Abstract
Catastrophizing exerts its deleterious effects on pain via multiple pathways, and some researchers have reported that high levels of catastrophizing are associated with enhanced physiological reactivity to painful stimulation. In this project, 42 generally healthy adults underwent a series of psychophysical pain testing procedures assessing responses to noxious mechanical, heat, and cold stimuli. Pain-catastrophizing cognitions were assessed prior to and then immediately after the various pain induction procedures. Blood samples were taken at baseline and then at several time points from the end of the procedures to 1 hour post-testing. Samples were assayed for serum levels of cortisol and interleukin-6 (IL-6). Both cortisol and IL-6 increased from baseline during the post-testing period (p’s< .05), with cortisol returning to baseline by 1 hour post-testing and IL-6 remaining elevated. Pain catastrophizing, measured immediately after the pain procedures, was unrelated to cortisol reactivity, but was strongly related to IL-6 reactivity (p< .01), with higher levels of catastrophizing predicting greater IL-6 reactivity. In multivariate analyses, the relationship between catastrophizing and IL-6 reactivity was independent of pain ratings. Collectively, these findings suggest that cognitive and emotional responses during the experience of pain can shape pro-inflammatory immune system responses to noxious stimulation. This pathway may represent one important mechanism by which catastrophizing and other psychosocial factors shape the experience of both acute and chronic pain in a variety of settings.
Keywords: Experimental Pain, Pro-Inflammatory, Interleukin-6, Cortisol, Catastrophizing
Introduction
Catastrophizing is a multidimensional, negatively-valenced, cognitive and affective response to pain that has emerged over the past decade as one of the most important determinants of short- and long-term pain-related outcomes(17;79;88;89). Individuals who report high levels of catastrophizing show enhanced musculoskeletal tenderness and amplified pain sensitivity both in non-patient samples(25;81) and in samples of patients with persistent pain(30;34;36;38;43;81). In longitudinal studies, moreover, high catastrophizers also appear to be at elevated risk for the onset and persistence of pain syndromes such as low back pain(71) and post-surgical pain(67;85). At present, the mechanisms by which catastrophizing exerts its deleterious effects have not been fully characterized, though cognitive-attentional processes, social interactions, and neural pain-modulatory systems have all been proposed as potential mediators of catastrophizingi’s effects(17;89).
We recently reviewed the literature on catastrophizing’s influence on pain in the context of rheumatic disease (17), and suggested that catastrophizing might be directly or indirectly associated with inflammatory processes. For example, studies in rheumatoid arthritis (RA) have reported positive relationships between catastrophizing (or helplessness, one component of catastrophizing) and elevated indices of disease activity and inflammation(64–66;82;103). Several of these investigations suggest directional relationships between catastrophizing and inflammation; for example, longitudinal RA studies have shown that high levels of catastrophizing prospectively predicted worsening erythrocyte sedimentation rates(1;27). Catastrophizing is also associated with elevations in patient-related pain severity in rheumatologic samples(11;22;49–52), and may contribute to interactions between pain and the immune system, which have become a recent focus of basic pain research(96;97). In general, the experience of pain appears to be associated with enhanced release of pro-inflammatory cytokines, which in turn sensitize the nervous system, promoting an amplification of pain transmission(4;5;13;44;84). Indeed, pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and others show positive associations with pain severity among samples of RA, osteoarthritis, back pain, cardiac, and fibromyalgia patients(2;33;62;68;86;94;95).
Collectively, these findings suggest that pain and inflammation are reciprocally linked, and hint that catastrophizing might be influential in shaping these interactions. To date, however, no studies have evaluated whether catastrophizing predicts aberrant inflammation-related responses to the experience of acute pain (in a controlled laboratory setting), although three published reports have documented the presence of cytokine reactivity to the application of calibrated noxious stimuli. Each of these studies noted significant increases in IL-6 following painful stimulation in healthy adults(58), patients with juvenile rheumatoid arthritis(80), and patients with persisting low back pain(32).
In the present project, we focus on assessing predictors of IL-6 and cortisol responses to acute painful stimulation in a sample of generally healthy adults. Both cortisol and IL-6 are stress-responsive parameters, and both are intimately involved in shaping short- and long-term inflammatory processes. We hypothesized that higher levels of pain-related catastrophizing would be associated with elevated IL-6 reactivity to pain, and potentially with blunted cortisol reactivity (i.e., since high pain tolerance is positively associated with cortisol reactivity to acute pain(16), and catastrophizing is associated with reduced tolerance for pain).
Materials and Methods
Participants
Participants were 42 healthy adults, recruited from the community using posted advertisements. The sample was predominantly middle-aged (mean age= 43.8 ± 14.2 years old; range= 19–64), with a preponderance of men (n=29, representing 69.0% of the sample). The majority indicated their race as “white” (n= 30, representing 71.4% of the sample), with the remainder being African-American (21.5%) or Asian (7.2%). Determination of eligibility for the study was made based on questionnaires and a medical history taken by a research nurse at a General Clinical Research Center (GCRC). Inclusion criteria for the study were age 18–64, and facility with the English language adequate to complete questionnaires; exclusion criteria were as follows: severe depression (BDI score > 34), history of myocardial infarction or other serious cardiovascular condition, peripheral neuropathy, pregnancy, Raynaud's syndrome, vasculitis or peripheral vascular disease, use of immune-system-modifying medications (including steroids), current infection, history of any autoimmune disorder, history of any painful rheumatologic condition such as fibromyalgia, use of antidepressants, adrenoreceptor antagonists, or opioids, recent history of substance abuse or dependence, current or recent chronic pain syndrome.
Session Protocol
All subjects provided verbal and written informed consent, and all procedures were approved by an Institutional Review Board. The setting for the study was a Clinical Research Center based within a university hospital. Participants arrived between 12:00 and 12:30; they had previously been requested to refrain from using over-the-counter medications or caffeine, smoking, or performing other than mild exercise prior to their arrival. After informed consent and screening for eligibility, participants completed questionnaires for approximately 10 minutes. Questionnaires included a medical history form, the Beck Depression Inventory, and the Pain Catastrophizing Scale (88), a well-validated, widely-used 14-item self-report measure of catastrophic thinking associated with pain (17).
Next, subjects were seated comfortably in a reclining chair and an indwelling catheter was inserted in the left forearm by a research nurse. After catheter placement, a 15-min period of rest was observed, following which 2 baseline blood samples (10 ml), separated by 5 min, were drawn. Subsequently, participants underwent the psychophysical stimulation procedure described below, after which additional (10 ml) blood samples were taken at several time points: immediately post-stimulation, 15 min post-stimulation, 30 min post-stimulation, and 60 min post-stimulation.
Psychophysical Pain Testing
Mechanical pain thresholds were assessed first using a digital pressure algometer (Somedic; Sollentuna, Sweden). As in previous studies (14;35;45), we selected several muscle/joint sites and bilaterally assessed pressure pain thresholds (PPTh). PPThs were determined twice at each of the following sites on the right and left sides of the body in a randomized order: the belly of the trapezius muscle, the metacarpophalangeal joint of the thumb, and the quadriceps muscle, near the insertion of the quadriceps femoris tendon. At each site, mechanical pressure was applied using a 0.5-cm2 probe covered with 1mm polypropylene pressure-transducing material; pressure was increased at a steady rate of 30 kPA/s until the subject indicated that the pressure was "first perceived as painful".
Next, contact heat stimuli were delivered using a Medoc Thermal Sensory Analyzer (TSA-2001, Ramat Yishai, Israel). Thermal assessment included sampling of heat pain thresholds (HPTH) on the ventral forearm using an ascending method of limits paradigm with a rate of rise of .5°C/Sec (23). Three trials of HPTH were performed first, followed by 4 trials of suprathreshold heat stimulation. In brief, 4 sequences of 10 rapid heat pulses were applied to the forearm, similar to prior studies (19;77). Within each sequence, the procedure was as follows: from a 38°C baseline temperature, ten successive thermal pulses were delivered. The rate of rise and fall of the thermode temperature was 10°C/sec, and target temperatures were delivered for approximately .5 sec each. The thermode remained in a fixed position during administration of the 10 pulses, and was then repositioned between sequences, with inter-sequence intervals of 2 min. Two different target temperatures (49°C, and 51°C) were utilized two times each in randomized order. Subjects verbally rated the painfulness of each thermal pulse on a 0–100 (0= “no pain”, 100= “most intense pain imaginable”) numeric rating scale.
Finally, responses to noxious cold were evaluated using a repeated cold pressor task (CPT), involving immersion of the right hand in a circulating cold water bath maintained at 4°C. The CPT is the most commonly-used method of pain induction in the laboratory, and has demonstrated clinical relevance (6;24). Several recent studies indicate that the CPT provokes increases in cortisol and norepinepherine, as well as producing increases in pro-inflammatory cytokine production (80). In the present protocol, participants underwent a series of five cold pressor tasks, with the first 4 consisting of serial immersions of the right hand for 30 sec, with 2 min between immersions. The 5th and final CPT involved an immersion of the right hand lasting until a participant reached pain tolerance (or a 3 min maximum). Participants rated the intensity of the cold pain on a 0–100 scale (“no pain” to “most intense pain imaginable”) every 15 sec. Following the final CPT, participants continued to relax in the chair.
Catastrophizing
Catastrophizing during these pain experiences was measured using a 6-item scale derived from the Pain Catastrophizing Scale (88); items query participants about feelings of helplessness, attention to pain, etc. during the laboratory pain tests that they have just undergone. This situation-specific measure of catastrophizing was assessed 3 times: immediately after the pressure pain testing, thermal pain testing, and cold pressor testing. Participants were asked to indicate the catastrophic thoughts they remembered experiencing during the pain procedures. We have previously validated this scale for use in laboratory settings (25); this situation-specific assessment of catastrophizing immediately following a pain experience is in contrast to the standard assessment of catastrophizing with the PCS or other measures, wherein subjects are cued to reflect on prior pain experiences, and then report on the degree to which they habitually have catastrophized.
Physiological Measures
Each blood sample was collected in a 10 ml tube and later transported to the GCRC Core Laboratory where it was centrifuged, aliquoted, and stored in a −80°C for later assay. Serum cortisol was assessed in duplicate using a radioimmunoassay (DSL Inc. Webstar, TX), with a lower-limit of detection of .5 µg/dl, a sensitivity of .11 µg/dl and an intra-assay coefficient of variation of < 10%. A standard high-sensitivity enzyme-linked immunosorbent assay (R& D Systems, Minneapolis) was utilized to assess serum levels of IL-6 in duplicate. This assay has a lower limit of detection of .16 ρg/ml, a sensitivity of .09 ρg/ml, and an intra-assay coefficient of variation of < 10%.
Data Analysis
Changes in serum levels of cortisol and IL-6 were evaluated using repeated measures analysis of variance (ANOVA). Inter-relationships among study variables were evaluated using Pearson correlations, while relationships between catastrophizing and physiological reactivity to pain were assessed using partial correlations (i.e., controlling for baseline levels of cortisol or IL-6). In addition, a measure of area-under-the-curve (AUC) (26;31;70) was used to summarize overall levels of cortisol and IL-6. Finally, hierarchical linear regression was used to assess the unique association of catastrophizing with AUC summary indices of reactivity. Analyses were performed using SPSS.
Results
Participants generally arrived at the Johns Hopkins General Clinical Research Center between 12:00 and 12:30 PM. The mean time of the two baseline blood draws was 1:30 and 1:35 respectively. The mean time of the post-testing blood sampling was 2:35 PM (indicating that the psychophysical pain testing session lasted one hour, on average), with the mean timing of subsequent blood samples at 2:49, 3:04, and 3:32.
Pressure pain thresholds were highest for the quadriceps (779.0 ± 318.6 KPa), followed by the trapezius (505.7 ± 229.0 KPa) and the thumb (395.3 ± 146.9 KPa). Heat pain thresholds, assessed on the volar forearm, were 44.9 ± 3.9°C. Mean pain ratings for the suprathreshold thermal stimuli delivered to the arm were 56.0 ± 32.1 (on the 0–100 scale) for the 49°C stimuli and 67.1±29.1 (on the 0–100 scale) for the 51°C stimuli. For the repeated cold pressor tests, mean cold pain ratings were 70.6 ± 24.1 (on the 0–100 scale), and the mean cold pain tolerance for the final CPT was 129.3 ± 63.5 seconds.
In terms of psychological functioning, BDI scores for the sample were quite low, with a mean of 2.5 ± 3.9. Scores on the Pain Catastrophizing Scale showed substantial individual variability, with a sample mean of 12.3 and a standard deviation of 10.3 (range: 0–50). Similarly, situation-specific catastrophizing scores had a mean of 10.2 ± 9.0. These scores were inter-correlated across all 3 assessment points (mean Pearson r= .57, p< .001), and cronbach’s alpha for all 18 items was .89 indicating excellent internal consistency of these responses. Interestingly, and consistent with prior studies (18), situation-specific catastrophizing scores were not significantly correlated with either standard PCS scores (r= .02) or BDI scores (r= .12). Correlations of catastrophizing and BDI scores with pain responses are presented in Table 1; in general, situation-specific catastrophizing was associated with enhanced pain sensitivity while PCS and BDI scores were largely unrelated to pain responses.
Table 1.
Associations between catastrophizing, depressive symptoms, and pain responses.
| SS Catastrophizing | PCS | BDI | |
|---|---|---|---|
| Heat Pain Threshold | −.40** | .09 | .07 |
| Pressure Pain Threshold | −.25 | .13 | −.11 |
| Heat Pain Ratings | .35* | .29 | .06 |
| Cold Pain Ratings | .48** | −.12 | −.01 |
| Cold Pain Tolerance | −.29 | −.01 | −.38* |
SS= “situation-specific”; PCS= Pain Catastrophizing Scale; BDI= Beck depression Inventory
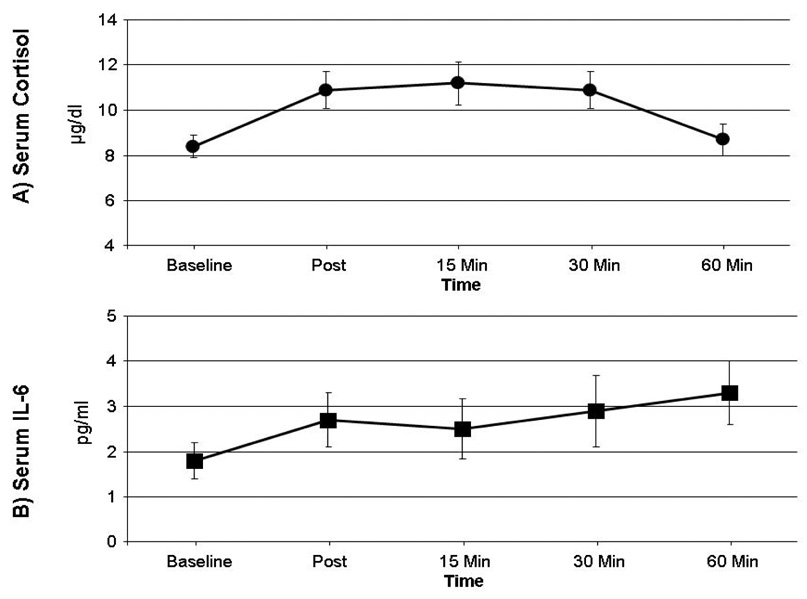
As expected, and consistent with prior research, repeated measures ANOVAs indicated that cortisol [F(4,164)= 6.5, P< .01] and IL-6 [F(4,164)= 3.6, p= .02] serum levels changed significantly over the course of the testing session, though the patterns of change differed somewhat. From baseline levels, cortisol increased roughly 33% at 15-min post-testing, then returned to baseline levels by the 60-min post-testing point. IL-6 increases, on the other hand, peaked at approximately 65% above baseline at the 60-min post-testing time point; cortisol and IL-6 data are depicted in Figure 1.
Figure 1.
Serum cortisol (Panel A) and IL-6 (Panel B) levels. Data points are group means; error bars represent SEM.
Interestingly, average pain ratings were not significantly correlated with either cortisol reactivity (r= .12 for the association of pain ratings with AUC for cortisol) or IL-6 reactivity (r= .08 for the association of pain ratings with AUC for IL-6). Partial correlations, controlling for baseline values of cortisol, indicated that situation-specific catastrophizing, PCS scores, and BDI scores were all unassociated with post-testing cortisol values (all p’s> .05). However, partial correlations, controlling for baseline values of IL-6, revealed that situation-specific catastrophizing was positively related to IL-6 levels at 15 minutes post-testing (r= .34, p< .05), 30 minutes post-testing (r= .52, p< .01), and 60 minutes post-testing (r= .49, p< .01), as well as the AUC summary measure of IL-6 (r= .43, p< .01). PCS scores were unrelated to IL-6 levels, and BDI scores showed marginal negative correlations with IL-6 levels immediately post-testing (r= −.26, p=.10), and 15 minutes post-testing (r= −.28, p= .08). Finally, situation-specific catastrophizing scores were examined as a unique predictor of IL-6 reactivity. The summary AUC measure of IL-6, with baseline IL-6 levels partialled out, was used as a dependent variable in the regression equation, and sex, average pain ratings during the testing session, PCS, and BDI scores were entered on the first steps, with situation-specific catastrophizing scores entered last as a predictor. Results are presented in Table 2; collectively, only situation-specific catastrophizing was associated with IL-6 reactivity, with higher levels of catastrophizing strongly predicting larger increases in IL-6 from baseline to the post-testing period. A similar analysis using the comparable AUC measure of cortisol revealed that none of these independent variables was a significant predictor of cortisol reactivity.
Table 2.
Results of hierarchical linear regression model predicting IL-6 reactivity to acute pain
| Step | Predictor Variable | R2 Change | F Change | Standardized β | p-value |
|---|---|---|---|---|---|
| 1 | Sex | .03 | 0.8 | −.14 | .35 |
| Mean Pain Rating | .07 | .68 | |||
| 2 | PCS | .02 | 0.4 | −.07 | .70 |
| BDI | .09 | .63 | |||
| 3 | Situation-Specific | .35 | 19.9 | .75 | .001 |
| Catastrophizing |
Note. IL-6= Interleukin-6, PCS= Pain Catastrophizing Scale; BDI= Beck Depression Inventory
Discussion
In the present study, situation-specific catastrophizing was associated with reduced pain thresholds and with elevated pain intensity ratings for noxious heat and cold. In addition, the administration of noxious stimuli increased serum levels of cortisol and IL-6, as others have reported previously(3;58), and higher levels of catastrophizing were related to greater pain-related increases in IL-6. These are, to our knowledge, the first data to demonstrate a link between catastrophizing-related cognitive/emotional processes and inflammatory responses to acute painful stimulation, though catastrophizing has been associated with enhanced cardiovascular responses to noxious stimulation(20) and two functional MRI studies have reported that catastrophizing is associated with amplified activation of the pain neuromatrix(37;83).
These findings support earlier research indicating that, in non-clinical samples, task-specific measures of catastrophizing are significantly associated with enhanced responsiveness to experimentally-administered noxious stimuli(25), while trait measures of an individual’s typical degree of catastrophizing show less robust associations(16;18). Similar findings have emerged recently in the depression literature, where day-to-day variability in “state” measures of depressed mood was associated with IL-6 levels while a “trait” measure of depressive symptomatology was not (78). As we reported previously, these two measures of catastrophizing (i.e., situation-specific catastrophizing scores and PCS scores) are minimally correlated(18), indicating the distinctness of these constructs. Important differences between the two catastrophizing measures include the specificity and universality of the referent pain event (i.e., for the situation-specific measure, all subjects have just undergone identical pain induction procedures and are queried specifically in reference to that experience) and the immediacy of the recall. Moreover, consistent with cognitive theory and with prior research from our group, catastrophizing might represent a type of latent construct, requiring sufficient activation to exert its effects (8). In this study, the unfamiliar and potentially minimally-threatening laboratory pain procedures, while they do induce some situation-specific catastrophizing, may not be sufficient to activate the catastrophizing constructs assessed by the PCS (which assesses responses to prior situations involving clinical pain). For example, the consent procedures specify that there is minimal risk of tissue damage or actual harm, whereas previous pain experiences (e.g., acute injuries, etc.) might well involve such threats.
Promoting elevated inflammatory responses to the experience of acute pain might constitute an important mechanism by which catastrophizing shapes long-term pain-related outcomes in a number of chronic conditions. IL-6 induces muscle and joint hyperalgesia(7;15) and mediates the development of injury-induced hyperalgesia(90). Moreover, IL-6 increases are associated with enhanced pain in healthy individuals exposed to the stresses of extended sleep deprivation(40). Following surgery, IL-6 levels are associated with postoperative pain(33;56;57) and reduced functioning(61). In samples of pain patients, IL-6 and inflammatory markers correlate with higher pain severity(12;53;62;68;86). Collectively, these findings support the conclusions of recent reviews, that proinflammatory cytokines are likely to play a facilitatory role in the development and maintenance of persistent pain syndromes, including neuropathic pains(59;60;91;98;100;101). In this study, catastrophizing predicted subsequent elevations in IL-6 well after the pain had ended; such prolonged facilitation of pro-inflammatory processes could play a role in shaping long-term pain outcomes across numerous pain states and disease processes. For example, catastrophizing has been implicated as an etiological or prognostic factor persistent pain syndromes such as postsurgical neuropathic pain(47;75), low back pain(71), and neck/shoulder pain(42;48;93).
The precise mechanism(s) by which catastrophizing might impact on inflammatory processes are far from clear, although the present findings do suggest that catastrophizing is not associated with blunted HPA-axis responses to pain. This result is consistent with other studies of cortisol responses to acute pain(16). The behavioral effects of catastrophizing, including less use of active coping strategies(87;88) and greater vigilance to bodily sensations(69), may play a role in producing greater physiological/inflammatory pain responses. On the other hand, it seems reasonable to suppose that such processes would primarily impact the degree of pain experienced by the participants, and while catastrophizing was positively correlated with pain ratings, individual differences in those pain ratings were unassociated with cortisol or IL-6 reactivity. This lack of relationship between pain ratings and physiological reactivity to pain could potentially be explained by a threshold-level effect whereby once a certain level of pain is reached, physiological reactivity is likely to ensue, and that reactivity may be driven by factors other than the perceived intensity of pain. Although this possibility is speculative, two recent joint replacement studies offer indirect support. Among patients undergoing total knee replacement (who were not treated with anti-inflammatory medications), a two-fold increase from baseline in plasma IL-6 levels was observed post-surgery, but those IL-6 levels were not correlated with the degree of reported knee pain (28). Similarly, patients undergoing total hip replacement (who were not given anti-inflammatory medications) showed an increase of several orders of magnitude in IL-6 within cerebrospinal fluid, and those increases were also uncorrelated with the degree of postoperative pain (9).
Physiologically, elevated catastrophizing is related to amplified responses to acute painful stimulation in multiple regions within the pain neuromatrix. In fibromyalgia patients, higher catastrophizing was associated with enhanced hemodynamic responses to mildly painful pressure in brain areas such as dorsolateral prefrontal cortex, anterior cingulate cortex, and medial frontal cortex(37). In addition, healthy subjects exposed to mildly painful electric stimulation showed a significant positive relationship between pain catastrophizing and pain responses in the prefrontal cortex, insula, and anterior cingulate cortex, though these relationships were altered during the experience of more intense pain(83). Though speculative, one possible mediating pathway for catastrophizing’s effects on acute inflammatory responses is via a potential influence on the functioning of endogenous opioid systems. Both exogenous(10;63) and endogenous(73;74;102) opioids have been shown to exert inhibitory influences on cytokine-mediated pro-inflammatory responses to injury or stress. Interestingly, catastrophizing has been indirectly associated with reduced efficacy of opioid pain-modulatory systems in several studies. Pain catastrophizing has been associated with greater post-operative opioid analgesic requirements(46), implying that catastrophizing was correlated with reduced benefit of opioids per unit of medication administered. In addition, among a subgroup of healthy adults, higher levels of catastrophizing were correlated with reduced analgesic benefit (measured as a function of changes in pain threshold and tolerance) of IV pentazocine, a kappa-opioid agonist(29). Finally, high levels of catastrophizing predict impairments in the functioning of opioid-mediated endogenous analgesic systems(39;99).
Some important limitations of this study will need to be addressed in future research. First, we applied multiple noxious stimuli during a rather lengthy psychophysical testing session and we are not able to identify which specific pain induction tasks were associated with cortisol and IL-6 reactivity. Indeed, without a control condition, we cannot exclude the possibility that it was not the pain itself, but rather the stress of participating in the study, that drove the observed increases in IL-6. In addition, none of our pain models involved inflammatory pain (e.g., capsaicin), which might have yielded stronger associations between pain responses and IL-6 levels. Second, the sample was relatively small, limiting its power, and larger replication studies will be necessary before these findings can be considered definitive. Third, we assessed catastrophizing immediately after, not during, the experience of pain, leaving open the possibility that pain intensity affected participants’ immediate recall of catastrophizing, rather than catastrophizing amplifying pain intensity at the time of stimulation. Fourth, our measure of IL-6 reactivity showed no sign of decline at our final assessment point, 1 hour after the end of painful stimulation. We are thus not able to determine the full time course of IL-6 reactivity to pain and it is possible that the increases in IL-6 continued, and/or that there were important individual differences in the recovery of IL-6 levels, that we were unable to measure. Finally, we do not have data addressing some of the potential mechanisms underlying the association between catastrophizing and IL-6 reactivity to pain. Additional methodologies, such as pharmacological challenges, will be necessary to determine the pathways through which catastrophizing exerts its influence.
In spite of these limitations, this study highlights the potential role of catastrophizing in modulating pain, supporting further research on the interface between psychological processes and central pain processing. We previously reported that catastrophizing is prospectively related to prolonged sensitization following resolution of acute pain(21), and other studies have also reported that catastrophizing predicts the future onset or worsening of chronic pain(41;55;71). The amplification of inflammatory responses to acute stress may partly underlie catastrophizing’s enduring effects. Future studies of interventions designed to reduce catastrophizing and improve pain-related outcomes may benefit from the evaluation of pro-inflammatory cytokines such as IL-6 in assessing the potentially beneficial effects of reducing catastrophizing. Finally, if these findings are replicated, it is possible that reductions in catastrophizing could be shown to have broadly positive health effects, as IL-6 levels are prospectively associated with the development of conditions such as Type II diabetes(72;92) and cardiovascular disease(54;76).
Acknowledgements
This work was supported by NIH Grant K23 AR051315 (to RRE), by awards from the American College of Rheumatology (to RRE) and Arthritis Foundation (to RRE), as well as the Johns Hopkins General Clinical Research Center (M01-RR002719). Thanks to Monika Haack for her extremely helpful comments and feedback. No authors have any conflict of interest regarding this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Affleck G, Tennen H, Urrows S, Higgins P. Neutroticism and the pain-mood relation in rheumatoid arthritis: Insights from a perspective daily study. J Consult Clin Psychol. 1992;60(1):119–126. doi: 10.1037//0022-006x.60.1.119. [DOI] [PubMed] [Google Scholar]
- 2.Ai AL, Kronfol Z, Seymour E, Bolling SF. Effects of mood state and psychosocial functioning on plasma Interleukin-6 in adult patients before cardiac surgery. Int J Psychiatry Med. 2005;35(4):363–376. doi: 10.2190/2ELG-RDUN-X6TU-FGC8. [DOI] [PubMed] [Google Scholar]
- 3.al'Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, et al. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66(2):198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- 4.Beilin B, Bessler H, Mayburd E, Smirnov G, Dekel A, Yardeni I, et al. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology. 2003;98(1):151–155. doi: 10.1097/00000542-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Beilin B, Shavit Y, Trabekin E, Mordashev B, Mayburd E, Zeidel A, et al. The effects of postoperative pain management on immune response to surgery. Anesth Analg. 2003;97(3):822–827. doi: 10.1213/01.ANE.0000078586.82810.3B. [DOI] [PubMed] [Google Scholar]
- 6.Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90(3):261–269. doi: 10.1016/S0304-3959(00)00406-1. [DOI] [PubMed] [Google Scholar]
- 7.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56(1):351–359. doi: 10.1002/art.22282. [DOI] [PubMed] [Google Scholar]
- 8.Buenaver LF, Edwards RR, Smith MT, Gramling SE, Haythornthwaite JA. Catastrophizing and pain-coping in young adults: associations with depressive symptoms and headache pain. J Pain. 2008;9(4):311–319. doi: 10.1016/j.jpain.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104(3):403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, et al. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol Pain. 2007;3:28. doi: 10.1186/1744-8069-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covic T, Adamson B, Hough M. The impact of passive coping on rheumatoid arthritis pain. Rheumatology (Oxford) 2000;39(9):1027–1030. doi: 10.1093/rheumatology/39.9.1027. [DOI] [PubMed] [Google Scholar]
- 12.Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88(11):1384–1393. doi: 10.1016/j.apmr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 13.De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;96(4):1096–1103. doi: 10.1213/01.ANE.0000055362.56604.78. table. [DOI] [PubMed] [Google Scholar]
- 14.Dhondt W, Willaeys T, Verbruggen LA, Oostendorp RA, Duquet W. Pain threshold in patients with rheumatoid arthritis and effect of manual oscillations. Scand J Rheumatol. 1999;28(2):88–93. doi: 10.1080/030097499442540. [DOI] [PubMed] [Google Scholar]
- 15.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152(2):521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112(1–2):188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, Bingham CO, III, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006;55(2):325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RR, Campbell CM, Fillingim RB. Catastrophizing and experimental pain sensitivity: only in vivo reports of catastrophic cognitions correlate with pain responses. J Pain. 2005;6(5):338–339. doi: 10.1016/j.jpain.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Edwards RR, Fillingim RB. Effects of age on temporal summation of thermal pain: clinical relevance in healthy older and younger adults. Journal of Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Fillingim RB. Styles of pain coping predict cardiovascular function following a cold pressor test. Pain Res Manag. 2005;10(4):219–222. doi: 10.1155/2005/216481. [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Fillingim RB, Maixner W, Sigurdsson A, Haythornthwaite J. Catastrophizing predicts changes in thermal pain responses after resolution of acute dental pain. J Pain. 2004;5(3):164–170. doi: 10.1016/j.jpain.2004.02.226. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RR, Goble L, Kwan A, Kudel I, McGuire L, Heinberg L, et al. Catastrophizing, Pain, and Social Adjustment in Scleroderma: Relationships With Educational Level. Clin J Pain. 2006;22(7):639–646. doi: 10.1097/01.ajp.0000210918.26159.94. [DOI] [PubMed] [Google Scholar]
- 23.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111(3):335–341. doi: 10.1016/j.pain.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114(3):315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22(8):730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 26.Evans E, Turley N, Robinson N, Clancy M. Randomised controlled trial of patient controlled analgesia compared with nurse delivered analgesia in an emergency department. Emerg Med J. 2005;22(1):25–29. doi: 10.1136/emj.2002.004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evers AW, Kraaimaat FW, Geenen R, Jacobs JW, Bijlsma JW. Stress-vulnerability factors as long-term predictors of disease activity in early rheumatoid arthritis. J Psychosom Res. 2003;55(4):293–302. doi: 10.1016/s0022-3999(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y, Ju H, Yang B, An H. Effects of a selective cyclooxygenase-2 inhibitor on postoperative inflammatory reaction and pain after total knee replacement. J Pain. 2008;9(1):45–52. doi: 10.1016/j.jpain.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69(1):97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 30.France CR, Keefe FJ, Emery CF, Affleck G, France JL, Waters S, et al. Laboratory pain perception and clinical pain in post-menopausal women and age-matched men with osteoarthritis: relationship to pain coping and hormonal status. Pain. 2004;112(3):274–281. doi: 10.1016/j.pain.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Ge HY, Madeleine P, Arendt-Nielsen L. Gender differences in pain modulation evoked by repeated injections of glutamate into the human trapezius muscle. Pain. 2005;113(1–2):134–140. doi: 10.1016/j.pain.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 32.Geiss A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F. Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114(1–2):104–117. doi: 10.1016/j.pain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci Lett. 1997;237(2–3):65–68. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- 34.Geisser ME, Casey KL, Brucksch CB, Ribbens CM, Appleton BB, Crofford LJ. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: association with mood, somatic focus, and catastrophizing. Pain. 2003;12(3):243–250. doi: 10.1016/S0304-3959(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 35.Gerecz-Simon EM, Tunks ER, Heale JA, Kean WF, Buchanan WW. Measurement of pain threshold in patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and healthy controls. Clin Rheumatol. 1989;8(4):467–474. doi: 10.1007/BF02032098. [DOI] [PubMed] [Google Scholar]
- 36.Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48(10):2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 37.Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(Pt 4):835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 38.Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003;17(4):593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 39.Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2007 doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 40.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haythornthwaite JA, Clark MR, Pappagallo M, Raja SN. Pain coping strategies play a role in the persistence of pain in post-herpetic neuralgia. Pain. 2003;106(3):453–460. doi: 10.1016/j.pain.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Hill JC, Lewis M, Sim J, Hay EM, Dziedzic K. Predictors of poor outcome in patients with neck pain treated by physical therapy. Clin J Pain. 2007;23(8):683–690. doi: 10.1097/AJP.0b013e3181468e67. [DOI] [PubMed] [Google Scholar]
- 43.Huppe A, Brockow T, Raspe H. Chronic widespread pain and tender points in low back pain: a population-based study. Z Rheumatol. 2004;63(1):76–83. doi: 10.1007/s00393-004-0531-5. [DOI] [PubMed] [Google Scholar]
- 44.Hutchinson MR, La Vincente SF, Somogyi AA. In vitro opioid induced proliferation of peripheral blood immune cells correlates with in vivo cold pressor pain tolerance in humans: a biological marker of pain tolerance. Pain. 2004;110(3):751–755. doi: 10.1016/j.pain.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Incel NA, Erdem HR, Ozgocmen S, Catal SA, Yorgancioglu ZR. Pain pressure threshold values in ankylosing spondylitis. Rheumatol Int. 2002;22(4):148–150. doi: 10.1007/s00296-002-0211-1. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen PB, Butler RW. Relation of cognitive coping and catastrophizing to acute pain and analgesic use following breast cancer surgery. J Behav Med. 1996;19(1):17–29. doi: 10.1007/BF01858172. [DOI] [PubMed] [Google Scholar]
- 47.Jensen MP, Ehde DM, Hoffman AJ, Patterson DR, Czerniecki JM, Robinson LR. Cognitions, coping and social environment predict adjustment to phantom limb pain. Pain. 2002;95(1–2):133–142. doi: 10.1016/s0304-3959(01)00390-6. [DOI] [PubMed] [Google Scholar]
- 48.Karels CH, Bierma-Zeinstra SM, Burdorf A, Verhagen AP, Nauta AP, Koes BW. Social and psychological factors influenced the course of arm, neck and shoulder complaints. J Clin Epidemiol. 2007;60(8):839–848. doi: 10.1016/j.jclinepi.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Keefe FJ, Affleck G, France CR, Emery CF, Waters S, Caldwell DS, et al. Gender differences in pain, coping, and mood in individuals having osteoarthritic knee pain: a within-day analysis. Pain. 2004;110(3):571–577. doi: 10.1016/j.pain.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Keefe FJ, Caldwell DS, Martinez S, Nulley J, Beckham J, Williams DA. Analyzing pain in rheumatoid arthritis patients. Pain coping strategies in patients who have had knee replacement surgery. Pain. 1991;46:153–160. doi: 10.1016/0304-3959(91)90070-E. [DOI] [PubMed] [Google Scholar]
- 51.Keefe FJ, Kashikar-Zuck S, Robinson E, Salley A, Beaupre P, Caldwell D, et al. Pain coping strategies that predict patients'and spouses' ratings of patients' self-efficacy. Pain. 1997;73(2):191–199. doi: 10.1016/S0304-3959(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 52.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87(3):325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 53.Koch A, Zacharowski K, Boehm O, Stevens M, Lipfert P, von Giesen HJ, et al. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm Res. 2007;56(1):32–37. doi: 10.1007/s00011-007-6088-4. [DOI] [PubMed] [Google Scholar]
- 54.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond) 2005;108(3):205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 55.Linton SJ. Do psychological factors increase the risk for back pain in the general population in both a cross-sectional and prospective analysis? Eur J Pain. 2005;9(4):355–361. doi: 10.1016/j.ejpain.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Lisowska B, Maldyk P, Kontny E, Michalak C, Jung L, Cwiek R. Postoperative evaluation of plasma interleukin-6 concentration in patients after total hip arthroplasty. Ortop Traumatol Rehabil. 2006;8(5):547–554. [PubMed] [Google Scholar]
- 57.Lisowska B, Maslinski W, Maldyk P, Zabek J, Baranowska E. The role of cytokines in inflammatory response after total knee arthroplasty in patients with rheumatoid arthritis. Rheumatol Int. 2007 doi: 10.1007/s00296-007-0508-1. [DOI] [PubMed] [Google Scholar]
- 58.Lutgendorf SK, Logan H, Costanzo E, Lubaroff D. Effects of acute stress, relaxation, and a neurogenic inflammatory stimulus on interleukin-6 in humans. Brain Behav Immun. 2004;18(1):55–64. doi: 10.1016/s0889-1591(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 59.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6(7):521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 60.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192(2):444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Miller RR, Cappola AR, Shardell MD, Hawkes WG, Yu-Yahiro JA, Hebel JR, et al. Persistent changes in interleukin-6 and lower extremity function following hip fracture. J Gerontol A Biol Sci Med Sci. 2006;61(10):1053–1058. doi: 10.1093/gerona/61.10.1053. [DOI] [PubMed] [Google Scholar]
- 62.Mukai E, Nagashima M, Hirano D, Yoshino S. Comparative study of symptoms and neuroendocrine-immune network mediator levels between rheumatoid arthritis patients and healthy subjects. Clin Exp Rheumatol. 2000;18(5):585–590. [PubMed] [Google Scholar]
- 63.Murphy GS, Szokol JW, Marymont JH, Avram MJ, Vender JS. The effects of morphine and fentanyl on the inflammatory response to cardiopulmonary bypass in patients undergoing elective coronary artery bypass graft surgery. Anesth Analg. 2007;104(6):1334–1342. doi: 10.1213/01.ane.0000264108.47280.f5. table. [DOI] [PubMed] [Google Scholar]
- 64.Parker J, Smarr K, Anderson S, Hewett J, Walker S, Bridges A, et al. Relationship of changes in helplessness and depression to disease activity in rheumatoid arthritis. J Rheumatol. 1992;19(12):1901–1905. [PubMed] [Google Scholar]
- 65.Parker JC, Smarr KL, Angelone EO, Mothersead PK, Lee BS, Walker SE, et al. Psychological factors, immunologic activation, and disease activity in rheumatoid arthritis. Arthritis Care Res. 1992;5(4):196–201. doi: 10.1002/art.1790050403. [DOI] [PubMed] [Google Scholar]
- 66.Parker JC, Smarr KL, Walker SE, Hagglund KJ, Anderson SK, Hewett JE, et al. Biopsychosocial parameters of disease activity in rheumatoid arthritis. Arthritis Care Res. 1991;4(2):73–80. doi: 10.1002/art.1790040204. [DOI] [PubMed] [Google Scholar]
- 67.Pavlin DJ, Sullivan MJ, Freund PR, Roesen K. Catastrophizing: a risk factor for postsurgical pain. Clin J Pain. 2005;21(2):83–90. doi: 10.1097/00002508-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Penninx BW, Abbas H, Ambrosius W, Nicklas BJ, Davis C, Messier SP, et al. Inflammatory markers and physical function among older adults with knee osteoarthritis. J Rheumatol. 2004;31(10):2027–2031. [PubMed] [Google Scholar]
- 69.Peters ML, Vlaeyen JW, van Drunen C. Do fibromyalgia patients display hypervigilance for innocuous somatosensory stimuli? Application of a body scanning reaction time paradigm. Pain. 2000;86(3):283–292. doi: 10.1016/S0304-3959(00)00259-1. [DOI] [PubMed] [Google Scholar]
- 70.Pham B, Cranney A, Boers M, Verhoeven AC, Wells G, Tugwell P. Validity of area-under-the-curve analysis to summarize effect in rheumatoid arthritis clinical trials. J Rheumatol. 1999;26(3):712–716. [PubMed] [Google Scholar]
- 71.Picavet HS, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156(11):1028–1034. doi: 10.1093/aje/kwf136. [DOI] [PubMed] [Google Scholar]
- 72.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 73.Raap T, Justen HP, Miller LE, Cutolo M, Scholmerich J, Straub RH. Neurotransmitter modulation of interleukin 6 (IL-6) and IL-8 secretion of synovial fibroblasts in patients with rheumatoid arthritis compared to osteoarthritis. J Rheumatol. 2000;27(11):2558–2565. [PubMed] [Google Scholar]
- 74.Refojo D, Kovalovsky D, Young JI, Rubinstein M, Holsboer F, Reul JM, et al. Increased splenocyte proliferative response and cytokine production in beta-endorphin-deficient mice. J Neuroimmunol. 2002;131(1–2):126–134. doi: 10.1016/s0165-5728(02)00268-0. [DOI] [PubMed] [Google Scholar]
- 75.Richardson C, Glenn S, Horgan M, Nurmikko T. A prospective study of factors associated with the presence of phantom limb pain six months after major lower limb amputation in patients with peripheral vascular disease. J Pain. 2007;8(10):793–801. doi: 10.1016/j.jpain.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5(2):77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Rohleder N, Miller GE. Acute deviations from long-term trait depressive symptoms predict systemic inflammatory activity. Brain Behav Immun. 2008;22(5):709–716. doi: 10.1016/j.bbi.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 79.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 80.Roupe vd V, Heijnen CJ, Wulffraat N, Kuis W, Kavelaars A. Stress induces increases in IL-6 production by leucocytes of patients with the chronic inflammatory disease juvenile rheumatoid arthritis: a putative role for alpha(1)-adrenergic receptors. J Neuroimmunol. 2000;110(1–2):223–229. doi: 10.1016/s0165-5728(00)00328-3. [DOI] [PubMed] [Google Scholar]
- 81.Schochat T, Raspe H. Elements of fibromyalgia in an open population. Rheumatology (Oxford) 2003;42(7):829–835. doi: 10.1093/rheumatology/keg199. [DOI] [PubMed] [Google Scholar]
- 82.Schoenfeld-Smith K, Petroski GF, Hewett JE, Johnson JC, Wright GE, Smarr KL, et al. A biopsychosocial model of disability in rheumatoid arthritis. Arthritis Care Res. 1996;9(5):368–375. doi: 10.1002/1529-0131(199610)9:5<368::aid-anr1790090505>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 83.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120(3):297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 84.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361(1–3):184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Stephens MA, Druley JA, Zautra AJ. Older adults' recovery from surgery for osteoarthritis of the knee: psychosocial resources and constraints as predictors of outcomes. Health Psychol. 2002;21(4):377–383. doi: 10.1037//0278-6133.21.4.377. [DOI] [PubMed] [Google Scholar]
- 86.Sturmer T, Brenner H, Koenig W, Gunther KP. Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann Rheum Dis. 2004;63(2):200–205. doi: 10.1136/ard.2003.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sullivan MJ, Adams H, Sullivan ME. Communicative dimensions of pain catastrophizing: social cueing effects on pain behaviour and coping. Pain. 2004;107(3):220–226. doi: 10.1016/j.pain.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 88.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychol Assess. 1995;7(4):524–532. [Google Scholar]
- 89.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 90.Summer GJ GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135(1–2):98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 91.Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105(3):838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- 92.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3–4):222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Voerman GE, Sandsjo L, Vollenbroek-Hutten MM, Larsman P, Kadefors R, Hermens HJ. Changes in cognitive-behavioral factors and muscle activation patterns after interventions for work-related neck-shoulder complaints: relations with discomfort and disability. J Occup Rehabil. 2007;17(4):593–609. doi: 10.1007/s10926-007-9109-9. [DOI] [PubMed] [Google Scholar]
- 94.Wallace DJ. Is there a role for cytokine based therapies in fibromyalgia. Curr Pharm Des. 2006;12(1):17–22. [PubMed] [Google Scholar]
- 95.Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxford) 2001;40(7):743–749. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- 96.Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 97.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82(4):981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 98.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257(2):139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 99.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186(1):79–85. doi: 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- 100.Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14(4):166–174. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- 101.Wieseler-Frank J, Maier SF, Watkins LR. Immune-to-brain communication dynamically modulates pain: physiological and pathological consequences. Brain Behav Immun. 2005;19(2):104–111. doi: 10.1016/j.bbi.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Yin H, Yu M, Cheng H, Zhang F, Gao Y, Lin J, et al. Beta-endorphin prevents collagen induced arthritis by neuroimmuno-regulation pathway. Neuro Endocrinol Lett. 2005;26(6):739–744. [PubMed] [Google Scholar]
- 103.Zautra AJ, Hamilton NA, Potter P, Smith B. Field research on the relationship between stress and disease activity in rheumatoid arthritis. Ann N Y Acad Sci. 1999;876:397–412. doi: 10.1111/j.1749-6632.1999.tb07664.x. [DOI] [PubMed] [Google Scholar]