Abstract
We have shown that dietary fish oil and pectin (FP) protects against radiation-enhanced colon cancer by upregulating apoptosis in colonic mucosa. To investigate the mechanism of action, we provided rats (n = 40) with diets containing the combination of FP or corn oil and cellulose (CC) prior to exposure to 1 Gy, 1 GeV/ nucleon Fe-ion. All rats were injected with a colon-specific carcinogen, azoxymethane (AOM; 15 mg/kg), 10 and 17 days after irradiation. Levels of colonocyte apoptosis, prostaglandin E2 (PGE2), PGE3, microsomal prostaglandin E synthase-2 (mPGES-2), total β-catenin, nuclear β-catenin staining (%) and peroxisome proliferator-activated receptor δ(PPAR δ) expression were quantified 31 weeks after the last AOM injection. FP induced a higher (P < 0.01) apoptotic index in both treatment groups, which was associated with suppression (P < 0.05) of antiapoptotic mediators in the cyclooxygenase (COX) pathway (mPGES-2 and PGE2) and the Wnt/β-catenin pathway [total β-catenin and nuclear β-catenin staining (%); P < 0.01] compared with the CC diet. Downregulation of COX and Wnt/β-catenin pathways was associated with a concurrent suppression (P < 0.05) of PPARδ levels in FP-fed rats. In addition, colonic mucosa from FP animals contained (P < 0.05) a proapoptotic, eicosapentaenoic acid-derived COX metabolite, PGE3. These results indicate that FP enhances colonocyte apoptosis in AOM-alone and irradiated AOM rats, in part through the suppression of PPARδ and PGE2 and elevation of PGE3. These data suggest that the dietary FP combination may be used as a possible countermeasure to colon carcinogenesis, as apoptosis is enhanced even when colonocytes are exposed to radiation and/or an alkylating agent.
Introduction
Colon cancer is the second leading cause of cancer mortality in the USA for men and women combined (1). The Harvard Report on Cancer Prevention (1999) stated that colon cancer may be ameliorated when risk factors such as diet, lifestyle and environment are modified. Prior exposure to environmental factors such as radiation may make colon cells more susceptible to chemical carcinogen-induced mutations (2). Studies have shown that airline pilots (3) and radiologists (4) have an elevated occurrence of cancers, including colon cancer. High colon cancer incidence rates also have been detected in Japanese atomic bomb survivors (5) and patients receiving radiotherapy directed to the pelvic region (6). Furthermore, there is evidence to suggest that radiation and chemical carcinogens synergistically promote colon carcinogenesis (7).
In addition, dietary factors have been shown to either protect against or promote colon carcinogenesis (8–13). Several epidemiological and animal tumorigenic studies have shown that diets rich in n-3 polyunsaturated fatty acids (PUFAs), such as those derived from fish oil [e.g. eicosapentaenoic acid (EPA), 20:5n-3 and docosahexanoic acid (DHA), 22:6n-3], protect against colon cancer [(8), reviewed in ref. 10], whereas diets containing high levels of n-6 PUFA, such as those derived from corn oil [e.g. arachidonic acid (AA), 20:4n-6], appear to promote cancer development in the colon (reviewed in refs 10,12,13). Furthermore, there appears to be an interactive effect of fat and fiber on colon tumorigenesis (9). Specifically, we have shown that a highly fermentable fiber such as pectin, which generates butyrate in the colon, exerts a chemoprotective effect only when fish oil is provided as the lipid source in rats injected with azoxymethane (AOM) by elevating apoptosis (11). However, it is unknown whether the combination of fish oil and pectin (FP) protects against both radiation and carcinogen-induced colon carcinogenesis.
Dietary fat and/or fiber influence the cyclooxygenase (COX) and Wnt/β-catenin pathways (8,14,15), which coordinate to suppress apoptosis during colon carcinogenesis in humans and rodent models of this disease (16,17). Prostaglandin E2 (PGE2), a product of the COX pathway, transactivates peroxisome proliferator-activated receptor δ (PPARδ), a transcription factor that belongs to the nuclear receptor superfamily (18). PPARδ has been shown to protect colonocytes from apoptosis, thus promoting colon carcinogenesis (18). In addition, β-catenin, a downstream effector of the Wnt/β-catenin pathway, transcriptionally upregulates PPARδ upon nuclear translocation (19). Interestingly, PGE2 can also upregulate β-catenin levels and its translocation into the nucleus (17).
Accumulating evidence suggests that 12-hydroxy-eicosatetraenoic acid (12-HETE), 15-HETE and 13-hydroxy-octadecadienoic acid (13-HODE), products of the lipoxygenase (LOX) pathway, may regulate apoptosis (20–22). LOX enzymes first convert AA, a product of linoleic acid (LA), to the intermediate product hydroperoxy-eicosatetraenoic acid. The hydroperoxy group is subsequently reduced to form metabolites such as 12- and 15-HETE. 13-HODE is the primary product of 15-LOX-1 acting on LA, a major fatty acid in corn oil. Whereas 12-HETE appears to be antiapoptotic (21), both 15-HETE and 13-HODE induce apoptosis in colon cancer cells (20,22).
In this study, we investigated whether dietary FP work together to enhance apoptosis through differential effects on the COX, LOX and Wnt/β-catenin pathways in colon carcinogenesis induced by both radiation (high-energy Fe-ions, one of the components of galactic cosmic radiation) and AOM.We also examined whether inhibition of the above pathways results in suppressed antiapoptotic PPARδ levels. We found that compared with a corn oil and cellulose (CC) diet, a FP diet suppressed molecular targets in the COX [microsomal prostaglandin E synthase-2 (mPGES-2) and PGE2] and Wnt/β -catenin (total β-catenin and percent nuclear β-catenin staining) pathways as well as PPARδ expression in both AOM-alone and irradiated AOM rats. Furthermore, the FP diet enhanced apoptosis irrespective of radiation treatment. We also provide evidence that the FP diet upregulates the biosynthesis of PGE3, a type III prostaglandin (PG) that can inhibit tumor cell growth (23,24), particularly in irradiated AOM rats. Moreover, in the case of AOM-alone treatment, n-6 metabolite (12-HETE, 15-HETE and 13-HODE) levels in FP-consuming rats were lower than that in CC-consuming rats, whereas irradiated AOM rats exhibited no differences in the above n-6 metabolites between the diet groups.
Materials and methods
Study design
The animal use protocol was approved by the University Laboratory Animal Care Committee of Texas A&M University (College Station, TX) and conformed to National Institutes of Health guidelines. This experiment utilized a 2 × 2 factorial design with two diet treatments (FP versus CC) and two radiation exposure levels (0 Gy or 1.0 Gy, 1 GeV/nucleon Fe-ion). All rats were injected with a colon-specific carcinogen, AOM.
Animals
Forty male Sprague–Dawley rats were obtained from Harlan Teklad (Houston, TX). Three-week-old rats (n = 20) belonging to the AOM-alone (0 Gy) treatment group were housed in the Laboratory Animal Research Resource facility at Texas A&M University, whereas an equal number of rats intended for irradiation treatment were transported to Brookhaven National Laboratory (Upton, NY). All rats were individually housed and maintained in a temperature- and humidity-controlled animal facility with a 12-h light cycle. Rats were provided with water and pellet diet ad libitum during a 5-day acclimatization period. The animals were then assigned to one of the two experimental diets. Rats were stratified by body weight so that the mean initial body weight was similar among the experimental groups (n = 10 rats per group).
Diets
Diet composition was as previously reported (13). All diets were composed of 6% fiber and 15% fat by weight, which equates to 30 g fiber and 30% of energy per day for humans, respectively. The major differences between the fatty acid compositions of the two lipid sources were the concentrations of EPA (18.2%) and DHA (11.3%) in fish oil and the concentration of LA (18:2n-6) in corn oil (55.4%). The FP diets contained 3.5 g of corn oil per 100 g of diet to deliver the level of LA necessary to protect against essential fatty acid deficiency. Additionally, 0.015 g of α-tocopherol (MT-70, Archer Daniels Midland, Decatur, IL) and 0.005 g of tertiary butylhydroquinone (20%, Gillco Ingredients, Vista, CA) were added per 100 g of FP diet as antioxidants as these are similar levels as those naturally found in corn oil. CC diets were supplemented with tertiary butylhydroquinone (0.019 g/100 g of diet) to obtain antioxidant levels equivalent to that in the FP diets. Citrus pectin, polygalacturonic acid methyl ester was obtained from Danisco Cultor (New Century, KS). Corn oil and vacuum-deodorized menhaden fish oil were procured from Degussa lipids (Degussa, Waukesha, WI).
Fresh diets were prepared as necessary and kept at −20°C for long-term storage (months) or 4°C for short-term storage (weeks) to prevent the formation of oxidized lipids.
Iron irradiation
Three weeks after starting the experimental diets (i.e. 7 weeks of age), the rats belonging to the irradiated groups were exposed to a single dose of ~1.0 Gy, 1 GeV/nucleon Fe-ion. This dose is not considered toxic to the rats, but enhances the tumorigenic effects of the colon carcinogen (2). The exposures were performed at the Alternating Gradient Synchrotron/Relativistic Heavy Ion Collider Facility, Brookhaven National Laboratory.
Animals were given uniform whole-body irradiation, two at a time, in animal holders that produced a minimum of radiation attenuation and were mounted perpendicular to the direction of the Fe-ion beam. Following a 1-day recovery period, rats were implanted with transponder chips containing information about ID number, diet and day of carcinogen treatment. Irradiated rats were shipped from Brookhaven National Laboratory to Texas A&M University immediately after the recovery period.
Carcinogen treatment
AOM (Sigma–Aldrich Corporation, St Louis, MO) was used as a targeted colon carcinogen and was administered by subcutaneous injection. The first dose of AOM was injected 32 days after the rats started receiving the experimental diets (10 days after the radiation exposure for the irradiated groups). The second dose of AOM was injected 1 week following the initial injection. In both cases, the injection volumes were adjusted to deliver 15 mg/kg body wt.
Tissue sample collection
Rats were terminated 31 weeks after the second AOM injection. Euthanasia was accomplished by CO2 asphyxiation followed by cervical dislocation and the colons were removed and cleaned with RNase-free phosphate-buffered saline (PBS). One centimeter sections of the most proximal and distal portions of the colon were fixed in 4% paraformaldehyde or 70% ethanol. The remaining midsection of the colon was cut vertically and the areas devoid of tumors were used for protein and PG analyses.
In vivo apoptosis measurement
The terminal uridine deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) procedure was performed to determine the effect of diet on apoptosis (25). Positive control sections were prepared by nicking DNA with DNase I (Ambion, Austin, TX) for 5 min. Rat colon sections obtained 9 h after injecting AOM served as a biological positive control. PBS was substituted for the terminal deoxynucleotidyl transferase enzyme in the working solution for developing negative control tissue sections. The total number of apoptotic cells and total number of cells per crypt column were determined in 50 crypt columns per rat. Apoptotic cells were identified by TUNEL staining in conjunction with characteristic morphological changes (cell shrinkage, membrane blebbing and chromatin condensation) to distinguish apoptotic cells and bodies from necrotic cells (26). An apoptotic index (apoptotic cells per crypt column cell numbers × 100) was subsequently calculated.
Nuclear β-catenin staining (%)
Nuclear β-catenin staining was measured using the methods described by Chang et al. (27) with slight modifications. Sections were deparaffinized in xylene and rehydrated through graded ethanol solutions to distilled water. For antigen detection, sections were microwaved in citrate buffer (pH 6) for 15 min at the medium setting. Sections were cooled for 30 min at room temperature and washed with PBS. To block endogenous peroxidase activity, sections were then incubated with 3% hydrogen peroxide in distilled water for 20 min. Primary antibody, mouse monoclonal anti- β-catenin (1:100 dilution; BD Transduction Laboratories, San Diego, CA), was incubated at room temperature for 1.5 h, and biotinylated rabbit anti-mouse IgG (1:200 dilution, 1 h incubation) served as a secondary antibody. An immunoenzymatic reaction was carried out using an avidin-biotinylated horseradish peroxidase complex (Vector Laboratories, Burlingame, CA). Brown staining was developed with 3,3′-diaminobenzidine as the chromogen substrate (Sigma Chemical, St Louis, MO). Twenty-five crypt columns were scored per rat for nuclear β-catenin, and the staining index was calculated as the number of punctated, darkly stained nuclei divided by the number of cells per crypt column × 100.
Quantification of mPGES-2, PPARδ and β-catenin by immunoblot
Protein was extracted from rat colon mucosa as described previously (25). Protein concentrations were determined by a BCA Protein Assay kit (Pierce, Rockford, IL). Colon mucosal lysates (30 µg) were incubated at 95°C for 5 min and separated by Novex® 4–12% Tris–glycine gels (Invitrogen, Carlsbad, CA) at 100 V for 3 h in 1× running buffer [25 mmol/l Tris, 192 mmol/l glycine and 0.1% sodium dodecyl sulfate (pH 8.3)] and electrophoretically transferred to Invitrolon polyvinylidene difluoride membranes (Invitrogen) at 95 V for 45 min in 1 × Tris–glycine transfer buffer (Novex, LC 3675, Invitrogen) with 0.025% sodium dodecyl sulfate. Polyvinylidene difluoride membranes were blocked with 2% bovine serum albumin (Fisher, Pittsburgh, PA) for 1 h at room temperature. The membranes were incubated with either rabbit polyclonal anti-mPGES-2 antibody (1:1000; Cayman Chemicals, Ann Arbor, MI), rabbit polyclonal anti-PPARδ antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), goat polyclonal anti- β-catenin antibody (1:1500; Santa Cruz Biotechnology) or goat polyclonal anti- β-actin antibody (1:10 000; Santa Cruz Biotechnology) overnight at 4°C. Membranes were subsequently probed with bovine anti-goat (β-catenin 1:100 000 and β-actin 1:100 000; Santa Cruz Biotechnology) or goat anti-rabbit IgG–horseradish peroxidase conjugate (mPGES-2 1:100 000, Cayman Chemicals; PPARδ 1:100 000, Santa Cruz Biotechnology). Target proteins were detected with SuperSignal West Dura Extended Duration Substrate (Pierce). Membranes were scanned and quantified with a Bio-Rad Fluor-Imager (Bio-Rad Laboratories, Hercules, CA) using Quantity One software (Bio-Rad Laboratories). β-Actin served as a loading control. Positive controls were as follows: mPGES-2 and β-catenin, MCF7 whole-cell lysate; PPARδ, Jurkat nuclear extracts (Santa Cruz Biotechnology).
Liquid chromatography/tandem mass spectrometry
For measurement of eicosanoids, an aliquot (10 µl) of enriched protein isolates from scraped colonic mucosa was mixed with 90 µl of homogenization buffer (500 mM Tris–HCl, pH 7.2, 0.5 M sucrose, 200 M ethylenediaminetetraacetic acid (EDTA), 100 mM ethylene glycol tetraacetic acid, 0.4 M sodium fluoride, 10% Triton X-100 and 10 mM sodium orthovanadate) and ice-cold PBS (150 µl) containing 1 mM EDTA and 0.1% butylated hydroxytoluene. Samples were extracted using the method of Yang et al. (28). Briefly, aliquots (20 µl) of 1 N citric acid and 2.5 µl of 10% butylated hydroxytoluene were added to samples to prevent free radical peroxidation. Prior to extraction, an aliquot (10 µl) of deuterated eicosanoids (PGE2-d4, 15-HETE-d8, 12-HETE-d8 and 13-HODE-d4) (100 ng/ml) were added to each sample as internal standards. Eicosanoids were subsequently extracted with 2 ml of hexane:ethyl acetate (1:1, vol/vol) and vortexed for 2 min. Samples were then centrifuged at 1800g for 10 min at 4°C. The upper organic layer was collected and the organic phases from three extractions were pooled, and then evaporated to dryness under a stream of nitrogen at room temperature. All extraction procedures were performed under low-light and low-temperature conditions to minimize potential photooxidation or thermal degradation of eicosanoid metabolites. Samples were then reconstituted in 100 µl methanol:10 mM ammonium acetate buffer, pH 8.5 (70:30, vol:vol) prior to liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis. The extracted PGs were quantitated by the LC/MS/MS method described by Yang et al. (28). Briefly, LC/MS/MS was performed using a Quattro Ultima tandemmass spectrometer (Waters Corporation, Milford, MA) equipped with an Agilent HP 1100 binary pump HPLC inlet (Agilent Technologies, Palo Alto, CA). The PGs were separated using a 2 × 150 mm Luna 3 µ phenyl–hexyl analytical column (Phenomenex, Torrance, CA). The mobile phase consisted of 10 mM ammonium acetate, pH 8.5, and methanol. The column temperature was maintained at 50°C, and samples were kept at 4°C during the analysis. Individual analytes were detected using electrospray negative ionization and multiple reaction monitoring of the transitions m/z 351 → 271 for PGE2, m/z 349 → 269 for PGE3 and m/z 355 → 275 for PGE2-d4. Fragmentation of all compounds was performed using argon as the collision gas at a collision cell pressure of 2.10 × 10−3 Torr. The identification of each PG was confirmed by comparison with reference standards obtained from Cayman Chemicals.
Statistics
The two diets (FP or CC) and two irradiation treatments (0 Gy, AOM alone; 1 Gy, 1 GeV/nucleon Fe-ion, irradiated AOM) represent a 2 × 2 factorial design. Apoptosis data were analyzed using the GLM and MIXED procedures in SAS (SAS Institute, Cary, NC). Data on n-3 PUFA-derived (PGE3) and n-6 PUFA-derived metabolites (PGE2, 12-HETE, 15-HETE and 13-HODE) and mPGES-2, β-catenin and PPARδ were analyzed using the same statistical procedures.
Results
FP diet enhances apoptotic index irrespective of radiation treatment
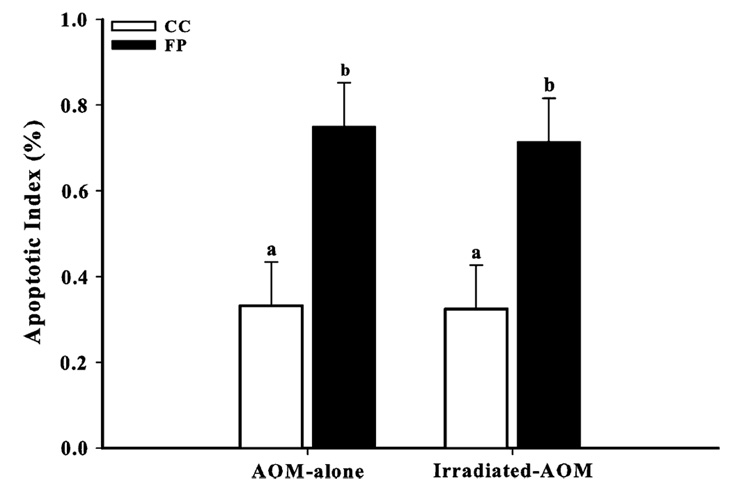
Decreased apoptosis is a significant risk factor for development of colon cancer (8).We found that the FP diet induced higher (P ≤ 0.01) apoptotic indices compared with the CC diet in both AOM-alone and irradiated AOM rats (Figure 1). This concurs with our previous findings that the combination of FP has a protective effect against colon carcinogenesis, partly through enhanced levels of apoptosis (13).
Fig. 1.
FP enhanced colonocyte apoptosis in AOM-alone and irradiated AOM rats compared with CC. The apoptotic index was calculated as the number of apoptotic cells/number of cells per crypt column × 100 (50 crypt columns per rat). Bars without similar letters differ (P < 0.01). Each bar indicates the least square means of 8–10 rats ± SEM.
FP suppresses mPGES-2 levels in both AOM-alone and irradiated AOM rats
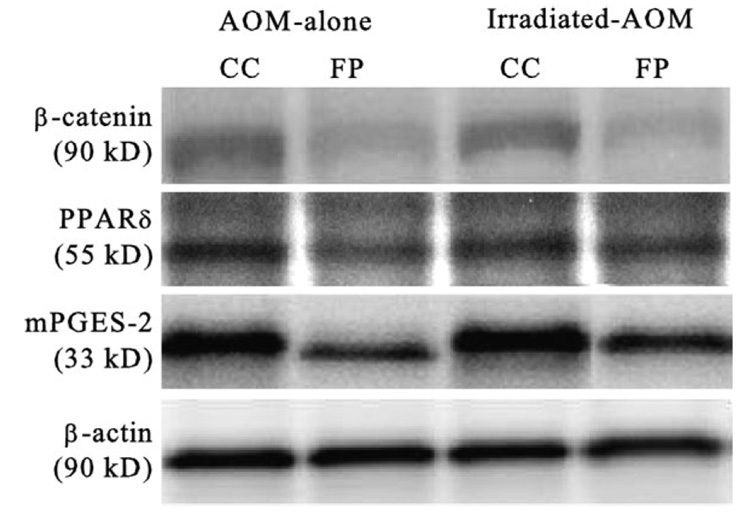
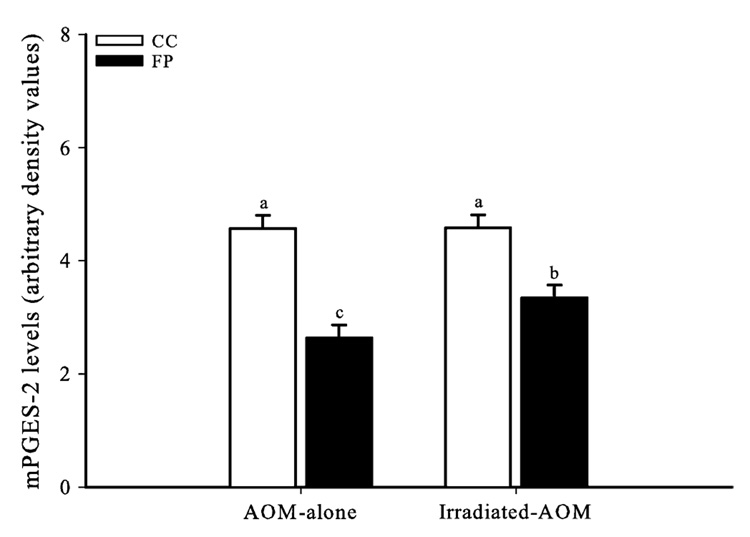
A representative immunoblot of mPGES-2, as well as β-catenin, PPARδ and β-actin, in the rat colonic mucosa is presented in Figure 2. mPGES-2 was recently identified as a second (along with mPGES-1) membrane-associated enzyme involved in the production of PGE2 (29). mPGES-2 is highly expressed in colorectal adenocarcinoma cells (30), and accumulating evidence suggests that the mPGES-2 participates in various COX-2-associated pathophysiological states, suggesting its potential as a novel target for chemoprevention and drug development (reviewed in ref. 31). Levels of mPGES-2 in both AOM-alone and irradiated AOM rats consuming the FP diet were lower (P ≤ 0.05) than levels observed in rats consuming the CC diet (Figure 3). In addition, irradiated AOM rats consuming the FP diet had higher levels of mPGES-2 compared with the AOM-alone rats.
Fig. 2.
Representative immunoblot of β-catenin, PPARδ, mPGES-2 and β-actin (loading control) in the colonic mucosal total cell lysates of both AOM-alone and irradiated AOM (1 Gy, 1GeV Fe-ion) rats fed either a CC or FP diet (8–10 rats per group).
Fig. 3.
FP reduced PGES (mPGES-2) levels in AOM-alone and AOM-irradiated rats compared with CC, though irradiation elevated mPGES-2 levels in rats consuming FP. Bars without similar letters differ (P < 0.05). Each bar indicates the least square means of 8–10 rats ± SEM.
FP diet suppresses PGE2 levels irrespective of radiation treatment
PGE2 is the major PG product of the COX-1 and COX-2 enzymes found in colorectal cancer (32), and it is implicated in the suppression of apoptosis (33). Levels of PGE2 were lower (P < 0.05) for rats consuming the FP diet versus the CC diet (Table I) in both the AOM-alone and irradiated AOM groups. Irradiation elevated (P < 0.05) PGE2 levels in both CC- and FP-consuming rats.
Table I.
Effect of diets on n-3 and n-6 fatty acid metabolites in AOM-alone (0 Gy) and irradiated AOM (1 Gy, 1 GeV/nucleon Fe-ion) rats
| Metabolite | AOM alone (pg/µg protein) |
Irradiated AOM (pg/µg protein) |
||
|---|---|---|---|---|
| CC | FP | CC | FP | |
| PGE2 | 15.18 ± 2.12b | 3.27 ± 0.99d | 22.07 ± 3.00a | 6.93 ± 1.59c |
| PGE3 | n.d. | 1.43 ± 0.21* | 0.03 ± 0.02 | 4.54 ± 1.27* |
| 12-HETE | 7.80 ± 0.71a | 3.68 ± 0.49c | 6.92 ± 0.78ab | 5.56 ± 0.66b |
| 15-HETE | 4.30 ± 0.59a | 1.81 ± 0.38b | 4.72 ± 0.72a | 4.51 ± 0.66a |
| 13-HODE | 19.81 ± 0.79a | 14.71 ± 0.68b | 20.10 ± 0.93a | 17.98 ± 0.83a |
Each value represents the least square mean of 8–10 rats ± SEM. n.d. stands for not detected.
Values in rows without similar superscripts differ (P < 0.05).
P < 0.05, diets differ within each treatment group.
FP diet differentially enhances PGE3 levels in AOM-alone and irradiated AOM rats
PGE3 is a type III PG that inhibits tumor cell growth (23,24). Hence, we measured the effect of the CC and FP diets on PGE3 production in both AOM-alone and irradiated AOM rats. Rats consuming the FP diet had elevated (P < 0.05) levels of PGE3 compared with the CC diet for both irradiation treatments (Table I). Furthermore, the FP diet enhanced (P < 0.05) levels of PGE3 in irradiated AOM rats compared with AOM-alone rats.
FP diet suppresses 12-HETE, 15-HETE and 13-HODE levels only in AOM-alone rats
Fatty acids can be metabolized along the LOX pathway in addition to the COX pathway, and LOXs are important regulators of cell survival and apoptosis. 12-HETE has demonstrated potent antiapoptotic activity in colon and various human cancer cells (21), whereas 15-HETE and 13-HODE have exhibited proapoptotic effects in human colon cancer cells (20,22). Levels of 12-HETE, 15-HETE and 13-HODE in AOM-alone rats consuming the FP diet were lower (P < 0.05) than levels in rats consuming the CC diet (Table I). Conversely, in irradiated AOM rats, levels of 12-HETE, 15-HETE and 13-HODE (P < 0.05) were similar to those found in rats consuming the CC diet as there was a significant increase in these metabolites due to radiation in the FP-consuming rats.
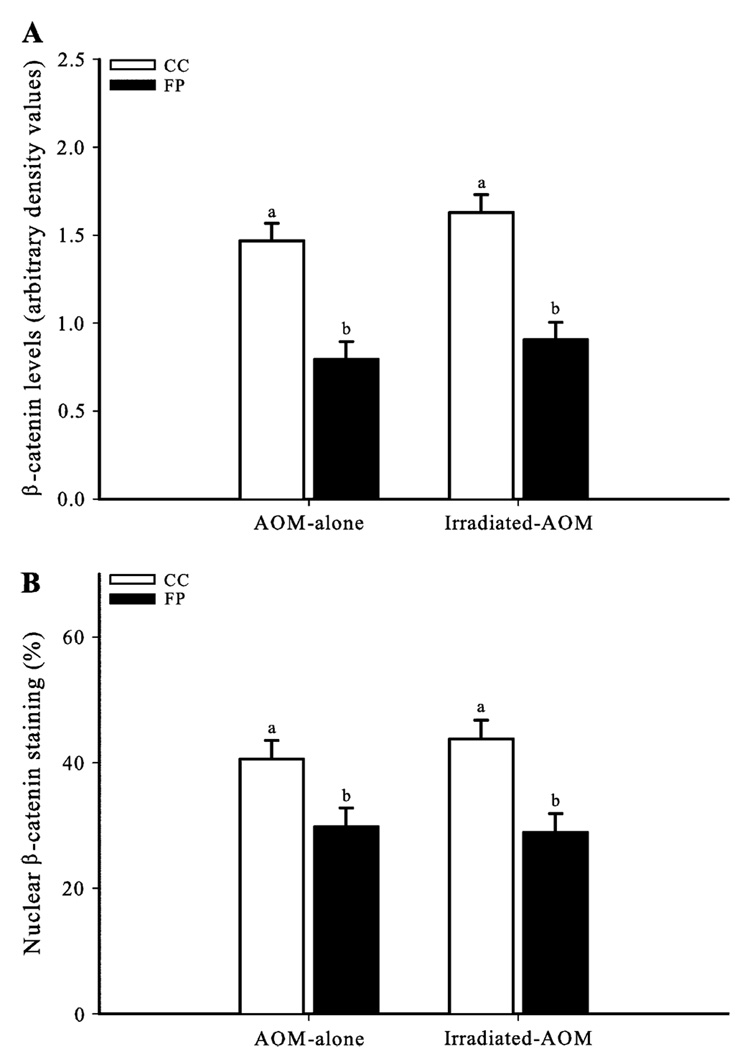
FP suppresses both total β-catenin and nuclear β-catenin staining levels in AOM-alone and irradiated AOM rats
The Wnt/ β-catenin pathway plays a central role in colon carcinogenesis (34). Dysregulation of this pathway by AOM and/or radiation leads to cytosolic β-catenin accumulation and subsequent translocation into the nucleus (16,35), where it activates the transcription of mitotic and antiapoptotic genes such as PPARδ (19,36). In the current study, rats in both irradiation groups consuming the FP diet had lower (P < 0.01) levels of total β-catenin (Figure 4A) and nuclear β-catenin staining (Figure 4B) compared with rats consuming the CC diet.
Fig. 4.
FP diet suppresses colonocyte total and nuclear β-catenin staining (%) levels. (A) FP reduced colonocyte β-catenin levels in AOM-alone and irradiated AOM rats compared with CC. (B) FP reduced the proportion of colonocytes with nuclear β-catenin staining (%). The nuclear β-catenin staining was calculated as the number of colonocyte nuclei stained for β-catenin/the number of cells per crypt column × 100 (25 crypt columns per rat). Bars without similar letters differ (P < 0.01). Each bar indicates the least square means of 8–10 rats ± SEM.
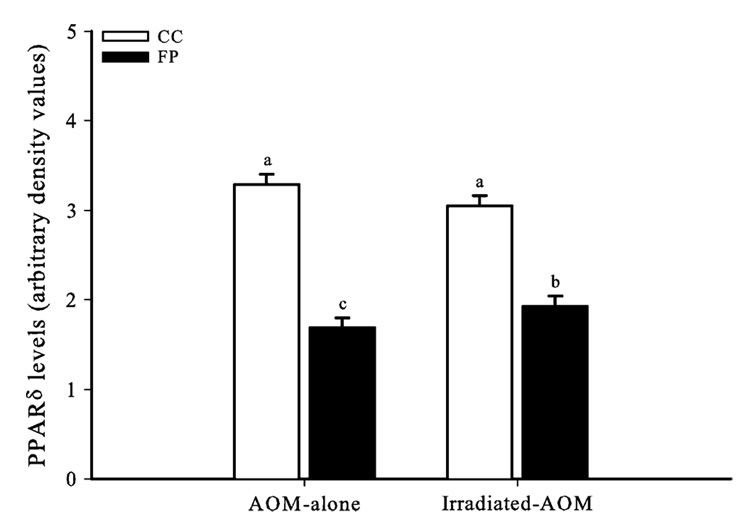
FP suppresses PPARδ levels in both AOM-alone and irradiated AOM rats
PPARδ, a nuclear transcription factor (18,19), has been shown to protect colonocytes from apoptosis, thus promoting colon carcinogenesis (18). PPARδ levels in both irradiation groups were lower (P < 0.05) in rats consuming FP compared with those consuming CC (Figure 5). Similar to the case with the antiapoptotic mediators mPGES-2 and 12-HETE, the FP diet was less effective at suppressing PPARδ levels in irradiated AOM rats. Thus, elevated levels of PPARδ are associated with greater levels of mPGES-2 and 12-HETE in irradiated AOM rats consuming FP.
Fig. 5.
FP reduced PPARδ expression in AOM-alone and irradiated AOM rats compared with CC, though irradiation elevated PPARδ expression in rats consuming FP. Bars without similar letters differ (P < 0.05). Each bar indicates the least square means of 8–10 rats ± SEM.
Discussion
The current in vivo study supports the hypotheses: (i) FP diet is effective in enhancing colonocyte apoptosis, in part through the suppression of the COX and Wnt/β-catenin pathways in rats exposed to radiation prior to injection with AOM and (ii) suppression of the above pathways results in the concomitant lowering of PPARδ, an antiapoptotic transcription factor. We tested these hypotheses using 40 weanling male Sprague–Dawley rats separated into two groups of 20 rats. One group was only injected with AOM, a colon-specific carcinogen, whereas the other group was both irradiated (1 Gy, 1 GeV/nucleon Fe-ion) and injected with AOM. We provided the rats with either a FP or CC diet for 37 weeks and found that at the tumor stage of colon carcinogenesis, apoptosis was enhanced only in the colonocytes of the FP group. This is significant because apoptosis is progressively suppressed during the development of colon cancer (37), making it one of the most critical targets in cancer prevention and treatment (8). We show for the first time that suppression of the COX (mPGES-2 and PGE2) and Wnt/β-catenin (total β-catenin and percent nuclear β-catenin staining) pathways by the FP diet are associated with a concurrent suppression of the nuclear transcription factor PPARδ compared with the CC diet. Our study is unique in that we have measured both the major type II (PGE2) and type III (PGE3) PGs using LC/MS/MS and found that colonic mucosa from FP animals contained PGE3, a EPA-derived COX metabolite. PGE3 is purported to have proapoptotic properties, and we and others are investigating its anticancerous properties. Therefore, a major finding of this study is that FP-consuming rats maintained greater levels of colonocyte apoptosis compared with CC-consuming rats even after irradiation, in part due to its favorable differential effects on antiapoptotic (PPARδ/PGE2) and proapoptotic (PGE3) mediators.
Fatty acids may differentially influence carcinogenesis via their metabolism into various PGs (10,38). For example, PGE2, a proinflammatory molecule that protects colonocytes from programmed cell death by activating antiapoptotic genes (18), is metabolized from n-6 PUFAs such as those found in corn oil. Conversely, fish oil is rich in n-3 PUFAs (e.g. EPA and DHA), which are metabolized into PGs such as PGE3 (23,24). While there is competition between n-6 and n-3 PUFAs for metabolic conversion, n-3 PUFAs are preferentially metabolized by COX enzymes (reviewed in ref. 10). Therefore, the effect of increased n-3 intake is decreased production of AA-derived antiapoptotic metabolites such as PGE2 (reviewed in ref. 10).
In addition to fish oil, butyrate, the major breakdown product of pectin fermentation, may also contribute to the downregulation of the COX pathway seen here. Butyrate reduced COX-2 expression in primary human colon cells obtained during surgery of colorectal tumors, diverticulitis and colon polyps and isolated from non-malignant/non-inflammatory tissue specimens adjacent to the resected colon segments (39). Interestingly, in colorectal cancer cells, butyrate suppresses the activity of nuclear factor-κB (40), which is a regulator of inducible nitric oxide synthase and COX-2 (41,42), as does DHA (43). Thus, a diet incorporating the combination of FP would be expected to reduce PGE2 levels.
In this study, we show that at the tumor stage of colon carcinogenesis, the FP diet reduced (P < 0.01) the levels of PGE2 and enhanced (P < 0.05) the levels of PGE3 in both AOM-alone and irradiated AOM rats compared with the CC diet. This is in agreement with reported in vivo and in vitro results (14,44). Even though we found that the levels of both PGE2 (P < 0.01) and PGE3 (P < 0.05) were higher in irradiated AOM rats consuming the FP diet compared with AOM alone, the elevation of PGE3 was greatly pronounced. This enhanced PGE2 and PGE3 content may be due to the ability of radiation and AOM to enhance the COX pathway (25,45), which in turn would enhance the ability of the colonocytes to produce PGs. The CC diet also conferred enhancement in PG production ability as PGE2 levels in irradiated AOM rats were higher than AOM-alone rats. These data demonstrate that with AOM-alone rats, the CC diet provides an abundance of antiapoptotic PGE2, as additional PGE2 production due to radiation did not suppress the apoptosis any further.
We further observed the levels of mPGES-2, one of the three known PGES enzymes (cytosolic PGES, mPGES-1 and mPGES-2) catalyzing the final step in PGE2 and PGE3 synthesis (30). Interestingly, the up- and downregulation of specific PGESs seems to be linked to that of specific COXs: cytosolic PGES couples preferentially with COX-1, and mPGES-1 and -2 predominately work in concert with COX-2 (30,31). Thus, it is expected that rats consuming the FP diet should experience suppressed mPGES-2 levels because fish oil suppresses COX-2 levels (46). While the FP diet did suppress (P < 0.05) mPGES-2 levels, as expected, in both AOM-alone and irradiated AOM rats compared with the CC diet, this suppression was less (P < 0.05) pronounced in irradiated AOM rats. Previous results suggest that irradiation chronically elevates the COX pathway (45); thus, suppression of mPGES-2 by fish oil may have been counteracted by the enhancement effects of radiation. Conversely, rats consuming the CC diet experienced no difference in mPGES-2 levels when treated with both radiation and AOM compared with treatment with AOM alone. These results indicate that changes in the PGE2 levels observed in this study cannot solely be explained by changes in mPGES-2 levels, which is to be expected considering that mPGES-1 and cytosolic PGES are also involved in the conversion of COX to PGE2.
We also investigated the effects of the diets and treatments on LOX pathway metabolites, which play an important role in the regulation of cell growth, survival and apoptosis in a variety of human cancer cells, including colon cancer (22,47,48). Compared with the CC diet, AOM-alone rats consuming the FP diet (which contained 3.5% CC as a source of essential fatty acids) produced lower (P < 0.05) levels of 12-HETE, 15-HETE and 13-HODE, as would be expected with the increased n-3 PUFA compared with n-6 PUFA content. Previous in vitro studies with colon cancer cells showed that 15-HETE and 13-HODE are proapoptotic (20,22), whereas 12-HETE appears to be antiapoptotic (21,48). Moreover, 15-HETE is a known inhibitor of 12-LOX, which catalyzes 12-HETE metabolism (49). Thus, we observed that even though the levels of 12-HETE increased in irradiated AOM rats consuming the FP diet compared with AOM-alone rats, the levels of 15-HETE also were elevated, perhaps as a compensatory mechanism.
Moreover, the observation that levels of 12-HETE, 15-HETE and 13-HODE increased in irradiated AOM rats consuming the FP diet compared with AOM alone suggests the ability of radiation to enhance the LOX pathway in these rats. The elevation of AA-derived LOX metabolites with radiation despite an unaltered supply of n-6 and n-3 PUFAs in rats consuming FP may point to relaxed competition between these substrates due to enhanced enzyme levels or activity. Interestingly, Chumak et al. (50), demonstrated that 12 year after the tragic Chernobyl nuclear power plant incident, 12-HETE, 15-HETE and 13-HODE levels in the individuals exposed to irradiation (≤0.32 or ≥0.32 Gy) were greater compared with non-irradiated individuals. These results indicate that irradiation elevates the LOX pathway in both animals and humans. It is also interesting to note that in FP-consuming rats, the levels of 12-HETE (P < 0.05), 15-HETE (P < 0.05) and 13-HODE (P < 0.05) increased such that they were not different from the levels found in irradiated AOM rats consuming the CC diet. Thus, even though administration of radiation to rats consuming the FP diet increased the levels of antiapoptotic 12-HETE, these levels were not higher than those in rats consuming the CC diet. Furthermore, despite experiencing higher levels of 12-HETE, the irradiated AOM rats consuming the FP diet also experienced higher levels of proapoptotic 15-HETE and 13-HODE in addition to drastically higher levels of proapoptotic PGE3. The overall effect of these alterations in apoptotic mediators was the maintenance of apoptotic index in rats consuming the FP diet despite the addition of radiation.
In addition to investigating the effects of an FP diet on the COX and LOX pathways in rats treated with both radiation and AOM, we also explored FP effects on critical targets of the Wnt/β-catenin pathway. The Wnt/β-catenin pathway plays a central role in colon carcinogenesis (34), and it is activated in both sporadic colon cancer in humans and experimentally induced rodent models of colon cancer (51,52). Under unperturbed homeostatic conditions, a stable pool of β-catenin is bound to α-catenin and cadherins in the plasma membrane (reviewed in ref. 53). Steady state levels of cytoplasmic or nuclear β-catenin are very low as it is strictly regulated (54). However, dysregulation of the Wnt/β-catenin pathway by radiation or AOM allows β-catenin to accumulate in the cytoplasm and subsequently translocate into the nucleus (16,35). Nuclear β-catenin binds to T-cell factor 4 and stimulates transcription of genes involved in proliferation (e.g. cyclin D1) (16) and apoptosis suppression (e.g. PPARδ) (19,36). In this study, we demonstrated that the FP diet suppressed both the total cellular β-catenin levels and the extent of colonocyte nuclei stained for β-catenin in both AOM-alone and irradiated AOM rats compared with the CC diet. This is supported by Fujise et al. (15), who recently showed that consumption of a 10% fish oil diet suppressed cytoplasmic β-catenin accumulation in AOM-injected male Sprague–Dawley rats compared with consumption of a 10% corn oil or control diet. Furthermore, Narayanan et al. (43) found that treating CaCo-2 human colon cancer cells with DHA (5 µM), one of the major fatty acids in oily fish, downregulated cytoplasmic and nuclear accumulation of β-catenin. Our study provides evidence that this relationship between fish oil and decreased β-catenin accumulation remains true even in rats exposed to both radiation and an alkylating agent. Even though the mechanism by which FP suppresses the Wnt/β-catenin pathway remains unclear, emerging evidence indicates that cross talk with the COX pathway may play an important role (17,52,55). PGE2 has been shown to induce T-cell factor 4 expression in LS-174T human colon cancer cells, indicating an increase in β-catenin nuclear translocation (55). Here, we demonstrate that the suppression of PGE2 by the FP diet compared with the CC diet is associated with a reduced percentage of cells with nuclei stained for β-catenin, irrespective of radiation treatment.
We also show that suppression of COX (PGE2) and Wnt/β-catenin (colonocyte nuclei stained for β-catenin) pathways resulted in concomitant suppression of PPARδ (19) in both AOM-alone and irradiated AOM rats. PPARδ has been shown to protect colonocytes from apoptosis, thus promoting colon carcinogenesis (18). Recently, Ouyang et al.. (36) showed that suppression of elevated PPARδ levels correlated (P < 0.05) with increased apoptosis in a mouse model of colon carcinogenesis. Interestingly, Xu et al. (56) demonstrated that AA can serve as a ligand activator of PPARδ to increase PGE2 production. Thus, replacement of AA derived from corn oil with EPA and DHA derived from fish oil can potentially reduce PGE2 production along two routes: a change in the substrate pool of available fatty acids that shifts PG production from type II to type III metabolites and/or suppression of ligand activation of PPARδ.
In summary, we expected that irradiation would suppress colonocyte apoptosis. However, further suppression of apoptosis was not observed in the CC-consuming rats on top of the significant apoptosis suppression conferred by the AOM-alone treatment. Moreover, irradiation did not suppress apoptosis in FP-consuming rats beyond the suppression observed for the AOM-alone treatment, perhaps due in part to the significant elevation of proapoptotic PGE3. Furthermore, we show for the first time that a FP diet suppresses antiapoptotic PPARδ levels, perhaps by suppressing the COX and Wnt/β-catenin pathways, even after exposure to both radiation and an alkylating agent (AOM). We also showed that irradiation elevates the LOX pathway (both anti- and proapoptotic agents) in rats consuming the FP diet, but these rats nevertheless maintain similar apoptotic indices for the irradiated AOM and AOM-alone treatments. Thus, the current study suggests that dietary FP may be used as a countermeasure against radiation-enhanced colon carcinogenesis because of its ability to maintain potentially protective levels of apoptosis.
Acknowledgements
We thank Dr Lavanya Reddivari and Mr Chris Tarver for their help with manuscript preparation.
Funding
American Institute for Cancer Research (05B094); National Institutes of Health (CA61750, CA82907); National Space Biomedical Research Institute, National Aeronautics and Space Administration (NCC 9-58, CA59034); National Institute of Environmental Health Sciences (P30-ES09106, CA57030 and CA104620).
Abbreviations
- AA
arachidonic acid
- AOM
azoxymethane
- CC
corn oil and cellulose
- DHA
docosahexanoic acid
- EPA
eicosapentaenoic acid
- 12-HETE
12-hydroxy-eicosatetraenoic acid
- 13-HODE
13-hydroxy-octadecadienoic acid
- LA
linoleic acid
- LC/MS/MS
Liquid chromatography/tandem mass spectrometry
- LOX
lipoxygenase
- mPGES-2
microsomal prostaglandin E synthase-2
- PBS
phosphate-buffered saline
- PG
prostaglandin
- PGE2
prostaglandin E2
- PPARδ
peroxisome proliferator-activated receptor δ
- PUFA
polyunsaturated fatty acid
Footnotes
Conflict of Interest Statement: None declared.
References
- 1.American Cancer Society. Cancer Facts and Figures 2007. Atlanta, GA: 2007. [Google Scholar]
- 2.Tanaka T, et al. Synergistic effect of radiation on colon carcinogenesis induced by methylazoxymethanol acetate in ACI/N rats. Jpn. J. Cancer Res. 1993;84:1031–1036. doi: 10.1111/j.1349-7006.1993.tb02797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholas JS, et al. Mortality among US commercial pilots and navigators. J. Occup. Environ. Med. 1998;40:980–985. doi: 10.1097/00043764-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Faisal A. Causes of radiologists’ deaths: a survey of 400 cases in the literature. Radiat. Med. 2003;21:108–111. [PubMed] [Google Scholar]
- 5.Shimizu Y, et al. Risk of cancer among atomic bomb survivors. J. Radiat. Res. (Tokyo) 1991;32 suppl. 2:54–63. doi: 10.1269/jrr.32.supplement2_54. [DOI] [PubMed] [Google Scholar]
- 6.Brinkley D, et al. The late effects of artificial menopause by X-radiation. Br. J. Radiol. 1969;42:519–521. doi: 10.1259/0007-1285-42-499-519. [DOI] [PubMed] [Google Scholar]
- 7.Sharp JG, et al. Apparent synergism between radiation and the carcinogen 1,2-dimethylhydrazine in the induction of colonic tumors in rats. Radiat. Res. 1989;117:304–317. [PubMed] [Google Scholar]
- 8.Chang WL, et al. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J. Nutr. 1998;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 9.Lee DY, et al. Dietary fat and fiber modulate colonic cell proliferation in an interactive site-specific manner. Nutr. Cancer. 1993;20:107–118. doi: 10.1080/01635589309514277. [DOI] [PubMed] [Google Scholar]
- 10.Roynette CE, et al. n-3 polyunsaturated fatty acids and colon cancer prevention. Clin. Nutr. 2004;23:139–151. doi: 10.1016/j.clnu.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Sanders LM, et al. An increase in reactive oxygen species by dietary fish oil coupled with the attenuation of antioxidant defenses by dietary pectin enhances rat colonocyte apoptosis. J. Nutr. 2004;134:3233–3238. doi: 10.1093/jn/134.12.3233. [DOI] [PubMed] [Google Scholar]
- 12.Chang WC, et al. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 13.Davidson LA, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagga D, et al. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl Acad. Sci. USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujise T, et al. Long-term feeding of various fat diets modulates azoxymethane-induced colon carcinogenesis through Wnt/beta-catenin signaling in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1150–G1156. doi: 10.1152/ajpgi.00269.2006. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M, et al. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellone MD, et al. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.He TC, et al. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen GG, et al. 15-hydroxy-eicosatetraenoic acid arrests growth of colorectal cancer cells via a peroxisome proliferator-activated receptor gamma-dependent pathway. Int. J. Cancer. 2003;107:837–843. doi: 10.1002/ijc.11447. [DOI] [PubMed] [Google Scholar]
- 21.Pidgeon GP, et al. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62:2721–2727. [PubMed] [Google Scholar]
- 22.Shureiqi I, et al. The 15-lipoxygenase-1 product 13-S-hydroxyocta-decadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc. Natl Acad. Sci. USA. 2003;100:9968–9973. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang P, et al. Formation and antiproliferative effect of prostaglandin E(3) from eicosapentaenoic acid in human lung cancer cells. J. Lipid Res. 2004;45:1030–1039. doi: 10.1194/jlr.M300455-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie KE, et al. Omega-3 and omega-6 fatty acids and PGE2 stimulate the growth of normal but not tumor mouse mammary epithelial cells: evidence for alterations in the signaling pathways in tumor cells. Prostaglandins Leukot. Essent. Fatty Acids. 1994;51:437–443. doi: 10.1016/0952-3278(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 25.Vanamala J, et al. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis. 2006;27:1257–1265. doi: 10.1093/carcin/bgi318. [DOI] [PubMed] [Google Scholar]
- 26.Kerr JF, et al. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Chang H, et al. Using a combination of cytochrome P450 1B1 and beta-catenin for early diagnosis and prevention of colorectal cancer. Cancer Detect. Prev. 2005;29:562–569. doi: 10.1016/j.cdp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Yang P, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:385–395. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Tanikawa N, et al. Identification and characterization of a novel type of membrane-associated prostaglandin E synthase. Biochem. Biophys. Res.Commun. 2002;291:884–889. doi: 10.1006/bbrc.2002.6531. [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, et al. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J. Biol. Chem. 2003;278:37937–37947. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- 31.Murakami M, et al. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat. 2002;68–69:383–399. doi: 10.1016/s0090-6980(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 32.Rigas B, et al. Altered eicosanoid levels in human colon cancer. J. Lab. Clin. Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 33.Sheng H, et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 34.Polakis P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima M, et al. Altered expression of beta-catenin during radiation-induced colonic carcinogenesis. Pathol. Res. Pract. 2002;198:717–724. doi: 10.1078/0344-0338-00326. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang N, et al. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR)delta expression in APC(min/+)mice proportionally to their tumor inhibitory effect: implications for the role of PPARdelta in carcinogenesis. Carcinogenesis. 2006;27:232–239. doi: 10.1093/carcin/bgi221. [DOI] [PubMed] [Google Scholar]
- 37.Bedi A, et al. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–1816. [PubMed] [Google Scholar]
- 38.Chapkin RS, et al. Colon cancer, fatty acids and anti-inflammatory compounds. Curr. Opin. Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- 39.Sauer J, et al. Physiological concentrations of butyrate favorably modulate genes of oxidative and metabolic stress in primary human colon cells. J. Nutr. Biochem. 2007;18:736–745. doi: 10.1016/j.jnutbio.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Schwab M, et al. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NF kappa B signalling. Mol. Immunol. 2007;44:3625–3632. doi: 10.1016/j.molimm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Kujubu DA, et al. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J. Biol. Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- 42.Lowenstein CJ, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc. Natl Acad. Sci. USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narayanan BA, et al. Effects of a combination of docosahexaenoic acid and 1,4-phenylene bis(methylene) selenocyanate on cyclooxygenase 2, inducible nitric oxide synthase and beta-catenin pathways in colon cancer cells. Carcinogenesis. 2004;25:2443–2449. doi: 10.1093/carcin/bgh252. [DOI] [PubMed] [Google Scholar]
- 44.Dommels YE, et al. Effects of high fat fish oil and high fat corn oil diets on initiation of AOM-induced colonic aberrant crypt foci in male F344 rats. Food Chem. Toxicol. 2003;41:1739–1747. doi: 10.1016/s0278-6915(03)00201-1. [DOI] [PubMed] [Google Scholar]
- 45.Keskek M, et al. Increased expression of cyclooxygenase-2 (COX-2) in radiation-induced small bowel injury in rats. J. Surg. Res. 2006;135:76–84. doi: 10.1016/j.jss.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 46.Rao CV, et al. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 2001;61:1927–1933. [PubMed] [Google Scholar]
- 47.Tang DG, et al. Arachidonate lipoxygenases as essential regulators of cell survival and apoptosis. Proc. Natl Acad. Sci. USA. 1996;93:5241–5246. doi: 10.1073/pnas.93.11.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillis RC, et al. Regulation of apoptosis in eicosapentaenoic acid-treated HL-60 cells. J. Surg. Res. 2007;137:141–150. doi: 10.1016/j.jss.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Sekiya F, et al. Feedback regulation of platelet function by 12S-hydroxyeicosatetraenoic acid: inhibition of arachidonic acid liberation from phospholipids. Biochim. Biophys. Acta. 1990;1044:165–168. doi: 10.1016/0005-2760(90)90232-m. [DOI] [PubMed] [Google Scholar]
- 50.Chumak A, et al. Monohydroxylated fatty acid content in peripheral blood mononuclear cells and immune status of people at long times after the Chernobyl accident. Radiat. Res. 2001;156:476–487. doi: 10.1667/0033-7587(2001)156[0476:mfacip]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Wang D, et al. WNT and cyclooxygenase-2 cross-talk accelerates adenoma growth. Cell Cycle. 2004;3:1512–1515. doi: 10.4161/cc.3.12.1288. [DOI] [PubMed] [Google Scholar]
- 52.Castellone MD, et al. Cyclooxygenase-2 and colorectal cancer chemoprevention: the beta-catenin connection. Cancer Res. 2006;66:11085–11088. doi: 10.1158/0008-5472.CAN-06-2233. [DOI] [PubMed] [Google Scholar]
- 53.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Bienz M, et al. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 55.Shao J, et al. Prostaglandin E2 Stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J. Biol. Chem. 2005;280:26565–26572. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- 56.Xu L, et al. Cross-talk between peroxisome proliferator-activated receptor delta and cytosolic phospholipase A(2)alpha/cyclooxygenase-2/prostaglandin E(2) signaling pathways in human hepatocellular carcinoma cells. Cancer Res. 2006;66:11859–11868. doi: 10.1158/0008-5472.CAN-06-1445. [DOI] [PubMed] [Google Scholar]