Abstract
Objectives
By using proteomics we isolated and identified proteins that were expressed/retained in stable and unstable human carotid artery atherosclerotic plaques.
Methods
The criteria for plaque instability were the presence of a thin fibrous cap or fissured cap covering the foamy or necrotic core, and the presence of overt, hemorrhagic, ulcerated or thrombotic plaques. Proteins were extracted from finely minced endarterectomy specimens (19 stable, 29 unstable plaques) and separated by 2-dimensional gel electrophoresis. Coomassie Blue-stained gels were analysed using PD-Quest software.
Results
A total of 57 distinct spots corresponding to 33 different proteins were identified by matrix assisted laser desorption/ionization mass spectrometry using the NCBI database. Most of the spots were present in both types of extracts, although significantly (p<0.05) differing in abundance between them. Compared to stable plaque, unstable ones showed reduced abundance of: protective enzymes SOD3 and GST, small heat shock proteins HSP27 and HSP20, annexin A10, and Rho GDI. In unstable plaques the more abundant proteins were: ferritin light subunit, SOD 2 and fibrinogen fragment D. For fibrinogen fragment D, the increased levels in unstable versus stable plaques was confirmed by Western blot analysis.
Conclusions
Since many of the differentially expressed proteins are known to have a functional role in inflammation and oxidative stress, we speculate that they may be involved in events relating to plaque stability.
Introduction
Atherosclerosis is a multifactorial disease in which hypertension, diabetes, hyperlipidemia and other risk factors are thought to play a role. However, the mechanisms underlying plaque formation and progression are not completely known. The rupture of the atherosclerotic plaque is the predominant underlying process in the pathogenesis of acute coronary syndromes and peripheral vascular disease (1–3). There are uncertainties about the definition of plaque instability and availability of reliable specific markers to identify plaques prone to rupture in vivo although it is generally held that plaque instability is caused by a substantial increase in proteolytic activity and inflammatory state.
We previously provided evidence that in unstable and much less in stable carotid endarterectomy plaques, there is a wide fragmentation of some apolipoproteins derived from the plasma and of arterial proteoglycans in a background of a pro-inflammatory and proteolytic microenvironment (4).
The current studies were carried out on the premise that plaque stability/instability is associated with distinct patterns of protein expression (5–7) and to this effect we initiated a detailed study of proteomic maps of plaques utilizing extracts. Until now, proteomics of atherosclerotic plaques has been carried out on either secretomes or homogenates. Martin Ventura et al. (8) by studying the protein secretion profiles obtained from cultured atherosclerotic plaques have identified heat shock protein 27 as biomarker of atherosclerosis and, more recently, some other proteins comprising cathepsin D (9), that could have a role in plaque instability. The proteomic studies that have been conducted on plaque homogenates did not take into account the heterogeneity in cellular composition of the atherosclerotic plaques. An elevated expression of α1-antitrypsin has been reported in advanced endarterectomy carotid lesions (10) and ferritin light chain in atherosclerotic coronary arteries obtained at autopsy (11). Recently, Sung et al. (12) reported a panel of proteins highly expressed in homogenates from atherosclerotic aortas. While this manuscript was in preparation, Bagnato et al. (13), by using direct tissue proteomics, provided a large scale map of proteins expressed within atherosclerotic lesions of human coronary arteries. We have analyzed plaque extracts, rather than secretomes or homogenates, in order to permit an enrichment in both topically expressed and filtered/retained proteins. Among the positive features of our “in situ” study are the utilization of a relatively large number of samples, their uniform origin from surgical endarterectomy rather than from post-mortem material, so avoiding the occurrence of proteolytic modifications prior to analysis, and most important, their careful histological characterization. Moreover, all of the more represented proteins were identified.
In the present study, by applying proteomics to human carotid artery plaque extracts, we have identified a panel of proteins that are differently represented in stable or unstable plaques, thus providing an additional means for analyzing the molecular processes involved in plaque destabilization in vivo. Western blotting was used to confirm different levels of selected proteins in plaque extracts. The results obtained are the subject of this report.
Material and methods
Tissue sampling
Proteomic analyses were conducted on carotid plaque specimens from forty eight patients (stenosis >70%; 35 man/13 women; age, 70±7; 91.5% hypertensive; 20% diabetic; 45.8% dyslipidemic) undergoing carotid endarterectomy, enrolled in a previous study and screened for plaque stability (4). Informed consent was obtained before enrolment. The study was approved by the local Ethical Committee of the University of Milan in accordance with institution guidelines and conformed with the principles outlined in the Declaration of Helsinki. The clinical and lipid data did not differ between the two subgroup of patients, as previously reported (4).
Carotid endarterectomy samples were dissected and histologically classified as stable or unstable plaques, as described previously (4). The surgically excised plaques were exhaustively rinsed in ice-cold saline (10 mL, three changes), supplemented with 100μM APMSF (4-amidinophenylmethane-sulfonylfluoride), 2μg/ml Kallikrein Inactivator (cyclohexylacetyl-Phe-Arg-Ser-Val-Gln amide), 50 μM leupeptin (Acetyl-Leu-Leu-Arg-Hydrochloride) and dissected into 2 segments: one for biochemical studies and the other for pathology and plaque classification.
Smooth muscle cells, histiocytes/macrophages and endothelial cells in stable and unstable plaques have been evaluated by immunocytochemistry. To identify the different cellular lines present in the plaques, immunocytochemical detection of Alpha Smooth Muscle Actin (1A4 clone, 1:1000, BioGenex), CD68 (KP1 clone, 1:10000, Dako) and CD31 (JC70A clone, 1:20, Dako) has been performed. Briefly, after incubation with the primary antibodies, the reaction was performed using the Novolink Polymer Detection System (Novocastra) and developed by diaminobenzidine.
According to Stary’s classification, stable plaques were considered those having a thick fibrous or fibrocalcific component, lined by endothelium, covering a possible central foamy or necrotic core (type IV, Vb and Vc). The criteria for plaque instability were the presence of a thin fibrous cap or fissured cap covering the foamy or necrotic core (some type IV and Va), and the presence of overt, hemorrhagic, ulcerated or thrombotic plaques (type VI).
Sample preparation for proteomics
The segments for biochemical analyses were placed in liquid nitrogen and stored at −80°C. Before analysis, the plaque segments were thawed to 4°C, washed in PBS, weighed, and finely minced with a tissue slicer blade. The minces were extracted with a solubilization buffer (8 M urea, 4% (w/v) CHAPS, 45 mM Tris) containing the same antiproteolytic agents as those used for washing solution at a ratio of 1 g wet weight tissue for 7 mL of extraction buffer under continuous shaking for 1 hour at room temperature. The resulting suspension was centrifuged at 65000 g in a TL-100 Beckman centrifuge for 30 minutes at 20°C, and both supernatants and pellets were collected.
Extracts were delipidated by adding ice-cold tri-n-butylphosphate:acetone:methanol (1:12:1) to a final acetone concentration of 80% and incubated at 4°C for 90 min. Precipitates were re-solubilized in the same extraction buffer by repeated sonications. Before and after delipidation, the protein concentration was determined by a DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA) using bovine serum albumin as a standard.
2D electrophoresis analysis
IEF was performed using 70 mm, immobilised linear pH 4–8 gradient strips (Nurex srl, Sassari, Italy). IPG strips were re-hydrated overnight at 20°C in a re-swelling tray with 300 μg of protein diluted in solubilization buffer containing 1% w/v DTT and 2% v/v Pharmalyte (pH 3.5–10) and subsequently focused at 50 μA/IPG strip for 22 kVh at 18°C.
Once IEF was completed, the strips were equilibrated under continuous shaking for 15min in Tris-HCl 50mM containing 6 M urea, 30% v/v glycerol, and 3% w/v SDS with the addition of 1% w/v DTT, followed by an equilibration for 15 min in the same buffer without DTT, but with the addition of 2.5% w/v iodoacetamide.
The IPG strips were then sealed with 0.5% low melting point agarose in SDS running buffer at the top of slab gels (8 ×7 ×0.1 cm).
SDS-PAGE was performed using 10%T, 3%C separating polyacrylamide gels without a stacking gel, in a MiniProtean II cell vertical slab gel electrophoresis apparatus (Bio-Rad, Hercules, CA, USA).
The second dimension was carried out at 50V for 15 min and subsequently at 150V until the Bromophenol dye front had reached the lower limit of the gel.
Gels were stained with Mass Compatible Super Blue Stain Kit (Nurex srl, Sassari) and scanned at 36.3 μm resolution using a GS-800 densitometer (Bio-Rad, Hercules, CA, USA). The dynamic range for resolved protein was 150-0.03 μg.
Image analysis was performed using the PDQuest 2-D V 6.2.0 software (Bio-Rad, Hercules, CA, USA) and the spots of interest were processed for MALDI-TOF MS analysis.
In-gel digestion and MALDI-TOF MS analysis
Spots of interest were excised with a scalpel or a steril pipette tip and destained with 100 μl of 5mM NH4HCO3/50% acetonitrile.
Gel pieces were then dehydrated with acetonitrile, dried at room temperature and reswollen with 10μl of 5mM NH4HCO3 containing 10ng/μl trypsin for 40 minutes on ice. Subsequently, excess of digestion buffer was removed and substituted with an equal volume of 5mM NH4HCO3. Tryptic digestion was conducted over night at 37°C. The peptide mixtures were stored at 4 °C until assayed. The peptides extracted from the gel pieces were mixed with an equal volume of 10 mg/mL α-cyano-4-hydroxy-cinnamic acid in 40% v/v acetonitrile/0.1% v/v trifluoroacetic acid and the mixed solution was applied as a microcrystalline thin film onto a 96-spot MALDI target. MALDI-MS was performed using a Micromass TofSpec 2E spectrometer (Manchester, UK), equipped with a 337 nm nitrogen laser. The instrument operated in the positive ion reflectron mode at 20 kV accelerating voltage with time-lag focusing.
Each mass spectrum was generated by accumulating data from 100 to 120 laser shots.
Spectra were calibrated by close spot internal calibration using trypsin, and the proteins were identified by peptide mass fingerprinting with the search program ProFound provided by Rockefeller University (www.unb.br/cbsp/paginiciais/profound.htm) which compares the experimentally determined tryptic peptide masses with theoretical peptide masses calculated for proteins contained in the nonredundant National Center for Biotechnology Information (NCBI) database. The search parameters included: taxa Homo sapiens, trypsin digest, protein molecular mass and pI ranges from the 2-D gels, iodoacetamide modifications, monoisotopic peptide masses, one missed cleavage by trypsin, and a mass deviation of 50–100 ppm. Identification of protein spots was obtained from triplicate analysis.
Statistical analysis was performed with t test or Rank Sum test. Values were considered significant at p≤0.05.
Western blot analysis
Western blotting was used to compare expression of selected proteins between stable and unstable extracts. 20 μg of extracted proteins were resolved by SDS-PAGE in 10%T, 3%C gels and electroblotted at 0.25 constant A onto PVDF membranes (GE Healthcare, UK). After transfer, membranes were blocked for 1 h at room temperature with 3% non-fat dry milk in PBS-Tween-20 (0.1% Tween-20 in PBS) and incubated overnight at 4 °C with anti-fibrinogen β antibody (Santa Cruz Biotechnology, CA, USA) or anti-transferrin antibody (Santa Cruz Biotechnology, CA, USA) both diluted 1:200 in the same blocking buffer. Then, membranes were washed with PBS-Tween-20 and incubated for 1 h at room temperature with the (horseradish peroxidase) HRP-conjugated secondary antibody (Santa Cruz Biotechnology, CA) diluted 1:4000 in blocking buffer. After membrane washing, proteins were visualized by ECL/autography followed by Q-One software densitometric analysis (Bio-Rad Hercules, CA, USA).
Results
Smooth muscle cells, histiocytes/macrophages and endothelial cells have been detected in both stable and unstable plaques. Nevertheless, all cellular types are more represented in unstable plaques in comparison to stable ones. In particular, smooth muscle cells and histiocytes are largely present in the necrotic core of unstable plaques, admixed with neo-formed vessels. On the other hand, in stable plaques the cellular component is much less represented, and hyalinized collagen is largely prevalent. Examples of immunocytochemical detection in stable and unstable plaques are given as supplementary files.
Our proteomic strategy used 2D gel electrophoresis to separate the extractable proteins from minced endarterctomy samples. We found no significant difference in total protein extractability between stable and unstable plaques (40.9±12.3 versus 48.3±14.1 μg protein/mg weight of wet tissue, p=0.068). Reproducible 2D gel patterns were obtained from both stable and unstable plaque extracts. A typical example of 2D gel profile is shown in Fig.1(a). A high resolution image analysis detected approximately 130 protein spots on each gel after Coomassie Blue staining, the most representative of which were identified by peptide mass fingerprinting analysis.
Figure 1.
(a) Representative 2D PAGE map of a plaque extract after Blue Coomassie G-250 staining. The molecular weight (MW) scale was constructed from protein standards (Invitrogen BenchMark Protein Ladder) run alongside the focused strip in the second dimension. The pI scale was constructed from the dimensions of the linear pH gradient strip (pH range 4–8 linear over 7 cm). The numbers indicated on the gels correspond to the spot numbers in Table 1 and in the table given as supplementary file. For experimental conditions, see text. (b) Close-up of portions of the 2-DE gel images showing proteins differently represented in unstable and stable plaque extracts. (c) Western blot analysis of plaque extracts using an antibody raised against a peptide mapping near the C-terminus of fibrinogen β of human origin and an anti-transferrin antibody as control.
All the identified proteins, their theoretical molecular weight (Mr), isoelectric point (pI), number of matched peptides, and the percentage of sequence coverage are listed in a supplementary file. The sequence coverage ranged from 10% for spot 34 to 62% for spot 57.
Image analysis data permitted us to establish that most of extracted proteins were of plasma origin (about 70%). The distributions of the identified proteins in both plasma-derived and constitutive protein subpopulations are reported in fig. 2 (panels a and b, respectively).
Figure 2.
Distribution of the identified proteins in plaque extracts after sorting for plasma-derived (panel a) and constitutive (panel b) subpopulations. Proteins in panel a represented about the 70% of the total, irrespective from plaque tipology.
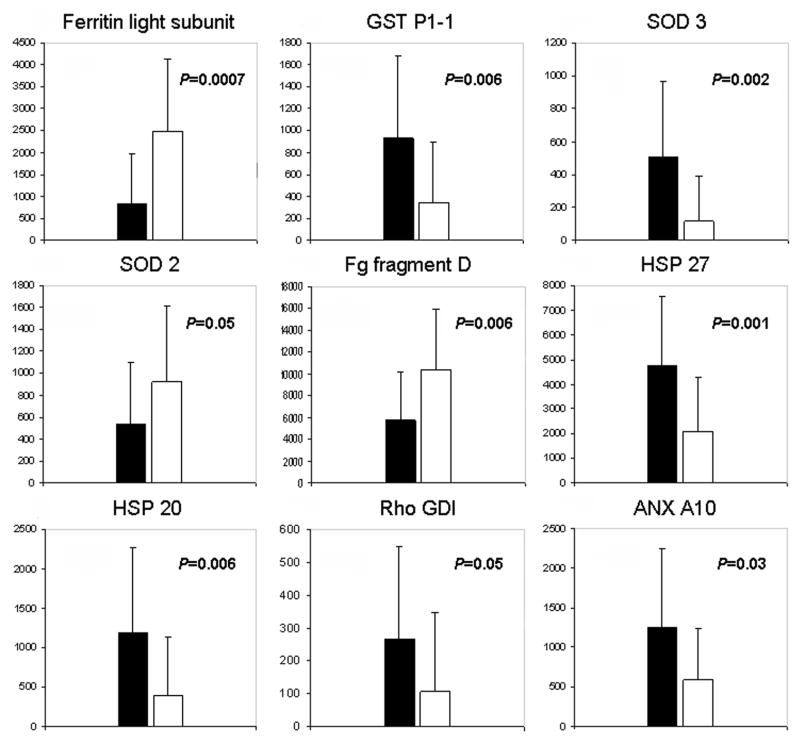
The analysis of 2D patterns obtained from 19 stable and 29 unstable plaques identified nine proteins with a level of expression significantly different between the two types of plaques (see Fig. 3 and table 1). Compared to the extracts of stable plaques, the unstable ones showed an increased concentrations of ferritin light subunit (UN/ST=2.98, p=0.0007), superoxide dismutase 2 (SOD2) (UN/ST=1.71, p=0.05) and fibrinogen fragment D (UN/ST=1.81, p=0.006) and a reduced expression of superoxide dismutase 3 (SOD3) (UN/ST=0.22, p=0.002), glutathione S-transferase (GST) (UN/ST=0.36, p=0.006), Rho GDP-dissociation inhibitor 1 (Rho-GDI) (UN/ST=0.41, p=0.05), annexin A10 (ANX A10) (UN/ST=0.47, p=0.038), HSP20 (UN/ST=0.33, p=0.006) and HSP27 (UN/ST=0.44, p=0.001). Table 1also lists the main physiological functions described in the literature for the above mentioned proteins.
Figure 3.
Graphic representation of the results obtained by 2D map differential analysis of proteins extracted from stable (black bars) and unstable (empty bars) plaques. On the y axis the spot intensities in arbitrary units are reported. Error bars represent standard deviations.
Table 1.
List of proteins differentially expressed in unstable and stable plaque extracts
| Spot no. | Identified protein | UN/ST | p | Known function | References |
|---|---|---|---|---|---|
| 57 | Ferritin light subunit | 2,98 | 0,0007 | Pro- or anti-oxidant agent? | [14] |
| 52 | Glutathione Transferase P1-1 | 0,36 | 0,006* | Protection from reactive electrophiles in the vascular media. | [16,17] |
| 43 | SOD3 (Cu-Zn) | 0,22 | 0,002* | Defence against the superoxide anion. | [18] |
| Preservation of NO bioactivity. | [19] | ||||
| 55 | SOD2 protein (Mn) | 1,71 | 0,05 | Defence against the superoxide anion in the mitochondria. | [18] |
| 25,26,27,28,29 | Fibrinogen Fragment D | 1,81 | 0,006 | Vascular constriction via ICAM-1. | [22] |
| Disorganization of cultured vascular endothelial cell monolayers. | |||||
| Increased cytokine/chemokine production and macrophage adhesion in vivo. | [23,24] | ||||
| 44,45,46 | HSP27 | 0,44 | 0,001* | Regulation of smooth muscle tone. | [25] |
| Chaperone activity. | [26] | ||||
| Anti-apoptotic agent. | [28] | ||||
| 56 | HSP20 | 0,33 | 0,006* | Regulation of smooth muscle tone. | [25] |
| Chaperone activity. | [26] | ||||
| Inhibition of platelet aggregation. | [27] | ||||
| 48 | Rho GDI | 0,41 | 0,05* | Regulation of cell growth and motylity. | [28,29] |
| 34 | Annexin A10 protein | 0,47 | 0,038* | Unknown. |
Proteins are organised by the number assigned on the gel.
Statistical analysis was performed with t test or Rank Sum test (*)
For Western blotting-differential proteomics confirmatory studies a selected antibody against the C-terminus of fibrinogen β was used to examine protein levels in unstable compared with stable plaque extracts. Blots were also stained with anti-transferrin antibody as a control (see Fig.1(c)).
Discussion
The main focus of this study was to determine whether atherosclerotic plaques from human carotid arteries classified as either stable or unstable by histological and immunocytochemical criteria, exhibited differences in expressed proteins by a proteomic approach. Extracted tissues in urea containing solvents were used in a sub-proteomic technology intended to enrich the samples in both topical and filtered proteins in order to improve their identification in the vascular tissue by mass spectrometry.
We identified 95.6% of total extractable proteins resolved by 2D electrophoresis: about 1/3 constitutive and about 2/3 filtered. The high percentage of plasma proteins that accumulated within the atherosclerotic plaques comprising albumin, transferrin, hemoglobin, apo A1, fibrinogen, suggests the existence of an impaired endothelial barrier function independent of plaque type. In this regard, the ratios transferrin (spot 1) to albumin (spot 2) were approximately three-fold higher than in the corresponding plasma samples. Moreover, our differential expression analyses, permitted the detection of significant differences among proteins having a level of expression below 1% with reference to the total extractable proteins. On the other hand, and in support of the specificity of our findings, the most abundant extractable functional proteins, whether filtered or constitutive, occurred in about equal abundance in both stable and unstable plaques.
Of note, the important differences in expressed proteins between the extracts of stable and unstable plaques were equally exhibited in all patients undergoing elective endarterectomy for carotid artery disease. Moreover, we have further validate our differential proteomics results by immunoblot analysis.
As indicated in Fig 3 and Table 1 two main classes of proteins were identified both recognized to play a role in either oxidative or inflammatory processes and in the formation and progression of the atherosclerotic plaque.
Proteins involved in oxidative processes
The analysis of 2D maps of plaque extractable proteins proved to be informative in terms of differentiating stable from unstable plaques.
Unstable plaque extracts showed an overexpression of ferritin light chain. An either pro-oxidant or anti-oxidant role for this protein in atherosclerosis has been suggested (14). In this regard, a positive correlation between plasma ferritin levels and both carotid artery wall thickening and coronary artery disease has been reported previously (14). Since ferritin synthesis is upregulated by some proinflammatory cytokines, ferritin light chain expression might be viewed as a marker of inflammation. Interestingly, iron loaded macrophages primed with pro-atherogenic oxidized LDL have been shown to display an increased synthesis and secretion of ferritin (15). The significant association between increased ferritin light chain levels in plaque extracts and plaque instability supports the view that ferritin light chain is a candidate marker of atherosclerosis progression (10).
We also found that plaque instability is associated with decreased levels of glutathione S-transferase (GST), that is known to be an important vascular defence factor by protecting blood vessels against vascular toxins α,β unsaturated carbonyl and 4-hydroxy-2-nonenale, and that has been implicated in the early phase of atherosclerotic lesions (16, 17). Further, in unstable plaques the differential expression of GST suggests an impairment of the ROS by-products scavenging.
Plaque instability was also associated with a reduced expression of the extracellular isoform of superoxide dismutase (SOD3) and an increased expression of its mithocondrial isoform (SOD2).
The functional importance of the individual SOD isoforms within the vessel wall, either under normal condition or during vascular disease, is yet unclear. In the vascular extracellular matrix, SOD3 is thought to represent the major defence against the superoxide anion radical (18) and in macrophage-rich areas of the atherosclerotic lesions, has been reported to co-localize with oxidized lipoproteins and peroxynitrite-modified proteins (19). Proteomic studies have also shown an increased level of SOD2 in monocytes primed with lipopolysaccharide (20), whereas an upregulated SOD3 was noted in the secretome of THP1 cells primed with ox-LDL (21). Both isoenzymes are variably regulated by inflammatory cytokines, therefore, their differential expression in relation to plaque instability may reflect more pronounced pro-oxidative and pro-inflammatory conditions (see also below).
Proteins involved in inflammatory processes
A broad spectrum of inflammatory mediators are expressed at the site of plaque rupture in human atherosclerotic lesions (2,3).
In our current study, we found plaque instability associated with increased levels of fibrinogen fragment D and with reduced levels of HSP20, HSP 27, Rho GDI, and ANX A10.
Several effects of fibrinogen fragment D on vascular cells have been described, namely increased vascular tone, endothelial disorganization, and increased endothelial permeability to albumine. It is also known that fibrin(ogen) directly stimulates the in vitro secretion of cytokines and chemokines by several cellular type and mediation of firm attachment of monocytes/leukocytes to endothelium (22,23). Moreover, the binding of fibrinogen and fragment D to the vascular wall has been shown to stimulate the release in vivo of pro-inflammatory and chemotactic factors, such as MCP-1 and IL-6 (24). We may speculate that our observed increased levels of fibrinogen fragment D in unstable plaques may be related to an increased propensity for intraplaque hemorrage and to contribute to plaque instability.
The small heat shock proteins HSP27 and HSP20 are constitutively highly expressed in muscle cells where they have a role chaperone activity and in the regulation of smooth muscle tone (25). It has been suggested that the function of the small HSPs in the vascular smooth muscle cells may be dependent on degree of phosphorylation and macromolecular associations (25). In our studies, we resolved three HSP27 isoforms, probably corresponding to different phosphorylation states, and one HSP20 isoform at basic pH. In cardiovascular diseases, HSP expression is modulated both at the lesion site and in the plasma and is thought to interfere with the atherosclerotic inflammatory response (26). It has also been reported that HSP20, but not HSP27, affect thrombin-mediated platelet aggregation preventing thrombus formation (27), whereas HSP27 can down regulate the apoptotic signalling pathway and thus contribute to stabilize the atherosclerotic lesions (28).
Our findings in unstable plaque extracts regarding the reduced levels of HSP27 corroborate the results by Martin-Ventura et al. (8), showing decreased level of HSP27 in secretomes from cultured complicated atherosclerotic plaques. In those studies, the patients with carotid atherosclerosis had decreased plasma levels of HSP 27 relative to healthy subjects (8).
Regarding Rho GDI, it is known that the inhibition of its dissociation is a powerful modulator of a great number of cellular responses to pro-inflammatory cytokines. By proteomic approaches, it has been reported that in cultured SMC, Rho GDI is down regulated by TNF-α (29) and that it is reduced in its expression in monocyte-derived macrophages primed with both LDL and ox-LDL (30). Our in situ proteomic observation on Rho GDI expression, is in keeping with the above in vitro results.
Our results on annexin A10 are difficult to interpret since it is the least known member of the annexin (ANX) family for which a function is still unknown. Since ANX A10 is more expressed in stable plaques, we may speculate that it is involved in events leading to plaque stabilization, probably in relation to the anti-coagulant, anti-apoptotic and anti-inflammatory activities of the ANX superfamily.
The limitation of our current data rests on the fact that we studied the whole plaques that are known to be structurally heterogeneous, as also shown by our previous studies conducted in human carotid artery plaques from a different source using sections generated by laser micro-dissection (31).
Nonetheless, we believe that the information derived from the current studies on plaque extracts is novel in that it add information to the data obtained on homogenates (10–12) or “in vitro” secretomes (8,9) from total arterial tissue.
In this respect, it should be noticed that, in a single lesion, a variable degree of histological heterogeneity is present. This probably accounts for different gene and protein expression results obtained either in different regions of the same plaque sample or in plaques showing different histological typology (5,7). Recent studies showed the feasibility to analyze laser micro-dissected tissue samples to partially overcome cell heterogeneity, but proteomic data on dissected tissues are not yet available. To address this problem we focused our study on the “in situ” filtered/secreted protein fraction.
In summary, the results of our present study have provided evidence for the usefulness of the proteomic approach in differentiating stable from unstable human carotid artery plaques and uncovered the presence, in unstable plaques, of a panel of proteins with pro-oxidant and pro-inflammatory potentials in accord with our current understanding of the molecular basis of the atherosclerotic process. Our results also invite further direct exploration of the biological role of the identified proteins including their potential post-translational modifications and involvement in athero-relevant signal pathways.
Supplementary Material
Supplementary figures. Panel 1: Stable plaque (Hematoxylin&Eosin, upper left): inside the fibrotic core, only scanty cells are positive to smooth muscle actin (a-SMA, upper right), CD68 (lower right) and CD31 (lower left). Actin staining also demonstrated the residual muscular wall of the vessel. Panel 2: Unstable plaque (Hematoxylin&Eosin, upper left): the necrotic core of the plaque is largely composed of cells immunoreactive to smooth muscle actin (a-SMA, upper right), CD68 (lower right) and CD31 (lower left).
Acknowledgments
Funding
This study was supported by Fondazione Banco di Sardegna grants # 684/2006.0424 (Formato), and #738/2007.0671(Cherchi), Sassari, Italy and by NIH-NHLBI grant# 63209 (Scanu).
We thank dr. Anna Guarino for technical assistance.
References
- 1.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8(11):1211–7. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 2.Peter Libby. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Lutgens E, van Suylen RJ, Faber BC, Gijbels MJ, Eurlings PM, Bijnens AP, Cleutjens KB, Heeneman S, Daemen MJ. Atherosclerotic plaque rupture: local or systemic process? Arterioscler Thromb Vasc Biol. 2003;23(12):2123–30. doi: 10.1161/01.ATV.0000097783.01596.E2. [DOI] [PubMed] [Google Scholar]
- 4.Formato M, Farina M, Spirito R, Maggioni M, Guarino A, Cherchi GM, Biglioli P, Edelstein C, Scanu AM. Evidence for a proinflammatory and proteolytic environment in plaques from endarterectomy segments of human carotid arteries. Arterioscler Thromb Vasc Biol. 2004;24(1):129–35. doi: 10.1161/01.ATV.0000104013.71118.53. [DOI] [PubMed] [Google Scholar]
- 5.Duran MC, Mas S, Martin-Ventura JL, Meilhac O, Michel JB, Gallego-Delgado J, Lázaro A, Tuñon J, Egido J, Vivanco F. Proteomic analysis of human vessels: application to atherosclerotic plaques. Proteomics. 2003;3(6):973–8. doi: 10.1002/pmic.200300389. [DOI] [PubMed] [Google Scholar]
- 6.Blanco-Colio LM, Martín-Ventura JL, Vivanco F, Michel JB, Meilhac O, Egido J. Biology of atherosclerotic plaques: what we are learning from proteomic analysis. Cardiovasc Res. 2006;72(1):18–29. doi: 10.1016/j.cardiores.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Krupinski J, Turu MM, Martinez-Gonzalez J, Carvajal A, Juan-Babot JO, Iborra E, Slevin M, Rubio F, Badimon L. Endogenous expression of C-reactive protein is increased in active (ulcerated noncomplicated) human carotid artery plaques. Stroke. 2006;37(5):1200–4. doi: 10.1161/01.STR.0000217386.37107.be. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Ventura JL, Duran MC, Blanco-Colio LM, Meilhac O, Leclercq A, Michel JB, Jensen ON, Hernandez-Merida S, Tuñón J, Vivanco F, Egido J. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 2004;110(15):2216–9. doi: 10.1161/01.CIR.0000136814.87170.B1. [DOI] [PubMed] [Google Scholar]
- 9.Durán MC, Martín-Ventura JL, Mohammed S, Barderas MG, Blanco-Colio LM, Mas S, Moral V, Ortega L, Tuñón J, Jensen ON, Vivanco F, Egido J. Atorvastatin modulates the profile of proteins released by human atherosclerotic plaques. Eur J Pharmacol. 2007;562(1–2):119–29. doi: 10.1016/j.ejphar.2007.01.077. [DOI] [PubMed] [Google Scholar]
- 10.Donners MM, Verluyten MJ, Bouwman FG, Mariman EC, Devreese B, Vanrobaeys F, van Beeumen J, van den Akker LH, Daemen MJ, Heeneman S. Proteomic analysis of differential protein expression in human atherosclerotic plaque progression. J Pathol. 2005;206(1):39–45. doi: 10.1002/path.1749. [DOI] [PubMed] [Google Scholar]
- 11.You SA, Archacki SR, Angheloiu G, Moravec CS, Rao S, Kinter M, Topol EJ, Wang Q. Proteomic approach to coronary atherosclerosis shows ferritin light chain as a significant marker: evidence consistent with iron hypothesis in atherosclerosis. Physiol Genomics. 2003;13(1):25–30. doi: 10.1152/physiolgenomics.00124.2002. [DOI] [PubMed] [Google Scholar]
- 12.Sung HJ, Ryang YS, Jang SW, Lee CW, Han KH, Ko J. Proteomic analysis of differential protein expression in atherosclerosis. Biomarkers. 2006;11(3):279–90. doi: 10.1080/13547500500525458. [DOI] [PubMed] [Google Scholar]
- 13.Bagnato C, Thumar J, Mayya V, Hwang SI, Zebroski H, Claffey KP, Haudenschild C, Eng JK, Lundgren DH, Han DK. Proteomics analysis of human coronary atherosclerotic plaque: a feasibility study of direct tissue proteomics by liquid chromatography and tandem mass spectrometry. Mol Cell Proteomics. 2007;6(6):1088–102. doi: 10.1074/mcp.M600259-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.You SA, Wang Q. Ferritin in atherosclerosis. Clin Chim Acta. 2005;357(1):1–16. doi: 10.1016/j.cccn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Yuan XM, Li W, Baird SK, Carlsson M, Melefors O. Secretion of ferritin by iron-laden macrophages and influence of lipoproteins. Free Radic Res. 2004;38(10):1133–42. doi: 10.1080/10715760400011692. [DOI] [PubMed] [Google Scholar]
- 16.He NG, Awasthi S, Singhal SS, Trent MB, Boor PJ. The role of glutathione S-transferases as a defense against reactive electrophiles in the blood vessel wall. Toxicol Appl Pharmacol. 1998;152(1):83–9. doi: 10.1006/taap.1998.8511. [DOI] [PubMed] [Google Scholar]
- 17.Cao Z, Hardej D, Trombetta LD, Li Y. The role of chemically induced glutathione and glutathione S-transferase in protecting against 4-hydroxy-2-nonenal-mediated cytotoxicity in vascular smooth muscle cells. Cardiovasc Toxicol. 2003;3(2):165–177. doi: 10.1385/ct:3:2:165. [DOI] [PubMed] [Google Scholar]
- 18.Faraci FM, Didion SP. Vascular Protection Superoxide Dismutase Isoforms in the Vessel Wall. Arterioscler Thromb Vasc Biol. 2004;24(8):1367–73. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 19.Luoma JS, Strålin P, Marklund SL, Hiltunen TP, Särkioja T, Ylä-Herttuala S. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arterioscler Thromb Vasc Biol. 1998;18(2):157–67. doi: 10.1161/01.atv.18.2.157. [DOI] [PubMed] [Google Scholar]
- 20.Gadgil HS, Pabst KM, Giorgianni F, Umstot ES, Desiderio DM, Beranova-Giorgianni S, Gerling IC, Pabst MJ. Proteome of monocytes primed with lipopolysaccharide: analysis of the abundant proteins. Proteomics. 2003;3(9):1767–80. doi: 10.1002/pmic.200300532. [DOI] [PubMed] [Google Scholar]
- 21.Fach EM, Garulacan LA, Gao J, Xiao Q, Storm SM, Dubaquie YP, Hefta SA, Opiteck GJ. In vitro biomarker discovery for atherosclerosis by proteomics. Mol Cell Proteomics. 2004;3(12):1200–10. doi: 10.1074/mcp.M400160-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Harley SL, Sturge J, Powell JT. Regulation by fibrinogen and its products of intercellular adhesion molecule-1 expression in human saphenous vein endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20(3):652–8. doi: 10.1161/01.atv.20.3.652. [DOI] [PubMed] [Google Scholar]
- 23.Sriramarao P, Languino LR, Altieri DC. Fibrinogen mediates leukocyte-endothelium bridging in vivo at low shear forces. Blood. 1996;88(9):3416–23. [PubMed] [Google Scholar]
- 24.Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002;99(3):1053–59. doi: 10.1182/blood.v99.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs LC, Giulumian AD, Knoepp L, Pipkin W, Dickinson M, Hayles C, Brophy C. Stress causes decrease in vascular relaxation linked with altered phosphorylation of heat shock proteins. Am J Physiol Regul Integr Comp Physiol. 2000;279(2):R492–8. doi: 10.1152/ajpregu.2000.279.2.R492. [DOI] [PubMed] [Google Scholar]
- 26.Pockley AG. Heat shock proteins, inflammation, and cardiovascular disease. Circulation. 2002;105(8):1012–7. doi: 10.1161/hc0802.103729. [DOI] [PubMed] [Google Scholar]
- 27.Kanno Y, Matsuno H. The possibility of novel antiplatelet peptides: the physiological effects of low molecular weight HSPs on platelets. Curr Pharm Des. 2006;12(7):887–92. doi: 10.2174/138161206776056047. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Ventura JL, Nicolas V, Houard X, Blanco-Colio LM, Leclercq A, Egido J, Vranckx R, Michel JB, Meilhac O. Biological significance of decreased HSP27 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(6):1337–43. doi: 10.1161/01.ATV.0000220108.97208.67. [DOI] [PubMed] [Google Scholar]
- 29.Jang WG, Kim HS, Park KG, Park YB, Yoon KH, Han SW, Hur SH, Park KS, Lee IK. Analysis of proteome and transcriptome of tumor necrosis factor alpha stimulated vascular smooth muscle cells with or without alpha lipoic acid. Proteomics. 2004;4(11):3383–93. doi: 10.1002/pmic.200400972. [DOI] [PubMed] [Google Scholar]
- 30.Kang JH, Kim HT, Choi MS, Lee WH, Huh TL, Park YB, Moon BJ, Kwon OS. Proteome analysis of human monocytic THP-1 cells primed with oxidized low-density lipoproteins. Proteomics. 2006;6(4):1261–73. doi: 10.1002/pmic.200500290. [DOI] [PubMed] [Google Scholar]
- 31.Joy ST, Patel PN, Pfaffinger D, Edelstein C, Scanu AM. Apolipoprotein(a) in the carotid artery plaque: evidence for proteolytic and pro-inflammatory modifications. Vascular Disease Prevention. 2008;5:1–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures. Panel 1: Stable plaque (Hematoxylin&Eosin, upper left): inside the fibrotic core, only scanty cells are positive to smooth muscle actin (a-SMA, upper right), CD68 (lower right) and CD31 (lower left). Actin staining also demonstrated the residual muscular wall of the vessel. Panel 2: Unstable plaque (Hematoxylin&Eosin, upper left): the necrotic core of the plaque is largely composed of cells immunoreactive to smooth muscle actin (a-SMA, upper right), CD68 (lower right) and CD31 (lower left).