Abstract
Diisopropylfluorophosphate (DFP) elicits cholinergic toxicity by inhibiting acetylcholinesterase, leading to accumulation of the neurotransmitter acetylcholine and excessive stimulation of cholinergic receptors throughout the body. Endocannabinoids inhibit the release of neurotransmitters including acetylcholine via a widely distributed retrograde signaling pathway. Endocannabinoid signaling is therefore a potential therapeutic target for the management of OP poisoning. We first evaluated the relative in vitro and in vivo (2.5 mg/kg, sc) effects of DFP on cholinesterase, fatty acid amide hydrolase (FAAH, an endocannabinoid degrading enzyme), monoacylglycerol lipase (MAGL, another endocannabinoid degrading enzyme) and cannabinoid receptor (CB1) binding in rat hippocampus. The effects of WIN 55212-2 (cannabinoid receptor agonist, 1.5 mg/kg), URB597 (FAAH inhibitor, 3 mg/kg), URB602 (MAGL inhibitor, 10 mg/kg) or AM404 (endocannabinoid uptake inhibitor, 10 mg/kg) on DFP toxicity were then examined. Adult male rats (n=5/treatment group) were given either peanut oil or DFP followed immediately by vehicle or one of the four cannabinomimetic drugs. Functional signs of toxicity were evaluated for 24 hours and then rats were sacrificed for neurochemical measurements. DFP inhibited cholinesterase, FAAH, MAGL and CB1 receptor binding in vitro in a concentration-dependent manner, with highest and lowest potency against cholinesterase and FAAH, respectively. In vivo, DFP inhibited hippocampal cholinesterase (89%) and FAAH (42%), but had no significant effect on MAGL or CB1 binding. Rats treated with DFP alone showed typical signs of cholinergic toxicity including involuntary movements and excessive secretions (SLUD signs). WIN 55212-2, URB597, URB602 and AM404 all significantly reduced involuntary movements following DFP exposure in a time-dependent manner, and most (URB597, URB602 and AM404) also significantly reduced DFP-induced SLUD signs. These results suggest that enhancing endocannabinoid signaling can attenuate the acute toxicity of DFP and provide rationale for further investigations on the role of endocannabinoids in cholinergic toxicity.
Introduction
Organophosphorus (OP) compounds are used worldwide as insecticides. Unfortunately, some of these OP compounds (e.g., sarin), have been also used as nerve agents in chemical warfare and terrorism. OPs elicit acute toxicity by inhibiting the enzyme acetylcholinesterase (AChE). Inhibition of AChE leads to accumulation of synaptic acetylcholine levels and excessive stimulation of cholinergic receptors throughout the body. Acute cholinergic toxicity is expressed as signs such as increased involuntary movements (e.g., tremors), autonomic dysfunction (e.g., excessive salivation), respiratory depression, cardiovascular alterations and others.
Natural cannabinoids are alkaloids obtained from the plant Cannabis sativa. Various forms of Cannabis have been used for both medicinal and recreational purposes for centuries (Frazzetto, 2003; Di Marzo et al., 2004; Hart, 2005). The principal psychoactive component of Cannabis was identified as Δ-9 tetrahydrocannabinol (Gaoni and Mechoulam, 1971; Hively et al., 1966). Tetrahydrocannabinol produces its psychotropic effects by binding to and activating specific cannabinoid receptors in the brain (Devane et al., 1988).
Cannabinoid receptors are members of the G protein coupled receptor superfamily. The cannabinoid receptors were cloned and classified into two subtypes, the cannabinoid type 1 (CB1R) (Devane et al., 1988) and the cannabinoid type 2 (CB2R) receptor (Munro et al., 1993). CB1 receptors are predominantly expressed in the nervous system (Herkenham et al., 1991; Tsou et al., 1998; Katona et al., 2001; Wilson and Nicoll, 2002) while CB2 receptors are primarily found in immune cells. Identification of specific cannabinoid receptors led to the subsequent discovery of endogenous ligands (i.e., endocannabinoids), anandamide and 2-arachidonyl glycerol (Devane et al., 1992; Stella and Piomelli, 1997). Endocannabinoids are synthesized “on demand” from membrane lipids by the action of N-acylphosphatidyl specific phospholipase D and diacylglycerol lipase (Basavarajappa, 2007). The enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) were found to inactivate the endocannabinoids, with FAAH being primarily responsible for inactivating anandamide and MAGL appearing to be predominant in degradation of 2-arachidonyl glycerol (Hashimotodani et al., 2007). The endocannabinoids and their receptors, a transporter, and enzymes involved in both synthesis and inactivation make up the endocannabinoid signaling system.
Endocannabinoids modulate neurotransmission in a retrograde fashion wherein depolarization of the postsynaptic cell leads to their synthesis and release (Piomelli et al., 2000; Maejima et al., 2001), after which they diffuse to the presynaptic terminal to activate cannabinoid receptors (Maejima et al., 2001; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001; Kreitzer and Regehr, 2002). Endocannabinoids appear to play an important role in the maintenance of synaptic plasticity through their widespread neuromodulatory actions (Zhu, 2006). Several studies have shown that endocannabinoids modulate the pre-synaptic release of many neurotransmitters including dopamine (Steffens et al., 2004; Cheer et al., 2007), glutamate (Shen et al., 1996; Sullivan, 1999; Levenes et al., 1998), acetylcholine (Gifford and Ashby, 1996; Steffens et al., 2003), GABA (Miller and Walker, 1995) and norepinephrine (Schlicker et al., 1997). The synthetic cannabinoid receptor agonist WIN 55,212-2 inhibited acetylcholine release both in brain slices (Gifford and Ashby, 1996) and in rat hippocampus in vivo (Tzavara et al., 2003). Blocking the CB1 receptor with the antagonist SR141716A increases acetylcholine release in hippocampal slices (Gifford and Ashby, 1996; Kathmann et al., 2001). As OP toxicity is ultimately related to the release and accumulation of acetylcholine, we hypothesized that enhancing endocannabinoid signaling could block the expression of OP toxicity.
We previously reported that acute administration of the synthetic cannabinoid receptor agonist WIN 55,212-2 reduced toxicity elicited by the organophosphate cholinesterase inhibitor paraoxon, while repeated pre-exposure to WIN 55212-2 (daily for seven days) actually increased acute paraoxon toxicity (Nallapaneni et al., 2006). In the present studies, we compared the effects of the direct cannabinoid receptor agonist WIN 55212-2 with indirect cannabinomimetics (URB597, a FAAH inhibitor; URB602, a MAGL inhibitor; and AM404, a transport inhibitor) on cholinergic toxicity elicited by the prototype serine hydrolase inhibitor, diisopropylfluorophosphate. Knowledge of interactions between endocannabinoid and cholinergic signaling may lead to better treatment strategies for OP intoxications as well as other disorders involving cholinergic neurotransmission.
Materials and Methods
Chemicals
Diisopropylflurophosphate (DFP) and WIN 55212-2 were purchased from Sigma Chemical Co. (St. Louis, MO). URB597, AM404 and URB602 were purchased from Cayman Chemical (Ann Arbor, MI). Radiolabeled acetylcholine iodide (acetyl-3H, specific activity 82 mCi/mmol) and [3H]CP 55,940 (side chain-2,3,4-3H (N), specific activity 158 Ci/mmol) were purchased from Perkin Elmer Life Sciences, Inc. (Boston, MA). Anandamide (ethanolamine 1-3H, specific activity 60 Ci/mmol) and 2-oleoyl [3H]glycerol (glycerol-1,2.3-3H, specific activity 10–20 Ci/mmol) were purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). All other chemicals were reagent grade.
Animals
All procedures involving animals were performed according to protocols approved by Oklahoma State University Institutional Animal Care and Use Committee, in accordance with the guidelines in the NIH/NRC Guide for the Care and Use of Laboratory Animals. Adult (2 month-old) male, Sprague Dawley rats were purchased from Harlan Sprague Dawley (Indianapolis, IN) and used in all studies. Rats were maintained in polycarbonate cages under a 12h:12h light:dark cycle and allowed to acclimatize for at least 5 days prior to study. Rats were allowed ad libitum access to feed (PMI® Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN) and tap water.
Tissue Preparations
The hippocampus contains a high density of cannabinoid receptors and enzymes required for both the synthesis and inactivation of endocannabinoids (Fride, 2005). Furthermore, cannabinoids have been shown to reduce hippocampal acetylcholine release (Gifford and Ashby, 1996; Tzavara et al., 2003). We therefore selected the hippocampus to evaluate neurochemical effects of DFP and the cannabinergic compounds. Hippocampus was dissected essentially as described by Glowinsky and Iversen (1966) and the samples were frozen at −70°C until use. One half of the hippocampus was used for preparation of membranes for measuring ChE, FAAH and CB1 receptor binding, while the other half was used to prepare soluble proteins for MAGL assay.
For membranes, tissues were homogenized in 10 volumes (w/v) of 100 mM Tris buffer, pH 9.0 containing 1 mM EDTA for 20 seconds at 28,000 rpm using a Polytron® homogenizer, and were subsequently centrifuged at 700 × g for 10 minutes at 4°C. Supernatants were then centrifuged at 10,000 × g for 20 minutes and pellets re-suspended in the original homogenate volume with 50 mM Tris buffer, pH 7.4 containing 1 mM MgCl2 and 1 mM EDTA just prior to assay.
For soluble proteins, tissues were homogenized in 20 volumes (w/v) of 50 mM sodium phosphate buffer, pH 7.0 containing 0.32 M sucrose. Homogenates were then centrifuged at 100,000 ×g for 60 minutes at 4°C and supernatants containing soluble proteins were stored at −70°C until assay.
ChE Assay
Total cholinesterase activity was measured by the radiometric method of Johnson and Russell (1975). The radiolabeled substrate ([3H]acetylcholine, 1 mM final concentration) was added to hippocampal membranes (80–100 ug protein) in reaction buffer containing 50 mM potassium phosphate buffer, pH 7.0 containing 0.1% Triton X-100 in a final reaction volume of 100 µl. The timed reactions were terminated by an acidic stop solution (100 µl) followed by addition of 5 ml of organic counting fluid. Contents were mixed thoroughly and radioactivity was counted for five minutes using a Wallac 1409 scintillation counter at 58–60% efficiency (Perkin Elmer, Gaithersburg, MD). Radioactivity was converted into nmol substrate hydrolyzed based on counts generated in control vials containing excess electric eel AChE. Preliminary assays were conducted to determine the incubation time and tissue amount required for linear rates of reaction.
FAAH Assay
FAAH was measured essentially by the method of Quistad and coworkers (2001). Substrate ([3H]arachidonyl ethanaloamine) was added to hippocampal membranes (20–30 µg protein) in 50 mM Tris buffer, pH 7.4 containing 1 mM MgCl2 and 1 mM EDTA and incubated at 37°C for 30 minutes in a final reaction volume of 0.5 ml. Reactions were terminated by adding 0.5 ml of chloroform:methanol (2:1). Organic and aqueous phases were separated by centrifugation at 7,000 rpm for 5 minutes in a microcentrifuge. A sample (0.2 ml) of the upper aqueous phase was aliquoted into a scintillation vial and 4 ml aqueous counting fluid (Scintisafe 30%, Fisher Scientific) was added. Vials were then vortexed and counted.
MAGL Assay
MAGL activity was measured essentially as described by Ghafouri and colleagues (2004). Substrate (2-oleoyl-[3H]glycerol, 10µM final concentration) was added to hippocampal soluble proteins (30–50 µg protein) in 10 mM Tris HCl buffer, pH 7.2 containing 1 mM EDTA. Reactions were incubated for 37°C for 10 minutes and stopped by adding 400 µl of chloroform:methanol (1:1). Tubes were centrifuged (2500 rpm for 10 min) and the upper aqueous phase collected. A 200 µl aliquot was counted.
CB1 Receptor Binding Assay
CB1 receptor binding was performed essentially as described by Quistad and coworkers (2002). Hippocampal membranes (125–175 µg protein) were pre-incubated for 15 min at room temperature in Tris buffer, pH 7.4 containing 1 mM MgCl2, 1 mM EDTA and 0.3 % BSA, with or without WIN 55212-2 (1 µM final concentration). Radioligand ([3H]CP 55940, 1 nM final concentration) was then added to all tubes and reactions were incubated at 30°C for 90 minutes. Tissues were collected onto GF/B filter (Brandel, Gaithersburg, MD) using a 24-well receptor harvestor (Brandel M-24) by vacuum filtration and washed three times with ice cold 0.9% NaCl containing 0.2% BSA. Filters were then transferred to scintillation vials and 4 ml of aqueous counting fluid (Scintisafe®) was added. Reactions were counted the following day. Specific binding (generally around 70% of total) was calculated as the difference in binding in tubes pre-incubated in the presence and absence of WIN 55212-2. Protein concentration of the samples was measured by the method described by Lowry and coworkers (1951). All biochemical values were related to protein concentration.
In vitro Studies
We compared the in vitro sensitivity of ChE, FAAH, MAGL and CB1 receptor binding to DFP. For in vitro enzyme inhibition assays, the tissues were pre-incubated with vehicle or one of a range of DFP concentrations (1 × 10−9 – 1 ×10−3 M) at 37°C for 30 minutes, followed by assay of the residual activity as described above. For CB1 receptor binding, tissues were pre-incubated with vehicle or one of a range of DFP concentrations (1 × 10−9 – 1 ×10−3 M) at room temperature for 15 minutes and in the presence or absence of WIN 55212-2 (1 µM), followed by addition of [3H]CP55,940 and incubation for 90 minutes at 30 °C before vacuum filtration and analysis as above.
In vivo Studies
Rats (n=5–10/group) were given vehicle (peanut oil, 1 ml/kg, sc) or DFP (2.5 mg/kg, sc in peanut oil). Subgroups of DFP-treated rats were co-exposed to either vehicle (96% saline/2% Cremophor EL/2% DMSO, 3 ml/kg, ip) or WIN 55,212-2 (1.5 mg/kg, ip), URB597 (3 mg/kg, ip), URB602 (10 mg/kg, ip) or AM404 (10 mg/kg, ip). Involuntary movements (e.g., tremors) and SLUD (an acronym for salivation, lacrimation, urination and defecation) signs were graded by an observer blinded to treatment groups essentially as described before (Liu and Pope, 1996; Shaikh et al., 2003; Nallapaneni et al., 2006). Involuntary movements were scored as: 2 = normal quivering of vibrissae, head and limbs; 2.5 = muscle fasciculations, occasional fine tremor in forelimbs and head; 3 = more consistent, fine tremor in forelimbs and head; 3.5 = tremor extending more caudally; 4 = whole body tremor; 5 = myoclonic jerks. SLUD signs were scored as 1 = normal, no excessive secretions; 2 = two mild or one more extensive secretion; 2.5 = multiple mild or two more extensive secretions; 3 = multiple moderate secretions; 4 = multiple severe secretions. Observers were blinded to treatment groups. Rats were sacrificed after the 24-hour observations and hippocampus was dissected for neurochemical evaluations.
Statistical Analyses
IC50 values and 95% confidence interval were calculated using Graphpad Prism® statistical software. For cholinesterase and FAAH activity, a “true’ IC50 was calculated based on total activities whereas for MAGL activity and CB1 binding, the IC50 was calculated only on the amount of activity/binding sensitive to DFP. Involuntary movements and SLUD signs are reported as median +/− interquartile range and analyzed by MANOVA with repeated measure of time. All neurochemical results were reported as mean group values ± standard error of mean (S.E.M) and analyzed using one-way ANOVA with post-hoc comparisons. The JMP statistical package (SAS, 1995) was used for analysis of both functional and neurochemical endpoints, with a p value of 0.05 being considered significant.
Results
Neurochemical effects of DFP in vitro
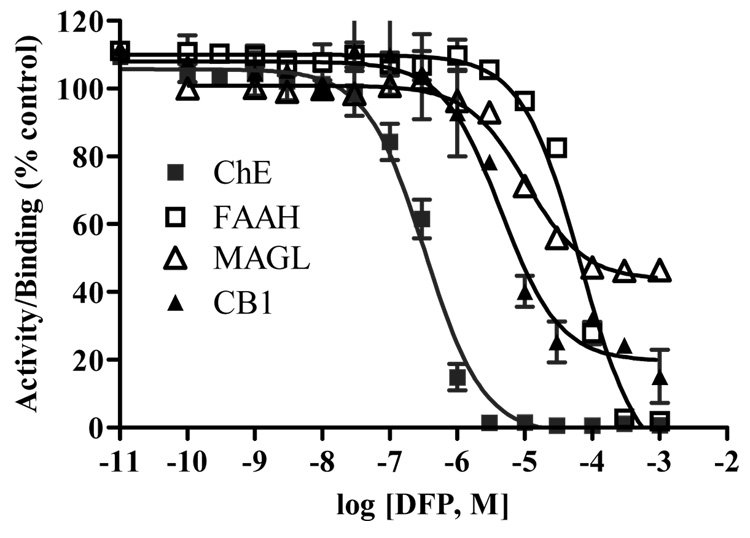
Figure 1 shows the in vitro effects of DFP on hippocampal ChE, FAAH, MAGL and CB1 receptor binding. DFP inhibited all three enzyme activities in a concentration-dependent manner. ChE was most sensitive to inhibition by DFP in vitro, with an IC50 of 343 nM (30 minutes, 37°C). DFP was a much less potent inhibitor of MAGL (IC50 = 10 µM) and FAAH (IC50 = 56 µM). While DFP appeared to inhibit essentially all of the total ChE and FAAH activity, only about 50% of the MAGL activity was sensitive to inhibition by DFP. CB1 receptor ([3H]CP55940) binding was also blocked by DFP in a concentration-dependent manner (IC50 = 4 µM, approximately 80% displacement).
Figure 1. Effect of DFP on cholinergic and cannabinergic markers in vitro.
Membrane preparations from rat hippocampal tissues were either pre-incubated at 37°C for 30 minutes (ChE, MAGL and FAAH activities) or at room temperature for 15 minutes (CB1 receptor binding) with different concentrations of DFP and residual activity/binding evaluated as described in methods section.
Neurochemical effects of DFP in vivo
Table 1 shows the effects of DFP (2.5 mg/kg, sc) on ChE, FAAH, MAGL and CB1 binding. Hippocampal ChE activity was extensively inhibited (89%) 24 hours after DFP dosing. Interestingly, while FAAH activity was the least sensitive of all markers to DFP in vitro, FAAH was inhibited about 40% by DFP in vivo, while CB1 receptor binding and MAGL activity were unaffected by in vivo dosing.
Table 1.
In vivo effects of DFP on hippocampal ChE, FAAH, MAGL and CB1 receptor binding.
| Treatmenta | ChEb | FAAHc | MAGLd | CB1e |
|---|---|---|---|---|
| Vehicle | 78.1 ± 3.4 (100) | 0.72 ± 0.02 (100) | 1.10 ± 0.07 (100) | 0.14 ± 0.01 (100) |
| DFP | 8.7 ± 0.6 (11)* | 0.42 ± 0.03 (58)* | 1.08 ± 0.14 (98) | 0.13 ± 0.01 (93) |
Rats (n=5/treatment group) were treated with either peanut oil (1 ml/kg, sc) or DFP in peanut oil (2.5 mg/kg) and sacrificed 24 hours later. Both sides of the hippocampus were dissected and frozen separately until assay. An asterisk indicates a significant difference between control and DFP-treated tissues. Values in parentheses represent percent of control.
Cholinesterase activity is reported as nmol acetylcholine hydrolyzed/min˙mg protein (mean ± SE).
FAAH activity is reported as nmol anadamide hydrolyzed/min˙mg protein (mean ± SE).
MAGL activity is reported as nmol 2-oleoyl glycerol hydrolyzed/min˙mg protein (mean ± SE).
CB1 receptor binding data are presented as pmol [3H]CP 55,940 bound/mg protein ± SE.
Effects of DFP on cholinergic signs of toxicity and their modulation by cannabinergic drugs
Figure 2 shows A) involuntary movements and B) SLUD signs from 0–24 hours after DFP dosing. Involuntary movements (i.e., tremors) typical of OP intoxication were elicited by DFP, peaking at 1 hour after treatment (median score = 4.0). There were significant effects of treatment with WIN55212-2 (p = 0.003), URB597 (p < 0.001), URB602 (p = 0.002) and AM404 (p < 0.001) at reducing DFP-induced involuntary movements, as well as significant treatment × time interactions in all cases. Thus, post-hoc analyses were not conducted on these data. DFP elicited relatively minor automomic effects (i.e., SLUD signs, Figure 2B). WIN 55212-2 had no significant effect on DFP-induced SLUD signs, whereas there were main effects of URB597 (p = 0.0002), URB602 (p = 0.003) and AM404 (p = 0.02) yielding less extensive SLUD signs. There were significant treatment × time interactions with URB597 and URB602, but not with AM404. Post-hoc analysis suggested significant differences between DFP alone and DFP/AM404 groups only at the 2 hr time-point.
Figure 2. Effects of WIN 55212-2, URB597, AM404 or URB602 on DFP-induced signs of cholinergic toxicity.
Adult male rats (n = 5/treatment group) were treated with DFP (2.5 mg/kg, sc, open square) and immediately exposed to either vehicle, WIN 55,212-2 (1.5 mg/kg, closed square), URB597 (3 mg/kg, open triangle), AM404 (10 mg/kg, open circle) or URB602 (10 mg/kg, open diamond) as described in methods. Controls (n = 5) received only peanut oil. Functional signs of cholinergic toxicity (involuntary movements and SLUD signs) were observed for 24 hours and are shown as median scores ± interquartile range (IQR). Figure 2A and Figure 2B represent involuntary movements and SLUD signs, respectively. No signs of toxicity were noted in vehicle controls (data not shown). All four cannabinomimetics significantly reduced involuntary movements elicited by DFP (Figure 2A). WIN 55212-2 had no significant effect, while URB597, URB602 and AM404 significantly reduced DFP-induced SLUD signs (Figure 2B). See Results for details. Note that while all five treatment groups are shown on the figure, some of the curves overlap.
Neurochemical interaction between DFP and cannabinergic drugs
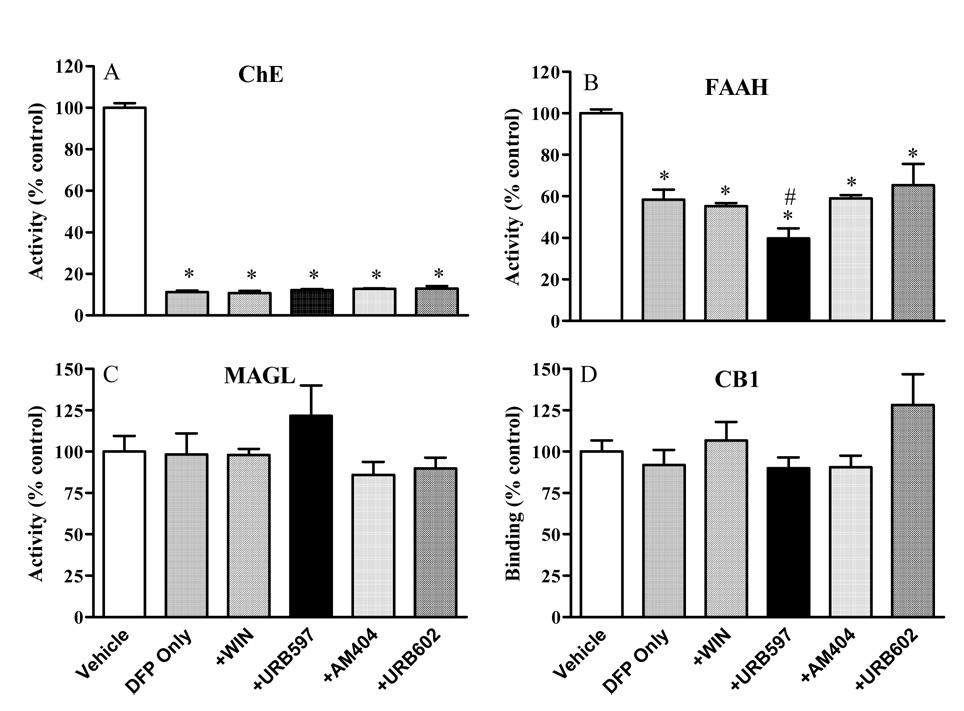
Following the 24-hour functional observations, rats were sacrificed and neurochemical changes evaluated. Figure 3 shows neurochemical changes in rats exposed to DFP, with or without co-exposure to one of the cannabinergic drugs. DFP (2.5 mg/kg, sc) elicited extensive (about 90%) ChE inhibition, and co-exposure to either of the cannabinergic drugs had no apparent influence. Similar to our initial studies (see Table 1), DFP led to significant FAAH inhibition (about 40%), and this inhibition was not affected in rats treated concurrently with WIN 55212-2, URB602 or AM404. There was, however, significantly greater FAAH inhibition (62%) in the DFP+URB597 treatment group. In contrast, no significant treatment-related reduction in MAGL or CB1 receptor binding was noted in DFP alone or DFP-cannabinergic drug treatment groups.
Figure 3. Interactive effects DFP and WIN 55212-2, URB597, AM404 or URB602 on hippocampal neurochemistry.
Rats were treated as described above. Tissues were collected 24 hours after treatment for measurement of cholinesterase, fatty acid amide hydrolase, monoacylglycerol lipase and CB1 receptor binding. ChE activity was calculated as mean nmol acetylcholine hydrolyzed/min.mg protein ± SE. FAAH activity was measured as nmol anandamide hydrolyzed/min.mg protein ± SE. MAGL activity was measured as nmol 2-oleoyl glycerol hydrolyzed/min.mg protein ± SE. CB1 receptor binding pmol/mg protein ± SE. All values were shown as percent of control. An asterisk indicates a significant difference from control (Vehicle) whereas a pound sign indicates a significant difference from the DFP only group.
Discussion
Endocannabinoids have been recently shown to be global neuromodulators that can regulate neurotransmission by decreasing the release of neurotransmitters including acetylcholine (Acquas et al., 2000, 2001; Fride, 2005; Tzavara et. al., 2003). We hypothesized that pharmacological enhancement of endocannabinoid signaling could reduce acetylcholine release and cholinergic signs of toxicity following exposure to an anticholinesterase. We previously reported that the synthetic cannabinoid receptor agonist WIN 55212-2 reduced functional signs of toxicity following exposure to the organophosphate anticholinesterase paraoxon (Nallapaneni et al., 2006). The objective of the present studies was to compare the effects of the direct receptor agonist WIN55212-2 with indirect cannabinoid receptor agonists (i.e., drugs that either block endocannabinoid degrading enzymes or inhibit cannabinoid transport) on expression of cholinergic toxicity elicited by diisopropylfluorophosphate (DFP).
Quistad and coworkers (2001, 2002, 2006) reported that some organophosphorus compounds including DFP can inhibit endocannabinoid degrading enzymes (fatty acid amide hydrolase, FAAH; monoacylglycerol lipase, MAGL) and block CB1 receptor binding in tissues from mouse brain. In our studies, DFP was a more potent in vitro inhibitor of rat hippocampal ChE activity compared to FAAH and MAGL, with approximately 160-fold and 30-fold differences in IC50 values (Figure 1). DFP also displaced [3H]CP 55940 binding from hippocampal cannabinoid receptors, with approximately 80% maximum displacement of ligand binding and an IC50 of 4 µM. These findings are relatively similar to those reported by Quistad and colleagues (2001, 2002, 2006) in mouse brain. In vivo, DFP caused extensive inhibition of rat hippocampal ChE activity (Table 1). While in vivo DFP had no effect on hippocampal MAGL activity or CB1 receptor binding, there was marked inhibition of FAAH activity. Based on its relatively weak in vitro potency against FAAH (Figure 1), it was surprising to see substantial inhibition following in vivo dosing. We previously noted, however, more extensive FAAH inhibition by the organophosphate paraoxon in vivo than was expected based on its relative in vitro inhibitory potency (Nallapaneni et al., 2006). These findings suggest that some anticholinesterases may both directly and indirectly affect FAAH activity.
DFP (2.5 mg/kg, sc) elicited classical signs of cholinergic toxicity (involuntary movements, Figure 2A; SLUD signs, Figure 2B). Co-administration of either WIN 55212-2, URB597, URB602 or AM404 significantly decreased involuntary movements elicited by DFP. Some reports have questioned the efficacy and or selectivity of URB602 at inhibiting MAGL, but recent studies confirm that URB602 can inhibit brain MAGL and increase 2-arachidonyl glycerol levels in brain slices (King et al., 2007; Vandevoorde et al., 2007). DFP had relatively minor effects on SLUD signs (Figure 2B). WIN 55212-2 had no effect while the indirect cannabinomimetics significantly reduced DFP-induced SLUD signs (Figure 2B). The more extensive involuntary movements elicited by DFP may be mediated by changes in cholinergic neurotransmission in the CNS, as opposed to a more predominant role of peripheral cholinergic signaling in elicitation of SLUD signs. There has been considerable interest in the therapeutic potential of cannabinergic drugs in neuroprotection for a variety of conditions (Alger, 2004; Lutz, 2004; Shafaroodi et al., 2004; Blair et al., 2006; Deshpande et al., 2007). Compared to many organophosphates (e.g., soman, sarin, VX), DFP is not a classical seizure-inducing compound (Gupta et al., 1985; Misulis et al., 1987), and is thought to elicit predominantly peripheral cholinergic effects. Under the present conditions, however, enhancing endocannabinoid signaling in the CNS appears to have protective actions against excessive cholinergic signaling that may initiate such critical CNS responses associated with severe OP intoxication.
DFP elicited extensive hippocampal cholinesterase inhibition, with no differences in treatment groups co-exposed to any of the cannabinergic drugs (Figure 3). As seen in the initial studies (Table 1), DFP also inhibited hippocampal FAAH activity (Figure 3). Furthermore, in rats co-exposed to DFP and URB597, significantly greater FAAH inhibition was noted. Fegley and coworkers (2005) reported that a single dose of URB597 had long-lasting effects on FAAH (i.e., there was still significant inhibition 24 hours after dosing), thus greater inhibition in rats 24 hours after treatment with both DFP and URB597 compared to DFP alone would be expected. Although DFP was a more potent in vitro inhibitor of hippocampal MAGL compared to FAAH (Figure 1), no MAGL inhibition was noted 24 hours after DFP treatment in vivo. As only one time point (24 hours after dosing) was used in our studies, it could be that the time course of inhibition is different for MAGL and FAAH. MAGL inhibition could have occurred earlier but with recovery of enzyme activity by the time of sacrifice. Both URB597 and URB62 are carbamates, however, and thus would be expected to elicit relatively long term (for hours) enzyme inhibition. It should be noted that rats treated with both DFP and URB602 also showed no MAGL inhibition. Similarly, CB1 receptor binding was unaffected by either DFP alone, or DFP and co-exposure to any of the cannabinergic compounds tested.
It has been previously reported that both anticholinesterases and cannabinoids can modulate hippocampal acetylcholine release (Fosbraey et al., 1990; Gifford et al., 1997; Carta et al., 1998; Tzavara et al., 2003; Steffens et al., 2003; Degroot et al., 2006). The toxicity of anticholinesterases is due to the inhibition of the enzyme acetylcholinesterase, thereby leading to accumulation of the neurotransmitter acetylcholine (for a review, see Pope et al., 2005). Thus, chemicals which enhance endocannabinoid signaling may inhibit the release of acetylcholine and thereby influence the expression of OP toxicity. Furthermore, anticholinesterase-mediated disruption of endocannabinoid degradation (e,g, by inhibiting FAAH or MAGL) or direct binding to cannabinoid receptors could be contributing factors in the differential toxicity of some organophosphorus anticholinesterases (Nomura et al., 2008). With DFP, it appears that these non-cholinesterase actions on components of the endocannabinoid signaling pathway would have minimal toxicological consequence, with the possible exception of FAAH inhibition.
Overall, it is clear that chemicals which enhance endocannabinoid signaling can reduce DFP toxicity. Motor signs (e.g., tremors) elicited by DFP seemed somewhat more sensitive to modulation by endocannabinoid signaling than autonomic signs of toxicity (e.g., excessive lacrimation). Along with our previous findings demonstrating that WIN 55212-2 reduces the acute toxicity of paraoxon, these results suggest that endocannabinoid signaling may be a therapeutic or prophylactic target for managing acute OP intoxication. Understanding cannabinergic-cholinergic interactions may be useful in the design of more effective therapeutic strategies for anticholinesterase toxicity and other clinical disorders involving cholinergic signaling.
Acknowledgements
This work was partially supported by research grant R01 ES009119 from the National Institute of Environmental Health Sciences, NIH (C.N.P.), and by the Oklahoma State University Board of Reagents. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interest.
References
- Acquas E, Pisanu A, Marrocu P, Di Chiara G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur J Pharmacol. 2000;401:179–185. doi: 10.1016/s0014-2999(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Acquas E, Pisanu A, Marrocu P, Goldberg SR, Di Chiara G. Delta-9 tetrahydrocannabinol enhances cortical and hippocampal acetylcholine release in vivo: a microdialysis study. Eur J Pharmacol. 2001;419:155–161. doi: 10.1016/s0014-2999(01)00967-0. [DOI] [PubMed] [Google Scholar]
- Alger BE. Endocannabinoids and their implications in epilepsy. Epilepsy Curr. 2004;4:169–173. doi: 10.1111/j.1535-7597.2004.04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS. Neuropharmacology of the endocannabinoid signaling system-molecular mechanisms, biological actions and synaptic plasticity. Curr. Neuropharmacol. 2007;5:81–97. doi: 10.2174/157015907780866910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RE, Deshpande LS, Sombati S, Falenski KW, Martin BR, DeLorenzo RJ. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther. 2006;317:1072–1078. doi: 10.1124/jpet.105.100354. [DOI] [PubMed] [Google Scholar]
- Carta G, Nava F, Gessa GL. Inhibition of hippocampal acetylcholine release after acute and repeated Delta9-tetrahydrocannabinol in rats. Brain Res. 1998;809:1–4. doi: 10.1016/s0006-8993(98)00738-0. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Köfalvi A, Wade MR, Davis RJ, Rodrigues RJ, Rebola N, Cunha RA, Nomikos GG. The endocannabinoids anandamide and 2-arachidonoylglycerol inhibit cholinergic contractility in the human colon. Mol. Pharmacol. 2006;70:1236–1245. [Google Scholar]
- Deshpande LS, Blair RE, Ziobro JM, Sombati S, Martin BR, DeLorenzo RJ. Endocannabinoids block status epilepticus in cultured hippocampal neurons. Eur J Pharmacol. 2007;558:52–59. doi: 10.1016/j.ejphar.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Sombati S, Blair RE, Carter DS, Martin BR, DeLorenzo RJ. Cannabinoid CB1 receptor antagonists cause status epilepticus-like activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci. Lett. 2007;411:11–16. doi: 10.1016/j.neulet.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug. Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fosbraey P, Wetherell JR, French MC. Neurotransmitter changes in guinea-pig brain regions following soman intoxication. J Neurochem. 1990;54:72–79. doi: 10.1111/j.1471-4159.1990.tb13284.x. [DOI] [PubMed] [Google Scholar]
- Frazzetto G. Does marijuana have a future in pharmacopoeia? EMBO Rep. 2003;4:633–642. doi: 10.1038/sj.embor.embor893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E. Endocannabinoids in the central nervous system: from neuronal networks to behavior. Curr Drug Targets CNS Neurol Disord. 2005;4:633–642. doi: 10.2174/156800705774933069. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Ghafouri N, Tiger G, Razdan RK, Mahadevan A, Pertwee RG, Martin BR, Fowler CJ. Inhibition of monoacylglycerol lipase and fatty acid amide hydrolase by analogues of 2-arachidonoylglycerol. Br J Pharmacol. 2004;143:774–784. doi: 10.1038/sj.bjp.0705948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford AN, Ashby CR. Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J Pharmacol Exper Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- Gifford AN, Tang Y, Gatley SJ, Volkow ND, Lan R, Makriyannis A. Effect of the cannabinoid receptor SPECT agent, AM 281, on hippocampal acetylcholine release from rat brain slices. Neurosci Lett. 1997;238:84–86. doi: 10.1016/s0304-3940(97)00851-3. [DOI] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Gupta RC. Classification and uses of organophosphates and carbamates. In: Gupta RC, editor. Toxicology of organophosphate and Carbamate Compounds. Sand Diego, CA: Academic Press; 2006. pp. 5–24. [Google Scholar]
- Gupta RC, Patterson GT, Dettbarn WD. Mechanisms involved in the development of tolerance to DFP toxicity. Fundam Appl Toxicol. 1985;5:S17–S28. doi: 10.1016/0272-0590(85)90111-3. [DOI] [PubMed] [Google Scholar]
- Hart CL. Increasing treatment options for cannabis dependence: a review of potential pharmacotherapies. Drug Alcohol Depend. 2005;80:147–159. doi: 10.1016/j.drugalcdep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacyglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hively RL, Mosher WA, Hoffmann FW. Isolation of trans-delta-tetrahydrocannabinol from marijuana. J Am Chem Soc. 1966;88:1832–1833. doi: 10.1021/ja00960a056. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Russell RL. Radiometric method of acetylcholinesterase measurement. Anal Biochem. 1975;64:229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Weber B, Zimmer A, Schlicker E. Enhanced acetylcholine release in the hippocampus of cannabinoid CB(1) receptor-deficient mice. Br J Pharmacol. 2001;132:1169–1173. doi: 10.1038/sj.bjp.0703987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely T, Donaldson D, Grube A. Pesticides Industry Sales and Usage. Washington, DC: Office of Prevention, Pesticides and Toxic Substances, United States Environment Protection Agency; 2004. p. 16. [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, Astarita G, Geaga JA, Luecke H, Mor M, Tarzia G, Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–330. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Lévénés C, Daniel H, Soubrié P, Crépel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J Physiol. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004;68:1691–1698. doi: 10.1016/j.bcp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Maejima T, Ohno-Shosaku T, Kano M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci. Res. 2001;40:205–210. doi: 10.1016/s0168-0102(01)00241-3. [DOI] [PubMed] [Google Scholar]
- Miller AS, Walker JM. Effects of a cannabinoid on spontaneous and evoked neuronal activity in the substantia nigra pars reticulata. Eur J Pharmacol. 1995;279:179–185. doi: 10.1016/0014-2999(95)00151-a. [DOI] [PubMed] [Google Scholar]
- Misulis KE, Clinton ME, Dettbarn WD, Gupta RC. Differences in central and peripheral neural actions between soman and diisopropyl fluorophosphate, organophosphorus inhibitors of acetylcholinesterase. Toxicol Appl Pharmacol. 1987;89:391–398. doi: 10.1016/0041-008x(87)90158-x. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ. Analyzing Data with GraphPad Prism. San Diego CA: GraphPad Software, Inc; 1995. [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C. Modulation of paraoxon toxicity by the cannabinoid receptor agonist WIN 55,212-2. Toxicology. 2006;227:173–183. doi: 10.1016/j.tox.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Blankman JL, Simon GM, Fujioka K, Issa RS, Ward AM, Cravatt BF, Casida JE. Activation of the endocannabinoid system by organophosphorus nerve agents. Nat Chem Biol. 2008;4:373–378. doi: 10.1038/nchembio.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Giuffrida A, Calignano A, Rodríguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol. Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- Pope C, Karanth S, Liu J. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ Toxicol Pharmacol. 2005;19:433–446. doi: 10.1016/j.etap.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol Appl Pharmacol. 2001;173:48–55. doi: 10.1006/taap.2001.9175. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE. Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol Lett. 2002;135:89–93. doi: 10.1016/s0378-4274(02)00251-5. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE. Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2- arachidonylglycerol and the associated hypomotility in mice. Toxicol Appl Pharmacol. 2006;211:78–83. doi: 10.1016/j.taap.2005.10.007. [DOI] [PubMed] [Google Scholar]
- SAS. Statistics and Graphics Guide, Version 3. Carey, NC: SAS Institute; 1995. [Google Scholar]
- Schlicker E, Timm J, Zentner J, Göthert M. Cannabinoid CB1 receptor-mediated inhibition of noradrenaline release in the human and guinea-pig hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:583–589. doi: 10.1007/pl00005093. [DOI] [PubMed] [Google Scholar]
- Shafaroodi H, Samini M, Moezi L, Homayoun H, Sadeghipour H, Tavakoli S, Hajrasouliha AR, Dehpour AR. The interaction of cannabinoids and opioids on pentylenetetrazole-induced seizure threshold in mice. Neuropharmacology. 47:390–400. doi: 10.1016/j.neuropharm.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 2004;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens M, Szabo B, Klar M, Rominger A, Zentner J, Feuerstein TJ. Modulation of electrically evoked acetylcholine release through cannabinoid CB1 receptors: evidence for an endocannabinoid tone in the human neocortex. Neuroscience. 2003;120:455–465. doi: 10.1016/s0306-4522(03)00318-x. [DOI] [PubMed] [Google Scholar]
- Steffens M, Engler C, Zentner J, Feuerstein TJ. Cannabinoid CB1 receptor-mediated modulation of evoked dopamine release and of adenylyl cyclase activity in the human neocortex. Br J Pharmacol. 2004;141:1193–1203. doi: 10.1038/sj.bjp.0705706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- Tsou K, Nogueron MI, Muthian S, Sañudo-Pena MC, Hillard CJ, Deutsch DG, Walker JM. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett. 1998;254:137–140. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Wade M, Nomikos GG. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. J Neurosci. 2003;23:9374–9384. doi: 10.1523/JNEUROSCI.23-28-09374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoorde S, Jonsson KO, Labar G, Persson E, Lambert DM, Fowler CJ. Lack of selectivity of URB602 for 2-oleoylglycerol compared to anandamide hydrolysis in vitro. Br J Pharmacol. 2007;150:186–191. doi: 10.1038/sj.bjp.0706971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Zhu PJ. Endocannabinoid signaling and synaptic plasticity in the brain. Crit Rev Neurobiol. 2006;18:113–124. doi: 10.1615/critrevneurobiol.v18.i1-2.120. [DOI] [PubMed] [Google Scholar]