Abstract
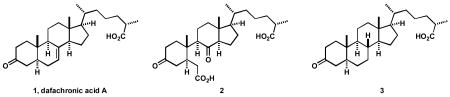
Synthesis and testing of dafachronic acid A (1) and its derivatives 2 and 3 have revealed that 1, and not a further oxidation product, is the natural ligand for the DAF-12 receptor of C. elegans.
Remarkably, the life span of the nematode C. elegans can be increased significantly by loss of function of a handful of genes that affect endocrine function. Amongst them, the daf-9 gene encodes a cytochrome P450 enzyme which is responsible for the biosynthesis of the bile acid-like steroid, dafachronic acid A (1). Based on various analytical techniques, it has been recently proposed by Mangelsdorf and Antebi that 1 is the major ligand for the nuclear receptor DAF-12, which in its ligand bound form regulates genes that prevent entry into the dauer stage, a long lived quiescent mode.1 However, synthesis of the proposed ligand remained elusive until a later work, in which the 25-(S) structure of 1 and its 25-(R)-diastereomer were made.2,3
In this research we address the question of whether dafachronic acid A is the true ligand for the nuclear hormone receptor DAF-12 or just a precursor of a further biooxidation product which is the actual ligand. We were intrigued by the fact that dafachronic acid A, with its Δ7-olefinic linkage, might be further oxidized biologically to a seco acid structure resembling that of glycinoeclepin A,4,5 a potent hatching factor for the eggs of the nematode Heterodera glycines. Consequently, we became interested in exploring the biological activity of the β-seco dafachronic acid A derivative 2, as an analog of glycinoeclepin A, which might even be a more active metabolite of 1. In this letter we describe the synthesis and biological evaluation of 2. For comparaison, we have also synthesized the 7,8-dihydro derivative of dafachronic acid A, 3, which would be expected to be devoid of activity if the seco acid 2 were the real ligand for DAF-12, rather than dafachronic acid A (1).
The synthesis of the diketo diacid 2 started with the previously reported 6-keto steroid 4.2 Baeyer-Villiger oxidation of 4 with trifluoroperacetic acid ((CF3CO)2O, H2O2, 0 °C, CHCl3) afforded the desired 7-membered lactone 5 in 94% yield and as a sole regioisomer. Lactone 5 was cleaved to a ketoacid intermediate by treatment with Jones’ reagent (2 equiv, 23 °C, acetone) which was esterified by diazomethane (CH2N2, Et2O) to give ketoester 6 in essentially quantitative yield over two steps. Saponification of the 3β-acetate, oxidation of the resulting alcohol to the ketone, and hydrolysis gave the diketo diacid 2 in 52% overall yield (three steps, Scheme 1). Our initial strategy for the synthesis of 2 involved the oxidation of the Δ7-olefinic linkage in 1 by various methods. Surprisingly, all attempts to directly oxidize the Δ7 bond to the diketo diacid 2 using O3 then H2O2, KMnO4, NBu4MnO4 and RuCl3-NaIO4 were unsuccessful.
Scheme 1.
Synthesis of analogs 2 and 3 from β-stigmasterol
To synthesize the 7,8-dihydro analog 3, we have also used an intermediate from our synthesis of 1.2 Thus, the Δ5-double bond in 7 was reduced (H2, 1 atm, Pd-C, EtOAc) to give the fully saturated steroid and the same three steps as above were performed to give analog 3 in 33% overall yield for the four steps. It should also be mentioned that the hydrogenation of 1 to 3 failed under several conditions.4
Next, samples of the synthetic dafachronic acid A 1, the seco-diacid 2, and 7,8-dihydrodafachronic acid A 3 were evaluated for their bioactivity. First, the ability of synthetic ligands to rescue daf-9 hormone biosynthetic mutants from the dauer state was measured. Consistent with 1 being a natural ligand for DAF-12, dafachronic acid A rescued dauer formation in the nanomolar range, with half maximal activity of 18.5 nM (Figure 2). Similarly, the 7,8-dihydrodafachronic acid A also gave substantial rescue with half maximal rescue at 292 nM. By contrast, the seco-diacid 2 was found not to rescue C. elegans from the dauer state, indicating that it is not a ligand. Second the ability of synthetic ligands to activate DAF-12 in transcriptional assays on a target gene, lit-1, was measured. To do this, plasmid constructs containing the daf-12 gene and the lit-1 gene fused to a luciferase reporter were co-transfected into human embryonic kidney cells (HEK293T), treated with various doses of the compounds, and luciferase induction measured by light emission.1 In accord with the dauer rescue results, 2 showed no activity even at 100 μM concentration (Figure 3), whereas 7,8-dihydrodafachronic acid A (3) showed similar activity as dafachronic acid A (1). Specifically, measurement of the dose response revealed EC50 values for daf-12 activation to be: for 7,8-dihydrodafachronic acid A, 114 nM and for dafachronic acid A, 26 nM. These results taken together allow the following conclusions: (1) dafachronic acid A is a natural ligand for DAF-12 nuclear receptor (2) in contrast to the soybean nematode case, ring B oxidative cleavage products are not the active agents, for gene activation of C. elegans DAF-12 and (3) Δ7,8 double bond is not essential for dafachronic acid activity on C. elegans.
Figure 2.
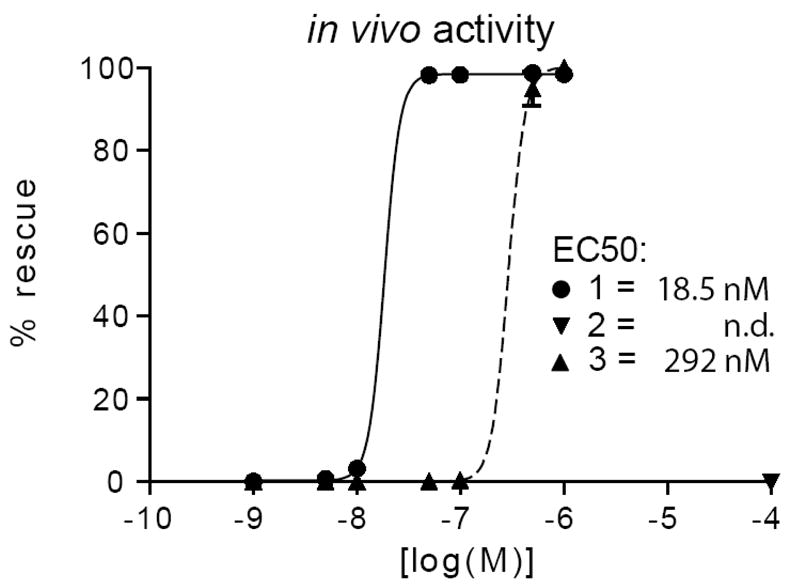
In vivo activity of sterols 1, 2, and 3 measured as the percentage of rescue of daf-9(dh6) null worms from dauer to wild-type gravid adults.
Figure 3.

Transcriptional activation of DAF-12 by 1, 2 and 3 on lit-1::ptk-luciferase reporter constructs, measuring relative luciferase units with and without ligand (RLU) vs concentration.
Supplementary Material
Experimental protocols and characterization for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Figure 1.
Structure of glycinoeclepin A
Acknowledgments
S.G. is grateful to NSERC (Canada) for a postdoctoral fellowship. A.A. is grateful for support from NIH and the Ellison Foundation. We thank Dongling Li for technical assistance.
Contributor Information
Simon Giroux, Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, Massachusetts 02138.
Axel Bethke, Huffington Center on Aging, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas 77030.
Nicole Fielenbach, Huffington Center on Aging, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas 77030.
Adam Antebi, Huffington Center on Aging, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas 77030.
E.J. Corey, Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, Massachusetts 02138
References
- 1.(a) Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]; (b) Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A. Proc Natl, Acad Sci USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, Mangelsdorf DJ, Antebi A. Developmental Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]; (d) (c) For an online resource on C. elegans, see: http://www.wormbook.org.
- 2.Giroux S, Corey EJ. J Am Chem Soc. 2007;129:9866–9867. doi: 10.1021/ja074306i. [DOI] [PubMed] [Google Scholar]
- 3.Giroux S, Corey EJ. Org Lett. 2008;10:801–802. doi: 10.1021/ol702936f. [DOI] [PubMed] [Google Scholar]
- 4.To the best of our knowledge, no succesful hydrogenation of isolated Δ7 double bonds have been reported in the literature.
- 5.Glycinoeclepin A, a natural product that is released into soil from the roots of the soybean plant, is active at 10−12 g/mL as hatching factor for H. glycines, see: (a) Fukuzawa A, Furusaki A, Ikura M, Masamune T. J Chem Soc Chem Commun. 1985;221–222:748.Masamune T, Anetai M, Takasugi M, Katsui N. Nature. 1982;297:495–496.
- 6.For the syntheses of glycinoeclepin A, see: (a) Murai A, Tanimoto N, Sakamoto N, Masamune T. J Am Chem Soc. 1988;110:1985–1986.Mori K, Watanabe H. Pure Appl Chem. 1989;61:543–546.Corey EJ, Houpis IN. J Am Chem Soc. 1990;112:8997–8998.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental protocols and characterization for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.




