Abstract
The new U.S. National Osteoporosis Foundation Clinician's Guide to Prevention and Treatment of Osteoporosis includes criteria for recommending pharmacologic treatment based on history of hip or vertebral fracture, femoral neck (FN), or spine BMD T-scores ≤−2.5 and presence of low bone mass at the FN or spine plus a 10-yr risk of hip fracture ≥3% or of major osteoporotic fracture ≥20%. The proportion of women who would be recommended for treatment by these guidelines is not known. We applied the NOF criteria for treatment to women participating in the Study of Osteoporotic Fractures (SOF). To determine how the SOF population differs from the general U.S. population of white women ≥65 yr of age, we compared women in SOF with women who participated in the National Health and Nutrition Examination Survey (NHANES) III on criteria included in the NOF treatment guidelines that were common to both cohorts. Compared with NHANES III, women in SOF had higher FN BMD and were younger. Application of NOF guidelines to SOF data estimated that at least 72% of U.S. white women ≥65 yr of age and 93% of those ≥75 yr of age would be recommended for drug treatment. Application of the new NOF Guidelines would result in recommending a very large proportion of white women in the United States for pharmacologic treatment of osteoporosis.
Key words: osteoporosis, treatment, guidelines, epidemiology, BMD
INTRODUCTION
The U.S. National Osteoporosis Foundation (NOF) recently released new guidelines, “Clinician's Guide to Prevention and Treatment of Osteoporosis,” that include comprehensive, useful, and thoughtful information about how to prevent fractures and mitigate their consequences.(1) The guidelines include recommendations about pharmacologic treatment to prevent fractures (Table 1). They recommend treatment based on history of hip or vertebral fracture, hip and spine BMD T-scores, and—among those with low bone mass—a prior fracture or a 10-yr risk of fracture estimated from the World Health Organization (WHO) risk index (FRAX).(2)
Table 1.
Criteria for Recommending Pharmacologic Treatment From the U.S. NOF Guidelines

Two approaches to treatment may be considered: one based on BMD alone and the other based on absolute fracture risk of fracture. Given that one half of all fractures occur in women without WHO BMD defined osteoporosis, treatment that is initiated based on BMD alone will not treat a large proportion of women who eventually go on to fracture.(3,4) In general, compared with treatment based on BMD alone, the emphasis on treating based on absolute risk of fracture will decrease the proportion of 50- to 60-yr-old women who are treated with drugs because they have a low risk of fractures. On the other hand, this approach may result in treating a large proportion of older people, particularly older white women who have higher absolute risk of fracture. However, regardless of ethnicity, increasing age is associated with increased fracture risk.(5) The proportions of various age groups that would be treated under the new NOF guidelines are not known. Therefore, we used data from the Study of Osteoporotic Fractures (SOF) and applied the new NOF treatment guidelines to estimate the proportion of white women :65 yr of age who would be recommended for pharmacologic treatment. SOF is a community-based sample of women from four urban areas. To confirm how well SOF represents the U.S. white female population ≥65 yr of age, we compared the SOF population to white females ≥65 yr of age who participated in the Third National Health and Nutrition Examination Survey (NHANES III) on treatment criteria listed in the NOF guidelines (including FRAX) common to both cohorts. This was done to gauge the similarities of the two cohorts on treatment criteria and to confirm the accuracy of the proportion that would be recommended for treatment in the U.S. white female population compared with SOF.
MATERIALS AND METHODS
We used data from the Study of Osteoporotic Fractures (SOF), a prospective study of community-dwelling white women ≥65 yr of age recruited from four communities in the United States: Baltimore, MD; Minneapolis, MN; Portland, OR; and Monongahela Valley near Pittsburgh, PA. Participants were recruited from population-based listings and mass mailings between 1986 and 1987.(6) SOF was initiated before widespread publicity about osteoporosis and women were not recruited on the basis of any risk factors for osteoporosis. All participants provided informed consent. This study was approved by the Institutional Review Board at each of the participating sites.
Demographic, anthropometric, lifestyle, and medical history
Baseline examinations took place from 1986 to 1988 (n = 9704). Women provided information regarding fracture history, smoking status, alcohol consumption, parental hip fracture history, rheumatoid arthritis, and corticosteroid use.
Height was measured with a wall-mounted Harpenden stadiometer (Holtain, DyFed, UK). Weight was measured with a balance beam scale. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
BMD
BMD was obtained between 1988 and 1990 (visit 2) at the proximal femur and lumbar spine by DXA using QDR 1000 densitometers (Hologic, Bedford, MA, USA). This was performed on 7959 of the 9451 (84%) surviving cohort (hip BMD by DXA was not available at baseline). T-scores for the femoral neck (FN), and total hip were calculated based on the means and SDs obtained from the NHANES III.(7) T-scores for the spine were calculated using the reference value provided by the manufacturer (Hologic).
WHO 10-yr absolute fracture risk
The WHO 10-yr absolute risk of both hip fracture and major osteoporotic fracture (hip, clinical spine, forearm, or shoulder) was calculated by the WHO Collaborating Center for Metabolic Bone Disease. Calculation of absolute risk was done following the FRAX algorithm.(2,8) FRAX is described in detail elsewhere. Briefly, the calculation of the 10-yr probabilities is based on nine risk factors (age, sex, BMI, previous history of fracture, parental history of hip fracture, current smoking, use of corticosteroids in past 3 mo, presence of rheumatoid arthritis, and three or more alcoholic beverages per day). The 10-yr probabilities for both hip and major osteoporotic fracture can be calculated with or without FN BMD. The NOF treatment guidelines use the 10-yr absolute risks (hip and major osteoporotic fracture) calculated using FN BMD.
NHANES III
NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the United States. The survey uses a stratified complex sampling strategy to identify and examine a nationally representative sample of ∼5000 persons each year. We used data from white women ≥65 yr of age who participated in NHANES III. A direct estimation of the proportion of white women ≥65 yr of age who would be recommended for treatment under the NOF guidelines cannot be performed using NHANES III because lumbar spine BMD, paternal history of fracture, and personal history of fracture at skeletal sites other than the hip, spine, and wrist were not assessed in NHANES III. For those factors in FRAX and/or the NOF guidelines that were measured in both SOF and NHANES III, we reported the mean values for continuous variables (age, BMI, and BMD) and proportions for dichotomous variables (personal history of fracture, maternal history of fracture, rheumatoid arthritis, current smoking, and consumption of ≥3 alcoholic drinks/d). NHANES III data were obtained from the publicly available data release (http://www.cdc.gov/nchs/nhanes.htm). Means and proportions were adjusted for the NHANES III sampling strategy as recommended in the NHANES analysis guidelines using the SURVEYMEANS and SURVEYFREQ procedures in SAS (SAS Institute, Cary, NC, USA).
Analysis
Women in SOF were excluded from the analysis if they had missing data for any of the factors required to apply the NOF guidelines, including those required to calculate FRAX. We applied the NOF guidelines as given in Table 1 to determine the proportion of women who would be recommended for treatment.
Radiographic vertebral fracture status was available in SOF. However, this information is not routinely collected in the usual care setting and therefore, to conservatively apply the guidelines, we did not include information about radiographic vertebral fractures in determining whether a woman should be treated.
In the primary analysis, the criterion “past history of hip fracture or clinical or radiographic vertebral fracture” was conservatively limited to past history of hip fracture. We also estimated the proportion of women who would be recommended for treatment if a history of clinical vertebral fractures was included. We also applied the criterion “T-score ≤−2.5 at femoral neck or spine” and “osteopenia and 10-yr risk of hip fracture ≥3% or osteopenia and major osteoporotic fracture ≥20%” alone. Finally, to determine if the proportion of women who would be recommended for treatment differed by age, we analyzed women ≥65 and ≥75 yr.
RESULTS
A total of 9704 women participated in the baseline examination. Between baseline and visit 2, 253 women died, 39 were terminated, 72 did not return for visit 2 but were monitored for fracture outcomes through postcard, and 1 was lost to follow-up. Among the 9339 women who returned for visit 2 (when DXA measurements of the hip and spine were completed), 7778 had complete BMD data available at the hip and spine. Among those with complete BMD data, 1682 were missing data for at least one criterion in FRAX or the NOF guidelines—most commonly paternal history of hip fracture. Thus, 6096 women were included in the analysis. Women who were missing at least one FRAX or NOF criteria had similar age, BMI, and femoral neck BMD.
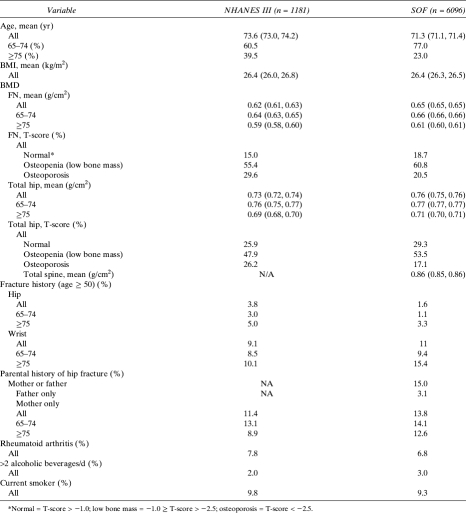
Women in SOF were younger (71 versus 74 yr) and had slightly higher BMD at the femoral neck and total hip compared with NHANES III. Therefore, women in SOF had a lower prevalence of T-scores below −2.5 and a higher number with T-scores >−1.0. Fewer women in SOF reported a previous hip fracture compared with the women in NHANES (11.4% versus 13.8%). However, a greater proportion of women in SOF ≥75 yr of age reported a history of previous wrist fracture compared with the woman in NHANES III (15.2 versus 10.1; Table 2).
Table 2.
Comparisons of Characteristics of White Women ≥65 yr of Age in NHANES III and in the Study of Osteoporotic Fractures
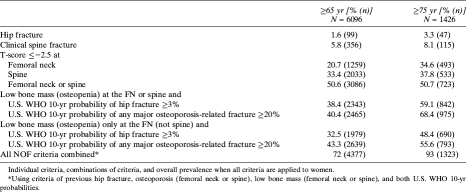
Overall, applying the modified NOF guidelines to women ≥65 yr of age, 71.8% would be recommended for treatment (Table 3). When history of clinical vertebral fracture is included, 72.5% of women would be recommended for treatment. When the guidelines are applied to women ≥75 yr of age, 93% of women would be recommended for treatment.
Table 3.
NOF Criteria for Recommending Pharmacologic Therapy
Applying only the criterion of “T-score ≤−2.5 at femoral neck or spine” would recommend 40.4% of women for drug treatment (Table 3). However, if T-score ≤−2.5 is limited to only the femoral neck, 20.7% would be recommended for treatment (Table 3). Applying only the criterion, “low bone mass and 10-yr risk of hip fracture ≥3% or major osteoporotic fracture ≥20%” would recommend 51.5% of women for drug treatment.
DISCUSSION
We estimate that the new NOF guidelines would recommend that pharmacologic treatment should be initiated for about three quarters of the participants in the SOF study and >90% of women >75 yr of age. These figures underestimate the proportions that would be recommended for treatment in the U.S. population because women in SOF had somewhat higher BMD values and were somewhat younger than the women in the U.S. population-based NHANES III survey. Furthermore, we did not include some criteria for treatment, such as a history or presence of a radiographic vertebral fracture—inclusion of this would further increase the number recommended for treatment. Our estimates are consistent with results from the Canadian Multicenter Osteoporosis Study that found that over two thirds of white women ≥65 yr of age would have a 10-yr probability of fracture ≥20% based on BMD and the risk factors used in the WHO model.(9) Although the NOF cost-effectiveness analysis did not report the proportion of women who would be treated under the NOF guidelines, they do conclude that pharmacologic treatment would be cost-effective for the average 68-yr-old white woman.(10) Thus, given that the average age of women who participated in SOF was 71 yr, it is consistent that we estimated that a high proportion (73%) of women would be recommended for drug treatment.
Our analysis suggests that about one half of the older white women recommended for pharmacologic treatment under the new guidelines would be included because they have low bone mass (osteopenia) at one of the two skeletal sites and at least one risk factor: a 10-yr estimated probability of hip fracture ≥3% or 10-yr estimated probability of major osteoporotic fracture ≥20%. When so many people, and such a large proportion of older women, are recommended for drug treatment, it is important that the assumptions underlying the recommendations be based on robust data. In particular, it is important that there be strong evidence that all of those people would substantially benefit from treatment. There have been few trials of the efficacy of treatments in this group, and it is uncertain whether treatment reduces the risk of nonvertebral fractures in people with osteopenia and no vertebral fracture.(11–13) A trial of clodronate in women ≥75 yr of age, not selected for osteoporosis, found a 20% decreased risk of clinical fractures.(14) On the other hand, the “Clinical Fracture Arm of FIT” found no significant reduction in risk of fracture in women with osteopenia (hip BMD T-scores > −2.5).(15) Moreover, in the risedronate hip study, no clear fracture reduction benefit compared with placebo was noted among women ≥80 yr of age who were selected on the basis of risk factors and not BMD.(16) Even in the Women's Health Initiative trial, it seemed that women with femoral neck BMD T-score <−2.5 had a greater reduction in nonspine fractures (RR, 0.53; 95% CI, 0.25–1.10) than did women with T-score ≥−2.5 (RR, 0.87%; 95% CI, 0.57–1.34, p for interaction = 0.15).(17)
We found that ∼21% of women in SOF had osteoporosis defined as a femoral neck BMD T-score ≤−2.5. In the FIT trial, alendronate decreased the risk of all clinical fractures by 31% in this group.(15) Expanding treatment by including women who had a spine BMD T-score ≤−2.5 added another 19% of white women. To date, all FDA-approved agents are effective in reducing vertebral fractures, and this effect does not seem to vary by BMD.
Our estimates have several limitations. SOF enrolled participants in 1986–1988 and measured hip and spine BMD in 1988–1990. The profile of risk factors and BMD in U.S. white women may have changed; in particular, there have been secular increases in weight. Our estimates were based on the WHO model that includes femoral neck BMD and BMI (which includes height and weight), a temporal population increase in weight may be associated with an increase in BMD over time. Thus, these population trends in body size might be associated with a decrease in the prevalence of osteoporosis. A second limitation of our analysis is that SOF is a cohort of community-dwelling volunteers and not a population-based sample. However, characteristics of the SOF participants are similar to, or healthier than, those of the population-based NHANES III, and therefore may underestimate the proportion of women who would be recommended for treatment. Third, FRAX may underestimate the 10-yr probability of major osteoporotic fracture among those who have a radiographic vertebral fracture. We conservatively excluded X-ray fractures when the FRAX 10-yr probabilities were calculated by the WHO (i.e., X-ray based fractures were not included as a “history of previous fracture” in the FRAX calculations). Fourth, because this analysis from SOF includes only white women ≥65 yr of age, this study is not able to estimate the proportion of younger women and men and women of other racial groups who would be recommended for pharmacologic treatment. Presumably, the proportion of people in these groups recommended for treated will be substantially less than the estimates from SOF because they have a lower risk of fracture and lower prevalence of osteoporosis. Finally, our estimate of the proportion of women who would be recommended for treatment is based on FRAX estimates from WHO as of early 2008. If new FRAX estimates become available, the proportion of women recommended for treatment may change.
We conclude that the new NOF guidelines for pharmacologic treatment for osteoporosis would recommend drug therapy to at least three quarters of white women ≥65 yr of age and 90% of those >75 yr. When such a large proportion of older women are recommended to receive drug treatment for osteoporosis, it is important that the assumptions that underlie that analysis be based on robust evidence. A trial of bisphosphonate therapy and/or other pharmaceutical agents in women and men with just “low bone mass” would be informative.
ACKNOWLEDGMENTS
The SOF is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Institute on Aging (NIA) under Grants AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, and 2 R01 AG027574-22A1.
REFERENCES
- 1.National Osteoporosis Foundation. Washington, DC, USA: National Osteoporosis Foundation; 2008. Clinician's Guide to Prevention and Treatment of Osteoporosis. [Google Scholar]
- 2.World Health Organization. Geneva, Switzerland: World Health Organization; 2008. FRAX WHO Fracture Risk Assessment Tool. [Google Scholar]
- 3.Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–1112. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- 4.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 5.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings S, Nevitt M, Browner W, Stone K, Fox K, Ensrud K, Carley J, Black D, Vogt T. Risk factors for hip fracture in white women: The Study of Osteoporotic Fractures research group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 7.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards JB, Leslie WD, Joseph L, Siminoski K, Hanley DA, Adachi JD, Brown JP, Morin S, Papaioannou A, Josse RG, Prior JC, Davison KS, Tenenhouse A, Goltzman D. Changes to osteoporosis prevalence according to method of risk assessment. J Bone Miner Res. 2007;22:228–234. doi: 10.1359/jbmr.061109. [DOI] [PubMed] [Google Scholar]
- 10.Dawson-Hughes B, Tosteson AN, Melton LJ, III, Baim S, Favus MJ, Khosla S, Lindsay RL. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 11.MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J. Systematic review: Comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;1:CD001155. doi: 10.1002/14651858.CD001155.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9:1–160. doi: 10.3310/hta9220. [DOI] [PubMed] [Google Scholar]
- 14.McCloskey EV, Beneton M, Charlesworth D, Kayan K, deTakats D, Dey A, Orgee J, Ashford R, Forster M, Cliffe J, Kersh L, Brazier J, Nichol J, Aropuu S, Jalava T, Kanis JA. Clodronate reduces the incidence of fractures in community-dwelling elderly women unselected for osteoporosis: Results of a double-blind, placebo-controlled randomized study. J Bone Miner Res. 2007;22:135–141. doi: 10.1359/jbmr.061008. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: Results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 16.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 17.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women's Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]