documentation of tumor-induced T cell expansion, the 2nd generation designer T cells offer the perspective of a self-sustained anti-tumor immune response in human anti-CEA cancer therapies. Because the Tandem CIR activates T cells through the normal TCR and co-stimulation pathways, this same feature is also self-limiting for T cell survival in the absence of antigen, a key safety feature for human use: when tumor is gone, the proliferative impulse is absent, after which the T cells gradually plateau and then decline in number over a period of a couple of weeks. Further, the designer T cells are entirely dependent upon IL2 for their survival. In vivo, the elimination of tumor will withdraw any stimulus for autogenous IL2 production, and termination of IL2 infusion at that time should therefore lead to decline of the surviving 2nd generation designer T cells in human therapies, providing a further safety assurance. With elimination of antigen and IL2, the CEA-specific T cells will die off with a small fraction returning to a resting, memory state, potentially maintaining an immune surveillance against recurrent tumor.
The 1st generation version of the anti-CEA designer T cells was applied in a phase I clinical trial without and with IL2 co-administration that showed adequate safety and proof-of-principle biologic responses, but which were time-limited in their activity [10]. By our laboratory correlate studies, it was inferred that AICD and a deficiency of co-stimulation underlay the decline of T cell activity in vivo in our trial [11]. These studies provided the impetus to engineer a 2nd generation (Tandem) version of this clinical product that could resist AICD and therefore persist longer in vivo to induce a meaningful response in human therapies. These preclinical tests establish essential data concordant with the intended features of a 2nd generation reagent. Clinical trials are under way in which the hypotheses of extended anti-tumor function of 2nd generation designer T cells will be assessed for actual human clinical utility. Future directions include the possibility of co-expressing additional molecules, supplementing Tandem with FLIP and/or Bcl-xl for the potential to augment the resistance of designer T cells to AICD and/or to render them immune to cytokine deprivation, obviating the need for systemic IL2 supplementation. Other maneuvers to include Signal 3 molecules (e.g., LFA-1) for more robust T cell activation and sustained IL2 secretion may be additional options. Such 3rd generation configurations would require additional tests of safety and efficacy before human translational studies.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants to RPJ from the National Cancer Institute/NIH, the US Army / Department of Defense Breast Cancer Program and the Geyer Foundation.
Footnotes
T cells must be activated and induced to proliferate before infecting them with retrovirus. The basal expansion is that of the T cell population after this initial stimulus and may continue for 2–4 weeks, after which T cell numbers decline, even with IL2 present at saturating levels (data not shown).
Proliferation tests longer than two weeks were not performed with Tandem, but the principle of an advantage of two-signals for proliferation was established for a minimum of 30 days in our earlier tests [11].
Prior 1st generation IgTCR assays of IL2 secretion [9] included phorbal ester (PMA) as a Signal 2-like agent, affording evidence for Signal 1, but not producing IL2 in a manner relevant to in vivo needs.
p = 0.014 by Fisher exact test (0/4 with tumor at 15 days for Tandem, versus 4/4 with tumor for IgTCR). When tumor sizes are examined by two-tailed t-test, Tandem has better response at p<0.002.)
Evidence for some cleavage and shedding of ECD of the receptor is seen with the forms slightly larger than ζ in the IgTCR (monomer and dimer). The cleaved non-reduced (NR) IgTCR remains dimeric because the linking cysteines are in the TM domain. A still larger cleaved form is seen in the reduced (R) Tandem that includes the cytoplasmic portion of CD28. This latter form does not show dimers in the NR lane, apparently cleaved proximal to the dimerizing cysteine in the CD28 ECD. These data suggest that the TM cys in CD28 does not participate in disulfide bonding as does its counterpart in TCRζ.
References
- 1.Rosenberg SA. Principles and applications of biologic therapy. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Lippincott; 1993. pp. 293–324. [Google Scholar]
- 2.Ma QZ, Gonzalo-Daganzo R, Junghans RP. Genetically engineered T cells as adoptive immunotherapy of cancer. In: Giaccone R, Schlinsky R, Sondel P, editors. Cancer Chemotherapy & Biological Response Modifiers - Annual 20. Oxford: Elsevier Science; 2002. pp. 319–345. [PubMed] [Google Scholar]
- 3.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88:100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sykulev Y, Cohen RJ, Eisen HN. The law of mass action governs antigen-stimulated cytolytic activity of CD8+ cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1995;92:11990–11992. doi: 10.1073/pnas.92.26.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 7.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636–642. [PubMed] [Google Scholar]
- 8.Radvanyi LG, Shi Y, Vaziri H, Sharma A, Dhala R, Mills GB, Miller RG. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J Immunol. 1996;156:1788–1798. [PubMed] [Google Scholar]
- 9.Nolan KF, Yun CO, Akamatsu Y, et al. Bypassing immunization: Optimized design of 'designer T cells' against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin Cancer Res. 1999;5:3928–3941. [PubMed] [Google Scholar]
- 10.Junghans RP, Safar M, Huberman MS, et al. Preclinical and phase I data of anti-CEA “designer T cell” therapy for cancer: A new immunotherapeutic modality. Proc Am Soc Clin Oncol. 2001;A1063 [Google Scholar]
- 11.Beecham EJ, Ma QZ, Ripley R, Junghans RP. Coupling of CD28 co-stimulation to IgTCR molecules: Dynamics of T cell proliferation and death. J Immunother. 2000;23:631–642. doi: 10.1097/00002371-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Vallina L, Harkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol. 1996;26:2304–2309. doi: 10.1002/eji.1830261006. [DOI] [PubMed] [Google Scholar]
- 13.Finney HM, Lawson ADG, Bebbington CR, Weir ANC. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 14.Krause A, Guo H, Latouche J, Tan C, Chueng NV, Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med. 1998;188:619–626. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beecham EJ, Ortiz-Pujols S, Junghans RP. Dynamics of tumor cell killing by human T lymphocytes armed with an anti-CEA chimeric immunoglobulin-T cell receptor. J Immunother. 2000;23:332–343. doi: 10.1097/00002371-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Romagnani S, Kapsenberg M, Radbruch A, Adorini L. Th1 and Th2 cells. Res Immunol. 1998;149:871–873. doi: 10.1016/s0923-2494(99)80016-9. [DOI] [PubMed] [Google Scholar]
- 17.Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol. 1985;134:157–166. [PubMed] [Google Scholar]
- 18.Noel PJ, Boise LH, Thompson CB. Regulation of T cell activation by CD28 and CTLA4. Adv Exp Med Biol. 1996;406:209–217. doi: 10.1007/978-1-4899-0274-0_22. [DOI] [PubMed] [Google Scholar]
- 19.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- 20.Schwartz MK. Cancer markers. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Lippincott; 1993. pp. 531–542. [Google Scholar]
- 21.Boucher D, Cournoyer D, Stanners CP, Fuks A. Studies on the control of gene expression of the carcinoembryonic antigen family in human tissue. Cancer Res. 1989;49:847–852. [PubMed] [Google Scholar]
- 22.Hansen H, Goldenberg DM, Newman ES, Grebenau R, Sharkey RM. Characterization of second generation monoclonal antibodies against carcinoembryonic antigen. Cancer. 1993;71:3478–3485. doi: 10.1002/1097-0142(19930601)71:11<3478::aid-cncr2820711104>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Scheinberg DA, Sgouros G, Junghans RP. Antibody-based immunotherapies for cancer. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. 4th Edition. Philadelphia: Lippincott; 2006. pp. 850–890. [Google Scholar]
- 24.Deeks SG, Wagner B, Anton PA, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5:788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 25.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor redirected cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 27.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 28.Sprent J. Stimulating naive T cells. J Immunol. 1999;163:4629–4636. [PubMed] [Google Scholar]
- 29.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MGO, Schlom J. A triad of costimulatory molecules synergize to amplify T cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 30.Boise LH, Minn AJ, Noel PJ, et al. CD28 co-stimulation can promote T cell survival by enhancing the expression of Bcl-xl. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhoff S, Muller WW, Li-Weber M, Krammer PH. Up-regulation of c-FLIPshort and reduction of activation-induced cell death in CD28-costimulated human T cells. Eur J Immunol. 2000;30:2765–2774. doi: 10.1002/1521-4141(200010)30:10<2765::AID-IMMU2765>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Dohrman A, Kataoka T, Cuenin S, Russell JQ, Tschopp J, Budd RC. Cellular FLIP (long form) regulates CD8+ T cell activation throughcaspase-8-dependent NF-kappa B activation. J Immunol. 2005;174:5270–5278. doi: 10.4049/jimmunol.174.9.5270. [DOI] [PubMed] [Google Scholar]
- 33.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 34.Giacomini P, Fisher PB, Duigou GJ, Gambari R, Natali PG. Regulation of class II MHC gene expression by interferons: insights into the mechanism of action of interferon. Anticancer Res. 1988;8:1153–1161. [PubMed] [Google Scholar]
- 35.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao BG, Zhang GX, Ma CG, Link H. Transforming growth factor-beta 1 (TGF-beta1)-mediated inhibition of glial cell proliferation and down-regulation of intercellular adhesion molecule-1 (ICAM-1) are interrupted by interferon-gamma (IFN-gamma) Clin Exp Immunol. 1996;103:475–481. doi: 10.1111/j.1365-2249.1996.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strober W, Kelsall B, Fuss I, et al. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 38.Haynes NM, Trapani JA, Teng MW, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- 39.Moeller M, Haynes NM, Kershaw MH, et al. Adoptive transfer of gene-engineered CD4+ helper T cells induces potent primary and secondary tumor rejection. Blood. 2005;106:2995–3003. doi: 10.1182/blood-2004-12-4906. [DOI] [PubMed] [Google Scholar]
- 40.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 41.Willemsen RA, Ronteltap C, Chames P, Debets R, Bolhuis RL. T cell retargeting with MHC class I-restricted antibodies: the CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J Immunol. 2005;174:7853–7858. doi: 10.4049/jimmunol.174.12.7853. [DOI] [PubMed] [Google Scholar]
- 42.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 43.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 44.Moeller M, Haynes NM, Trapani JA, et al. A functional role for CD28 costimulation in tumor recognition by single-chain receptor-modified T cells. Cancer Gene Ther. 2004;11:371–379. doi: 10.1038/sj.cgt.7700710. [DOI] [PubMed] [Google Scholar]
- 45.Yun CO, Nolan KF, Beecham EJ, Reisfeld RA, Junghans RP. Targeting of T lymphocytes to melanoma through chimeric anti-GD3 immunoglobulin-T cell receptors (IgTCR) Neoplasia. 2000;2:449–459. doi: 10.1038/sj.neo.7900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renner C, Jung W, Sahin U, et al. Cure of xenografted human tumors by bispecific monoclonal antibodies and human T cells. Science. 1994;264:833–835. doi: 10.1126/science.8171337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.