Abstract
Human exposure to the life-span developmental neurotoxicant, methylmercury (MeHg), is primarily via the consumption of fish or marine mammals. Fish are also excellent sources of important nutrients, including selenium and n-3 polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA). Laboratory models of developmental MeHg exposure can be employed to assess the roles of nutrients and MeHg and to identify potential mechanisms of action if the appropriate exposure measures are used. In describing chronic exposures, relationships between daily intake and brain mercury are consistent and orderly across species, even when large differences in blood:brain ratios exist.
It is well-established that low level developmental MeHg produces sensory deficits. Recent studies also show that perseveration in reversal-learning tasks occurs after gestational exposures that produce low micromolar concentrations in the brain. A no-effect level has not been identified for this effect. These exposures do not affect the acquisition or performance of discrimination learning, set shifting (extra-dimensional shift), or memory. Reversal learning deficits may be related to enhanced impact of reinforcers as measured using progressive ratio reinforcement schedules, an effect that could result in perseveration. Also reported is enhanced sensitivity to dopamine reuptake inhibitors and diminished sensitivity to pentobarbital, a GABAA agonist. Diets rich in PUFAs or selenium do not protect against MeHg's effects on reversal learning but, by themselves, may diminish variability in performance, enhance attention or psychomotor function and may confer some protection against age-related deficits in these areas.
It is hypothesized that altered reward processing, dopamine and GABAergic neurotransmitter systems, and cortical regions associated with choice and perseveration are especially sensitive to developmental MeHg at low exposure levels. Human testing for MeHg's neurotoxicity should emphasize these behavioral domains.
Keywords: Methylmercury, development, polyunsaturated fatty acid, docosahexaenoic acid, selenium, behavior, perseveration, reversal learning, aging, animal models
1. BACKGROUND
During the mid-twentieth century, inhabitants around Minamata Bay, Japan unwittingly consumed MeHg-contaminated fish. Children born to exposed mothers displayed cerebral palsy, athetosis, chorea, speech disorders and, in nearly 1/3 of the children born between 1955 and 1958, mental retardation (Harada, 1995). Since the Minamata episode, studies of populations exposed to MeHg have occurred in Canada, Peru, the Faroe Islands, the Seychelles Islands, New Zealand, the Amazon Basin, and Iraq. Developmental MeHg exposure has been associated with difficulties on cognitive measures including the Boston Naming Test, The WISC, and the California Verbal Learning Test in the Faroe Islands (Grandjean, et al., 1998; Grandjean, et al., 1997) and New Zealand (Crump, et al., 1998) cohorts. An intensive study of the Seychelles Islander cohort, a population that eats 12 fish meals/week, has reported a general absence of effects of MeHg exposure (Axtell, et al., 2000; Davidson, et al., 1998; Davidson, et al., 2000; Myers, et al., 1995; Myers, et al., 1997), apart from a single statistically significant relationship in pre-adolescents between exposure and performance on the grooved pegboard task (Myers, et al., 2003). A recent report from this population, however, suggests that effects on psychomotor development are present and detectable after controlling for polyunsaturated fatty acids (Davidson, et al., In Press; Strain, et al., In Press). Impaired acquisition of Differential-Reinforcement-of- Low-Rate (DRL) performance has been reported in a cohort of children exposed to MeHg via maternal consumption of Great Lakes fish (Stewart, et al., 2006). The reasons for discrepancies among these studies remain to be identified, but differences among these populations in other dietary constituents, including polychlorinated biphenyls (PCBs) and nutrients, have been noted (Myers and Davidson, 1998; Myers, et al., 2007).
Concerns remain about the consumption of shellfish and finfish, especially about potential cognitive deficits arising from developmental MeHg exposure. In the U.S., many States have issued advisories limiting the consumption of fish from lakes, rivers and ponds because of MeHg contamination. Regulatory agencies recommend that MeHg intake by pregnant women be limited to 0.1 (EPA, 1997) or about 0.22 (WHO, 2004) µgHg/kg/day, which correlates with about one to two cans of tuna per week. About 500,000 children are born each year with blood mercury levels in excess of 5.8 µg/L, a level that has been associated with cognitive deficits. This was said to produce a social cost of about $8.7 billion/year because of the estimated drop in IQ scores resulting from this exposure (Trasande, et al., 2005). As noted in that paper, this could underestimate the costs since IQ may not provide the best estimator of MeHg's effects. Recent work from our group, reviewed below, supports this suggestion. The animal literature, in which exposures can be controlled experimentally, has identified consistent sensory effects and some motor effects of developmental exposures (Newland and Rasmussen, 2000; Rice, 1996b; Weiss, et al., 2005) but has not detected cognitive effects of developmental MeHg exposure consistently on tests associated with memory or thought to be related to performance on IQ tests (Rice, 1996b; Rice, 1998c). Recent reports, reviewed below, indicate that development MeHg exposure causes perseveration and disrupted impact of reinforcing events in adult animals. In adult humans, perseveration on such procedures as Wisconsin Card Sorting Tasks is poorly correlated with scores on IQ tests (Ardila, et al., 2000; Arffa, 2007; Boone, et al., 1993), so the economic costs of developmental exposures may have been underestimated by a reliance on such tests.
Here we review this work and place it in context by 1) identifying the exposure-related considerations that contribute to a valid animal model, noting the risk-assessment dilemma posed by the joint presence of nutrients and MeHg in fish, 2) reviewing an experimental model of nutrient/MeHg interactions, 3) reviewing the case for potential nutritional modifiers, and 4) positing a potential mechanism of action.
2. MERCURY, DHA, AND SELENIUM IN DIFFERENT FISH SPECIES
One of the leading controversies in the MeHg literature lies in advisories concerning the consumption of fish (Carrington and Bolger, 2002; Egeland and Middaugh, 1997; Mahaffey, 2004a). Fish are an excellent source of protein without the high levels of saturated fats found in many meats. Many species are a rich source of long-chain polyunsaturated fatty acids (PUFAs), especially the n-3 (omega-3) fatty acid, docosahexaenoic acid (DHA), a 22 carbon PUFA that plays a role in neural (Carlson and Neuringer, 1999; Innis, 2001; Wainwright, et al., 1999) and visual (Neuringer, et al., 1994) development. DHA can come from two sources: directly from the diet or by conversion from alpha linolenic acid (ALA), an 18-carbon PUFA that must be obtained from the diet (Brenna, 2002; Jones and Kubow, 1999). The importance of fish as a source of DHA can be illustrated by noting that only about 5% of ALA is converted into DHA, making this plant-derived PUFA an inefficient source of DHA. (This value can vary depending on the presence of other dietary constituents (Brenna, 2002). Thus, directly consuming DHA from fish is roughly equivalent to consuming 20 times as much ALA. The synthesis of long-chain n-3 and n-6 PUFAs from dietary uses the same enzymes, but preference is given to the n-3 synthetic pathway at the expense of the n-6 pathway. Therefore, excessive n-3 PUFA supplementation can result in an imbalance between n-3 and n-6 PUFAs and, in turn, growth retardation, smaller brains, and behavioral deficits in animal models (Wainwright, et al., 1992; Wainwright, et al., 1999). This issue is important in designing balanced diets for experimental studies, but the likelihood of such imbalances occurring in humans consuming normal (unsupplemented) diets remains uncertain (Lapillonne, et al., 2003).
Fish are also a rich source of selenium, an essential element with antioxidant properties of its own, in addition to playing a role in the formation of glutathione (Burk, 2002; Burk and Laevander, 1999) and brain selenoproteins (Ralston, et al., 2007). Selenium deficiencies are associated with hair loss, growth retardation, reproductive failure, and kidney and liver damage. Keshan disease, a myopathy associated with chronic selenium deficiency in selenium-poor regions of China (Ge and Yang, 1993; Tan, et al., 2002), appears to entail an enhanced susceptibility to coxsackievirus B4 virus that disappears with selenium supplementation (Burk and Laevander, 1999; Peng, et al., 2000). Selenium toxicity, associated with consumption of foods grown in selenium-rich soils or consumption of selenium supplements, is also characterized by hair loss, growth retardation, diseased nails and skin lesions in human populations (Burk and Laevander, 1999), and in animal studies (Abdo, 1994). At least eleven selenoproteins have been identified, including the glutathione peroxidase family, iodothyronine deiodinase family, and selenium protein P, many of which play roles in CNS function (Behne and Kyriakopoulos, 2001; Burk and Laevander, 1999).
Selenium concentrations in the CNS are aggressively defended, even at the expense of other organ systems. After six generations of severe depletion of dietary selenium, brain selenium had declined only to 60% of control levels, even as those of liver, muscle, and blood were down to 1% of controls (Behne, et al., 2000; Chen and Berry, 2003). With respect to MeHg, it has long been demonstrated that Se can prevent or delay adult-onset MeHg exposure, either through its antioxidant actions or by directly binding mercury (Moller-Madsen and Danscher, 1991; Raymond and Ralston, 2004; Skerfving, 1978).
The nutrition vs. neurotoxicity controversy can be addressed first by noting that there are large differences in the content of MeHg and various nutrients in fish and little correlation between these (Chapman and Chan, 2000; Mahaffey, 2004a). Figure 1 (top) shows the mercury and DHA content of various fish species. Only DHA content is shown here because of the importance of this PUFA, but the correlation between this and total n-3 PUFAs was 0.77 in these species (not shown), so DHA is a fair proxy for other n-3 PUFAs. Points in the upper left are high in DHA and low in mercury. Points in the lower right are high in mercury and low in DHA. It is interesting that the long-lived, predatory fish identified by the USEPA as being of particular concern because of mercury content (swordfish, tilefish, shark, and king mackerel),are also especially low in DHA. See (Mahaffey, et al., 2008) for a similar treatment.
Figure 1.
Mercury content in selected fish species compared with DHA content (top) and Se content (bottom). A box is drawn around the species identified by the US EPA as especially high in MeHg. The line in the top figure connects the origin to shark and swordfish, the species targeted by the US EPA with higher DHA content. The line in the bottom figure represents a 1:1 Se:Hg molar ratio. These data derive from several sources (Ache, et al., 2000; EPA, 1997; Holland, et al., 1993; Mahaffey, 2004b; U.S. Department of Agriculture, 2007).
The bottom graph shows selenium content. The data are structured similarly to the top graph, with points in the upper left being nutrient rich. These data are expressed as ppm of selenium because of the common use of this unit of concentration. However, the relative molar content of these two elements may be a better measure (Ralston, et al., 2007), so a line is added to this graph showing the points at which the molar content of Hg equals that of selenium. The closer a point is to that line, the greater the content of Hg relative to selenium. As with DHA, the fish species that have the highest content of mercury relative to selenium are also those for which the USEPA has issued special warnings.
The differences across fish species in nutrient and MeHg content, coupled with the absence of a correlation between these two indicators means that it is possible to identify fish that are relatively beneficial and fish that are relatively hazardous. Fish that fall in the upper regions of the figure, and especially the upper left region, will be especially rich in the nutrient. In contrast, fish in the lower, and especially the lower right, regions will increase MeHg exposure while conferring relatively poor nutritional benefits. nutrient/MeHg graphin the nutritionally beneficial without containing mercury. This is relevant to the formation of fish advisories. See also (Mahaffey, et al., 2008).
3. MODELING NEUROTOXICITY IN THE LABORATORY
Studies of human populations are crucial since it is human health that is of direct interest, but they are inevitably correlational and rarely provide more than statistical control over dosing, nutrition, environmental influences, or other important variables. Studies conducted with laboratory animals can provide the necessary control, identify the mechanisms of action by which neurotoxicants act and characterize the functional consequences of low-level exposure. Behavioral studies provide an important bridge between mechanistic studies and studies of human populations. To be successful, laboratory models with behaving animals must use effect markers that reveal relevant functional domains. These studies must also use exposure markers that permit meaningful differentiation of low-level from high-level exposures and that permit comparisons across species, including humans.
3.1. Brain mercury and chronic daily intake are suitable markers of exposure
The key marker of exposure for MeHg neurotoxicity is the concentration of organic or inorganic mercury in the central nervous system. Mercury appears to enter the brain in its methylated form, but as long as it remains in that form, it can also exit the brain (Aschner and Aschner, 1990; Kerper, et al., 1992). Demethylated mercury persists as inorganic mercury, and accumulates. Its half-life of elimination appears to be on the order of years (Magos, 1987; Vahter, et al., 1994; Vahter, et al., 1995). With short exposures, the proportion of mercury that is inorganic is about 5% to 10%, but this value can increase with prolonged exposures as inorganic mercury accumulates (Day, et al., 2005; Vahter, et al., 1994).
Species differ in the relationships among intake, blood, and brain concentrations, and rats show remarkably high blood to brain ratios (Magos, 1987). As shown in Table 1, brain:blood ratios associated with developmental exposure range from 0.06 to 0.29 for the rat, depending on the chronicity of exposure (Magos, 1987; Newland and Reile, 1999a). By comparison, this ratio is greater than 1.0 for mice, monkeys, and humans (Cernichiari, et al., 1995; Magos, 1987; Stern, et al., 2001). The low brain:blood ratio in rats has been attributed to especially high levels of mercury-binding sulfur in rat blood, which serves as a mercury depot (Magos, 1987). The red-blood-cell to plasma ratio for rats is 145 to 1 as compared to 7, 25, and 21 to 1 for mice, rhesus monkeys, and humans respectively (Magos, 1987). Table 1 shows some of the species differences identified by (Magos, 1987) and updates it with recent data drawing from studies using chronic exposure regimens. The value provided for rats in the earlier review, 0.06, is frequently cited but this blood:brain ratio was observed with a relatively high dose of MeHg and with adult exposure. For developmental exposure in which exposure begins before mating and continues throughout gestation, this ratio increases to 0.15 to 0.25 at post-natal day (PND) 1 and becomes even higher at PND 21.
Table 1.
A comparison of Brain:Blood ratios across species and dosing regimens.
| Species | Dosing Regimen | Age | Brain:blood | Reference |
|---|---|---|---|---|
| Mouse | Chronic, drinking water, via dam. | PND 4 | 1.41–1.71 | (Stern, et al., 2001) |
| Mouse | Chronic, adult onset, drinking water, lifetime (> 14 month) exposure | Adult | 0.54–0.63 | (Stern, et al., 2001) |
| Mouse | 14 days after single, oral dose in adult. | Adult | 0.4–0.9 | (Nielsen, et al., 1994) |
| Mouse | Acute in adult. | Adult | 1.2–1.3 | (Magos, 1987) |
| Rat | 5 days/week, 3–13 weeks, adults. | Adult | 0.06 | (Magos and Butler, 1976) |
| Rat | Begin before mating, continue to parturition. | Birth. | 0.13–0.17 | (Newland and Reile, 1999a) |
| Rat | Begin before mating, continue to weaning (post natal exposure was nil) | Weanling | 0.21–0.29 | (Newland and Reile, 1999a) |
| Rat | Chronic, via maternal drinking water | Birth | 0.1–0.251 (Se dependent) | (Newland, et al., 2006) |
| Pregnant rat | Single inject into pregnant rat, determine 1–14 days later. | Fetal | 0.11–0.20 | (Wannag, 1976) |
| Pregnant rat | Single injection into pregnant rat, determine 1–14 days later. | Maternal | 0.02–0.1 | (Wannag, 1976) |
| Rat | Chronic, adult onset, drinking water | Adult | 0.08–0.13 | (Day, et al., 2005, Newland, et al., 2006) |
| Rat | Dietary, begin before, continue to PND 50 | Adult | 0.14 | (Lindstrom, et al., 1991) |
| Macaque | Daily, oral, adultonset for 6 to 18 months | Adult. | -- 3.2 (6 months) -- 5.1 (12, 18 months -- 5.4–8.3 for obese monkeys) | (Vahter, et al., 1994) |
| Macaca fascicularis | Four weekly doses | Infant | 2.5 | (Burbacher, et al., 2005b) |
| Squirrel Monkey | Weekly oral doses | Adult | 2.5 | (Berlin, et al., 1975) |
| Human | Neonatal | 1.7 | (Cernichiari, et al., 1995) |
These species differences in blood-binding can be circumvented by relating brain mercury to daily intake, rather than blood mercury. When these exposure markers are used only allometric considerations apply. Figure 2 shows the relationship between daily intakes of 3 to 500 µg/kg/day and neonatal brain concentration for rats, mice, and monkeys. In the studies used to fit the regression line, breeding females began MeHg exposure well before mating to give mercury levels time to stabilize (references in figure caption). The data for mice were collected on PND 4 (Stern, et al., 2001) so the concentrations were divided by 0.8 to account for brain growth during this period. Only blood concentrations were available for the monkey studies so a blood:brain ratio of 2.5 as reported in (Burbacher, et al., 2005c) was used to estimate brain concentration. A different ratio would shift the curve to the left or right but would not affect the slope. Neonatal brain mercury follows a power-function relationship (linear on log-log axes) between mercury intake and brain concentration in the mouse (Stern, et al., 2001), rat (Newland, et al., 2006; Newland and Reile, 1999a) and macaque neonate (Gilbert, et al., 1996; Rice, 1989a). The curve for monkeys is shifted to the left 10-fold from that for rodents, a species difference in sensitivity that is captured by the intercepts of 0.0023 and 0.03 for rodents and monkeys, respectively. Mice and rats fall on the same line, despite marked differences in brain:blood ratios. The slope of 1.3 describing the relationship between intake and brain concentration is identical for these species.
Figure 2.
Neonatal brain Hg vs estimated daily maternal intake for mice (Stern, et al., 2001), rats ((Newland and Reile, 1999a), unpublished data) and macaques (Burbacher, et al., 2005c; Gilbert, et al., 1996; Rice, 1989a). Lines shows power function fit to rodent or primate data. Slope is 1.3 for both lines. Intercepts are 0.0023 ppm and 0.03 ppm for rodents and monkeys, respectively. The left-most data point from (Burbacher, et al., 2005c) was not used in the regression but is included in the figure because it represents a very low dosing regimen and blood and brain concentrations were reported. It is lower than predicted by the regression because this entailed four doses administered weekly.
The exposures from animal studies shown in Figure 2 span brain concentrations of about 0.2 ppm (about 1 µM) to nearly 10 ppm (about 50 µM), concentrations that span the range described in studies of human populations. Autopsy data from Minamata revealed an average of 5 ppm Hg (range 0.4–15.4 ppm) in brains from 5 cases of fatal infantile Minamata Disease (Takeuchi, et al., 1962).The median brain mercury in occipital cortex from 27 infant autopsies in the Seychelles was 0.15 ppm (range 0.05 to 0.3 ppm). In that study, infant brain Hg was estimated to be 0.015 times that maternal hair (Cernichiari, et al., 1995). If this relationship holds in other populations, then brain mercury in Faroese infants would reach 0.4 ppm (Grandjean, et al., 1998), and brain mercury from non-fatal Minamata cases would range from 0.06 to 2.0 ppm, the higher levels being associated with mental retardation and cerebral palsy (Akagi, et al., 1998). Thus, the brain concentrations estimated from three important epidemiological studies (0.06 to 15.4 ppm) correspond to those from animal models of chronic, low-level developmental exposure. It might be noted that the brain concentrations from the rodent studies are not a NOAEL ("no observed adverse effect level") but a LOAEL ("lowest observed effect level") in the rodent brain. The effects are described below.
Two important conclusions can be drawn when extrapolating across species or across doses. First, the binding of mercury to blood is important when using blood as a marker of brain concentration. Apparently, this binding is of less relevance when comparing, across species, the relationship between daily intake and brain concentration, if exposure is sufficiently long to allow this binding to saturate or stabilize. This analysis leads to a hypothesis that, upon initial exposure, mercury is absorbed into the blood compartment due to binding to red blood cells, but once this compartment is filled the relationship between exposure and brain mercury in rats resembles that in other species so the differences among species are largely allometric. A particular daily intake produces a ten-fold larger brain concentration in the monkey than in the rat, suggesting that extrapolation across species can be accomplished using considerations of body mass or metabolic rate. These conclusions apply to neurotoxicity; their implications for species differences in MeHg's effects on blood physiology or other organs, such as kidney, have not been addressed here.
A second conclusion is important for low-dose extrapolation. The slope of the power function is greater than one. This means that a 10-fold decrease in exposure results in a greater-than 10-fold decrease in brain mercury, and brain mercury falls off rapidly as exposure declines. If this slope also applies to humans, then it means that a doubling of exposure results in a greater than doubling of brain mercury or, conversely, that halving exposure can reduce brain levels by more than two-fold.
In 1990, developmental exposures that resulted in brain concentrations of < 3 ppm were described as "low" and 3–11 ppm as "moderate" in animal models, regardless of species (Burbacher, et al., 1990). The supporting data on low-level exposures in rats are more solid now than when that review was conducted, and while the review remains a good guide it may be time to update the anchors on the scale. For example, reproducible behavioral, anatomical, and electrophysiological effects are associated with neonatal brain concentrations as low as 0.3 ppm, so the range described as "low" could reasonably be lowered by an order of magnitude.
3.2. Nutrition-MeHg effects can be studied experimentally
The strength of laboratory studies lies in their ability to control the key influences over toxicity, including diet, other co-exposures, as well as parameters like the dose, duration and period of exposure. For example, the potential for selenium or vitamin E (Beyrouty and Chan, 2006 Moller; Moller-Madsen and Danscher, 1991) to ameliorate MeHg neurotoxicity or for PCBs to enhance its toxicity (Roegge, et al., 2004; Widholm, et al., 2004) has been examined by comparing a single MeHg dosing regime with or without the other compound. An especially powerful approach is the full factorial design, in which multiple MeHg dosing regimens are crossed with different co-exposures such as selenium or DHA. This approach permits the detection of a main effect of diet and of MeHg in a single experiment. Most important, any protection conferred by diet, or elevated risk due to a dietary insufficiency, would appear as a diet X mercury interaction. Such an interaction indicates that the shape of the dose-effect curve for mercury would depend upon the diet or other co-exposures.
In a series of studies reviewed below, developmental and adult-onset exposure to MeHg was examined in rats exposed to a customized AIN-93 purified diet that was high or marginal in a targeted nutrient, selenium or n-3 polyunsaturated fatty acids. Nutrient levels were selected to be neither excessive nor deficient in these nutrients, according to current guidelines or recommendations (National Research Council, 1995; Wainwright, 2002; Wauben and Wainwright, 1999). The exposure regimen for the DHA studies is illustrated in Figure 3. Three-month old female rats began their assigned diet within days of arriving at the colony. After three weeks, they began exposure to one of three levels of MeHg, 0, 0.5, or 5.0 ppm in their drinking water and then, after about three weeks, they were mated with unexposed males. These drinking water concentrations produce exposures of about 0, 40, or 400 µg/kg/day of mercury (as MeHg), although during the later days of gestation, this exposure can increase somewhat because of increased fluid intake by pregnant rats (Newland and Reile, 1999a). Maternal exposure continued up through PND 15, when pups could reach the water spout, and was reinstated after weaning in order to study chronic exposures. The regimen for rats in a selenium-MeHg study was similar except that the animals were four months of age upon arrival at the colony. The regimen used permitted the examination of chronic, adult-onset exposure in the female breeders and developmental exposures in the offspring. Most studies used female offspring to facilitate comparisons with the adult-onset exposures,
Figure 3.
Exposure regimen used to examine developmental and adult-onset MeHg exposure in Auburn studies. Offspring exposure to MeHg ended at birth but they were maintained on their high- or low- nutrient (DHA or Se) diet throughout life. The vertical dashed line indicates that maternal exposure was interrupted from post-natal day 16 to 21 because pups could reach the water bottle, but there is no significant exposure via maternal milk (see text).
When these regimens began, we had just reported that no significant exposure occurred during lactation (Newland and Reile, 1999a). This finding has now been replicated (Sakamoto, et al., 2002; Stern, et al., 2001), so we are confident that, functionally, MeHg exposure to the offspring ended at birth. A recent paper suggested that there is lactational exposure during this important post-natal period, but pre-weanling rats may have been able to drink contaminated water directly in that study (Manfroi, et al., 2004).
The design of diets to model human fish consumption is complicated by the large number of nutrients contained in fish and the vast differences across fish species in the presence of these nutrients. One could try to model a particular fish diet, but such an approach would fail to address a slightly different diet. It is preferable to identify a particular dietary component and vary that systematically. Even this simplification is complicated when studying PUFAs because several are important to nervous system function and the two important classes, the n-3 and n-6 PUFAs, compete for the same enzymes in their biosynthesis from dietary precursors. It is difficult, but important, to develop diets that differ on only one dimension, such as n-3 PUFA content, while holding constant other characteristics, like saturated fats, n-6 PUFA content, or selenium content. It is impossible to design a perfect pair of diets, especially with PUFA studies, but the test diets should differ on only one variable as much as possible.
To study the role of DHA, a custom mixture of palm, safflower, and soybean oils was designed to comprise 67% of the oil mixture for all rats. For the Fish Oil (FO) group, the remaining 33% was a DHA-rich fish oil(mostly menhaden), and for the Coconut Oil (CO) group, it was coconut oil devoid of DHA. Table 2 shows the fatty acid makeup of these diets. An important feature of both diets was that the n-6 PUFAs and total monounsaturated fats were similar. The diets differed somewhat in saturated fats because of the use of coconut oil but the greatest difference between these diets lay in their composition of n-3 PUFAs. The CO diet was devoid of eicosapentaenoic acid (EPA) and DHA but contained 1% ALA so as to provide a minimal level of n-3 PUFAs. By comparison, the FO diet was very rich in these n-3 PUFAs. The n-6:n-3 ratio was 16.1 and 2.1 in the CO and FO diets, respectively. These ratios are on the margins of dietary recommendations for rodents (National Research Council, 1995; Reeves, et al., 1993), so these diets do not represent severe deficiency or excess in any constituent. Diets began six weeks and MeHg three weeks prior to mating.
TABLE 2.
Fatty Acid Content of Experimental Diets (% Fatty Acids, as weight)
| Diet | ||
|---|---|---|
| Coconut Oil | Fish Oil | |
| Total Saturated FAs | 59.1 | 38.4 |
| Total Monounsaturated FAs | 21.8 | 20.4 |
| 18:3 n-3 (α Linolenic) | 1.1 | 1.6 |
| 18:2 n-6 (Linoleic) | 18.1 | 23.2 |
| 20:5 n-3 (EPA) | - | 5.4 |
| 22:6 n-3 (DHA) | - | 4.2 |
| E n-3 | 1.1 | 11.2 |
| E n-6 | 18.1 | 23.2 |
| n-6:n-3 ratio | 16.5 | 2.1 |
The selenium diets are much easier to describe. The Low-selenium diet contained a nominal concentration of 0.06 ppm of selenium, the lowest possible with a casein based diet. The High-selenium diet was supplemented with sodium selenite to produce 0.6 ppm. Diet analyses revealed that the actual concentrations approximated these levels (Newland, et al., 2006). These diets were neither severely deficient nor excessive in selenium content.
The mercury concentrations in the brains of neonates exposed to MeHg and selenium during gestation are shown in Figure 4. Brain mercury levels (Figure 4, top) approximated 0.1 to 0.3 ppm (~1 µM) in the low-Hg-exposure groups and 3–5 ppm (~20 µM) in the high-Hg-exposure groups. Selenium levels (Figure 4, bottom) were influenced both by dietary selenium and mercury, but the difference was relatively small. The 10-fold difference in dietary selenium (0.06 to 0.6 ppm) produced only a 15% difference in brain selenium. Maternal mercury exposure produced an equally modest drop in neonatal selenium concentrations. These effects demonstrate that, even in the neonatal brain, brain selenium levels are tightly controlled.
Figure 4.
Mercury (top) and selenium (bottom) in the brains of neonates exposed during gestation to mercury and selenium.
Brain mercury in neonatal rats from the PUFA cohort exposed to 0.5 or 5 ppm of mercury as MeHg in their drinking water were close to those from the selenium cohort. The lower dose resulted in brain mercury of about 0.29 ppm. The 10-fold increase in drinking water MeHg increased brain mercury by nearly 20-fold, to 5.5 ppm (Newland, et al., 2006). Dietary PUFAs did not influence mercury concentration in blood or brain and mercury did not significantly affect PUFA concentrations in the neonatal brain (unpublished observations).
4. BEHAVIORAL EFFECTS OF DEVELOPMENTAL METHYLMERCURY EXPOSURE IN ANIMAL MODELS
The behavioral effects of developmental MeHg exposure pose an interesting challenge to neurotoxicology because they appear to lie outside the behavioral domains frequently examined in animal models. Perhaps the most common tests of neurotoxicity involve memory or procedures thought to model the general cognitive domains tapped in human IQ tests. As noted in the introductory comments, however, developmental MeHg exposure appears to affect behavioral domains that are nearly orthogonal to these processes, instead involving the regulation of reinforcer, or reward, function. Evidence for this claim is reviewed here, but first, some brief background.
The emerging MeHg literature is pointing to the value of a distinction between discrimination and reinforcement processes (Newland and Paletz, 2000). What does this mean? Operant, or voluntary, behavior can be distinguished along several dimensions. The most important is that operant behavior is affected, reinforced, by its previous consequences. Reinforcing events also define contexts that determine the likelihood of a response. For example, if turning on a light has signaled that lever-pressing produces food, and turning off the light signals extinction (no food is available), then the light, previously an irrelevant stimulus, will increase the probability of lever-pressing, and turning it off will decrease it. Two processes are present here. The first is that lever-pressing produces food, a response-consequence relationship that strengthens lever-pressing at the expense of other responses. The second process, discrimination, is evident in noting that the light signals the presence of that relationship between lever-pressing and reinforcement. Thus, we have the acquisition of a light-dark discrimination (Catania, 1992; Davison and Nevin, 1999). Both processes are always present, but experimental procedures can emphasize one set over another.
It is becoming recognized in the basic operant (Davison and Nevin, 1999; Donahoe, et al., 1993) and behavioral neuroscience (Schultz, et al., 1997; Schultz, et al., 2000) literatures that reinforcement and response acquisition processes are distinct from discrimination processes, are subject to different environmental events, and reflect different neural substrates. Many standard behavioral tests, memory tests, and even IQ testing reflect simple to highly refined discrimination processes and may not detect the outcome of exposure to toxicants like MeHg that affect the processing of reinforcing consequences. Such effects would appear as an impairment in behavioral adjustments following a change in the source of reinforcement. If lever-pressing is reinforced at a high rate for a long time and then reinforcement stops, the pursuant changes in behavior can then changing behavior after reinforcement stops can take some time (Nevin, 1979; Nevin, 1988). With developmental MeHg exposure, such adaptations take even longer, and the outcome appears as perseveration.
4.1. Non-Human primate models
A benchmark against which to compare rodent models lies in two long-term cohorts involving low-level developmental MeHg exposures and life-span development in nonhuman primates. One cohort, examined by Deborah Rice and colleagues at Health Canada, was exposed to 10, 25, or 50 µgHg/kg/day delivered chronically either pre- or pre-plus-post-natally. One infant in the 50 µg/kg/day group displayed nystagmus and hind-limb paralysis, and a second developed a wasting syndrome at two years of age. The second cohort, examined by Tom Burbacher and colleagues at the University of Washington, was exposed to 50 to 90 µgHg/kg/day during gestation. These dosing regimens produced dose-related maternal blood concentrations of 0.3 to 2 ppm (Rice, 1989a; Rice, 1989b; Stinson, et al., 1989; Vahter, et al., 1994) and likely infant brain concentrations of 0.75 to 5 ppm (Figure 2).
These studies have been reviewed previously and so will not be reviewed in detail here (Rice, 1996a; Rice, 1996b). There were reproducible sensory-motor deficits across auditory, visual, and somatosensory domains in the exposed monkeys (Rice, 1989c; Rice, 1998a; Rice and Gilbert, 1982; Rice and Gilbert, 1990; Rice and Gilbert, 1992; Rice and Hayward, 1999). Especially interesting is that these effects became more pronounced during aging, despite the absence of continued exposure (Burbacher, et al., 2005b; Rice, 1996a). On some cognitive tasks, discussed below, there were no effects of developmental MeHg, or effects were in the direction of improvement. When the Health-Canada animals were 20 years of age and showed diminished sensorimotor function, MeHg-related cognitive deficits were still not detected on simple and complex choice reaction time, tasks selected because of their relationship to scores on IQ tests in humans. The outcome led the author to question whether there is any cognitive impairment associated with low-level MeHg exposure, at least in nonhuman primates (Rice, 1998b), a bold conclusion that had solid support from the literature as it existed only ten years ago.
4.2. Developmental MeHg exposure does not consistently affect the initial acquisition and expression of discrimination or of memory
The acquisition and performance of discrimination tasks have also not consistently been sensitive to developmental MeHg exposure. Some infant and juvenile monkeys exposed to 10, 35 or 50 µg/kg/day during development showed a statistically significant, if subtle, improvement in performance on the acquisition of a complex, form-based discrimination as compared with unexposed controls, and they showed diminished influence by irrelevant stimuli (Rice, 1992). Even a monkey showing nystagmus and hind-limb paralysis at birth resembled controls on these tasks. As aging adults, no effects were noted on choice reaction times (Rice, 1998b).
Rats exposed developmentally to MeHg showed no discrimination-based effects on the acquisition of a visual, spatial, or olfactory discrimination (Buelke-Sam, et al., 1985; Goldey, et al., 1994; Schreiner, et al., 1986), even at doses in excess of 2 mg/kg/day. In these tasks, the animals are required to engage in one response in the presence of some stimulus and another response in the presence of another stimulus, the basic definition of discrimination learning. In these studies, exposed animals acquired and expressed these discriminations equally to unexposed animals. We have noted a similar absence of an effect of MeHg in the acquisition of discrimination but robust effects on reversals, as illustrated below in the section on discrimination reversals (Paletz, et al., 2007; Reed, et al., 2006).
MeHg-exposed rats and monkeys have been examined using a spatial delayed alternation task. In this task an animal experiences a series of trials during a session. On one trial, pressing the left lever produces a reinforcer, and on the next trial pressing the right lever does so. The correct lever alternates from trial to trial. To perform accurately, the animal must remember which lever was previously reinforced. A delay interval is imposed between opportunities to respond, thereby making this a task that emphasizes working memory. If a neurotoxicant is affecting memory, then its effects would be minimal at short delays but especially large at the longer delays that challenge memory.
Studies out of the University of Washington raised the possibility of cognitive effects during development but not necessarily in the direction of impairment, at least not in an obvious manner. Monkeys exposed to 0, 50, 70, or 90 µg/kg/day MeHg in utero were tested on a spatial delayed alternation task at 7 to 9 years of age. No impairment was noted and, in fact, MeHg exposure apparently facilitated performance as indicated by more correct trials and fewer incorrect responses than controls (Gilbert, et al., 1993).
Mice exposed up to 8 mg/kg/day during either gestational day (GD) 7–9 or GD 12–14 were examined on the spatial delayed alteration tasks (Dore, et al., 2001). The GD 7–9 exposure regimen retarded the acquisition of a spatial alternation task but did not affect delayed spatial alternation at any delay interval after acquisition had occurred. The GD 12–14 exposure period, GD 12–14, disrupted working and reference memory in a radial arm maze. In a second series of studies, mice consumed 4 to 8 ppm of mercury (as MeHg) in drinking water from GD 2 to weaning. There was no effect on the acquisition or expression of a simple discrimination or on delayed spatial alternation in a standard T maze. Deficits were reported in high-dose females in the acquisition, but not performance, of a more complex, two-stage modified Y maze (Goulet, et al., 2003). In a recent study, mice were exposed to 0.4 or 4 mg/kg of MeHg on PND 10 via gastric intubation and examined on locomotor activity, swim maze, or radial arm maze (Fischer, et al., 2008a). Effects on radial arm maze appeared at the high dose, which also diminished locomotor activity in this and a similar study (Fischer, et al., 2008b). Retardation of swim maze performance (detected as longer latencies) was seen in young adults after the 0.4 mg/kg dose.
Mice exposed to 3 ppm of MeHg in drinking water during gestation or chronically during adulthood had difficulty acquiring a delayed spatial alternation procedure (Weiss, et al., 2005). As with the aforementioned studies, this reflected an effect of acquisition, but unlike the previous studies, the effects apparently did not depend on exposure period, as developmental exposures could not be distinguished from chronic adult exposures. These training difficulties prevented the examination of delay-specific effects.
Rats exposed developmentally to 0.5 ppm MeHg via maternal drinking water, in which exposure began well before mating, displayed a small but statistically significant increase in errors on both delayed and nondelayed spatial alternation tasks. Since the MeHg effects were not related to the delay, however, the authors concluded that this probably did not reflect memory deficits (Widholm, et al., 2004). The pattern of errors was not random, however, and was consistent with perseveration on the lever that was reinforced on the previous trial. In another study by a different group, mice exposed to 4 ppm of MeHg, again with exposure beginning well before mating, showed cerebellar deficits but no effect on water maze performance (Markowski, et al., 1998).
Memory effects might have been detected in another study. Using a modified Fagan-type task, infant monkeys viewed photographs of monkey faces and the time spent looking at the faces was recorded (Gunderson, et al., 1986; Gunderson, et al., 1988). Sometimes novel faces were shown, and at other times, faces were shown that had been presented previously. In such a task, children spend more time looking at novel faces so a short gaze duration is interpreted as a measure of recognition memory, i.e., they remember seeing the face previously so look only briefly. Unexposed infant macaques spent more time looking at novel faces, as do children, but the exposed monkeys viewed novel and previously presented faces with equal gaze durations. Consistent with the interpretation of the neuropsychological test on which this was modeled, the effect was viewed as a failure of recognition memory. It can be noted, however, that with developmental exposure MeHg accumulates in regions of the occipital cortex serving vision and that such exposures permanently impair higher-order visual processing as detected in changes in contrast-sensitivity contours (Burbacher, et al., 2005a; Rice and Gilbert, 1990). These changes occur especially in the processing of high-spatial frequency visual information, even with high-illumination, the sort of visual information that would be required to detect differences in fine details that distinguish different faces. It is possible, therefore, that this effect on a Fagan-type task represents a deficit in higher order visual function, and perhaps even a primate form of prosopagnosia, as noted in a previous review (Newland and Paletz, 2000). Perhaps gaze was similar because impaired vision caused all of the faces to look similar. Such an interpretation would be consistent with other studies demonstrating the sensitivity of sensory function to MeHg (Berlin, et al., 1975; Choi, et al., 1978; Harada, 1995). In a study of exposed children from the Great Lakes, no effects of MeHg exposure were noted on a Fagan test (Darvill, et al., 2000).
Taken together, these studies provide little evidence of consistent or robust MeHg-related deficits in the acquisition or expression of discriminations using procedures said to involve "reference memory," or on tasks designed to reflect remembering during a particular session or trial ("working memory") such as the delayed spatial alternation or radial arm maze task. This is not to say that there are no effects. Deficits have been described in the acquisition of discrimination learning and in the radial arm maze, depending on exposure period, but effects on maze learning have not been consistently reproduced or tend to be high-dose effects (Goldey, et al., 1994; Rossi, et al., 1997; Vorhees, 1985; Weiss, et al., 2005). Swim maze performance was reported recently in mice after postnatal exposure to 0.4 mg/kg on PND 10 (Fischer, et al., 2008a). These tasks may be pertinent to the mental retardation noted in the high exposures experienced in Minamata, and as such, they anchor the right end of the dose-effect curve for environmentally relevant exposures.
4.3. Low-level developmental MeHg exposure results in perseveration on discrimination reversal and choice tasks
In a spatial discrimination reversal (SDR) procedure, the subject faces a panel containing two response devices. When a trial begins, the animal (or, in some cases, human) can respond on either side, but only responses on the left alternative (for example) result in reinforcer delivery; right-lever responses do not. After an accuracy criterion is reached, a reversal is implemented and only right lever-pressing results in reinforcer delivery, while left lever-pressing has no programmed consequences, i.e., it is under extinction. This procedure and variations on it have been very sensitive to developmental MeHg exposure.
In our implementation of this procedure with rats, some potentially important methodological components were included (Paletz, et al., 2007; Reed, et al., 2006). A response-initiated discrimination reversal procedure was used: the response devices were retractable levers located on the front wall of an operant chamber and the levers were unavailable to the animal between trials or at the beginning of a trial. The onset of a trial was signaled by the sounding of a tone, but the animal also had to press a (non-retractable) lever located on the back wall in order to insert the two front levers into the chamber. Requiring that the animal respond to initiate the trial insures that it is on-task and not engaged in grooming or some other behavior that precludes responding. It also positioned the animal at the back of the chamber at the beginning of each trial, preventing it from standing in front of the correct lever throughout the session.
Once the two levers were inserted, the animal could press either lever, but only one alternative resulted in reinforcer delivery. A final methodological detail can be noted. To insure that the discrimination is well-established, the animal had to meet a criterion of 85% accuracy on three successive sessions before a reversal occurred. This is potentially important because it ensures that responding on a particular lever has been reinforced frequently (at least 153 times across three sessions) before a reversal commences. A laxer criterion would have allowed us to impose a reversal earlier for some animals but might have diminished the sensitivity of the procedure. Finally, imposing an accuracy criterion rather than an experience criterion, such as 10 sessions on a particular arrangement, ensures consistent performance across subjects.
Two types of errors are possible. Errors of commission entail pressing the incorrect lever, which, after a reversal, is the previously reinforced lever. Errors of omission entail failing to press the back lever within 15 seconds of the trial onset (signaled by a tone) or failure to press the front lever within 15 seconds of pressing the back lever. The response-initiated procedure also permits the inclusion of two latency measures. The first is the latency to press the back lever starting the trial. More important, however, is that we can determine a response latency to press the front lever. Data on latency will not be discussed here but are described in the original papers (Paletz, et al., 2007; Reed, et al., 2006).
Some rats also experienced a visual discrimination reversal (VDR) after completing a SDR phase (Paletz, et al., 2007). The procedure was similar to the SDR except that a light indicated the correct lever. Each lever had an LED (light-emitting diode) over it. For the initial discrimination, the lever under an illuminated LED was correct. When criterion was met, a reversal was imposed so that the lever under a non-illuminated LED was correct. This procedure was difficult for the rats.
These procedures resemble a form of neuropsychological testing called intradimensional and extradimensional shift, used in tests such as the CANTAB (De Luca, et al., 2003; Fray and Robbins, 1996; Ozonoff, et al., 2004) and the Wisconsin Card Sort Task (Boone, et al., 1993; Dias, et al., 1997) to detect damage to the frontal cerebral cortex. Both the spatial and visual discrimination reversal tasks can be viewed as an intradimensional shift since the reversal was along the same dimension (spatial location or illumination) as the original discrimination. The animals that performed on the VDR after the SDR also experienced an extradimensional shift, sometimes referred to as a set-shift. The dimension that had been relevant, spatial location, became irrelevant and a new dimension, illumination, became relevant. The procedures implemented with the rats had fewer distracters and therefore were much simpler than those implemented with humans or non-human primates (Rice, 1992).
The results were striking. Figure 5 shows three animals from the studies of MeHg and selenium (Reed, et al., 2006). The top panel typifies control performance. For the original acquisition (four left-most data points), the number of correct responses exceeded 85% accuracy (51/60 trials) quickly, and the criterion of three successive sessions with at least 85% accuracy was met by session four for the original discrimination and all subsequent reversals. Most errors were of commission, in which the wrong lever was pressed. These dominated the first session of each reversal.
Figure 5.

Representative animals showing spatial discrimination reversal (SDR) learning. The ordinate shows the number of trials with a correct response, commission error, or omission error for each session (abscissa). A reversal is indicated by a break in the lines. The top panel is representative of control rats, all of which met criterion rapidly. The middle panel is illustrative of the 5 ppm-exposed rats. The bottom panel is an extreme case. After producing only one single correct response in 17 sessions in the first reversal, this animal was provided "behavior therapy" (details in text). From(Reed et al., 2006.)
The middle panel illustrates a pattern common with the developmentally exposed animals. The original discrimination was acquired quickly. In this case, five sessions were required, but exposed animals as a group did not differ from controls in their acquisition of the original discrimination. The effects of developmental exposure appeared on the first reversal. This animal completed five sessions with only one correct lever-press. Errors of commission were high early in this reversal but dropped as errors of omission (failing to respond) increased. After finally making correct lever-presses on session 11, this animal completed the reversal rapidly, and subsequent reversals were completed quickly.
The bottom panel, representing a different animal from the 5 ppm (higher dose) exposure group, represents an extreme, but not unique, case from our studies. This animal, which showed normal growth and otherwise appeared healthy, alert and responsive on handling, had considerable difficulty in acquiring the original discrimination, reaching criterion after 23 sessions. On the first reversal, this animal pressed the now incorrect (left) lever over 100 times during sessions 24–31 and pressed the correct (right) lever only once (session 36) during sessions 24–40. The high number of response omissions that eventually developed indicated that little responding occurred. At this point, a form of behavior therapy was imposed for one session. The incorrect, left, lever remained retracted, and only the correct, right, lever was inserted during this session. The animal began responding on the right lever and continued doing so in the subsequent sessions when both levers were again available. This and subsequent reversals were completed after this intervention, albeit slowly. Such an extreme case of perseveration was seen in a limited number of rats as well as in a squirrel monkey with a different sort of choice procedure (Newland, et al., 1994).
This perseveration on the formerly reinforced lever has been reported by us three times now as illustrated in Figure 6. For the PUFA cohort, it was seen with both the SDR procedure and the VDR procedure (Paletz, et al., 2007). For the selenium cohort, it was seen in the SDR procedure (Reed, et al., 2006). VDR was not examined in the selenium cohort. Note that the original discrimination and completion of the extradimensional shift, the shift from SDR to VDR, was acquired similarly by all exposure groups. The difficulty of the VDR can be seen in the number of errors that occurred before criterion was met.
Figure 6.
Commission errors to criterion in the spatial discrimination reversal (SDR) procedure for the selenium (Se) cohort (top) and the n-3 polyunsaturated fatty acid (PUFA) cohort (center). Errors from the visual discrimination reversal (VDR) procedure for the n-3 PUFA cohort are in the bottom. The points over "OD-Light" in the bottom panel represent an "extradimensional shift" from the SDR sessions in the middle panel. Errors are shown for the original discrimination (OD-left for SDR, OD-light for VDR) and the first three reversals are shown. Statistical differences are indicated with a (^) p<0.05 for the 5ppm group compared to controls; ($) p<0.01 for the 0.5 ppm group compared to controls; (#) p<0.05 for the 5 ppm group compared to the 0.5 ppm group. Error bars represent ±1 SEM. Note differences in scaling between SDR and VDR. Top graph adapted from Reed et al. (2006). Center and bottom graphs adapted from Paletz et al. (2007).
The reproducibility of the effects on spatial and visual discrimination reversals seen here contrast with earlier attempts at examining reversal learning in MeHg exposed animals. Methodological considerations may account for the discrepancies. In one study, rats were trained under a visual discrimination for five training sessions and four reversal sessions, with reversals imposed regardless of performance (Schreiner, et al., 1986). Trials were not response-initiated, and the criterion for a reversal was not based on individual performance. When the first reversal was imposed, group average accuracy on trials containing a response was 80%, but some trials did not contain a response. Since the average accuracy was 80%, approximately half of the animals that responded were performing at less than 80% at the end of the original acquisition and therefore more poorly than all of the animals illustrated in Figure 6. These methodological differences are important. Requiring the animal to respond to begin a trial ensures that the animal is not distracted. Perseveration entails persisting on a previously reinforced response (Boulougouris, et al., 2007; Robbins, 1996), so a strict accuracy criterion that is consistent across all animals provides similar reinforcement for correct responding immediately prior to a reversal.
In an earlier study conducted with infant and juvenile monkeys, visual discrimination was established and then reversal procedures were imposed (Rice, 1992). In that study, a performance criterion was determined individually before a reversal was imposed. For infants this criterion was 85% accuracy for five sessions (not necessarily consecutive), and for juveniles, this criterion was at least nine correct trials in a ten-session block, so reversals could occur within a session. Trials were not response-initiated. The discrepancy between the results of this study and our studies might be related to the accuracy criterion, absence of response initiation, or age at testing (infants or juveniles vs. fully adult in our studies).
4.4. Developmental MeHg exposure enhances the efficacy of primary reinforcers
Procedures that link response effort directly to reinforcement, like ratio schedules of reinforcement, are very sensitive to the impact or efficacy of reinforcing consequences. Under a fixed-ratio (FR) schedule of reinforcement, a fixed number of responses is required for reinforcement. When the response requirement is low (e.g., FR 5, or 5 responses/reinforcer), responding occurs at a high, steady rate. Under a large FR schedule, the high response effort and low reinforcement rate result in erratic behavior and long pauses (Baron and Derenne, 2000; Derenne and Baron, 2002; Ferster and Skinner, 1957), a phenomenon called "ratio strain." When the ratio becomes very large responding stops altogether. Under a progressive ratio (PR) schedule, the response requirement begins at a low value and increases gradually throughout the session until responding ceases.
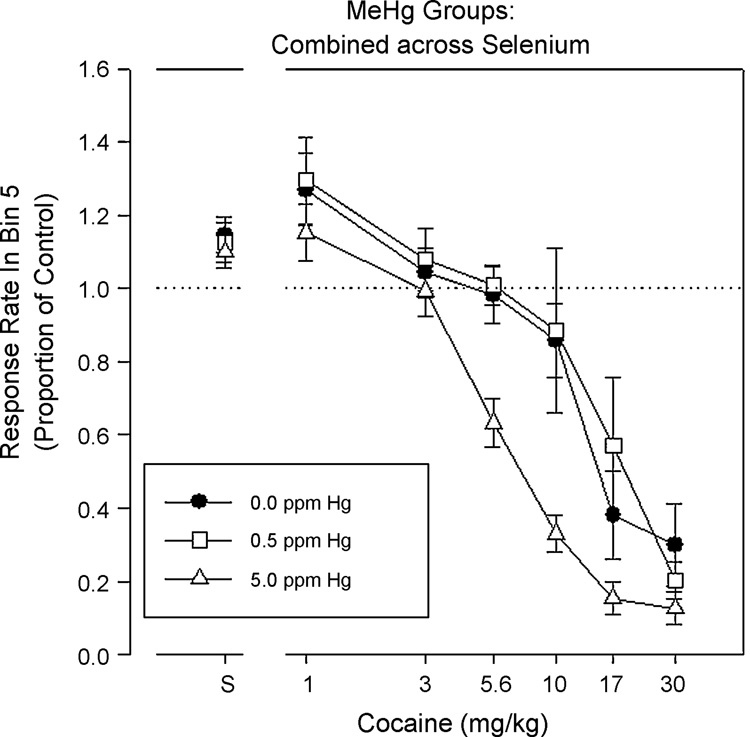
PR schedules have been used to assess the abuse liability and reinforcing efficacy of abused drugs or other reinforcers (Griffiths, et al., 1977; Stafford, et al., 1998). Highly reinforcing drugs, like cocaine, maintain hundreds and sometimes thousands of responses per reinforcer before responding ceases, while drugs with less abuse potential support much less responding.
The rapid acquisition of FR responding is sensitive to developmental exposure to cadmium (Newland, et al., 1986), tetrochlorodibenzo-p-dioxin (TCDD) (Hojo, et al., 2002), ethanol (Middaugh and Gentry, 1992), and MeHg (Paletz, et al., 2006; Reed, et al., 2008). With cadmium, TCDD, and MeHg, the effect is in the direction of enhanced acquisition. In a recent report, for example, adult rats were trained overnight to press a lever under a FR 1 schedule (each lever-press was reinforced with a sucrose pellet), and the FR 1 remained in effect for three days (Paletz, et al., 2006). The response requirement then changed every third session to an FR 5 (days 4–6), FR 25 (days 7–9), FR 75 (days 10–12), and FR 5 (days 13–15). The main finding, shown in Figure 7, was that rats exposed in utero to 5 ppm of MeHg responded more rapidly than control rats under the FR 25 and FR 75 (statistically significant under FR 75) but not under the FR 1 or FR 5 schedules.
Figure 7.
Response rates from the rapid acquisition of fixed-ratio (FR) procedure for rats exposed during gestation to MeHg. Data are collapsed across high- and low- DHA diet groups. The 5 ppm groups, which showed greater responding under the high-ratio conditions, were different from controls for the FR25 and FR 75 conditions (statistically significant for the FR75). (From Paletz et al., 2006).
In a later phase of this study, the same rats lever-pressed under a PR schedule for three, 150 minute sessions. The initial requirement for each session was 10 responses, and the requirement increased by 5%, 10%, or 20% after each reinforcer delivery. The maximum ratio obtained (MRO), the breakpoint, is the ratio at which the response requirement becomes so effortful and reinforcement so sparse that responding deteriorates (Hodos and Kalman, 1961; Stafford, et al.). Figure 8 shows that rats exposed to the high dose of MeHg had statistically higher MRO breakpoints than control rats for the 5% condition. While this group also showed quantitatively higher breakpoints for the other two conditions, there was too much variability to detect statistical significance. Evidently, a slow rate of increase produces greater experimental power.
Figure 8.
Maximum ratio obtained (MRO, ordinate) during a progressive ratio schedule for rats exposed during gestation to MeHg. Data are collapsed across high-and low-DHA diet groups. The ratio increased by 5%, 10%, or 20% after each reinforcer. The * shows a statistical difference between the 5-ppm group and controls. From (Paletz et al., 2006.)
Recently, the main finding of enhanced acquisition under increasing ratio schedules due to developmental MeHg exposure was replicated and extended with procedural variations in the implementation of the progressive ratio schedule (Reed, et al., 2008). Results of this study confirmed previous findings: 5-ppm rats had significantly higher MRO breakpoints than controls for the 5% condition (also for the 20% condition in this study). Interestingly, the MeHg effect occurred only in rats consuming a diet adequate in selenium content, suggesting some interaction with dietary selenium.
This experiment also contained a condition testing the validity of the PR schedule as a measure of reinforcer magnitude. If the PR schedule is sensitive to reinforcing magnitude, then a large reinforcer magnitude should support greater responding than a small one. In one condition, the number of sucrose pellets delivered increased from 1 to 3, and the MRO increased accordingly (Reed, et al., 2008).
The appearance of MeHg effects on discrimination reversals and, in littermates, on performance under progressive ratios or rapid acquisition of FR schedules suggests a link between the two. The link is hypothesized to lie in the impact of the reinforcer on the persistence of responding. A higher magnitude, or efficacious, reinforcer will maintain responding with greater persistence, and in the reversal learning procedure, this persistence would appear as perseveration. This hypothesis is developed more fully below.
5. EARLY DEVELOPMENTAL EXPOSURE AND AGING: A ROLE FOR DIET
5.1. MeHg effects emerge in aging animals
The Minamata cohort has revealed, not only the irreversibility of MeHg's neurotoxicity but also that other effects are uncovered during aging. Somatosensory effects persist decades after exposure ends in humans (Ninomiya, et al., 2005). Similarly, somatosensory as well as visual and auditory deficits have been reported in aging monkeys (Rice, 1996a; Rice, 1996b). In the Minamata cohort, late-emergent signs include very serious ones like difficulty bathing, eating, or dressing oneself, that are detected with the Activities of Daily Living questionnaire given to individuals who were probably young adults during the major outbreak (Kinjo, et al., 1993).
Even more troubling, animal studies have shown that exposure that occurs exclusively during gestation can result in motor and cognitive deficits that appear only during aging. Newland and colleagues exposed rats to MeHg during gestation using an exposure regimen similar to that shown in Fig 3, except that all animals were on the same chow diet. In one study, young adult rats were trained to lever-press at high response rates using a Differential Reinforcement of High-Rate (DRH) schedule of reinforcement. In this procedure, a rat had to press a lever 9 times within 4 seconds for reinforcement (DRH 9:4). All groups acquired the task similarly. Unexposed rats showed about a 20% reduction in the number of criterion response sequences (nine responses within four seconds) when they were over two years of age (Newland and Rasmussen, 2003). Animals exposed to MeHg during gestation showed significant difficulties in sustaining this performance, and for many, performance deteriorated to zero criterion response sequences well before they turned two years of age.
Littermates of these animals were tested in an acquisition-of-choice procedure (Newland and Reile, 1999b; Newland, et al., 2004). In this procedure, a single experimental session began with two levers for the rat to press. Pressing either lever provided a reinforcer every 3 minutes, on average, but actual reinforcer delivery was unpredictable. This is called a Concurrent Random Interval 180" Random Interval 180" schedule or Conc RI 180" RI 180" schedule of reinforcement. As expected, responding was distributed equally between the levers. After 30 minutes, one lever became several times richer than the other and behavior changed gradually so that most responding occurred on the richer lever. By comparison with controls, the change in preference for the rich lever was significantly slower in MeHg-exposed animals that underwent behavioral testing for the first time when were 2.3 years of age. Littermates tested at 1.7 years of age showed trends but no statistically significant effect of MeHg (Newland, et al., 2004).
The rats in the PUFA cohort, in which animals were exposed to MeHg during gestation and to diets low or high in DHA throughout life, underwent spatial- and visual-discrimination reversals when they were young adults, as described above. After completing SDR and VDR procedures, they remained idle in their home cages and did not receive any behavioral assessment until they were 2.3 years of age when they were retested on the SDR procedure (Paletz, et al., 2007). The effect of MeHg on commission errors during reversals seen when the rats were younger was not observed after the rats had aged.
The absence of an effect with the SDR procedure may seem inconsistent with the earlier study showing impaired acquisition of choice in 2.3 year-old rats (Newland, et al., 2004). There are, however, some important differences between the two studies. One is that in the earlier choice study, responding was reinforced intermittently at a rate of about 0.33 reinforcers/min, and choice was acquired repeatedly in single sessions under a free-operant (i.e., not a trials) procedure. In the SDR procedure, every correct lever-press was reinforced, transitions occurred across multiple sessions, and a response-initiated trials procedure was used. Perhaps a more important difference is that the aging SDR animals, which showed no MeHg-related effects, had had extensive behavioral experience. In contrast, the aging animals on the acquisition of choice procedure had had little experience and showed effects when they were old, as compared with younger littermates. For the SDR task, at least, behavioral experience apparently prevented MeHg's developmental neurotoxicity from being expressed, but the acquisition of a new behavior or a sedentary life, sitting in the home cage, exacerbated MeHg's neurotoxicity.
5.2. In adulthood and aging, low dietary selenium and n-3 PUFAs have behavioral effects independent of MeHg's developmental neurotoxicity
The design used in the MeHg-nutrition studies permitted the detection not just of the effects of developmental exposure to MeHg but also the effects of a diet chronically marginal or sufficient in selenium or DHA. DHA had no influence over MeHg's neurotoxicity, but dietary selenium may have, albeit not consistently. The diets themselves had behavioral effects of their own, and these effects were different from those of developmental MeHg.
In the SDR procedure, rats that received the diet low in either nutrient, n-3 PUFA or selenium showed response failures (errors of omission) more often than rats that received either of the nutrient-rich diets. Figure 9 illustrates this effect in adult and geriatric rats from the n-3 PUFA study. The number of errors made by the geriatric rats is lower than adults because they had been exposed to the SDR procedure when they were younger. The number of trials without a response, or omission errors, was higher for the animals on the low n-3 PUFA diet than for rats that were on the high diet. A similar effect was seen in the study of low and high levels of selenium, except that only adult, not geriatric, rats were used.
Figure 9.
Errors of omission under a spatial discrimination reversal procedure for rats on a diet low (coconut oil) or high (fish oil) in n-3 PUFAs during adulthood (A) or when geriatric (G). Data points over G represent re-testing under the SDR procedure. The data point over "OD-left G" followed the data point over "R3-right A" by about seven months, during which visual discrimination reversals were assessed. SDR performance was retained in these geriatric animals. For both ages, the fish oil diet resulted in fewer omitted responses. (From Paletz et al., 2007.)
A second effect of diet is illustrated in Figure 10, taken from aged rats from the n- 3 PUFA cohort (Paletz, et al., 2007) or adult rats from the selenium cohort (Reed, et al., 2006). The increased number of omission errors was not due to an overall shift in the distribution, but rather an increased variability among nutrient-deficient rats such that a subset of rats showed an especially high number of omissions. Nutrient-sufficient rats were, as a group, more consistent in their responding. This effect, seen with both Se and DHE diet cohorts, was independent of MeHg exposure. For both diet cohorts, the lowand high-nutrient groups were indistinguishable from one another with respect to commission errors (i.e., incorrect responses), an endpoint that was sensitive to MeHg. The increased variability may reflect increased susceptibility in a subset of these rats on the low nutrient diets.
Figure 10.
Omission errors to criterion during the original discrimination (OD) and three reversals (R1–R3) of the SDR. Top shows data from aged rats exposed to a diet low (top left) or high (top right) in n-3 PUFAs (from (Paletz, 2004) and described in (Paletz, et al., 2007)). The bottom panels show data from rats exposed to a diet either low (bottom left) or high (bottom right) in Se (adapted from Reed et al. (2006)). Horizontal lines inside the box plots identify the median, whiskers span the interquartile range, and outliers below the 5th and above the 95th percentiles are shown as open circles.
It is noteworthy that these nutrients had similar and specific behavioral effects despite no similarity in structure or chemistry. It is tempting to speculate that these effects might be related to their common physiological roles as antioxidants. Thus, the increased variability in the low-nutrient diets could reflect greater susceptibility to other disrupting events. Linking these dietary effects to the increased omissions is more difficult, but it might be related to diminished attention or psychomotor slowing associated with the low-nutrient diets. Finally, the observation that the adult low-selenium rats resembled the aged rats in the PUFA cohort suggests that chronic exposure to low-levels of selenium may accelerate age-related deficits in this dependent measure.
6. POTENTIAL NEURAL MECHANISMS BY WHICH DEVELOPMENTAL MeHg IS NEUROTOXIC
Many of the behavioral effects reviewed above occurred in animals for which MeHg exposure ended at birth or early in development, so MeHg concentrations in the brain would be very low at the time of testing. Therefore, these behavioral effects reflect permanent alteration in neural structure and function caused by the presence of MeHg during neural development. Clues to potential neural mechanisms by which these long-lasting effects occur can be found from in vitro studies of neurotransmitter systems that are especially sensitive to developmental MeHg exposure, drug challenges with behaving animals to confirm the functional relevance of this sensitivity, anatomical structures or cell types that are sensitive to MeHg, and their relationship to the behavioral measures that are sensitive to low-level, developmental MeHg exposure. Some potential mechanisms pertinent to changes in the monitoring of reinforcement function are reviewed briefly here, including dopamine and GABA neurotransmitter systems since they appear to be permanently altered by developmental MeHg exposure.
6.1. Dopamine neurotransmitter systems track reinforcers and participate in choice
Dopamine cell bodies are confined to midbrain regions, but receptors are distributed widely. Cell bodies in the ventral tegmental area (VTA) send fibers to cortical (mesocortical) and subcortical (mesolimbic) areas and their activity is correlated with the delivery of primary and secondary reinforcers (Spanagel and Weiss, 1999; Wise, 2004) . In recent years their functions have been extended to include the tracking of the source and relative magnitude of reinforcement (Carelli, 2002; Salamone, et al., 2003). In studies of the activity of single cells of the primate frontal cortex, dopamine neuron activity is involved in the acquisition and expression of choice (Schultz, et al., 1998; Schultz, et al., 2000). These neurons are also sensitive to changes in reinforcement contingencies (termed "error prediction"). Tursts of activity appear when an unexpected reinforcer is presented or an expected reinforcer is not presented. In rodent studies, both choice and progressive ratio performance are influenced by lesions of dopamine rich regions or pharmacological manipulation of dopamine systems in cortical regions (Evenden, 1999; Kheramin, et al., 2005; Kheramin, et al., 2002; Mobini, et al., 2002; Mobini, et al., 2000). Basal ganglia dopamine pathways may also be relevant here. Cell bodies in the substantia nigra send fibers to striatum and are involved with motor function as well as the expression of choice (Schultz, et al., 2000)
Thus, there is similarity in the functional domains associated with dopamine neuronal systems and those that are affected by low-level MeHg exposure during development. Mesocortical systems are involved with choice, reinforcer efficacy, and the monitoring of changes in reinforcement contingencies, behavioral processes that are sensitive to low-level, gestational MeHg exposure. Such correlations in function could occur, of course, be due to any of a number of other causes so more direct evidence that developmental MeHg exposure influences dopamine function is required. The correlations can, however, provoke hypotheses about potential mechanisms.
6.2 Dopamine systems are sensitive to MeHg
In adult synaptosomes, low-to-moderate micromolar concentrations of MeHg provoke spontaneous release of dopamine (Minnema, et al., 1989), decreases the reuptake of dopamine into neurons (Rajanna and Hobson, 1985), inhibits the function of monoamine oxidase (MAO), an enzyme that catalyzes the oxidation of dopamine(Chakrabarti, et al., 1998), and enhances the PCB-induced release of dopamine (Bemis and Seegal, 1999). . These studies indicate the vulnerability of adult dopamine neurons to the presence of MeHg, but do not show that these effects are long lasting or that these effects apply to developmental exposures. Developmental exposure to MeHg in greater than mg/kg doses produces both transient and delayed alterations in catecholamine, and especially dopamine, neurotransmitter systems in the central nervous system (Bartolome, et al., 1984; O'kusky, et al., 1988; Slotkin and Bartolome, 1987). Some delayed effects, including increased turnover and reduced extracellular dopamine, were not detected until after weaning, although the extent to which these continued past sexual maturity was not reported (Bartolome, 1982). Gestational exposure to MeHg produced persistent, regional-specific reductions in MAO activity in 45 day-old female offspring (Beyrouty, et al., 2006). This effect, if it persists into adulthood, would result in elevated levels of synaptic dopamine, and other monoamines, since MAO is the main enzyme involved in its degradation and removal from the synapse. GABAergic, cholinergic, glutamatergic, serotonergic and noradrenergic neurotransmitter systems have also shown sensitivity to MeHg in in vitro studies (Bartolome, et al., 1982; Mckay, et al., 1986; Minnema, et al., 1989; O'kusky, et al., 1988; O'kusky and Mcgeer, 1985; O'kusky and Mcgeer, 1989).
6.3. MeHg exposure influences the functionality of dopamine receptor systems in behaving animals
The above studies show that neurotransmission is affected by the presence of MeHg and that changes persist in young animals when MeHg exposure occurs during development but they do not indicate whether such effects continue into adulthood, whether they are seen after occur following chronic low-level, exposure regimens that better model human exposures and, especially, whether the effects also appear in behaviog. Since some studies also point to the sensitivity, at least in vitro of other neurotransmitter systems, it is possible that these effects are nonspecific. Acute drug challenges in behaving animals can address these issues (Walsh and Tilson, 1986). Properly designed, they can also address issues of pharmacological or behavioral specificity. By incorporating doses that range from inactive to overtly behaviorally disruptive and by testing drugs that are selective for different neurotransmitter systems, it is possible to test hypotheses about the specific role of a neurotransmitter system. Similarly, by examining different behavioral endpoints, the sensitivity of specific behavioral processes such as choice, memory, or motor function can be evaluated. These challenges often unmask neurotoxicity even if no overt signs or behavioral effects have been reported.
Early studies with behaving animals demonstrated diminished sensitivity to amphetamine for animals exposed to rather high doses of MeHg, upward of 5 to 40 mg/kg administered by maternal injection during gestation. Using simple autoshaping and lever-pressing tasks (Hughes and Sparber, 1978) or avoidance and DRL performance in adults (Eccles and Annau, 1982), rats exposed during gestation did not show any differences in baseline (non-drug) responding, but MeHg-exposed rats were less sensitive to amphetamine. In another report, young rats exposed prenatally showed greater sensitivity to amphetamine on locomotor activity, ultrasonic vocalizations, and passive avoidance (Cagiano, et al., 1990). Unfortunately, these were single-dose studies or they spanned only a narrow range of doses and frequently involved only a single drug. The MeHg exposures were quite high by current standards.
Drug challenges were conducted with animals exposed during gestation to MeHg in drinking water such that about 40 or 400 µg/kg/day of mercury was delivered to the pregnant dam (Rasmussen and Newland, 2001). Drugs were selected because of their activity on neurotransmitter systems examined using other, non-behavioral, assays. The drugs used were haloperidol, amphetamine, pentobarbital, scopolamine, and dizocilpine because they act with relative specificity through dopaminergic, GABAergic, cholinergic, or glutamatergic neurotransmitter systems. A full dose-effect curve was generated for each drug. The behavioral measure was high-rate operant behavior developed under a DRH 9:4 schedule of reinforcement as described earlier. Adult rats exposed gestationally to MeHg showed enhanced sensitivity to amphetamine, a dopaminenergic agonist, but not to haloperidol, a D2 inverse agonist or antagonist (Rasmussen and Newland, 2001). Amphetamine promotes the release and blocks the reuptake of dopamine (and norepinephrine) so the results indicate that MeHg’s effects on dopamine systems have behavioral significance. The haloperidol results suggest that MeHg does not alter postsynaptic dopamine receptors, at least not D2,receptors, or that co-activation of D1 and D2 receptors is required to detect MeHg's neurochemical effects (discussed below).
The sensitivity of dopamine has been examined recently in greater detail in a doctoral dissertation (Reed, 2007; Reed and Newland, Under Review). In that study, different doses of cocaine were administered to rats exposed during gestation to MeHg. Figure 11 shows one dose-effect relationship from that study. The behavior studied was lever-pressing maintained under a Fixed-Interval 120" schedule of reinforcement. In this procedure, the first lever-press after 120" is reinforced. Typically, a low rate of lever-pressing occurs early in the 120" interval and response rates increase as the interval nears the end. Only the high response rates that occurred in the last 24" of the interval are shown in Figure 11 (overall rates showed a similar effect). The higher MeHg exposure group displayed approximately a 2-fold increased sensitivity to cocaine, similar to the magnitude reported earlier with d amphetamine (Rasmussen and Newland, 2001). Interestingly, the exposed animals were not differentially sensitive to the noradrenergic reuptake inhibitor desipramine or to selective D1 or D2 agonists or antagonists. These data are consistent with hypotheses that MeHg-induced sensitivity is mediated by presynaptic mechanisms or by diminished effects of monoamine oxidase. A second possibility is that developmental methylmercury influences a post-synpatic process that is initiated by joint activation of D1 and D2 receptors, but not by either receptor alone. When activated alone, these receptor systems have opposite post-synaptic effects, with D1 receptors increasing and D2 receptors decreasing cAMP activity. Joint activation, however, increases the rate of spike firing in dopaminergic neurons in a slice preparation (Hopf, et al., 2003). Moreover, D1 and D2 agonists administered jointly into nucleus accumbens maintains lever-pressing that results in that administration (Chu B and Kelley, 1992), and is involved in reward processing (Koch, et al., 2000) and substance abuse (Hodge, et al., 1997; Ikemoto, et al., 1997).
Figure 11.
Cocaine challenges in adult animals exposed to MeHg during gestation. Data show response-rate reductions from the last 24" of a Fixed-Interval 120" schedules, during which relatively high response rates occurred. Gestational MeHg exposure resulted in a leftward shift in the acute dose-effect curve ( adapted from (Reed, 2007; Reed and Newland, Under Review)).
6.4. GABAergic neurotransmission is affected by developmental MeHg exposure
Of the other neurotransmitters mentioned above, GABA also stands out as one of potential significance. The cerebral cortex and cerebellum, both GABA rich regions, are sensitive to developmental and adult-onset MeHg exposure at low micromolar or high nanomolar levels (Atchison, 2005; Choi, et al., 1978; Sager, et al., 1982; Yuan, et al., 2005). Disruption of GABAA receptor function by MeHg has also been noted in adult animals in in vitro studies (Atchison, 2005; Fitsanakis and Aschner, 2005; Narahashi, et al., 1994). There is some suggestion that the developing GABA system in post-weanling animals is affected by MeHg exposure (O'kusky, 1985), but the long-term implications of this or the effects at low MeHg exposure levels were not described. Newland and Rasmussen, in addition to reporting increased sensitivity to amphetamine, reported diminished sensitivity in behaving animals to pentobarbital, a GABAA agonist.
6.5 Cortex and cerebellum are sensitive to developmental MeHg exposure
It has long been recognized that developmental MeHg exposure can cause significant disruption of laminated regions, including cortex and cerebellum (Berlin, et al., 1975; Choi, et al., 1978). It is now becoming apparent that this disruption occurs at low micromolar concentrations approaching those reached in our studies. Exposure of rats between gestational days 6 and 15 to 1 or 2 mg/kg/day of MeHg, doses higher than the doses used in recent studies reviewed above, distorted the morphology and size of cortical laminae at 10- and 21-days of age. This exposure interfered with the nerve growth factor (NGF) signal cascade, resulting in a dramatic loss of neurites, decreased cell density, and reduced cell size in the occipital cortex of rats (Parran, et al., 2003; Parran, et al., 2004; Parran, et al., 2001). In in vitro studies, neurite outgrowth was impaired at tissue mercury concentrations of 0.3 to 3 ppm (1.5 to 15 µM) (Barone, et al., 1998); (Meacham, et al., 2005). similar to brain mercury concentrations of 0.5 to 10 ppm in neonates used in our exposure protocol (Day, et al., 2005; Newland, et al., 2006; Newland and Reile, 1999a).
The cerebellum cortex, a GABA-rich region, is also sensitive to MeHg. The selective vulnerability of cerebellar granule cells to MeHg was identified long ago in adult Minamata victims (Takeuchi, et al., 1962) and has been confirmed in in vivo and in vitro animal models, some involving high-nanomolar/low-micromolar concentrations (Atchison, 2005; Castoldi, et al., 2003; Kunimoto and Suzuki, 1997; Nagashima, 1997). Pathology, cell loss, disrupted synaptic transmission, and altered calcium homeostasis are seen in granule cells at MeHg concentrations several-fold lower than concentrations that damage the larger Purkinje cells also located in the cerebellum (Castoldi, et al., 2001).
MeHg-induced disruption of migration and derangement of lamination has also been seen in cerebellar development after systemic exposure (Sager, et al., 1984). This damage may be due to disruption of granule cell migration and synaptogenes (Choi, et al., 1978; Kunimoto and Suzuki, 1997) or disruption of neural cell adhesion molecules, which are perturbed at low micromolar concentrations in mice (Dey, et al., 1999). Developmental exposure using a regimen similar to ours (0 or 4 ppm in water) produced no overt signs of toxicity but reduced the density of migrating cells in the molecular layer of PND 7 offspring, effects that persisted to 30 days of age (Markowski, et al., 1998).
Exposures to high doses of MeHg during development impair performance on tasks that are reflective (but not diagnostic) of cerebellar damage (Roegge and Schantz, 2006) but more subtle effects have been difficult to detect. Cerebellar dysmorphology has been linked to disruption of neural cell adhesion molecules caused by low micromolar exposure to MeHg, or to a developmental dosing, although under a regimen somewhat higher than those used in the studies of reversal learning (Dey, et al., 1999). MeHg-induced disruption of migration and derangement of lamination has also been seen in cerebellar development (Sager, et al., 1984). In both in vivo and in vitro models of cerebellar development, granule cell migration and synaptogenes are perturbed at low micromolar concentrations, effects that could interfere with cerebellar lamination (Choi, et al., 1978; Kunimoto and Suzuki, 1997). In mice, developmental exposure using a regimen similar to ours (0 or 4 ppm in water) produced no overt signs of toxicity but did affect cerebellar cortical development in a pattern showing regional selectivity (Markowski, et al., 1998). There was a reduced density of migrating cells in the molecular layer of PND 7 offspring, and these effects persisted to 30 days of age. A reduced width of the external granule cell layer was also noted.
Much less is known about the consequences of damage to cerebellar granule cells during development but procedures indicative of extensive cerebellar damage are not particularly sensitive to developmental MeHg exposure. Nonetheless, because of the cerebellum's role in orchestrating motor sequences, it might be hypothesized that this disruption could be related to motor deficits that have been associated with developmental exposure in humans (Clarkson, et al., 1976; Cox, et al., 1995) and animals (Newland and Rasmussen, 2000).
6.6. An hypothesis about MeHg's developmental neurotoxicity can be formulated
Studies reviewed here link developmental MeHg exposure to altered sensitivity to drugs that elevate synaptic dopamine or that promote activity at GABAA receptors, but not to noradrenergic agonists, cholinergic or glutamate antagonists, or even agonists at specific dopamine receptor subtypes (manuscript under review). Others have linked developmental exposure to reduced neurite outgrowth and disrupted lamination of the frontal cortex (Barone, et al., 1998; Parran, et al., 2004). The frontal cortex is a target for dopaminergic projections from midbrain cortical systems and is also rich in GABA. Frontal cortical regions are also involved in perseveration, impulsivity, and progressive ratio performance (Evenden, 1999; Kheramin, et al., 2005; Kheramin, et al., 2002; Mobini, et al., 2002; Mobini, et al., 2000). Perseveration on a discrimination reversal task has been seen in rats following excitotoxic damage to the orbital prefrontal cortex (Chudasama and Robbins, 2003), in marmosets after lesions to the frontal lobes (Ridley, et al., 1993) and in humans with frontal lobe damage (Robbins, 1996). Humans with age-related decreases in cortical size perseverated on tasks such as the Wisconsin Card Sorting Task (Head, et al., 2002; Raz, et al., 1998). Set shifting/extradimensional shift is impaired by lesions in basal ganglia or lateral cortical areas (Robbins, 1996). Similar functionality appears in rodents, too. Lesions of the medial frontal cortex produced perseveration but only had transient effects on overall accuracy (Passetti, et al., 2002). Lesions of the orbitofrontal cortex did not impair the acquisition of a discrimination but did impair reversal learning in rats (Boulougouris, et al., 2007),a pattern identical to what we have noted. These regions are rich in both dopamine and GABA receptors.
Taken together, these findings lead to a hypothesis that the perseveration and disruption of choice associated with developmental MeHg exposure may be mediated by disruptions in dopamine or GABA neurotransmitter systems. While findings may imply medial or orbitofrontal cortical regions as being particularly vulnerable, it is premature to offer conclusions about such anatomical specificity at present. It seems reasonable, however, to speculate that overactivity of dopaminergic systems coupled with underactivity of inhibitory control by GABAergic systems could be linked to the perserveration nder choice and discrimination reversal procedures as well as the elevated responding under large fixed ratio schedules in the studies of developmental exposure reviewed here.
7. SUMMARY
The presence of MeHg, selenium, and n-3 PUFAs in fish complicate the evaluation of risks and benefits of fish consumption. Experimental models of MeHg's neurotoxicity and of the benefits of these nutrients in the diet contribute to a deeper understanding of how these compounds act in development and adulthood. Our group has examined these compounds explicitly using a factorial design that permits the assessment of the benefits of nutrients alone, the neurotoxicity of MeHg alone, and the possibility that nutrients may protect against MeHg's developmental neurotoxicity. This has been accomplished in rodents using environmentally relevant exposure regimens that permit comparisons across laboratory models when brain mercury is the exposure marker.
Gestational exposure to MeHg produces a robust perseveration on choice tasks in adult animals. In fact, we have not identified a level of exposure that fails to produce this effect. This perseveration is reversible with experience or with an explicit therapeutic behavioral intervention. Both of these effects are reproducible, and both of these effects are unrelated to the presence of dietary n-3 PUFAs or selenium. While some potential interactions between selenium and MeHg have been noted with developmental exposure on other endpoints, these effects require further research to understand better. We hypothesize that MeHg's effects are related to impaired development of the cerebral cortex, enhanced dopamine activity, and diminished GABA activity in exposed animals. While it is likely that the cerebellum is also involved in MeHg's developmental neurotoxicity, it is not clear what role it plays.
Selenium and n-3 PUFAs had similar behavioral effects despite their structural differences. The presence of these compounds in the diet reduced the number of trials in which the animal failed to respond in the discrimination reversal procedure and conferred some protection against age-related effects on these tasks. It is possible that these outcomes are related to their antioxidant effects, but this must remain speculative at present. We saw no evidence that n-3 PUFAS confer protection against MeHg's developmental neurotoxicity, with some mixed evidence that selenium might do so on selected endpoints, but not on the major behavioral effect, perseveration of a previously reinforced response during reversal learning.
ACKNOWLEDGEMENTS
Supported by ES 10865.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. Christopher Newland, Department of Psychology, 226 Thach Hall, Auburn University, Auburn, AL. 36849-5214, 334 844 6479 (V), 334 844 4447 (F), chris.newland@auburn.edu.
Elliott M. Paletz, Department of Psychiatry, University of Wisconsin-Madison, 6001 Research Park Boulevard, Madison, WI 53719
Miranda N. Reed, Department of Neurology and Neuroscience, University of Minnesota School of Medicine, 420 Delaware St, Minneapolis, MN 55455
REFERENCES
- Ache BW, Boyle JD, Morse CE. Prepared by Battelle for the U. S. EPA Gulf of Mexico Program, Stennis Space Center, MS. 2000. A survey of the occurrence of mercury in the fishery resources of the gulf of mexico. [Google Scholar]
- Akagi H, Grandjean P, Takizawa Y, Weihe P. Methylmercury dose estimation from umbilical cord concentrations in patients with minamata disease. Environmental Research. 1998;77:98–103. doi: 10.1006/enrs.1997.3822. [DOI] [PubMed] [Google Scholar]
- Ardila A, Pineda D, Rosselli M. Correlation between intelligence test scores and executive function measures. Archives of Clinical Neuropsychology. 2000;15:31–36. [PubMed] [Google Scholar]
- Arffa S. The relationship of intelligence to executive function and non-executive function measures in a sample of average, above average, and gifted youth. Archives of Clinical Neuropsychology. 2007;22:969–978. doi: 10.1016/j.acn.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Aschner M, Aschner JL. Mercury neurotoxicity: Mechanisms of blood-brain barrier transport. Neuroscience and Biobehavioral Reviews. 1990;14:169–176. doi: 10.1016/s0149-7634(05)80217-9. [DOI] [PubMed] [Google Scholar]
- Atchison WD. Is chemical neurotransmission altered specifically during methylmercury-induced cerebellar dysfunction? Trends Pharmacol Sci. 2005;26:549–557. doi: 10.1016/j.tips.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Axtell CD, Cox C, Myers GJ, Davidson PW, Choi AL, Cernichiari E, et al. Association between methylmercury exposure from fish consumption and child development at five and a half years of age in the seychelles child development study: An evaluation of nonlinear relationships. Environmental Research. 2000;84:71–80. doi: 10.1006/enrs.2000.4082. [DOI] [PubMed] [Google Scholar]
- Baron A, Derenne A. Progressive-ratio schedules: Effects of later schedule requirements on earlier performances.[erratum appears in j exp anal behav. 2002 jul;78(1):94.] Journal of the Experimental Analysis of Behavior. 2000;73:291–304. doi: 10.1901/jeab.2000.73-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone S, Haykal-Coates N, Parran DK, Tilson HA. Gestational exposure to methylmercury alters the developmental pattern of trk-like immunoreactivity in the rat brain and results in cortical dysmorphology. Developmental Brain Research. 1998;109:13–31. doi: 10.1016/s0165-3806(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Bartolome J, Trepanier P, Chait EA, Seidler FJ, Deskin R, Slotkin TA. Neonatal methylmercury poisoning in the rat: Effects on development of central catecholamine neurotransmitter systems. Toxicol Appl Pharm. 1982;65:92–99. doi: 10.1016/0041-008x(82)90366-0. [DOI] [PubMed] [Google Scholar]
- Bartolome J, Whitmore WL, Seidler FJ, Slotkin TA. Exposure to methylmercury in utero: Effects on biochemical development of catecholamine neurotransmitter systems. Life Sciences. 1984;35:657–670. doi: 10.1016/0024-3205(84)90261-3. [DOI] [PubMed] [Google Scholar]
- Behne D, Kyriakopoulos A. Mammalian selenium-containing proteins. Annual Review of Nutrition. 2001;21:453–473. doi: 10.1146/annurev.nutr.21.1.453. [DOI] [PubMed] [Google Scholar]
- Behne D, Pfeifer H, Rothlein D, Kyriakopoulos A. Roussel AM, Favier AE, Anderson RA. Trace elements in man and animals 10. New York: Kluwer Academic/Plenum Publishers; 2000. Cellular and subcellular distribution of selenium and selenium-containing proteins in the rat; pp. 29–34. [Google Scholar]
- Bemis JC, Seegal RF. Polychlorinated biphenyls and methylmercury act synergistically to reduce rat brain dopamine content in vitro. Environmental Health Perspectives. 1999;107:879–885. doi: 10.1289/ehp.99107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin M, Grant CA, Hellberg J, Hellstrom J, Schultz A. Neurotoxicity of methylmercury in squirrel monkeys. Cerebral cortical pathology, interference with scotopic vision, and changes in operant behavior. Arch Environ Health. 1975;30:340–348. doi: 10.1080/00039896.1975.10666717. [DOI] [PubMed] [Google Scholar]
- Beyrouty P, Chan HM. Co-consumption of selenium and vitamin e altered the reproductive and developmental toxicity of methylmercury in rats. Neurotoxicology & Teratology. 2006;28:49–58. doi: 10.1016/j.ntt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Beyrouty P, Stamler CJ, Liu JN, Loua KM, Kubow S, Chan HM. Effects of prenatal methylmercury exposure on brain monoamine oxidase activity and neurobehaviour of rats. Neurotoxicol Teratol. 2006;28:251–259. doi: 10.1016/j.ntt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Boone KB, Ghaffarian S, Lesser IM, Hill-Gutierrez E, Berman NG. Wisconsin card sorting test performance in healthy, older adults: Relationship to age, sex, education, and iq. Journal of Clinical Psychology. 1993;49:54–60. doi: 10.1002/1097-4679(199301)49:1<54::aid-jclp2270490108>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural Brain Research. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Current Opinion in Clinical Nutrition & Metabolic Care. 2002;5:127–132. doi: 10.1097/00075197-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Buelke-Sam J, Kimmel CA, Adams J, Nelson CJ, Vorhees CV, Wright DC, et al. Collaborative behavioral teratology study: Results. Neurobehavioral Toxicology & Teratology. 1985;7:591–624. [PubMed] [Google Scholar]
- Burbacher TM, Grant KS, Mayfield DB, Gilbert SG, Rice DC. Prenatal methylmercury exposure affects spatial vision in adult monkeys. Toxicology and Applied Pharmacology. 2005a;208:21–28. doi: 10.1016/j.taap.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Grant KS, Mayfield DB, Gilbert SG, Rice DC. Prenatal methylmercury exposure affects spatial vision in adult monkeys. Toxicol Appl Pharmacol. 2005b;208:21–28. doi: 10.1016/j.taap.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Rodier PM, Weiss B. Methylmercury developmental neurotoxicity: A comparison of effects in humans and animals. Neurotoxicology and Teratology. 1990;12:191–202. doi: 10.1016/0892-0362(90)90091-p. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environmental Health Perspectives. 2005c;113:1015–1021. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk RF. Selenium, an antioxidant nutrient.[see comment] Nutr in Clin Care. 2002;5:75–79. doi: 10.1046/j.1523-5408.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- Burk RF, Laevander OA. Shils ME, Olson JA, Shike M, Ross AC. Modern nutrition in health and disease. Baltimore, MD: Williams and Williams; 1999. Selenium; pp. 265–276. [Google Scholar]
- Cagiano R, De Salvia MA, Renna G, Tortella E, Braghiroli D, Parenti C, et al. Evidence that exposure to methyl mercury during gestation induces behavioral and neurochemical changes in offspring of rats. Neurotoxicology & Teratology. 1990;12:23–28. doi: 10.1016/0892-0362(90)90108-o. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. 'natural' reinforcement. Physiology & Behavior. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carlson SE, Neuringer M. Polyunsaturated fatty acid status and neurodevelopment: A summary and critical analysis of the literature. Lipids. 1999;34:171–178. doi: 10.1007/s11745-999-0351-2. [DOI] [PubMed] [Google Scholar]
- Carrington CD, Bolger MP. An exposure assessment for methylmercury from seafood for consumers in the united states. Risk Analysis. 2002;22:689–699. doi: 10.1111/0272-4332.00061. [DOI] [PubMed] [Google Scholar]
- Castoldi A, Coccini T, Ceccatelli S, Manzo L. Neurotoxicity and molecular effects of methylmercury. Brain Research Bulletin. 2001;55:197–203. doi: 10.1016/s0361-9230(01)00458-0. [DOI] [PubMed] [Google Scholar]
- Castoldi A, Coccini T, Manzo L. Neurotoxic and molecular effects of methylmercury in humans. Reviews on Environmental Health. 2003;18:19–31. doi: 10.1515/reveh.2003.18.1.19. [DOI] [PubMed] [Google Scholar]
- Catania AC. Learning. Englewood Cliffs, NJ: Prentice Hall; 1992. [Google Scholar]
- Cernichiari E, Brewer R, Myers GJ, Marsh DO, Lapham LW, Cox C, et al. Monitoring methylmercury during pregnancy: Maternal hair predicts fetal brain exposure. Neurotoxicology. 1995;16:705–710. [PubMed] [Google Scholar]
- Cernichiari E, Myers GJ, Ballatori N, Zareba G, Vyas J, Clarkson T. The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology. 2007;28:1015–1022. doi: 10.1016/j.neuro.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, Loua KM, Bai C, Durham H, Panisset JC. Modulation of monoamine oxidase activity in different brain regions and platelets following exposure of rats to methylmercury. Neurotoxicology & Teratology. 1998;20:161–168. doi: 10.1016/s0892-0362(97)00104-9. [DOI] [PubMed] [Google Scholar]
- Chapman L, Chan HM. The influence of nutrition on methylmercury intoxication. Environmental Health Perspectives. 2000;108 Supplement 1:29–56. doi: 10.1289/ehp.00108s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Berry MJ. Selenium and selenoproteins in the brain and brain diseases. J Neurochem. 2003;86:1–12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW, Amin-Zaki L, Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: A major effect of methylmercury poisoning in utero. Journal of Neuropathology & Experimental Neurology. 1978;37:719–733. doi: 10.1097/00005072-197811000-00001. [DOI] [PubMed] [Google Scholar]
- Chu B, Kelley AE. Potential of reward-related responding by psychostimulant infusion into nucleus accumbens: Role of dopamine receptor subtypes. Psychobiology. 1992;20:153–162. [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J. Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson T, Amin-Zaki L, Al-Tikriti S. An outbreak of methylmercury poisoning due to consumption of contaminated grain. Federation Proceedings. 1976;35:2395–2399. [PubMed] [Google Scholar]
- Cox C, Marsh D, Myers G, Clarkson T. Analysis of data on delayed development from the 1971–1972 outbreak of methylmercury poisoning in iraq: Assessment of influential points. Neurotoxicology. 1995;16:727–730. [PubMed] [Google Scholar]
- Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: Benchmark analysis of a new zealand cohort. Risk Analysis. 1998;18:701–713. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- Darvill T, Lonky E, Reihman J, Stewart P, Pagano J. Prenatal exposure to pcbs and infant performance on the fagan test of infant intelligence. Neurotoxicology. 2000;21:1029–1038. [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: Outcomes at 66 months of age in the seychelles child development study [see comments] JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Palumbo D, Myers GJ, Cox C, Shamlaye CF, Sloane-Reeves J, et al. Neurodevelopmental outcomes of seychellois children from the pilot cohort at 108 months following prenatal exposure to methylmercury from a maternal fish diet. Environmental Research. 2000;84:1–11. doi: 10.1006/enrs.2000.4084. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. doi: 10.1016/j.neuro.2008.06.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, Nevin JA. Stimuli, reinforcers, and behavior: An integration. Journal of the Experimental Analysis of Behavior. 1999;71:439–482. doi: 10.1901/jeab.1999.71-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Reed MN, Newland MC. Neuromotor deficits and mercury concentrations in rats exposed to methyl mercury and fish oil. Neurotoxicology and Teratology. 2005;27:629–641. doi: 10.1016/j.ntt.2005.03.011. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, et al. Normative data from the cantab. I: Development of executive function over the lifespan. Journal of Clinical & Experimental Neuropsychology: Official Journal of the International Neuropsychological Society. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Derenne A, Baron A. Preratio pausing: Effects of an alternative reinforcer on fixed- and variable-ratio responding. Journal of the Experimental Analysis of Behavior. 2002;77:273–282. doi: 10.1901/jeab.2002.77-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P, Gochfeld M, Reuhl K. Developmental methylmercury administration alters cerebellar psa-ncam expression and golgi sialyltransferase activity. Brain Research. 1999;845:139–151. doi: 10.1016/s0006-8993(99)01887-9. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the wisconsin card sort test: Restriction to novel situations and independence from "On-line" Processing. J. Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe JW, Burgos JE, Palmer DC. A selectionist approach to reinforcement. Journal of the Experimental Analysis of Behavior. 1993;60:17–40. doi: 10.1901/jeab.1993.60-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore FY, Goulet S, Gallagher A, Harvey PO, Cantin JF, D'Aigle T, et al. Neurobehavioral changes in mice treated with methylmercury at two different stages of fetal development. Neurotoxicology & Teratology. 2001;23:463–472. doi: 10.1016/s0892-0362(01)00167-2. [DOI] [PubMed] [Google Scholar]
- Eccles CU, Annau Z. Prenatal methyl mercury exposure: Ii. Alterations in learning and psychotropic drug sensitivity in adult offspring. Neurobehav Toxicol Teratol. 1982;4:377–382. [PubMed] [Google Scholar]
- Egeland GM, Middaugh JP. Balancing fish consumption benefits with mercury exposure. Science. 1997;278:1904–1905. doi: 10.1126/science.278.5345.1904. [DOI] [PubMed] [Google Scholar]
- Evenden J. The pharmacology of impulsive behavior in rats v: The effects of drugs on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology. 1999;143:111–122. doi: 10.1007/s002130050926. [DOI] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of reinforcement. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Fischer C, Fredriksson A, Eriksson P. Coexposure of neonatal mice to a flame retardant pbde 99 (2,2',4,4',5-pentabromodiphenyl ether) and methyl mercury enhances developmental neurotoxic defects. Toxicol. Sci. 2008a;101l:275–285. doi: 10.1093/toxsci/kfm271. [DOI] [PubMed] [Google Scholar]
- Fischer C, Fredriksson A, Eriksson P. Neonatal co-exposure to low doses of an ortho-pcb (pcb 153) and methyl mercury exacerbate defective developmental neurobehavior in mice. Toxicology. 2008b;244:157–165. doi: 10.1016/j.tox.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Aschner M. The importance of glutamate, glycine, and gamma-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharmacol. 2005;204:343–354. doi: 10.1016/j.taap.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW. Cantab battery: Proposed utility in neurotoxicology. Neurotoxicology and Teratology. 1996;18:499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Ge K, Yang G. The epidemiology of selenium deficiency in the etiological study of endemic diseases in china. Am J Clin Nutr. 1993;57:259S–263S. doi: 10.1093/ajcn/57.2.259S. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Burbacher TM, Rice DC. Effects of in utero methylmercury exposure on a spatial delayed alternation task in monkeys. Toxicol Appl Pharmacol. 1993;123:130–136. doi: 10.1006/taap.1993.1229. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Rice DC, Burbacher TM. Fixed interval/fixed ratio performance in adult monkeys exposed in utero to methylmercury. Neurotoxicology & Teratology. 1996;18:539–546. doi: 10.1016/0892-0362(96)00081-5. [DOI] [PubMed] [Google Scholar]
- Goldey ES, O'Callaghan JP, Stanton ME, Barone S, Jr, Crofton KM. Developmental neurotoxicity: Evaluation of testing procedures with methylazoxymethanol and methylmercury. Fundamental & Applied Toxicology. 1994;23:447–464. doi: 10.1006/faat.1994.1127. [DOI] [PubMed] [Google Scholar]
- Goulet S, Dore FY, Mirault ME. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicology & Teratology. 2003;25:335–347. doi: 10.1016/s0892-0362(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F. Cognitive performance of children prenatally exposed to "Safe" Levels of methylmercury. Environmental Research. 1998;77:165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicology & Teratology. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bradford LD. Advances in behavioral pharmacology. New York: Academic Press; 1977. Predicting the abuse liability of drugs with animal drug self-administration procedures: Psychomotor stimulants and hallucinogens; pp. 164–208. [Google Scholar]
- Gunderson V, Grant K, Burbacher T, Fagan J, Mottet N. The effect of low-level prenatal methylmercury exposure on visual recognition memory in infant crab-eating macaques. Child Development. 1986;54:1076–1083. [PubMed] [Google Scholar]
- Gunderson VM, Grant-Webster KS, Burbacher TM, Mottet NK. Visual recognition memory deficits in methylmercury-exposed macaca fascicularis infants. Neurotoxicology & Teratology. 1988;10:373–379. doi: 10.1016/0892-0362(88)90041-4. [DOI] [PubMed] [Google Scholar]
- Harada M. Minamata disease: Methylmercury poisoning in japan caused by environmental pollution. Critical Reviews in Toxicology. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: The role of regional cortical shrinkage and cognitive resources. Psychol Aging. 2002:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: Further examination of the role of dopamine receptors in the nucleus accumbens. Alcoholism: Clinical & Experimental Research. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hojo R, Stern S, Zareba G, Markowski VP, Cox C, Kost JT, et al. Sexually dimorphic behavioral responses to prenatal dioxin exposure. Environ Health Persp. 2002;110:247–254. doi: 10.1289/ehp.02110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B, Brown J, Buss DH. Fish and fish products. Cambridge, United Kingdon: The Royal Society of Chemistry; 1993. [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine d1 and d2 receptors increases spike firing of nucleus accumbens neurons via g-protein {beta}{gamma} subunits. J. Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JA, Sparber SB. D-amphetamine unmasks postnatal consequences of exposure to methylmercury in utero: Methods for studying behavioral teratogenesis. Pharmacol Biochem Behav. 1978;8:365–375. doi: 10.1016/0091-3057(78)90072-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine d1 and d2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis S, de La Presa , Owens S. Dietary fatty acid composition in pregnancy alters neurite membrane fatty acids and dopamine in newborn rat brain. Journal of Nutrition. 2001;131:118–122. doi: 10.1093/jn/131.1.118. [DOI] [PubMed] [Google Scholar]
- Jones PJH, Kubow S. Shils ME, Olson JA, Shike M, Ross AC. Modern nutrition in health and disease. Baltimore, MD: Williams and Williams; 1999. Lipids, sterols, and their metabolites; pp. 67–94. [Google Scholar]
- Kerper LE, Ballatori N, Clarkson TW. Methylmercury transport across the blood-brain barrier by an amino acid carrier. American Journal of Physiology. 1992;267:761–765. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, et al. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: Implications for models of inter-temporal choice. Behav Brain Res. 2005;156:145–152. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, et al. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on intertemporal choice: A quantitative analysis. Psychopharmacology. 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Higashi H, Nakano A, Sakamoto M, Sakai R. Profile of subjective complaints and activities of daily living among current patients with minamata disease after 3 decades. Environmental Research. 1993;63:241–251. doi: 10.1006/enrs.1993.1144. [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of muscles accumbens dopamine d1 and d2 receptors in instrumental and pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Kunimoto M, Suzuki T. Migration of granule neurons in cerebellar organotypic cultures is impaired by methylmercury. Neurosci Lett. 1997;226:183–186. doi: 10.1016/s0304-3940(97)00273-5. [DOI] [PubMed] [Google Scholar]
- Lapillonne A, Clarke SD, Heird WC. Plausible mechanisms for effects of long-chain polyunsaturated fatty acids on growth. The Journal of Pediatrics. 2003;143:9–16. doi: 10.1067/s0022-3476(03)00397-4. [DOI] [PubMed] [Google Scholar]
- Magos L. Eccles CU, Annau Z. The toxicity of methyl mercury. Baltimore: Johns Hopkins; 1987. The absorption, distribution, and excretion of methyl mercury; pp. 24–44. [Google Scholar]
- Mahaffey KR. Fish and shellfish as dietary sources of methylmercury and the [formula: See text] -3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: Risks and benefits. Environ Res. 2004a;95:414–428. doi: 10.1016/j.envres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR. Fish and shellfish as dietary sources of methylmercury and the [omega]-3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: Risks and benefits. Environmental Research. 2004b;95:414–428. doi: 10.1016/j.envres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Jeffries RA. Methylmercury and omega-3 fatty acids: Co-occurrence of dietary sources with emphasis on fish and shellfish. Environmental Research. 2008;107:20–29. doi: 10.1016/j.envres.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, et al. Maternal milk as methylmercury source for suckling mice: Neurotoxic effects involved with the cerebellar glutamatergic system. Toxicological Sciences. 2004;81:172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- Markowski VP, Flaugher CB, Baggs RB, Rawleigh RC, Cox C, Weiss B. Prenatal and lactational exposure to methylmercury affects select parameters of mouse cerebellar development. Neurotoxicology. 1998;19:879–892. [PubMed] [Google Scholar]
- McKay SJ, Reynolds JN, Racz WJ. Differential effects of methylmercuric chloride and mercuric chloride on the l-glutamate and potassium evoked release of [3h]dopamine from mouse striatal slices. Canadian Journal of Physiology & Pharmacology. 1986;64:656–660. doi: 10.1139/y86-108. [DOI] [PubMed] [Google Scholar]
- Meacham CA, Freudenrich TM, Anderson WL, Sui L, Lyons-Darden T, Barone S, Jr, et al. Accumulation of methylmercury or polychlorinated biphenyls in in vitro models of rat neuronal tissue. Toxicol Appl Pharmacol. 2005;205:177–187. doi: 10.1016/j.taap.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Middaugh L, Gentry GD. Prenatal ethanol effects of reward efficacy for adult mice are gestation stage specific. Neurotoxicol Teratol. 1992;14:365–370. doi: 10.1016/0892-0362(92)90044-b. [DOI] [PubMed] [Google Scholar]
- Minnema DJ, Cooper GP, Greenland RD. Effects of methylmercury on neurotransmitter release from rat brain synaptosomes. Toxicol Appl Pharmacol. 1989;99:510–521. doi: 10.1016/0041-008x(89)90158-0. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology. 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- Moller-Madsen B, Danscher G. Localization of mercury in cns of the rat. Iv. The effect of selenium on orally administered organic and inorganic mercury. Toxicol Appl Pharm. 1991;108:457–473. doi: 10.1016/0041-008x(91)90092-s. [DOI] [PubMed] [Google Scholar]
- Myers G, Davidson P, Cox C, Shamlaye C, Tanner M, Marsh D, et al. Summary of the seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicology. 1995;16:711–716. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW. Prenatal methylmercury exposure and children: Neurologic, developmental, and behavioral research. Environmental Health Perspectives. 1998;106 Suppl 3:841–847. doi: 10.1289/ehp.98106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study.[see comment] Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Shamlaye CF, Axtell CD, Cernichiari E, Choisy O, et al. Effects of prenatal methylmercury exposure from a high fish diet on developmental milestones in the seychelles child development study. Neurotoxicology. 1997;18:819–829. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Strain JJ. Nutrient and methyl mercury exposure from consuming fish. J. Nutr. 2007;137:2805–2808. doi: 10.1093/jn/137.12.2805. [DOI] [PubMed] [Google Scholar]
- Nagashima K. A review of experimental methylmercury toxicity in rats: Neuropathology and evidence for apoptosis. Toxicol Pathol. 1997;25:624–631. doi: 10.1177/019262339702500613. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Ma JY, Arakawa O, Reuveny E, Nakahiro M. Gaba receptor-channel complex as a target site of mercury, copper, zinc, and lanthanides. Cellular & Molecular Neurobiology. 1994;14:599–621. doi: 10.1007/BF02088671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Nutrient requirements of laboratory animals. Washington, D.C.: National Academy Press; 1995. [Google Scholar]
- National Research Council. Toxicological effects of methylmercury. Washington, D.C.: National Academy Press; 2000. [Google Scholar]
- Neuringer M, Reisbick S, Janowsky J. The role of n-3 fatty acids in visual and cognitive development: Current evidence and methods of assessment. Journal of Pediatrics. 1994;125:S39–S47. doi: 10.1016/s0022-3476(06)80735-3. [DOI] [PubMed] [Google Scholar]
- Nevin JA. Quantitative analyses of behavior: Reinforcement and the organisation of behavior. New York: Wiley; 1979. Reinforcement schedules and response strength; pp. 117–158. [Google Scholar]
- Nevin JA. Behavioral momentum and the partial reinforcement effect. Psychological Bulletin. 1988;103:44–56. [Google Scholar]
- Newland MC, Ng WW, Baggs RB, Gentry GD, Weiss B, Millar RK. Operant behavior in transition reflects neonatal exposure to cadmium. Teratology. 1986;34:231–241. doi: 10.1002/tera.1420340302. [DOI] [PubMed] [Google Scholar]
- Newland MC, Paletz EM. Animal studies of methylmercury and pcbs: What do they tell us about expected effects in humans? Neurotoxicology. 2000;21:1003–1027. [PubMed] [Google Scholar]
- Newland MC, Rasmussen EB. Aging unmasks adverse effects of gestational exposure to methylmercury in rats. Neurotoxicology and Teratology. 2000;22:819–828. doi: 10.1016/s0892-0362(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Newland MC, Rasmussen EB. Behavior in adulthood and during aging is affected by contaminant exposure in utero. Current Directions in Psychological Science. 2003;12:212–217. [Google Scholar]
- Newland MC, Reed MN, LeBlanc A, Donlin WD. Brain and blood mercury and selenium after chronic and developmental exposure to methylmercury. Neurotoxicology. 2006;27:710–720. doi: 10.1016/j.neuro.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicol. Sci. 1999a;50:106–116. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA. Tilson HA, Harry J. Target organ series: Neurotoxicology. New York: Raven Press; 1999b. Learning and behavior change as neurotoxic endpoints; pp. 311–338. [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicology and Teratology. 2004;26:179–194. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposure to lead or methyl mercury: Reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicology and Applied Pharmacology. 1994;126:6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Imamura K, Kuwahata M, Kindaichi M, Susa M, Ekino S. Reappraisal of somatosensory disorders in methylmercury poisoning. Neurotoxicology and Teratology. 2005;27:643–653. doi: 10.1016/j.ntt.2005.03.008. [DOI] [PubMed] [Google Scholar]
- O'Kusky J. Synaptic degeneration in rat visual cortex after neonatal administration of methylmercury. Experimental Neurology. 1985;89:32–47. doi: 10.1016/0014-4886(85)90263-8. [DOI] [PubMed] [Google Scholar]
- O'Kusky JR, Boyes BE, McGeer EG. Methylmercury-induced movement and postural disorders in developing rat: Regional analysis of brain catecholamines and indoleamines. Brain Research. 1988;439:138–146. doi: 10.1016/0006-8993(88)91470-9. [DOI] [PubMed] [Google Scholar]
- O'Kusky JR, McGeer EG. Methylmercury poisoning of the developing nervous system in the rat: Decreased activity of glutamic acid decarboxylase in cerebral cortex and neostriatum. Brain Research. 1985;353:299–306. doi: 10.1016/0165-3806(85)90219-6. [DOI] [PubMed] [Google Scholar]
- O'Kusky JR, McGeer EG. Methylmercury-induced movements and postural disorders in developing rat: High-affinity uptake of choline, glutamate, and gamma-aminobutyric acid in the cerebral cortex and caudate-putamen. Neurochemistry. 1989;53:999–1006. doi: 10.1111/j.1471-4159.1989.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, et al. Performance on cambridge neuropsychological test automated battery subtests sensitive to frontal lobe function in people with autistic disorder: Evidence from the collaborative programs of excellence in autism network. Journal of Autism & Developmental Disorders. 2004;34:139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Craig-Schmidt MC, Newland MC. Gestational exposure to methylmercury and n-3 fatty acids: Effects on high- and low-rate operant behavior in adulthood. Neurotoxicology and Teratology. 2006;28:59–73. doi: 10.1016/j.ntt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Day JJ, Craig-Schmidt MC, Newland MC. Spatial and visual discrimination reversals in adult and geriatric rats exposed during gestation to methylmercury and n - 3 polyunsaturated fatty acids. Neurotoxicology. 2007;28:707–719. doi: 10.1016/j.neuro.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parran DK, Barone S, Jr, Mundy WR. Methylmercury inhibits trka signaling through the erk1/2 cascade after ngf stimulation of pc12 cells. Brain Research. Developmental Brain Research. 2004;149:53–61. doi: 10.1016/j.devbrainres.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Parran DK, Barone S, Mundy WR. Methylmercury decreases ngf-induced trka autophosphorylation and neurite outgrowth in pc12 cells. Developmental Brain Research. 2003;141:71–81. doi: 10.1016/s0165-3806(02)00644-2. [DOI] [PubMed] [Google Scholar]
- Parran DK, Mundy WR, Barone S., Jr Effects of methylmercury and mercuric chloride on differentiation and cell viability in pc12 cells. Toxicological Sciences. 2001;59:278–290. doi: 10.1093/toxsci/59.2.278. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: Dissociable functions of distinct medial prefrontal subregions. Cerebral Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Peng T, Li Y, Yang Y, Niu C, Morgan-Capner P, Archard LC, et al. Characterization of enterovirus isolates from patients with heart muscle disease in a selenium-deficient area of china. J. Clin. Microbiol. 2000;38:3538–3543. doi: 10.1128/jcm.38.10.3538-3543.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanna B, Hobson M. Influence of mercury on uptake of [3h]dopamine and [3h]norepinephrine by rat brain synaptosomes. Toxicology Letters. 1985;27:7–14. doi: 10.1016/0378-4274(85)90114-6. [DOI] [PubMed] [Google Scholar]
- Ralston NVC, Blackwell JL, Raymond LJ. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol Trace Elem Res. 2007;119:255–268. doi: 10.1007/s12011-007-8005-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, Newland MC. Developmental exposure to methylmercury alters behavioral sensitivity to d amphetamine and pentobarbital in adult rats. Neurotoxicol Teratol. 2001;23:45–55. doi: 10.1016/s0892-0362(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC. Mercury:Selenium interactions and health implications. Seychelles Medical and Dental Journal (SMDJ) 2004;7:72–77. doi: 10.1016/j.neuro.2020.09.020. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Reed MN, Banna KM, Donlin WD, Newland MC. Effects of gestational exposure to methylmercury and dietary selenium on reinforcement efficacy in adulthood. Neurotoxicology and Teratology. 2008;30:29–37. doi: 10.1016/j.ntt.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Newland MC. Gestational methylmercury exposure selectively increases the sensitivity of operant behavior to cocaine. doi: 10.1037/a0014595. Under Review. [DOI] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: Effects on a spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27:721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr Ain-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the ain-76a rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rice DC. Blood mercury concentrations following methyl mercury exposure in adult and infant monkeys. Environmental Research. 1989a;49:115–126. doi: 10.1016/s0013-9351(89)80026-x. [DOI] [PubMed] [Google Scholar]
- Rice DC. Brain and tissue levels of mercury after chronic methylmercury exposure in the monkey. Journal of Toxicology & Environmental Health. 1989b;27:189–198. doi: 10.1080/15287398909531290. [DOI] [PubMed] [Google Scholar]
- Rice DC. Delayed neurotoxicity in monkeys exposed developmentally to methylmercury. Neurotoxicology. 1989c;10:645–650. [PubMed] [Google Scholar]
- Rice DC. Effects of pre- plus postnatal exposure to methylmercury in the monkey on fixed interval and discrimination reversal performance. Neurotoxicology. 1992;13:443–452. [PubMed] [Google Scholar]
- Rice DC. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicology. 1996a;17:583–596. [PubMed] [Google Scholar]
- Rice DC. Sensory and cognitive effects of developmental methylmercury exposure in monkeys, and a comparison to effects in rodents. Neurotoxicology. 1996b;17:139–154. [PubMed] [Google Scholar]
- Rice DC. Age-related increase in auditory impairment in monkeys exposed in utero plus postnatally to methylmercury. Toxicological Sciences. 1998a;44:191–196. doi: 10.1006/toxs.1998.2487. [DOI] [PubMed] [Google Scholar]
- Rice DC. Issues in developmental neurotoxicology: Interpretation and implications of the data. Canadian Journal of Public Health. Revue Canadienne de Sante Publique. 1998b;89 Suppl 1:S31–S36. [PubMed] [Google Scholar]
- Rice DC. Lack of effect of methylmercury exposure from birth to adulthood on information processing speed in the monkey. Neurotoxicology & Teratology. 1998c;20:275–283. doi: 10.1016/s0892-0362(97)00099-8. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Early chronic low-level methylmercury poisoning in monkeys impairs spatial vision. Science. 1982;216:759–761. doi: 10.1126/science.7079739. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Effects of developmental exposure to methyl mercury on spatial and temporal visual function in monkeys. Toxicol Appl Pharmacol. 1990;102:151–163. doi: 10.1016/0041-008x(90)90092-9. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Exposure to methyl mercury from birth to adulthood impairs high-frequency hearing in monkeys. Toxicol Appl Pharmacol. 1992;115:6–10. doi: 10.1016/0041-008x(92)90361-u. [DOI] [PubMed] [Google Scholar]
- Rice DC, Hayward S. Comparison of visual function at adulthood and during aging in monkeys exposed to lead or methylmercury. Neurotoxicology. 1999;20:767–784. [PubMed] [Google Scholar]
- Ridley RM, Clark BA, Durnford LJ, Baker HF. Stimulus-bound perseveration after frontal ablations in marmosets. Neuroscience. 1993:595–604. doi: 10.1016/0306-4522(93)90409-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. discussion 1470-1. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Schantz SL. Motor function following developmental exposure to pcbs and/or mehg. Neurotoxicology and Teratology. 2006;28:260–277. doi: 10.1016/j.ntt.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Wang VC, Powers BE, Klintsova AY, Villareal S, Greenough W, et al. Motor impairment in rats exposed to pcbs and methylmercury during early development. Toxicological Sciences. 2004;77:315–324. doi: 10.1093/toxsci/kfg252. [DOI] [PubMed] [Google Scholar]
- Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Experimental Brain Research. 1997;117:428–436. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- Sager PR, Aschner M, Rodier PM. Persistent differential alterations in developing cerebellar cortex of male and female mice after methylmercury exposure. Developmental Brain Research. 1984;12:1–11. doi: 10.1016/0165-3806(84)90170-6. [DOI] [PubMed] [Google Scholar]
- Sager PR, Doherty RA, Rodier PM. Effects of methylmercury on developing mouse cerebellar cortex. Experimental Neurology. 1982;77:179–193. doi: 10.1016/0014-4886(82)90152-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Kakita A, Wakabayashi K, Takahashi H, Nakano A, Akagi H. Evaluation of changes in methylmercury accumulation in the developing rat brain and its effects: A study with consecutive and moderate dose exposure throughout gestation and lactation periods. Brain Research. 2002;949:51–59. doi: 10.1016/s0006-8993(02)02964-5. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: Implications for studies of natural motivation, psychiatry, and drug abuse. Journal of Pharmacology & Experimental Therapeutics. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Schreiner G, Ulbrich B, Bass R. Testing strategies in behavioral teratology: Ii. Discrimination learning. Neurobehavioral Toxicology & Teratology. 1986;8:567–572. [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. [review] [30 refs] Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Skerfving S. Interaction between selenium and methylmercury. Environmental Health Perspectives. 1978;25:57–65. doi: 10.1289/ehp.782557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bartolome J. Biochemical mechanisms of developmental neurotoxicity of methylmercury. Neurotoxicology. 1987;8:65–84. [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: A review. Psychopharmacology. 1998;139 doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stern S, Cox C, Cernichiari E, Balys M, Weiss B. Perinatal and lifetime exposure to methylmercury in the mouse: Blood and brain concentrations of mercury to 26 months of age. Neurotoxicology. 2001;22:467–477. doi: 10.1016/s0161-813x(01)00047-x. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, et al. Response inhibition during differential reinforcement of low rates (drl) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environmental Health Perspectives. 2006;114:1923–1929. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson CH, Shen DM, Burbacher TM, Mohamed MK, Mottet NK. Kinetics of methyl mercury in blood and brain during chronic exposure in the monkey macaca fascicularis. Pharmacology & Toxicology. 1989;65:223–230. doi: 10.1111/j.1600-0773.1989.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Strain J, Davidson P, Bonham M, Duffy E, Stokes-Riner A, Thurston S, et al. Associations of maternal long chain polyunsaturated fatty acids, methyl mercury, and infant development in the seychelles child development and nutrition study. Neurotoxicology. doi: 10.1016/j.neuro.2008.06.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Morikawa N, Matsumoto H, Shiraishi Y. A pathological study of minamata disease in japan. Acta Neuropathologica. 1962;2:40–57. [Google Scholar]
- Tan Ja, Zhu W, Wang W, Li R, Hou S, Wang D, et al. Selenium in soil and endemic diseases in china. The Science of The Total Environment. 2002;284:227–235. doi: 10.1016/s0048-9697(01)00889-0. [DOI] [PubMed] [Google Scholar]
- Trasande L, Landrigan PJ, Schecter C. Public health and economic consequences of methylmercury toxicity to the developing brain. Environmental Health Perspectives. 2005;113:590–596. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Mottet NK, Friberg L, Lind B, Shen DD, Burbacher T. Speciation of mercury in the primate blood and brain following long-term exposure to methyl mercury. Toxicol Appl Pharmacol. 1994;124:221–229. doi: 10.1006/taap.1994.1026. [DOI] [PubMed] [Google Scholar]
- Vahter ME, Mottet NK, Friberg LT, Lind SB, Charleston JS, Burbacher TM. Demethylation of methylmercury in different brain sites of macaca fascicularis monkeys during long-term subclinical methylmercury exposure. TAP. 1995;134:273–284. doi: 10.1006/taap.1995.1193. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Behavioral effects of prenatal methylmercury in rats: A parallel trial to the collaborative behavioral teratology study. Neurobehavioral Toxicology & Teratology. 1985;7:717–725. [PubMed] [Google Scholar]
- Wainwright P. Dietary essential fatty acids and brain function: A developmental perspective on mechanisms. Proceedings of the Nutrition Society. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Huang YS, Bulman-Fleming B, Dalby D, Mills DE, Redden P, et al. The effects of dietary n-3/n-6 ratio on brain development in the mouse: A dose response study with long-chain n-3 fatty acids. Lipids. 1992;27:98–103. doi: 10.1007/BF02535807. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Jalali E, Mutsaers LM, Bell R, Cvitkovic S. An imbalance of dietary essential fatty acids retards behavioral development in mice. Physiology & Behavior. 1999;66:833–839. doi: 10.1016/s0031-9384(99)00028-1. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Tilson HA. Neurobehavioral toxicology. Baltimore: Johns Hopkins; 1986. The use of pharmacological challenges; pp. 244–267. [Google Scholar]
- Wauben IP, Wainwright PE. The influence of neonatal nutrition on behavioral development: A critical appraisal. Nutrition Reviews. 1999;57:35–44. doi: 10.1111/j.1753-4887.1999.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Weiss B, Stern S, Cox C, Balys M. Perinatal and lifetime exposure to methylmercury in the mouse: Behavioral effects. Neurotoxicology. 2005;26:675–690. doi: 10.1016/j.neuro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to aroclor 1254 and/or methylmercury in rats. Toxicol Sci. 2004;82:577–589. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Otero-Montanez JK, Yao A, Herden CJ, Sirois JE, Atchison WD, et al. Inwardly rectifying and voltage-gated outward potassium channels exhibit low sensitivity to methylmercury. Neurotoxicology. 2005;26:439–454. doi: 10.1016/j.neuro.2005.03.005. [DOI] [PubMed] [Google Scholar]