To the Editor:
Atopic dermatitis (AD) is a chronic skin condition that affects 10% to 20% of children and 1% to 3% of adults.1 Individuals with atopic dermatitis are at an increased risk for cutaneous infections with Staphylococcus aureus, herpes simplex, and the small pox or vaccinia virus.2 Recently, it has been shown that defects in the innate immune system, such as the capacity to increase the production of broad spectrum antimicrobial peptides like cathelicidin, may account for this increase in infections.3,4
Important insight into the mechanism responsible for the production of antimicrobial peptides came with the discovery of the vitamin D response element in the cathelicidin promoter and the finding that the conversion of 25-hydroxyvitamin D to the active 1,25 dihydroxyvitamin D by CYP27B1 can occur in keratinocytes and monocytes and is under the control of Toll-like receptor 2.4–6 Thus, with infection or wounding, activation of Toll-like receptor 2 results in expression of CYP27B1, causing conversion of 25-hydroxyvitamin D to the active 1,25 dihydroxy-vitamin D, and subsequent induction of cathelicidin.5,6 Our study sought to examine whether supplementation with oral cholecalciferol could provide the CYP27B1 enzyme enough substrate to overcome this relative deficiency in induction of cathelicidin in atopic patients.
This was a single-center, controlled study of 14 normal controls and 14 atopic subjects with moderate to severe atopic dermatitis with an average Rajka-Langeland score of 6 (range, 4–9). Two-millimeter punch biopsies of uninvolved skin were collected from all subjects, and subjects with AD also received 2-mm punch biopsies of lesional skin. Baseline calcium and 25-hydroxyvitamin D levels were obtained. Supplementation with oral vitamin D3 (cholecalciferol) at 4000 IU per day was given for 21 days. At 21 days, subjects with AD received a 2-mm punch of involved skin, and all subjects had repeat biopsies of uninvolved skin, repeat serum calcium, and vitamin D levels. The study was approved by the Human Research Protection Program at the University of California, San Diego. All subjects gave written informed consent.
Analysis of cathelicidin expression in skin was performed by qRT-PCR and normalized to GAPDH. Total RNA was isolated from the 2-mm skin biopsy samples from the subjects by homogenization. Briefly, the biopsy samples were placed into 2.0-mL polypropylene tubes (Biospec Products, Bartlesville, Okla) with 1.0 mL Trizol (Invitrogen Corp, Carlsbad, Calif). Zirconia/Silica beads (Biospec Products) were added to the tubes, and the skin samples were homogenized by using a Mini-beadbeater-8 (Biospec Products). Homogenized Trizol solution was transferred to a ribonuclease-free tube for RNA extraction according to the manufacturer’s instructions, and RNA was reversed-transcribed by using iScript (Bio-Rad, Munchen, Germany). Real-time, quantitative PCR assay for cathelicidin was performed with the ABI 7000 Sequence Detection system (PE Applied Biosystems, Foster City, Calif). Expression of cathelicidin was evaluated by using a FAM-CAGAGGATTGTGACTTCA-MGB probe with primers 5′-CTTCACCAGCCCGTCCTTC-3′ and 5′-CCAGGACGACACAGCAGTCA-3′. For GAPDH expression, a VIC-CATCCATGACAACTTTGGTA-MGB probe with primers 5′-CTTAGCACCCCTGGCCAAG-3′ and 5′-TGGTCATGAGTCCTTCCACG-3′ was used. Fold induction was calculated by using the 2-delta CT method, where delta CT is CT cathelicidin – CT GAPDH and CT is the cycle at which arbitrary detection threshold is crossed. The 1-way ANOVA test from the GraphPad Prism statistical package (La Jolla, Calif) was used to determine significant differences. We constrained error rates to the standard α level of 0.05 by the Newman-Keuls method.
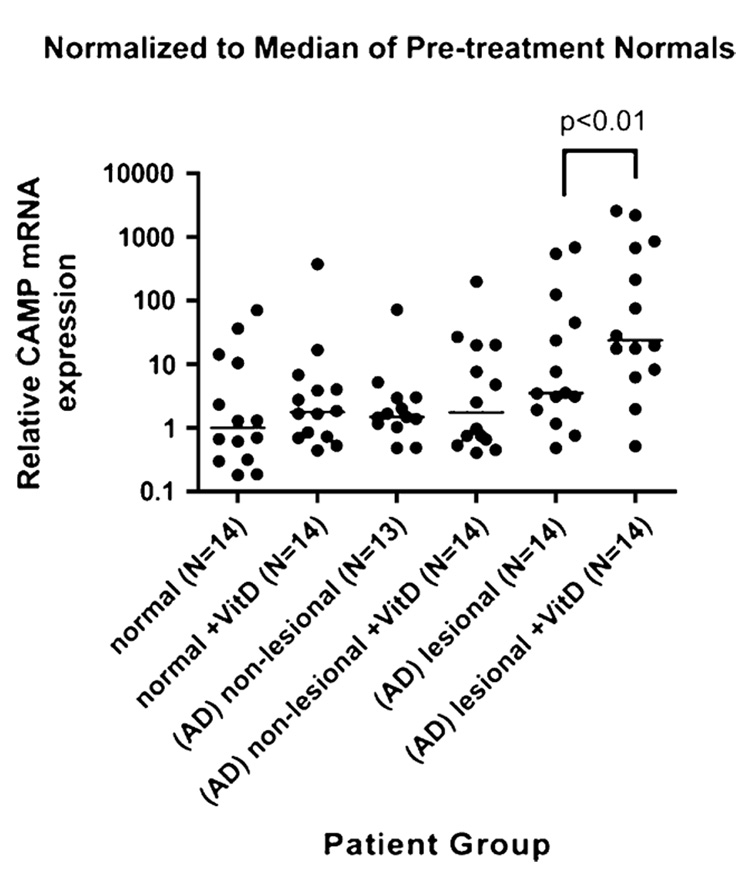
After supplementation with 4000 IU/d oral vitamin D for 21 days, AD lesional skin showed a statistically significant increase in cathelicidin expression from a median of 3.53 relative copy units (RCU) before supplementation to a median of 23.91 RCU postsupplementation (P <.01; Fig 1). Normal skin showed a more modest increase from a median of 1.0 RCU to 1.78 RCU (P >.05), and AD nonlesional skin, mildly increased from a median value of 1.50 RCU to 1.75 RCU postsupplementation (P >.05). These results suggest that supplementation with oral vitamin D dramatically induces cathelicidin production in AD lesional skin and may also induce production in normal skin. Immunofluorescence staining of duplicate skin biopsies from lesional skin confirmed results from RT-PCR and demonstrated an increase in cathelicidin protein after oral vitamin D supplementation.
FIG 1.
Increase in cathelicidin expression in lesional atopic skin. Cathelicidin mRNA measured by qRT-PCR in lesional and nonlesional skin of atopic subjects and nonlesional skin of normal subjects. Atopic involved skin showed significantly higher hCAP18 mRNA expression with vitamin D supplementation compared with normal skin and AD nonlesional skin (P < .01 by 1-way ANOVA). Data are shown as relative expression as normalized by the median of pretreatment controls (bars, median of each group).
Serum calcium and vitamin D levels were measured in atopic and normal subjects at baseline and after 3 weeks of supplementation with 4000 IU/d of oral vitamin D3. 4000 IU of vitamin D3 has been well documented to effectively increase 25-hydroxyvitamin D levels7 while maintaining normal serum calcium levels, suggesting that the current recommendation for vitamin D supplementation may be too conservative. Indeed, serum calcium in our normal control subjects did not rise but actually decreased from a median of 9.8 mg/dL to a median of 9.4 mg/dL postsupplementation (P > .05). During this time, their serum 25-hydroxyvitamin D levels rose from a median of 24.5 mg/mL to 37 mg/mL (P > .05). Similarly, atopic subjects’ serum calcium levels also decreased from a median of 9.6 mg/dL to a median of 9.4 mg/dL postsupplementation (P > .05), and their serum 25-hydroxyvitamin D levels rose from a median of 22.5 mg/mL to 35.5 mg/mL (P > .05). No subject complained of any adverse event during supplementation.
Analysis of previous data has shown that with wounding or disruption of the epidermal barrier, cathelicidin is induced.6 Our data show that there is a small (but not statistically significant) increase in cathelicidin in AD lesional skin before treatment with vitamin D (Fig 1) indicative of some ability of the atopic subject to induce cathelicidin with disruption of the epidermal barrier. This small increase is aided immensely with oral vitamin D3, allowing the AD lesional skin value to rise significantly. Because this increase is primarily seen in lesional skin, it is hypothesized that only with the supplementation with oral vitamin D can AD lesional skin be allowed to increase cathelicidin to its normal level seen postinjury. The smaller induction of normal skin and nonlesional skin suggests that these are already in their normal uninduced state (nonwounded and noninfected).
Vitamin D deficiency has been linked to increased rates of multiple cancers, autoimmune diseases, infectious diseases, cardiovascular diseases, and hypertension.8 We believe our study has shown that supplementation of oral vitamin D can result in correction of defects in cathelicidins in the innate immune system of atopic subjects. Larger studies examining the incidence and risks of infections in atopic subjects while on vitamin D supplementation, and supplementation for a longer duration, will be necessary in the future to see if this increase in cathelicidin is adequate in the prevention of infections in these patients.
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
REFERENCES
- 1.Schultz-Larsen FV, Hanifin JM. Epidemiology of atopic dermatitis. Immunol Allergy Clin North Am. 2002;22:1–24. [Google Scholar]
- 2.Boguniewicz M, Leung DY. Atopic dermatitis. J Allergy Clin Immunol. 2006;117:S475–S480. doi: 10.1016/j.jaci.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 4.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P, Stenger S, Li H, Wenzel L, Tan B, Krutzik S, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Schauber J, Dorschner R, Coda A, Büchau A, Liu P, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vieth R, Chan P, MacFarlane G. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73:288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 8.Holick M. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]