Abstract
Paget's disease of bone (PDB) is a focal disorder of bone remodeling that leads to overgrowth of affected bone, with rare progression to osteosarcoma. Extensive studies of familial PDB showed that a majority of cases harbor germline mutations in the Sequestosome1 gene (SQSTM1). In contrast, little is known about the mutational status of SQSTM1 in sporadic PDB. We hypothesized that somatic SQSTM1 mutations might occur in the affected tissues of sporadic PDB and pagetic osteosarcoma. We used laser capture microdissection to capture homogeneous populations of cells from the affected bone or tumor of patients with sporadic PDB or pagetic osteosarcoma, respectively. DNA from these samples and appropriate controls was used for sequence analysis and allelic discrimination analysis. Two of five patients with sporadic PDB had SQSTM1C1215T mutations detected in their affected bone but not in their blood samples, indicating a somatic origin of the mutations. Samples from three of five sporadic pagetic osteosarcoma patients had the SQSTM1C1215T mutation, whereas the normal adjacent tissue from two of these tumors clearly lacked the mutation, again indicating an occurrence of somatic events. No SQSTM1 mutations were found in primary adolescent osteosarcomas. The discovery of somatic SQSTM1 mutations in sporadic PDB and pagetic osteosarcoma shows a role for SQSTM1 in both sporadic and inherited PDB. The discovery of somatically acquired mutations in both the diseased bone and tumor samples suggests a paradigm shift in our understanding of this disease.
Key words: Paget's disease of bone, pagetic osteosarcoma, Sequestosome 1, somatic mutations, laser capture microdissection
INTRODUCTION
Paget's disease of bone (PDB; OMIM 602080) is a focal disorder of bone remodeling characterized by accelerated bone turnover at affected sites.(1) It is a relatively common disorder, affecting 1–3% population in the United States, United Kingdom, and other European countries, increasing in prevalence with age.(1) PDB can be monostotic or polyostotic and has a predilection for the axial skeleton, skull, pelvis, and long bones of the lower extremities.(2) The accelerated rate of bone resorption that marks the earliest lesion in PDB is coupled to bone formation and over time results in the overgrowth of the affected bone causing structural impairment, bone deformity, and localized bone pain.(3) The histology is one of chaotic cancellous bone formation; stromal elements replace normal marrow, and there is heightened vascularity of bone.(4) Unexplained to date is why PDB does not spread to adjacent or new bone sites in the lifetime of an individual.(5) Since the first description of PDB by Sir James Paget in 1877, a salient feature of the disease has been the potential for osteosarcoma to arise in the pagetic bone. Reports have placed the risk of osteosarcoma in PDB between 0.7% and 5%.(6–9) The epidemiology of pagetic osteosarcoma is striking in that it accounts for 20% of osteosarcoma patients older than 40 yr of age(7) and as high as 50% of osteosarcoma patients ≥60 yr of age.(10) In the majority of patients, there is evidence of symptomatic, polyostotic PDB long before the identification of tumor within pagetic bone.(11) Although there is certainly an increased risk for osteosarcoma in PDB, the molecular basis for this remains unclear. It has been suggested that the malignant change, which occurs in association with PDB, is the result of prolonged abnormal cellular activity.(12) Despite advances in the treatment of osteosarcomas, the prognosis for pagetic osteosarcoma patients remains poor.(13,14)
The familial form of PDB is inherited in an autosomal dominant fashion with high but incomplete penetrance.(15,16) PDB is a heterogeneous disease with linkage to at least five loci.(17–21) Putative predisposition genes have been identified at two of the loci; PDB2 is associated with TNFRSF11A gene on chromosome 18q22,(18) whereas PDB3 is associated with the SQSTM1 gene on chromosome 5q35.(19) Another form of early-onset PDB associated with inclusion body myopathy and frontotemporal dementia has been linked to the VCP gene (9p13-p12),(22) whereas juvenile PDB is linked to the TNFRSF11B gene.(23) Approximately 40% of the familial PDB cases have been linked to inherited mutations in the SQSTM1 locus on human chromosome 5q35.(17,24–33) Mutations in SQSTM1 have also been identified in the peripheral blood of affected individuals with no family history of PDB,(25,32) suggesting that these “sporadic” cases are, in fact, de novo familial cases. In all but one case,(24) the constitutional mutations were found in the heterozygous state.
As mentioned, SQSTM1 mutations have been studied extensively in the constitutional tissues of both familial and sporadic cases.(24–29) We were curious whether PDB might arise in aging bone as a result of somatic mutations in the tissues and whether subsequent changes in the microenvironment of bone might lead to the development of a pagetic osteosarcoma. We therefore chose to examine the status of SQSTM1 in the affected bones and tumors from PDB patients with no evidence of familial disease.
MATERIALS AND METHODS
Patient samples
All work was done under the approval of each institution's institutional review board. All of the pagetic bone samples were from patients with no known family history of PDB. Five samples of snap-frozen, mineralized, nondecalcified pagetic bone and matched peripheral blood (PGDZ1–5) were obtained from the New England Registry for Paget's Disease. Five samples of formalin-fixed paraffin-embedded (FFPE) pagetic osteosarcoma and normal adjacent bone (PGOS1–5) were obtained from pathology archives of the Mayo Clinic in Rochester, MN, USA. Twenty samples of snap-frozen nondecalcified adolescent osteosarcoma were obtained from the Cooperative Human Tissue Network. DNA from a peripheral blood sample of a healthy person (WILDTYPE CONTROL) and from a patient with a constitutional heterozygous SQSTM1C1215T mutation (MUTANT CONTROL) was used as controls. Random study numbers were assigned to ensure anonymity of all samples.
Laser capture microdissection
Samples were prepared for laser capture microdissection (LCM) by a modification of Jacquet et al.(34) The frozen bone tissues were placed in 20% sucrose overnight at 4°C to preserve tissue architecture. After a thorough wash in PBS, the bone samples were placed into plastic molds and embedded in cryomatrix gel inside a supercooled chamber comprised of 2-methylbutane in a foam cup surrounded by dry ice. Serial 7.5-μm sections of each frozen tissue sample were cut and transferred onto multiple adhesive-coated slides using the CryoJane Tape-Transfer System (Instrumedics, Hackensack, NJ, USA). Serial 5-μm sections of the FFPE pagetic tumor and matched normal samples were cut and placed on multiple uncoated slides. After lightly staining all slides with H&E, one set that represented each of the experimental tissue samples was coverslipped to obtain microscopic images, and the rest were used for LCM.
Before LCM, representative stained sections were viewed by pathology (Elizabeth A. Saria, MD, DABP), identifying the pagetic cells to capture and mapping out the area of the tissue showing histological characteristics of PDB (Fig. 1A). After LCM (Fig. 1B), electronic images of the captured material were sent back to pathology to confirm accuracy of capture and rule out contamination with normal tissue. LCM was done using a PixCell II Laser Capture Microdissector (Molecular Devices, Sunnyvale, CA, USA). Approximately 1000 cells, accumulated from up to three sections on a slide, were captured in triplicates for each tissue sample. DNA was independently isolated from each LCM capture using the PicoPure DNA isolation kit (Molecular Devices) and evaluated for purity and concentration. Based on these parameters, optimal DNA isolates (two for each tissue sample) were used for experimental applications.
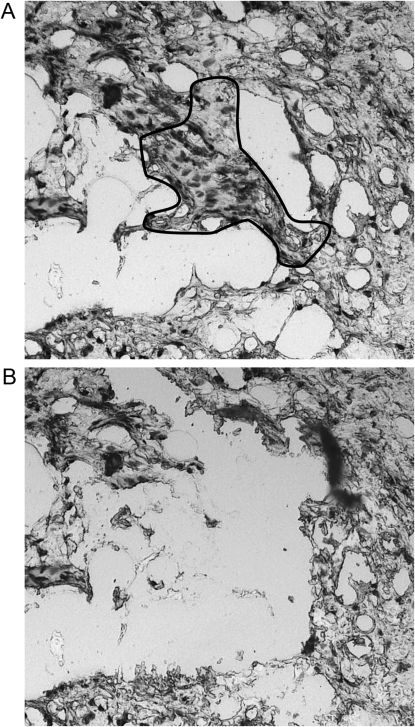
FIG. 1.
Example of PDB tissue before and after LCM. (A) A portion of an H&E-stained section of bone photographed before LCM exhibiting active Paget's disease with characteristic histological features of chaotic bone formation resulting in a lacelike cancellous bony structure. (B) Photograph of the same part of the tissue section after performing LCM. The osteoblast-like cells within the pagetoid bone have been selectively captured. This method allowed capture of the relevant cells while avoiding contamination from surrounding noninvolved regions. The captured portion of this tissue was used to extract DNA for sequencing and allelic discrimination. Approximately 1000 cells were captured per slide. Magnification for both images, ×100.
The entire protocol was repeated at least three times using independent serial sections of the same tissue to ensure reproducibility. As in all procedures in which PCR amplification may be used, careful attention was paid through all steps from sectioning to isolation to prevent carryover contamination. A fresh cutting blade was used for each sample.
DNA from the peripheral blood of PDB patients was extracted using DNA FlexiGene Kit, and the DNeasy Tissue Kit (both from Qiagen, Valencia, CA, USA) was used for extraction of DNA from the adolescent osteosarcoma samples. None of the samples were pooled.
Sequence analysis
Exons 7 and 8 of the SQSTM1 gene were amplified in each sample by 30 rounds of PCR using the following primers designed using MacVector 7.2.2 (Accelrys Software, San Diego, CA, USA): exon 7F, 5′-TGTCTCCTGTGTGCTCATGGTG-3′; exon 7R, 5′-CCTTCTGTTTGTGTCGCTGAAATC-3′; exon 8F, 5′-CACTGTGGCCTGTGAGGAC-3′; exon 8R, 5′-TCAGCACACACACACACAGG-3′.
The amplified DNA samples were purified using the Exo/SAP-IT kit (USB, Cleveland, OH, USA) and sequenced using the Big Dye Terminator v3.1 Cycle Sequencing Kit (ABI, Foster City, CA, USA), and the sequences were read on an ABI Model 3100 DNA sequencer. The data were analyzed using CodonCode Aligner (CodonCode, Dedham, MA, USA).
Allelic discrimination (5′ nuclease) assay
The allelic discrimination (AD) assay used a TaqMan assay-based chemistry method (ABI) to amplify and detect specific alleles in purified genomic DNA. Custom TaqMan probes were designed to detect the SQSTM1C1215T mutation using File Builder 3.0 software (ABI). A 600-bp sequence spanning exon 8 of SQSTM1, including the intronic regions on the 5′ and 3′ ends, was chosen from the SQSTM1 genomic sequence (NCBI accession number NM_003900.3) as a template to target the point mutation (C1215T), which was near the center of the sequence. The region was further analyzed to ensure that the chosen template was specific to the SQSTM1 gene and did not contain any other single nucleotide polymorphisms or repetitive sequences. The custom primers designed to yield a PCR product of 66 bp for exon 8 of SQSTM1 were as follows: SQSTM1EX8-C2TF, 5′-CCTCATGGCTTCCTTACTGTTTCG-3′; SQSTM1EX8-C2TR, 5′-GCATCTGGGAGAGGGACTCA-3′.
The following were the reporter probes for the wildtype and mutant sequence with their fluorescent dyes, respectively: VIC, CAGCCGCGGGTCA; FAM (6-carboxyfluorecein), TCAGCCGCAGGTCA.
For consistency and accurate readouts, duplicate samples containing 20 ng of genomic DNA were used for all experiments. Experimental controls for each assay, in duplicates or triplicates, were RNase- and DNase-free water (GIBCO, Carlsbad, CA, USA) as negative template (NTC), wildtype DNA (+/+), and heterozygous mutant DNA (+/C1215T).
The 2× TaqMan Universal Master Mix without UNG Amperase (ABI Part 4324018) was used for the assay, which was done in an 7500 Real-Time PCR System (ABI) with AD and absolute quantification (AQ) options. The amplification was carried out in a total reaction volume of 50 μl under the following PCR conditions: stage I, 95°C for 8.5 min, followed by 50 cycles of stage II (92°C for 15 s) and stage III (60°C for 1.5 min).
The data were analyzed using ABI's proprietary Sequence Detection Software (SDS Version 1.3). For analysis of the AQ data, an amplification plot was generated by manually setting threshold intensity for the fluorescence signal and automated selection of start and end cycles, as recommended by the protocol. The ratio of the fluorescence intensity of the reporter dye, which discriminates its allele specifically, to that of the internal passive reference dye, gives a value that indicates normalized reporter intensity (Rn). The amplification graph allows analysis of the change in magnitude of the fluorescence signal from baseline (Delta Rn) for each sample plotted against the cycle number of the real-time PCR. The cycle threshold (Ct) is the cycle number at which the fluorescence intensity of a sample exceeds the threshold intensity, indicating that a given sample that displays a Ct value carries the allele corresponding to its fluorescent reporter dye.
Detection of measles virus nucleocapsid protein expression
Tissue samples of pagetic bone, pagetic osteosarcoma, and normal bone were used for laser capture as described above, and RNA was extracted using the High Pure RNA Paraffin Kit (catalog 03270289001; Roche Diagnostics, Indianapolis, IN, USA) following the manufacturer's instructions. As a positive control for measles virus nucleocapsid protein (MVNP), NIH-3T3 cells transfected with either MVNP or the empty vector (EV) were used (kind gift from Drs GD Roodman and DL Galson, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA). The cells were grown in 100 × 20 mm round culture dishes (catalog 5666–4160; USA Scientific, Ocala, CA, USA) in DMEM (GIBCO catalog 11995-065), completed with 10% FBS (catalog S11150; Atlanta Biologicals) and 1% penicillin-streptomycin (GIBCO catalog 15140-122). The cultures were grown to confluence before harvesting with 1 ml of 0.25% 1× Hyclone Trypsin-EDTA (catalog SH30042.01; Thermo Scientific). The trypsin was neutralized with complete media and centrifuged at 1750 rpm for 5 min. The supernatant was suctioned off without disturbing the cell pellet, and the tube containing the cell pellet was immediately placed in ice and subjected to RNA isolation. RNA was isolated from the cells using the Illustra RNAspin Mini RNA Isolation Kit (product 25-050070; GE Healthcare, Piscataway, NJ, USA), following the manufacturer's protocol.
Reverse transcription of the RNA from the patient samples and control samples was done following the manufacturer's protocol using the TaqMan Reverse Transcription Reagents (P/N N808-0234; Applied Biosystems) with MultiScribe Reverse Transcriptase (catalog 4311235; ABI) to generate cDNA. The resulting cDNA products were subjected to real-time PCR in an ABI Model 7500 Real-Time system. The MVNP primer sequences used were as follows: sense, 5′-AGTCGAATTCAAAGTGAGAATGAGCTAC-3′; antisense, 5′-CTGAAGCTTGGCTGGACTCCGATGCA-3′.(35) Primer sets for mouse β-actin (forward: CCTAAGGCCAACCGTGAAAAG, reverse: TCTTCATGGTGCTAGGAGCCA) and human β-actin (forward: CAGCAGATGTGGATCAGCAAG, reverse: GCATTTGCGGTGGACGAT) were used as positive controls for the presence of RNA in each sample. The final volume of the reaction was 25 μl and contained SYBR Green Master Mix at 1× concentration (supplied at 2×, Power SYBR Master Mix, product 4368577; ABI), the forward and reverse primers, cDNA template, and RNase-free water. The reaction conditions were as follows: 50°C × 2 min and activation step at 95°C × 10 min, followed by 40 cycles at 92°C × 15 s with annealing at 60°C × 1 min. The output data were analyzed using proprietary ABI SDS software.
RESULTS
Sequence analysis of bone tissue and blood samples from patients with sporadic PDB
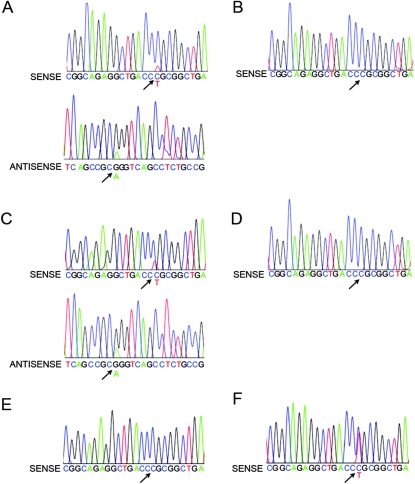
We sequenced DNA from the affected bone samples and DNA from matched blood samples and a constitutional heterozygous SQSTM1C1215T control blood sample. A C1215T mutation in exon 8 of SQSTM1 was identified in affected bone tissue of two patients (PGDZ1 and PGDZ2) with sporadic PDB. This mutation was not present in their matched blood DNA samples (Figs. 2A–2D) or in the affected bone from the other sporadic PDB patients (PGDZ3–5; PGDZ3 shown in Fig. 2E). The sequence of the control blood sample (Fig. 2F) shows the constitutional SQSTM1C1215T mutation in this patient with familial PDB. The sequence data for exon 7 and the rest of exon 8 did not show any mutations.
FIG. 2.
Sequence analysis of the C1215T mutation in exon 8 of SQSTM1 in pagetic bone and matched peripheral blood. The arrows point to the corresponding location of the C1215T mutant peak in each of the samples. (A) PGDZ1: affected bone, sense and antisense sequences. (B) PGDZ1: peripheral blood, sense sequence. (C) PGDZ2: affected bone, sense and antisense sequences. (D) PGDZ2: peripheral blood, sense sequence. (E) PGDZ3: affected bone, sense sequence. (F) MUTANT CONTROL: peripheral blood from a patient with familial PDB carrying a constitutional SQSTM1+/C1215T heterozygous mutation, sense sequence. The mutant allele is present in the affected bone of PGDZ1 and PGDZ2 but not in the matched peripheral blood samples or in the affected bone of PGDZ4. Note that the peak height of the mutant allele in DNA from the constitutional SQSTM1+/C1215T heterozygous mutation sample (F) is substantially higher than the corresponding peak height in the DNA from the affected bone from patients PGDZ1 and PGDZ2 (A and C). This suggests that the mutant allele is present in a higher percentage of cells in the germline heterozygote and that PGDZ1 and PGDZ2 are likely mosaic for the mutation in the affected bone.
Curiously, in contrast to the constitutionally heterozygous SQSTM1C1215T control sample, which showed both the wildtype and mutant alleles in roughly equal peak areas, in the pagetic bone samples from PGDZ1 and PGDZ2, the peak areas of the mutant allele were routinely significantly less than the wildtype peak areas. This was true for repeated independent samples and for sequencing in both sense and antisense directions.
Published data have shown that PCR amplification is unbiased with respect to incorporating bases at single nucleotide polymorphism (SNP) sites(36); therefore, if a polymorphism is present in all cells of a tissue, the relative frequency of the two bases at the specific SNP variable base (or at any single base substitution mutation) should be in equal representation. If one of the allele is in greater relative frequency, it is likely because the minor allele is present in only a fraction of the cells in the sample. This seemed to be the case for the two pagetic bone samples containing the SQSTM1C1215T mutant allele.
Allelic discrimination analysis of affected bone from sporadic PDB patients
We next used the allelic discrimination assay for the SQSTM1C1215T mutation to confirm whether the affected bone samples carried a somatic mutation. First, the assay was performed on the control blood sample from a familial PDB patient known to have the heterozygous C1215T mutant allele (MUTANT CONTROL) and a blood sample from a normal person with wildtype sequence (WILDTYPE CONTROL). The amplification plots and Ct versus sample graphs for both these samples are shown in Fig. 3.
FIG. 3.
Results of quality assessment of the allelic discrimination assay performed using control samples. (A) Amplification plot (Delta Rn vs. cycle number) for the heterozygous mutant sample (MUTANT CONTROL), which amplified both the mutant and the wildtype allele. (B) Amplification plot shown for the wildtype control sample (WILDTYPE CONTROL), which amplified only the wildtype allele. The dashed line indicates the cycle threshold (Ct) set for each real-time PCR experiment. (C) Ct vs. sample graph shown for triplicate amplification experiments of the control samples, along with a no template control (NTC).
Next, the two sporadic PDB samples (PGDZ1 and PGDZ2) in which the C1215T mutation was detected by sequencing and the sporadic PDB sample PGDZ3 in which only wildtype SQSTM1 was detected were tested using the AD assay (Fig. 4). DNA from the affected bone samples from PGDZ1 and PGDZ2 amplified both the mutant and the wildtype alleles (Figs. 4A and 4C) and confirmed the presence of the SQSTM1C1215T heterozygous mutant allele. In contrast, matched blood samples from each of these sporadic PDB patients amplified only the wildtype allele (Figs. 4B and 4D). In contrast, DNA samples from the affected bone and matched blood samples of PGDZ3 amplified only the wildtype allele (data not shown).
FIG. 4.
Somatic SQSTM1C1215T mutations found in affected bone of sporadic PDB patients but not in matched blood samples. (A–D) Amplification plots showing Delta Rn vs. cycle number analysis of DNA from affected bone of sporadic PDB patients and their matched blood samples. The dashed line indicates the cycle threshold (Ct) set for each real-time PCR experiment. The affected bone tissue of two PDB patients, PGDZ1 (A) and PGDZ2 (C), shows amplification of both the SQSTM1C1215T mutant and the wildtype allele. Matched peripheral blood samples for these patients, PGDZ1 (B) and PGDZ2 (D), respectively, amplified only the wildtype allele. (E and F) Ct vs. sample graphs show analysis of the pagetic bone tissues (E) and the blood samples (F). The heterozygous mutant sample (MUTANT CONTROL), a homozygous wildtype sample (WILDTYPE CONTROL), and a no template control (NTC) used as controls for the experiment are also shown.
Allelic discrimination assay analysis of DNA from pagetic osteosarcoma samples
Pagetic osteosarcoma arises from within pagetic bone presumably as cells undergo a malignant transformation. To test whether pagetic osteosarcomas from patients without a family history of PDB had somatic mutations in SQSTM1, we performed allelic discrimination analysis on DNA samples from laser-captured pagetic osteosarcoma tumors and matched normal adjacent tissue from five patients. The analysis showed that the mutant SQSTM1C1215T allele was amplified in the DNA from three of the five pagetic osteosarcomas (PGOS1 TUMOR [Fig. 5A], PGOS2 TUMOR [Fig. 5C], and PGOS3 TUMOR [Fig. 5E]), whereas DNA from two of the matched LCM-captured normal adjacent tissues (PGOS2 NAT and PGOS3 NAT) showed amplification of only the wildtype allele (Figs. 5D and 5F). The matched normal adjacent tissue from the third pagetic osteosarcoma patient (PGOS1 NAT; Fig. 5B) did show a trend in amplifying the mutant allele but did not cross threshold significance, showing that this patient was possibly an example of a low-level somatic mosaicism mutation. Alternatively, the archived normal tissue section may have included tumor cells at the time of the original biopsy.
FIG. 5.
Somatic SQSTM1C1215T mutations found in tumor tissue of patients with sporadic pagetic osteosarcoma but not in normal adjacent bone tissue. (A–F) Amplification plots showing Delta Rn vs. cycle number analysis of DNA from pagetic osteosarcoma tumors (PGOS1 TUMOR, PGOS2 TUMOR, and PGOS3 TUMOR) and normal adjacent tissue (PGOS1 NAT, PGOS2 NAT, and PGOS3 NAT) from three PDB patients with no family history. The dashed line indicates the cycle threshold (Ct) set for each real-time PCR experiment. The tumor tissue of all three samples showed amplification of both the SQSTM1C1215T mutant and the wildtype allele (A, C, and E, respectively), whereas the NAT for two samples (PGOS2 NAT and PGOS3 NAT) amplified only the wildtype allele (D and F, respectively). Interestingly, the PGOS1 NAT sample (B) showed a trend in amplifying the mutant allele but did not cross the Ct, suggesting that either the archived “normal” tissue section might have included tumor cells as well or that the patient had a mutation that resulted in a somatic mosaicism in the normal bone tissue that gave rise to the tumor.
Analysis for SQSTM1C1215T mutations in primary adolescent osteosarcoma samples
To test whether mutations in SQSTM1 were also found in adolescent osteosarcoma, we sequenced exons 7 and 8 of the SQSTM1 gene (which encompass the regions in which all known mutations of SQSTM1 in PDB have been found) in DNA from 20 samples of nonpagetic adolescent osteosarcoma tumors. None of the adolescent osteosarcoma tumors were found to have mutations in the SQSTM1 sequence (data not shown). We also used the allelic discrimination assay to test whether the C1215T mutation in SQSTM1 was present at low frequencies in DNA from tumor and NAT of nonpagetic adolescent osteosarcoma samples (ADOS1–3). Only the pagetic osteosarcoma tumor tissue showed heterozygous SQSTM1C1215T mutations (Fig. 6A), whereas none of the adolescent osteosarcoma samples (ADOS1–3 tumor and ADOS1–3 NAT) showed amplification of the SQSTM1 mutant allele (Fig. 6B). Comparing the frequency of SQSTM1 mutations detected by AD in the pagetic osteosarcoma samples with the absence of detected mutations in the 20 adolescent osteosarcoma samples, Fisher's exact test suggested that this absence of mutations was significant (p = 0.0043) and that mutations in SQSTM1 were therefore likely not a factor in adolescent osteosarcoma.
FIG. 6.
SQSTM1C1215T mutations are present only in osteosarcoma associated with PDB and not in primary adolescent osteosarcoma. (A) Cycle threshold (Ct) vs. samples analysis of DNA from pagetic osteosarcoma tumors (T) and normal adjacent tissue (N) of three patients (PGOS1, PGOS2, and PGOS3) with no family history of PDB. The tumor samples amplified both the SQSTM1C1215T mutant and the wildtype allele, whereas the corresponding normal adjacent tissue from these samples amplified only the wildtype allele. (B) Ct vs. samples analysis of DNA from nonpagetic adolescent osteosarcoma tumors (T) and matched normal adjacent tissue (N) of three patients (ADOS1, ADOS2, and ADOS3). Neither the tumor or normal adjacent tissue samples amplified the mutant allele. Results from the heterozygous mutant sample (MUTANT CONTROL), a homozygous wildtype sample (WILDTYPE CONTROL), and a no template control (NTC) used as controls for the experiment are also shown for comparison.
Correlation of SQSTM1 mutations with MVNP expression
One of the controversial etiologic elements of PDB is the role of paramyxoviruses such as measles in PDB. It has been >30 yr since the first report of nuclear inclusions in affected osteoclasts of PDB patients.(37) Since that time, there have been a number of reports that the nucleocapsid protein from measles virus (MVNP) or from closely related paramyxoviruses is expressed in osteoclasts and hematopoietic stem cells of patients with PDB,(35,38) although some laboratories have failed to detect measles virus in PDB patients.(39) Additionally, experimental evidence has shown that transfection of osteoclast precursors with the MVNP cDNA resulted in a pagetic-like osteoclast phenotype,(40) whereas mouse models expressing MVNP in their osteoclasts also show a phenotype similar in many respects to PDB.(41) Thus, the preponderance of evidence seems to support a role for MVNP in PDB.(42) To see whether MVNP was expressed in our samples and whether there was a correlation with the somatic mutations in SQSTM1, we used real-time PCR to examine RNA from three of our pagetic bone and three of our osteosarcoma samples for expression of MVNP. We used LCM to capture samples from each of our pagetic bone and tumor samples as well as normal bone and isolated RNA from the captured cells. As positive controls, we grew and isolated RNA from NIH3T3 cells that had been transfected with a plasmid containing the MVNP cDNA. We grew and isolated RNA from the NIH3T3 cells after we had isolated RNA from the human samples to ensure that no accidental contamination of the LCM-captured material would be possible during the RNA isolation. The sample and control RNA were subjected to real-time PCR for MVNP expression with either human or mouse β-actin as a control for the real-time PCR assay. As can be seen in Fig. 7, two of the three pagetic bone samples and two of three pagetic osteosarcoma samples were positive for MVNP expression. Curiously, PGDZ1, which is positive for the SQSTM1C1215T mutation, was negative for MVNP expression, whereas PGDZ3, which is wildtype for SQSTM1, was positive for MVNP.
FIG. 7.
MVNP expression found in pagetic bone and pagetic osteosarcomas but not in normal bone. Real-time PCR analysis of RNA isolated from normal bone, pagetic bone (PGDZ1–3), pagetic osteosarcoma (PGOS1–3), and NIH3T3 cells transfected with either MVNP (NIH3T3 MVNP) or empty vector (NIH3T3 EV). The figure shows the cycle threshold (Ct) vs. sample for each sample. PGDZ2 and PGDZ3 and PGOS1 and PGOS2 were positive for MVNP expression, whereas PGDZ1 and PGOS3 were negative, as were the normal bone control samples. Mouse β-actin and human β-actin were used as control for the presence of RNA in the NIH3T3 cells and human samples, respectively. NTC, no template control.
DISCUSSION
Constitutional SQSTM1 mutations causing variable penetrance and expressivity in familial PDB and some cases of de novo PDB have been found and described in the literature. This is the first paper to describe somatic SQSTM1 mutations found in the pagetic bones of patients with sporadic PDB and pagetic osteosarcoma.
Somatically acquired mutations are relatively common in cancer. In contrast, such mutations are uncommon in noncancer syndromes. In these rare noncancer syndromes, the disease is the result of somatically acquired mutations occurring in a subpopulation of hematopoietic stem cells or occurring early in embryonic development and leading to somatic mosaicism in the patient. Examples of the first type of somatically acquired noncancer syndrome are lymphoproliferative and autoimmune diseases, such as autoimmune lymphoproliferative syndrome and paroxysmal nocturnal hemoglobinuria, which result from somatic mutations in hematopoietic stem cells.(43,44) Examples of the second type are McCune-Albright syndrome(45) and atrial fibrillation,(46) which are characterized by somatically acquired mutations that occur during early development. In the first type, the somatic mutation can be detected in the circulating cells, whereas in the second type, the disease manifests at an early age.
In our analysis of the somatic mutations we identified in the SQSTM1 gene, it seems that sporadic PDB does not fit the model of somatically acquired mutations found in the other noncancer syndromes. That the SQSTM1 mutations were somatically acquired was consistent with the analysis of the relative frequency of the mutant and wildtype allele, which showed that the mutation seemed to be present in only a subset of cells in the affected tissue. One possible explanation for this would be that the mutation was present only in the pagetic osteoclasts or osteoclast precursors in the affected tissue. This model would be consistent with the lymphoproliferative and autoimmune disease model of somatic mutation occurring in the hematopoietic stem cell precursors of the pagetic osteoclasts and would account for the altered phenotype of pagetic osteoclasts and the early osteolytic lesions that are the hallmark of PDB. However, we did not detect the mutant allele in the peripheral blood of the sporadic PDB patients in whom the pagetic bone was shown to contain the C1215T mutation by either sequencing or allelic discrimination assay. Thus, it was unlikely that sporadic PDB was the result of somatic mosaicism in the hematopoietic precursors that gave rise to the pagetic osteoclasts. Nor does sporadic PDB fit the model of somatically acquired mutations occurring during early embryonic development giving rise to somatic mosaicism in the affected tissue because PDB does not manifest until much later in life.
Thus, PDB fits more closely with the cancer model of somatically acquired mutations, acquired throughout life, occurring at the site of the disease. Like the hematopoietic system, bone undergoes constant renewal throughout life from bone remodeling. Thus, the potential for somatic mutations is present. In this model, the mutation would occur initially in an adherent cell at the affected site. This cell could be either a stromal cell or an osteoblast. This mutant cell would proliferate and could stimulate increased osteoclastogenesis through intercellular signaling pathways that involved the SQSTM1 protein. This model is more consistent with the observed focal nature of the lesion, because it would not involve circulating precursor cells. This model is also consistent with what is known of the clinical progression of PDB, which is characterized by a net focal overgrowth of bone over time. This model is also consistent with the fact that the pagetic lesion remains localized to where it began, usually within subchondral bone and moves in one direction through bone but does not cross joint spaces.
It is not possible to completely explain the etiology of PDB by SQSTM1 mutations alone. If SQSTM1 mutations were sufficient to initiate PDB, all patients with the inherited form of the disease would likely develop polyostotic disease or manifest the disease at a significantly earlier age than patients with the sporadic form of the disease. However, the epidemiology of familial PDB compared with sporadic PDB shows that only some patients with familial disease have polyostotic disease and/or earlier onset than those with sporadic disease.(16) Moreover, the polyostotic phenotype and the earlier onset have been correlated to a certain extent with presence of germline nonsense mutations in p62SQSTM1 but not with the missense mutations observed most commonly in patients of familial and sporadic PDB.(30) Thus, the effect of either somatically acquired or inherited mutations might also depend on other environmental factors, such as the presence of measles virus,(42) which could affect aging bone or osteoclast precursors making them permissive to the clinical expression of PDB.
One of the sequelae of PDB is the malignant transformation to osteosarcoma. Here, we described the occurrence of heterozygous somatic SQSTM1 mutations in the pagetic tumor. This is consistent with the presence of the mutation in the precursor osteoblastic cells. It remains to be tested to see whether the presence of these mutations in the osteosarcoma tumors was simply a legacy of the origin of the pagetic bone disease or whether there was a selection process whereby a pagetic tumor can only arise from osteoblastic cells that carry an SQSTM1 mutation.
Finally, because of the possible dependence of PDB on environmental factors such as the presence of measles virus,(42) we examined our samples for the presence of MVNP expression. We found MVNP expression in two of three of our pagetic bone samples as well as in two of three of our pagetic osteosarcoma samples. Although the small number of samples precludes a statistically significant analysis, this is consistent with previous findings of MVNP expression in PDB patients.(38) However, there did not seem to be an absolute relationship between SQSTM1 mutations and MVNP expression, although again, because the sample numbers are low, statistical analysis was uninformative.
Our study does have several caveats. First, although our sequence analysis of the pagetic bone samples and adolescent osteosarcoma tumors covered the entire region in which mutations of the SQSTM1 gene involved in PDB have been reported, the technical design of the allelic discrimination assay required us to focus on one mutation at a time, and we chose to focus on the SQSTM1C1215T mutation. Because we used the allelic discrimination assay to analyze our pagetic osteosarcomas, this may have resulted in underestimating the frequency of other SQSTM1 mutations in the pagetic osteosarcoma tumors, which does not lessen the significance of our discovery of the somatically acquired nature of the mutations that we did find.
Second, we chose to examine mononuclear cells in the affected bone and tumor rather than the polynuclear osteoclasts. We did this because of our interest in the progression from pagetic bone to osteosarcoma, a tumor of osteoblastic origin; we were interested in the question of whether there was a genetic basis for predisposition to osteosarcoma in PDB patients. It is possible that the laser captured material we used included mononuclear osteoclast precursors as well as osteoblast lineage cells. Again, whereas this precludes definitive association of the mutation with the osteoblastic cells, it does not lessen the significance of the discovery that somatic mutations were present in the affected bone and not in the circulating cells.
Next, although we failed to find the mutation in the peripheral blood of PDB patients, it is possible that there are circulating cells containing the mutation; it is impossible to absolutely prove a negative. However, there did not seem to be widespread presence of cells in the peripheral blood containing the mutation, and it was certainly true that the mutations were not present as de novo constitutional mutations.
Finally, because we were unable to obtain samples of pagetic bone from our pagetic osteosarcoma patients, we examined normal adjacent tissue in these individuals to determine whether the mutation was present as a constitutional mutation. A likely scenario would be that the somatic mutation had occurred in the affected pagetic bone of these patients and that the malignant transformation took place after the somatic mutation. This would be consistent with the long latency period between initial detection of PDB and the occurrence of the pagetic osteosarcoma.(11) Again, this does not lessen the importance of the discovery of somatic mutations in sporadic Paget's disease and sporadic PDB associated osteosarcoma.
In conclusion, we set out to determine the status of SQSTM1 in the pagetic bone of patients with no family history of PDB to test whether somatically acquired mutations at the SQSTM1 locus could contribute to the etiology of PDB and PDB-associated osteosarcoma. What we found was that sporadic PDB is an example of a nonmalignant disease in which somatically acquired mutations in SQSTM1 occurring in cells at the site of the disease may play an important role in the disease etiology. Somatically acquired mutations in SQSTM1 were also found in sporadic PDB-associated osteosarcoma. Thus, PDB provides a new model by which mutations somatically acquired over the life of the individual are linked to the disease phenotype. This may shed insight into the pathogenesis of chronic diseases of aging, in which there is a slow shift in the integrity of the skeletal tissue as a consequence of somatic mutations affecting the specific environment of bone cells.
ACKNOWLEDGMENTS
We thank the New England Registry for Paget's Disease, the Paget's Foundation for Paget's Disease of Bone and Related Disorders, and the Cooperative Human Tissue Network for support. We thank Drs G David Roodman and Deborah L Galson for the NIH3T3 cells containing MVNP; Drs Joseph Lorenzo, Yu-Feng Huang, Carmen Sapienza, and Andrew Arnold for helpful discussions and insights; and Pamela Vachon, Anita Marren, Vicki DuPaul-McGloin, and Theresa George for administrative work. This work was supported by grants from the NIAMS (AR054161, AR044904, and AR047684) and support from the Murray-Heilig Endowment.
REFERENCES
- 1.Siris ES, Roodman GD. Paget's disease of bone. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th ed. Washington, DC, USA: ASBMR; 2006. pp. 320–329. [Google Scholar]
- 2.Fraser WD. Paget's disease of bone. Curr Opin Rheumatol. 1997;9:347–354. doi: 10.1097/00002281-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Siris ES. Paget's disease of bone. J Bone Miner Res. 1998;13:1061–1065. doi: 10.1359/jbmr.1998.13.7.1061. [DOI] [PubMed] [Google Scholar]
- 4.Unni KK, Dahlin DC. Premalignant tumors and conditions of bone. Am J Surg Pathol. 1979;3:47–60. doi: 10.1097/00000478-197902000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Maldague B, Malghem J. Dynamic radiologic patterns of Paget's disease of bone. Clin Orthop Relat Res. 1987:126–151. [PubMed] [Google Scholar]
- 6.Freydinger JE, Duhig JT, Mc DL. Sarcoma complicating Paget's disease of bone. A study of seven cases with report of one long survival after surgery. Arch Pathol. 1963;75:496–500. [PubMed] [Google Scholar]
- 7.Wick MR, Siegal GP, Unni KK, McLeod RA, Greditzer HG., III Sarcomas of bone complicating osteitis deformans (Paget's disease): Fifty years' experience. Am J Surg Pathol. 1981;5:47–59. doi: 10.1097/00000478-198101000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Greditzer HG, III, McLeod RA, Unni KK, Beabout JW. Bone sarcomas in Paget disease. Radiology. 1983;146:327–333. doi: 10.1148/radiology.146.2.6571760. [DOI] [PubMed] [Google Scholar]
- 9.Hadjipavlou A, Lander P, Srolovitz H, Enker IP. Malignant transformation in Paget disease of bone. Cancer. 1992;70:2802–2808. doi: 10.1002/1097-0142(19921215)70:12<2802::aid-cncr2820701213>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer. 1986;57:1442–1449. doi: 10.1002/1097-0142(19860401)57:7<1442::aid-cncr2820570734>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Moore TE, King AR, Kathol MH, el-Khoury GY, Palmer R, Downey PR. Sarcoma in Paget disease of bone: Clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199–1203. doi: 10.2214/ajr.156.6.2028867. [DOI] [PubMed] [Google Scholar]
- 12.Smith J, Botet JF, Yeh SD. Bone sarcomas in Paget disease: A study of 85 patients. Radiology. 1984;152:583–590. doi: 10.1148/radiology.152.3.6235535. [DOI] [PubMed] [Google Scholar]
- 13.Klein RM, Norman A. Diagnostic procedures for Paget's disease. Radiologic, pathologic, and laboratory testing. Endocrinol Metab Clin North Am. 1995;24:437–450. [PubMed] [Google Scholar]
- 14.Mankin HJ, Hornicek FJ. Paget's sarcoma: A historical and outcome review. Clin Orthop Relat Res. 2005:97–102. doi: 10.1097/01.blo.0000180053.99840.27. [DOI] [PubMed] [Google Scholar]
- 15.Siris ES, Ottman R, Flaster E, Kelsey JL. Familial aggregation of Paget's disease of bone. J Bone Miner Res. 1991;6:495–500. doi: 10.1002/jbmr.5650060511. [DOI] [PubMed] [Google Scholar]
- 16.Seton M, Choi HK, Hansen MF, Sebaldt RJ, Cooper C. Analysis of environmental factors in familial versus sporadic Paget's disease of bone: The New England Registry for Paget's Disease of Bone. J Bone Miner Res. 2003;18:1519–1524. doi: 10.1359/jbmr.2003.18.8.1519. [DOI] [PubMed] [Google Scholar]
- 17.Daroszewska A, Ralston SH. Genetics of Paget's disease of bone. Clin Sci (Lond) 2005;109:257–263. doi: 10.1042/CS20050053. [DOI] [PubMed] [Google Scholar]
- 18.Cody JD, Singer FR, Roodman GD, Otterund B, Lewis TB, Leppert M, Leach RJ. Genetic linkage of Paget disease of the bone to chromosome 18q. Am J Hum Genet. 1997;61:1117–1122. doi: 10.1086/301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurin N, Brown JP, Lemainque A, Duchesne A, Huot D, Lacourciere Y, Drapeau G, Verreault J, Raymond V, Morissette J. Paget disease of bone: Mapping of two loci at 5q35-qter and 5q31. Am J Hum Genet. 2001;69:528–543. doi: 10.1086/322975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fotino M, Haymovits A, Falk CT. Evidence for linkage between HLA and Paget's disease. Transplant Proc. 1977;9:1867–1868. [PubMed] [Google Scholar]
- 21.Good DA, Busfield F, Fletcher BH, Duffy DL, Kesting JB, Andersen J, Shaw JT. Linkage of Paget disease of bone to a novel region on human chromosome 18q23. Am J Hum Genet. 2002;70:517–525. doi: 10.1086/338658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 23.Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S. Osteoprotegerin deficiency and juvenile Paget's disease. N Engl J Med. 2002;347:175–184. doi: 10.1056/NEJMoa013096. [DOI] [PubMed] [Google Scholar]
- 24.Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002;70:1582–1588. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hocking LJ, Lucas GJ, Daroszewska A, Mangion J, Olavesen M, Cundy T, Nicholson GC, Ward L, Bennett ST, Wuyts W, Van Hul W, Ralston SH. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget's disease. Hum Mol Genet. 2002;11:2735–2739. doi: 10.1093/hmg/11.22.2735. [DOI] [PubMed] [Google Scholar]
- 26.Johnson-Pais TL, Wisdom JH, Weldon KS, Cody JD, Hansen MF, Singer FR, Leach RJ. Three novel mutations in SQSTM1 identified in familial Paget's disease of bone. J Bone Miner Res. 2003;18:1748–1753. doi: 10.1359/jbmr.2003.18.10.1748. [DOI] [PubMed] [Google Scholar]
- 27.Eekhoff EW, Karperien M, Houtsma D, Zwinderman AH, Dragoiescu C, Kneppers AL, Papapoulos SE. Familial Paget's disease in The Netherlands: Occurrence, identification of new mutations in the sequestosome 1 gene, and their clinical associations. Arthritis Rheum. 2004;50:1650–1654. doi: 10.1002/art.20224. [DOI] [PubMed] [Google Scholar]
- 28.Falchetti A, Di Stefano M, Marini F, Del Monte F, Mavilia C, Strigoli D, De Feo ML, Isaia G, Masi L, Amedei A, Cioppi F, Ghinoi V, Bongi SM, Di Fede G, Sferrazza C, Rini GB, Melchiorre D, Matucci-Cerinic M, Brandi ML. Two novel mutations at exon 8 of the Sequestosome 1 (SQSTM1) gene in an Italian series of patients affected by Paget's disease of bone (PDB) J Bone Miner Res. 2004;19:1013–1017. doi: 10.1359/JBMR.040203. [DOI] [PubMed] [Google Scholar]
- 29.Good DA, Busfield F, Fletcher BH, Lovelock PK, Duffy DL, Kesting JB, Andersen J, Shaw JT. Identification of SQSTM1 mutations in familial Paget's disease in Australian pedigrees. Bone. 2004;35:277–282. doi: 10.1016/j.bone.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Hocking LJ, Lucas GJ, Daroszewska A, Cundy T, Nicholson GC, Donath J, Walsh JP, Finlayson C, Cavey JR, Ciani B, Sheppard PW, Searle MS, Layfield R, Ralston SH. Novel UBA domain mutations of SQSTM1 in Paget's disease of bone: Genotype phenotype correlation, functional analysis, and structural consequences. J Bone Miner Res. 2004;19:1122–1127. doi: 10.1359/JBMR.0403015. [DOI] [PubMed] [Google Scholar]
- 31.Layfield R, Ciani B, Ralston SH, Hocking LJ, Sheppard PW, Searle MS, Cavey JR. Structural and functional studies of mutations affecting the UBA domain of SQSTM1 (p62) which cause Paget's disease of bone. Biochem Soc Trans. 2004;32:728–730. doi: 10.1042/BST0320728. [DOI] [PubMed] [Google Scholar]
- 32.Beyens G, Van Hul E, Van Driessche K, Fransen E, Devogelaer JP, Vanhoenacker F, Van Offel J, Verbruggen L, De Clerck L, Westhovens R, Van Hul W. Evaluation of the role of the SQSTM1 gene in sporadic Belgian patients with Paget's disease. Calcif Tissue Int. 2004;75:144–152. doi: 10.1007/s00223-004-0244-4. [DOI] [PubMed] [Google Scholar]
- 33.Lucas GJ, Hocking LJ, Daroszewska A, Cundy T, Nicholson GC, Walsh JP, Fraser WD, Meier C, Hooper MJ, Ralston SH. Ubiquitin-associated domain mutations of SQSTM1 in Paget's disease of bone: Evidence for a founder effect in patients of British descent. J Bone Miner Res. 2005;20:227–231. doi: 10.1359/JBMR.041106. [DOI] [PubMed] [Google Scholar]
- 34.Jacquet R, Hillyer J, Landis WJ. Analysis of connective tissues by laser capture microdissection and reverse transcriptase-polymerase chain reaction. Anal Biochem. 2005;337:22–34. doi: 10.1016/j.ab.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Reddy SV, Menaa C, Singer FR, Cundy T, Cornish J, Whyte MP, Roodman GD. Measles virus nucleocapsid transcript expression is not restricted to the osteoclast lineage in patients with Paget's disease of bone. Exp Hematol. 1999;27:1528–1532. doi: 10.1016/s0301-472x(99)00097-1. [DOI] [PubMed] [Google Scholar]
- 36.Kuppuswamy MN, Hoffmann JW, Kasper CK, Spitzer SG, Groce SL, Bajaj SP. Single nucleotide primer extension to detect genetic diseases: Experimental application to hemophilia B (factor IX) and cystic fibrosis genes. Proc Natl Acad Sci USA. 1991;88:1143–1147. doi: 10.1073/pnas.88.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills BG, Singer FR. Nuclear inclusions in Paget's disease of bone. Science. 1976;194:201–202. doi: 10.1126/science.959849. [DOI] [PubMed] [Google Scholar]
- 38.Reddy SV, Singer FR, Roodman GD. Bone marrow mononuclear cells from patients with Paget's disease contain measles virus nucleocapsid messenger ribonucleic acid that has mutations in a specific region of the sequence. J Clin Endocrinol Metab. 1995;80:2108–2111. doi: 10.1210/jcem.80.7.7608263. [DOI] [PubMed] [Google Scholar]
- 39.Matthews BG, Afzal MA, Minor PD, Bava U, Callon KE, Pitto RP, Cundy T, Cornish J, Reid IR, Naot D. Failure to detect measles virus ribonucleic acid in bone cells from patients with Paget's disease. J Clin Endocrinol Metab. 2008;93:1398–1401. doi: 10.1210/jc.2007-1978. [DOI] [PubMed] [Google Scholar]
- 40.Kurihara N, Reddy SV, Menaa C, Anderson D, Roodman GD. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest. 2000;105:607–614. doi: 10.1172/JCI8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurihara N, Zhou H, Reddy SV, Garcia Palacios V, Subler MA, Dempster DW, Windle JJ, Roodman GD. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget's disease-like bone lesions in mice. J Bone Miner Res. 2006;21:446–455. doi: 10.1359/JBMR.051108. [DOI] [PubMed] [Google Scholar]
- 42.Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005;115:200–208. doi: 10.1172/JCI24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puck JM, Straus SE. Somatic mutations–not just for cancer anymore. N Engl J Med. 2004;351:1388–1390. doi: 10.1056/NEJMp048116. [DOI] [PubMed] [Google Scholar]
- 44.Luzzato L. Paroxysmal nocturnal hemoglobinuria: An acquired X-linked genetic disease with somatic-cell mosaicism. Curr Opin Genet Dev. 2006;5:242–251. doi: 10.1016/j.gde.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein LS. G(s)alpha mutations in fibrous dysplasia and McCune-Albright syndrome. J Bone Miner Res. 2006;21(Suppl 2):120–124. doi: 10.1359/jbmr.06s223. [DOI] [PubMed] [Google Scholar]
- 46.Gollob MH, Jones DL, Krahn AD, Danis L, Gong XQ, Shao Q, Liu X, Veinot JP, Tang AS, Stewart AF, Tesson F, Klein GJ, Yee R, Skanes AC, Guiraudon GM, Ebihara L, Bai D. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]