Abstract
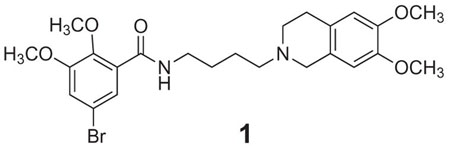
5-Bromo-N-[4-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-butyl)]-2,3-dimethoxy-benzamide (1) is one of the most potent and selective σ2 receptor ligands reported to date. Previous structure-activity relationship studies of such tetrahydroisoquinolinyl benzamides have focused on the linker that connects the ring systems and the effects of benzamide ring substituents. The present study explores the effects of fusing methylene-, ethylene- and propylenedioxy rings onto the tetrahydroisoquinoline in place of the two methoxy groups. These modifications decreased σ2 affinity by 8- to 12-fold, with no major differences noted with ring size. By contrast, the methylenedioxy analog showed a 10-fold greater σ1 affinity than 1, and progressively lower σ1 affinities were then noted with increasing ring size. We also opened the tetrahydroisoquinoline ring of 1 to study the effects of greater conformational fluidity on σ receptor binding. The σ2 affinity of the open-ring compound decreased by 1700-fold, while σ1 affinity was not changed. Thus, a constrained tetrahydroisoquinoline ring system is key to the exceptional σ2 receptor binding affinity and selectivity of this active series.
Keywords: σ receptors, tetrahydroisoquinoline
Functional sigma (σ) receptors are located throughout the brain and periphery, and can be differentiated into σ1 and σ2 subtypes.1–4 These subtypes play distinct functional roles, and have different pharmacological characteristics. Both σ1 and σ2 subtypes are involved in central nervous system disorders such as schizophrenia, depression and dementia.1,2 Certain σ receptor antagonists can ameliorate the effects of cocaine and other psychostimulant drugs of abuse, and have potential as medications.1–3 Moreover, σ receptors are over-expressed by many cancers.4 Some σ receptor ligands induce apoptosis in cancer cells,5–7 and one is in a clinical trial for prostate cancer treatment.8 Thus, there is much interest in subtype selective σ receptor ligands as molecular probes and as therapeutic agents.
A variety of structural classes are avid binders to both σ receptor subtypes which has hampered the development of selective binding models.9,10 Although a number of studies have investigated the effects of structure on relative σ1 / σ2 receptor binding affinity and selectivity, few truly selective compounds are known. Recently, Mach and colleagues11 identified a series of tetrahydroisoquinolinyl benzamides that rank among the most selective σ2 receptor ligands known to date. For example, 1 displays high apparent affinity, Ki = 8.2 nM, for σ2 sites in vitro accompanied by 1573-fold selectivity over σ1 sites.
Published structural modifications have concentrated on the length of the alkyl spacer connecting the two different ring systems, and the effects of various benzamide substituents.11–13 To extend the structure-activity relationships for this active series, we report on the effects of fusing methylene-, ethylene- and propylenedioxy rings onto the tetrahydroisoquinoline. This stems from work on 1,4-disubstituted piperazines, where we found that σ receptor subtype affinity and selectivity can be modulated by similar manipulations of dimethoxybenzene moieties.14 In addition, we noticed that C-N bond rotation in 1 is limited by the tetrahydroisoquinoline ring. Thus, we wished to open this ring to gain insight into the contributions of conformational fluidity to σ receptor binding.
Compound 1 was obtained for reference using the reported methods.11 The novel congeners were prepared as shown in Schemes 1–3. For methylenedioxy analog 2, the corresponding tetrahydroisoquinoline was synthesized from piperonal using an established route that culminates with the Pictet-Spengler reaction.15–17 Alkylation with 4-bromobutanenitrile, followed by reduction and amidation with 5-bromo-2,3-dimethoxybenzoyl chloride, afforded 2 which was characterized as the oxalate salt (Scheme 1).
Scheme 1.
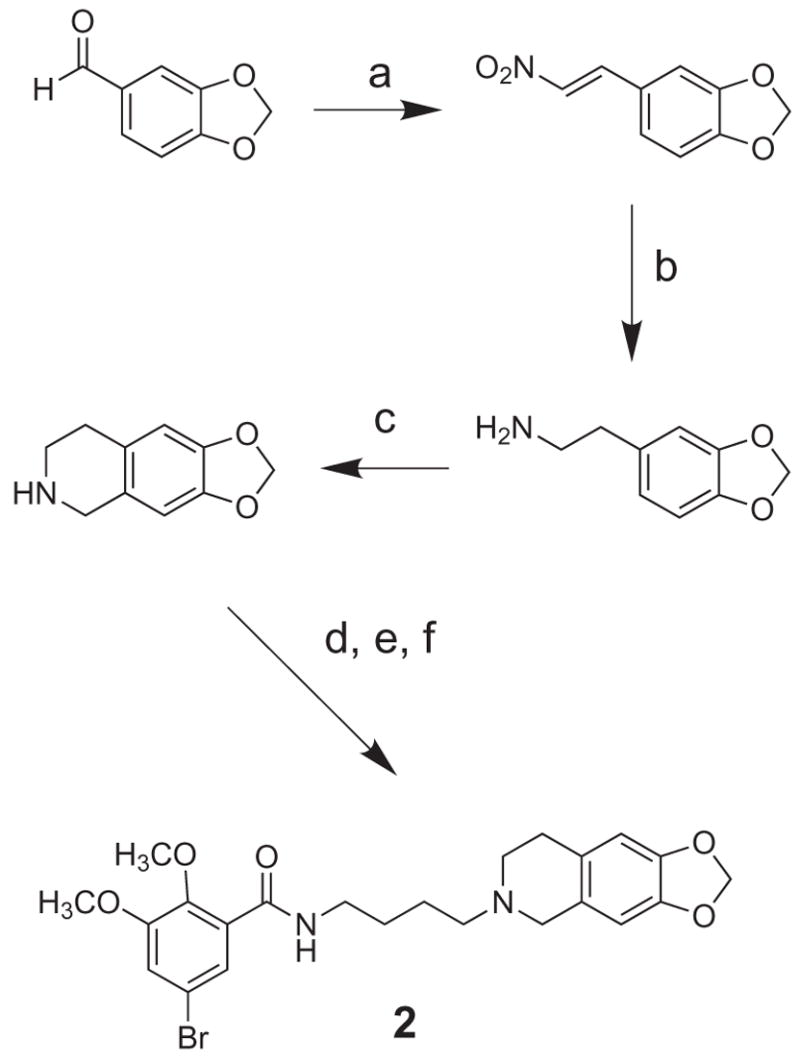
(a) CH3NO2, MeOH, NaOH; (b) LiAlH4; (c) paraformaldehyde; (d) 4-bromobutanenitrile, K2CO3, NaI, DMF; (e) LiAlH4; (f) 5-bromo-2,3-dimethoxybenzoyl chloride.
Scheme 3.
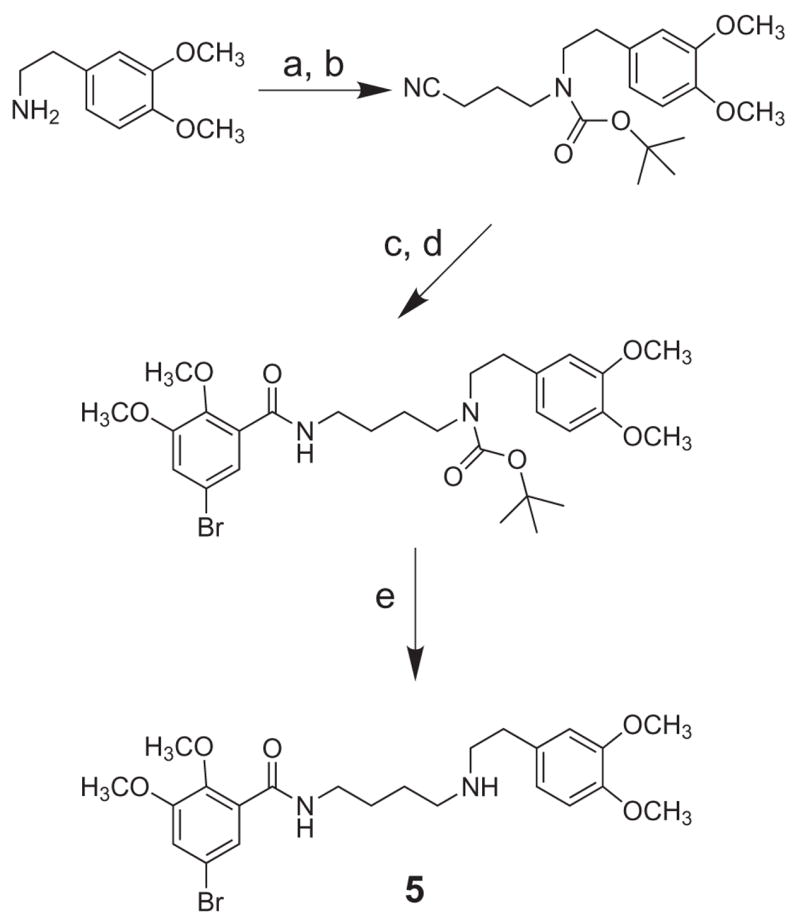
(a) 4-bromobutanenitrile; (b) (Boc)2O, MeOH, Et3N; (c) LiAlH4; (d) 5-bromo-2,3-dimethoxybenzoyl chloride; (e) 10% TFA, CH2Cl2.
Ethylenedioxy (3) and propylenedioxy (4) analogs were synthesized in parallel fashion from their corresponding tetrahydroisoquinolines (Scheme 2). In turn, these three-ring heterocycles were obtained from N-Boc protected tetrahydroisoquinoline diol by base-promoted cycloalkylation with the appropriate dibromoalkane catalyzed by tetrabutylammonium bromide (Scheme 2).
Scheme 2.
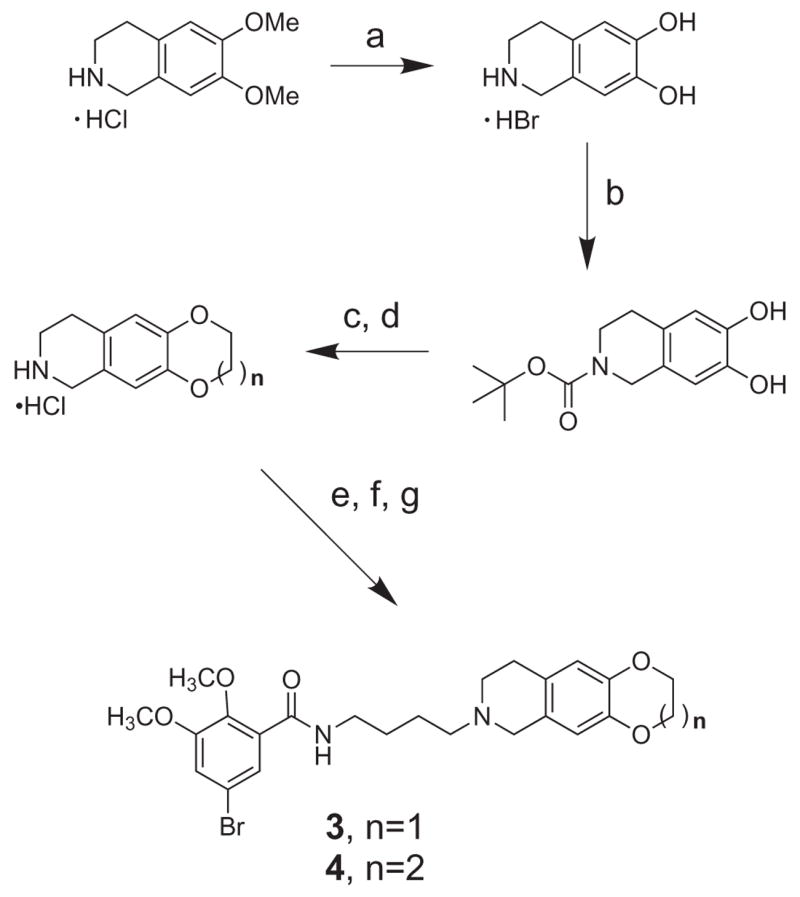
(a) 48% HBr, 120 °C, 2 h; (b) (Boc)2O, MeOH, Et3N; (c) Br-(CH2)n-Br: n = 2, 3, TBAB; (d) 4N HCl; (e) 4-bromobutanenitrile, K2CO3, NaI, DMF; (f) LiAlH4; (g) 5-bromo-2,3-dimethoxybenzoyl chloride.
As shown in Scheme 3, open-ring compound 5 was prepared by alkylation of the commercially available 2-(3,4-dimethoxyphenyl)ethanamine, followed by N-Boc protection, reduction, amidation and deprotection.
As expected, compound 1 displayed very high affinity and selectivity for σ2 sites in vitro (Table 1). The degree of σ2 selectivity, based upon Ki ratios, was somewhat less than previously found11 as a consequence of a higher apparent affinity for σ1 sites. The σ1 receptor assay in guinea pig brain membranes is susceptible to slight changes in conditions. So, we also tested 1 using the previously reported regimen (pH 8.0 vs. pH 7.4 buffer, 3.0 nM vs. 1.0 nM [3H](+)-pentazocine, 25 vs. 37 °C, 120 vs. 150 min, and 10 μM (+)-pentazocine vs. 1.0 μM haloperidol to define nonspecific binding). The σ1 receptor IC50 value of 1273 ± 22 nM found for 1 under the present conditions increased substantially, about 50%, to 1895 ± 110 nM. Comparing this lower affinity σ1 receptor IC50 with the σ2 receptor IC50 of 3.0 ± 0.11 for 1 under the present conditions would double the selectivity assigned. Also, the σ2 receptor binding was assessed using rat liver membranes in the previous work, while guinea pig brain membranes were employed in the present study. In such ways, experimental factors can impact the σ1 / σ2 subtype selectivity determinations from various laboratories.
Table 1.
Binding properties of compounds 1 – 5 at σ1 and σ2 receptors.
| Compound |
Ki (nM)
|
ratio σ1/σ2 | |
|---|---|---|---|
| σ1 | σ2 | ||
| 1 | 881 ± 15 | 2.7 ± 0.1 | 326 |
| 2 | 82.2 ± 5.6 | 20.7 ± 2.0 | 4 |
| 3 | 338 ± 8.4 | 21.7 ± 1.2 | 16 |
| 4 | 1430 ± 36 | 32.6 ± 1.5 | 44 |
| 5 | 880 ± 60 | 4616 ± 247 | 0.2 |
Values are means ± SEM (n = 3 – 5) from competition assays against [3H](+)-pentazocine (σ1) and [3H]DTG / (+)-pentazocine (σ2) in membranes from male guinea pig brains.
Replacement of the two methoxy groups by a methylene-, ethylene- or propylenedioxy ring decreased σ2 affinity by 8- to 12-fold, with no major effects attributable to the specific sizes of the rings (Table 1). By contrast, methylenedioxy analog 2 showed a 10-fold greater σ1 affinity than the parent scaffold 1. Further effects of ring size were well defined, with progressively 4-fold lower σ1 affinities noted for the ethylenedioxy (2) and propylenedioxy (3) analogs. Thus, σ1 binding exhibits the most sensitivity to these perturbations. Together, the data indicate that σ1 / σ2 receptor binding affinity and selectivity can be modulated by subtle changes in molecular volumes, ring conformations, and the precise orientations of the oxygen atoms in this region.
Remarkably, the σ2 affinity of open-ring compound 5 decreased by 1700-fold, while the σ1 affinity was not changed (Table 1). It is difficult to provide a molecular explanation for such an interesting result. Nevertheless, this observation may aid in developing σ receptor binding models for tetrahydroisoquinolinyl benzamides. Clearly, the greater conformational freedom of 5 with respect to 1 is detrimental to σ2 receptor binding but has no influence on binding interactions with σ1 receptors. The effect is pronounced, and leads to a low affinity compound having 5-fold selectivity for binding to σ1 receptors. Thus, the constrained tetrahydroisoquinoline ring is critically important to high σ2 receptor binding affinity and selectivity.
In conclusion, we determined that modifications of the two methoxy groups of the tetrahydroisoquinolinyl benzamides can be used to modulate the relative affinities and selectivities of ligand binding to σ1 and σ2 receptor subtypes. We also demonstrated that a constrained tetrahydroisoquinoline ring system is key to the exceptional σ2 receptor binding affinity and selectivity observed for this active series.
Acknowledgments
We thank the National Cancer Institute (P50 CA 103130: Center for Single Photon-Emitting Cancer Imaging Agents) for partial support of this research. We also acknowledge facilities provided by Truman Memorial Veterans’ Hospital, and NSF CHE-95-31247 and NIH 1S10RR11962-01 grant awards for NMR instrumentation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Guitart X, Codony X, Monroy X. Psychopharmacol. 2004;174:301. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi T, Su T-P. CNS Drugs. 2004;18:269. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Eur J Pharmacol. 2003;469:1. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 4.Aydar E, Palmer CP, Djamgoz MB. Cancer Res. 2004;64:5029. doi: 10.1158/0008-5472.CAN-03-2329. [DOI] [PubMed] [Google Scholar]
- 5.Colabufo NA, Berardi F, Contino M, Niso M, Abate C, Perrone R, Tortorella V. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:106. doi: 10.1007/s00210-004-0961-2. [DOI] [PubMed] [Google Scholar]
- 6.Azzariti A, Colabufo NA, Berardi F, Porcelli L, Niso M, Simone GM, Perrone R, Paradiso A. Mol Cancer Ther. 2006;5:1807. doi: 10.1158/1535-7163.MCT-05-0402. [DOI] [PubMed] [Google Scholar]
- 7.Rothfuss J, Zeng C, Vangveravong S, Chu W, Tu Z, Hotchkiss R, Chang KC, Mach RH. MEDI; Abstracts of Papers 232nd National Meeting of the American Chemical Society; San Francisco CA. September 10–14, 2006; Washington, DC: American Chemical Society; 2006. p. 040. [Google Scholar]
- 8.Casellas P, Galiegue S, Bourrie B, Ferrini JB, Jbilo O, Vidal H. Anticancer Drugs. 2004;15:113. doi: 10.1097/00001813-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Glennon RA. Mini-Rev Med Chem. 2005;5:927. doi: 10.2174/138955705774329519. [DOI] [PubMed] [Google Scholar]
- 10.Glennon RA. Brazil J Pharm Sci. 2005;41:1. [Google Scholar]
- 11.Mach RH, Huang Y, Freeman RA, Wu L, Vangveravong S, Luedtke RR. Bioorg Med Chem Lett. 2004;14:195. doi: 10.1016/j.bmcl.2003.09.083. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Tu Z, Jones LA, Vangveravong S, Wheeler KT, Mach RH. Eur J Pharmacol. 2005;525:8. doi: 10.1016/j.ejphar.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 13.Rowland DJ, Tu Z, Xu J, Ponde D, Mach RH, Welch MJ. J Nucl Med. 2006;47:1041. [PubMed] [Google Scholar]
- 14.Xu R, Lever JR, Lever SZ. MEDI; Abstracts of Papers 233rd National Meeting of the American Chemical Society; Chicago IL. March 25–29; Washington, DC: American Chemical Society; 2007. 2007. p. 462. [Google Scholar]
- 15.Bowman RK, Johnson JS. J Org Chem. 2004;69:8537. doi: 10.1021/jo0485536. [DOI] [PubMed] [Google Scholar]
- 16.Koseki Y, Katsura S, Kusano S, Sakata H, Sato H, Monzene Y, Nagasaka T. Heterocycles. 2003;59:527. [Google Scholar]
- 17.Ruchirawat S, Chaisupakitsin M, Patranuwatana N, Cashaw JL, Davis VE. Synth Commun. 1984;14:1221. [Google Scholar]
- 18.Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. Synapse. 2006;59:350. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- 19.Data for 2. 1H NMR (free base, CDCl3, δ) 1.67 (m, 4H, CH2); 2.51 (t, 2H, CH2); 2.65 (t, 2H, CH2); 2.75 (t, 2H, CH2); 3.44–3.48 (m, 4H, CH2); 3.84–3.86 (2s, 6H, OCH3); 5.86 (s, 2H, OCH2O); 6.44 (s, 1H, aromatic CH); 6.52 (s, 1H, aromatic CH); 7.08 (d, 1H, CH); 7.74 (d, 1H, aromatic CH); 8.01 (t, 1H, amide NH). Mp. 174–175°C (mono-oxalate salt); Anal. Calcd for C23H27BrN2O5 • C2H2O4: C, 51.64; H, 5.03; N, 4.82. Found: C, 51.80; H, 5.15; N, 4.80.Data for 3. 1 H NMR (free base, CDCl3, δ) 1.68 (p, 4H, CH2); 2.52 (t, 2H, CH2); 2.66 (t, 2H, CH2); 2.75 (t, 2H, CH2); 3.49 (m, 4H, CH2). 3.86 (2s, 6H, OCH3); 4.20 (s, 4H, OCH2CH2O); 6.49 (s, 1H, CH); 6.57 (s, 1H, CH); 7.10 (d, 1H, CH); 7.75 (d, 1H, CH); 7.99 (t, 1H, amide NH). Mp. 146–147°C (mono-oxalate salt); Anal. Calcd for C24H29BrN2O5 • C2H2O4: C, 52.45; H, 5.25; N, 4.70. Found: C, 52.66; H, 5.30; N, 4.61.Data for 4. 1 H NMR (free base, CDCl3, δ) 1.66 (m, 4H, CH2); 2.13 (m, 2H, CH2); 2.50 (t, 2H, CH2); 2.65 (t, 2H, CH2); 2.77 (t, 2H, CH2); 3.40–3.48 (m, 4H, CH2). 3.84–3.86 (2s, 6H, OCH3); 4.12 (s, 4H, CH2O); 6.61 (s, 1H, CH); 6.69 (s, 1H, CH); 7.10 (d, 1H, CH); 7.75 (d, 1H, CH); 7.98 (t, 1H, amide NH). Mp. 163–164°C (mono-oxalate salt); Anal. Calcd for C25H31BrN2O5 • C2H2O4: C, 53.21; H, 5.46; N, 4.60. Found: C, 53.26; H, 5.48; N, 4.60.Data for 5. 1H NMR: (free base, CDCl3, δ) 1.66 (br m, 4H, CH2); 2.85 (t, 2H, CH2); 2.96 (t, 2H, CH2); 3.43 (t, 2H, CH2); 3.83–3.86 (4s, 12H, OCH3); 5.75 (br s, 1H, NH); 6.70–6.80 (m, 3H, aromatic CH); 7.11 (d, 1H, aromatic CH); 7.74 (d, 1H, aromatic CH); 7.99 (t, 1H, amide NH). Mp. 176–177°C (mono-oxalate salt); Anal. Calcd for C23H31BrN2O5 • C2H2O4: C, 51.29; H, 5.68; N, 4.79. Found: C, 51.07; H, 5.72; N, 4.75.
- 20.The σ receptor binding assays were performed as previously described in detail,18 except membranes were prepared exclusively from male guinea pig brains. In brief, σ1 assays used [3H](+)-pentazocine (1.0 nM) in 50 mM Tris-HCl buffer (pH 7.4, 25 °C) with nonspecific binding defined by haloperidol (1.0 μM). Assay tubes were incubated for 150 min at 37°C using 0.25 mg protein in a final volume of 1.0 mL. The σ2 assays used [3H] ditolylguanidine ([3H]DTG, 3.0 nM) with 200 nM (+)-pentazocine added as a σ1 receptor mask. Incubations were performed using 50 mM Tris-HCl buffer (pH 8.0, 25 °C) with nonspecific binding defined by DTG (100 μM). Assay tubes were incubated for 120 min at 25 °C using 0.25 mg protein in a final volume of 0.5 mL. Test compounds were dissolved in water containing 0.1% HOAc and 1.0% EtOH, and comprised 10% of the final assay volumes. Ten concentrations were used that were centered on the IC50 and spaced equally on log scale. Assays were terminated by addition of ice-cold incubation buffer followed by rapid filtration through Whatman GF/B glass fiber filters presoaked in 0.5% polyethylenimine using a Brandel cell harvester. Filters were washed three times with 3–4 mL of ice-cold buffer, dried, and extracted with Hi-Safe 2 scintillation cocktail. Radioactivity was measured using a Wallac 1409 liquid scintillation counter at a tritium efficiency of 44%. Binding data were analyzed with curve-fitting programs Prism 4.0b and Radlig 6.0. Ki values were computed from IC50’s using the Cheng-Prusoff relationship, with Kd input values of 2.3 nM for [3H](+)-pentazocine and 23.9 nM for [3H]DTG.18


