Abstract
Critical role of mitochondria in programmed cell death leads to the design of mitochondriotropic agents as a strategy in regulating apoptosis. For anticancer therapy, stimulation of proapoptotic mitochondrial events in tumor cells and their suppression in surrounding normal cells represents a promising paradigm for new therapies. Different approaches targeting regulation of components of mitochondrial antioxidant system such as Mn-SOD demonstrated significant antitumor efficiency, particularly in combination therapy. This review is focused on a newly discovered early stage of mitochondria-dependent apoptosis - oxidative lipid signaling involving a mitochondria-specific phospholipid cardiolipin (CL). Cytochrome c (cyt c) acts as a CL-specific peroxidase very early in apoptosis. At this stage, the hostile events are still secluded within the mitochondria and do not reach the cytosolic targets. CL oxidation process is required for the release of pro-apoptotic factors into the cytosol. Manipulation of cyt c interactions with CL, inhibition of peroxidase activity, and prevention of CL peroxidation are prime targets for the discovery of anti-apoptotic drugs acting before the “point-of-no-return” in the fulfillment of the cell death program. Therefore, mitochondria-targeted disruptors and inhibitors of cyt c/CL peroxidase complexes and suppression of CL peroxidation represent new strategies in anti-apoptotic drug discovery.
Keywords: Apoptosis, Cardiolipin, Cytochrome c, Drug delivery, Mitochondria-targeting
1 Introduction
If categories of emotions could be applied to relationships between mitochondria and cells, the best description may be by the words of the well known lyrics “It’s a thin line between love and hate.” Unavoidable transition from anaerobic to aerobic life on our planet about two billions year ago, forced interactions between eukaryotic single-cellular and multicellular organisms and aerobic bacteria with their electron transporting oxygen reducing capacities. These apparently “symbiotic” relationships evolved as an essential mechanism in the fight against a hostile oxygen-rich environment and allowed organisms to effectively utilize the high oxidizing potential of oxygen as an acceptor of electrons in reactions coupled with the production of energy in specialized organelles, mitochondria.
Separation of the bacteria-containing compartments and insulating them with a specialized additional outer membrane may be perceived as a sign of “mistrust or suspicion” between the parties involved, yet contributing to the mutually beneficial co-existence of the host and the guest organisms. This suspicion - on the borderline with animosity - was enhanced and maintained by the presence in the mitochondrial intermembrane space of different cell death-inducing protein factors such as cytochrome c (cyt c), apoptosis-inducing factor (AIF), Smac/Diablo [1]. During peaceful co-existence, these proteins are involved in essential functions, particularly the shuttling of electrons by cyt c. AIF has a pronounced similarity in aminoacid sequence with several mitochondrial oxidoreductases [2]; the role of Smac/Diablo under normal conditions is unknown. However, the fragility of these well coordinated relationships and strictly controlled functions are readily revealed (during famine) by unfavorable conditions which unmask the real calamitous interplay between mitochondria and host cells. In a process called programmed (interestingly - programmed by which of the two partners?) cell death, these proteins, pro-apoptotic factors, are released from mitochondria and activate the machinery of hydrolysis and disassembly of the major macromolecules - proteins and DNA [1]. Reciprocally, host cells are permanently prepared to initiate the program of elimination of damaged (weakened) or useless (e.g., in starvation conditions) mitochondria using a specifically designed program, mitophagy.
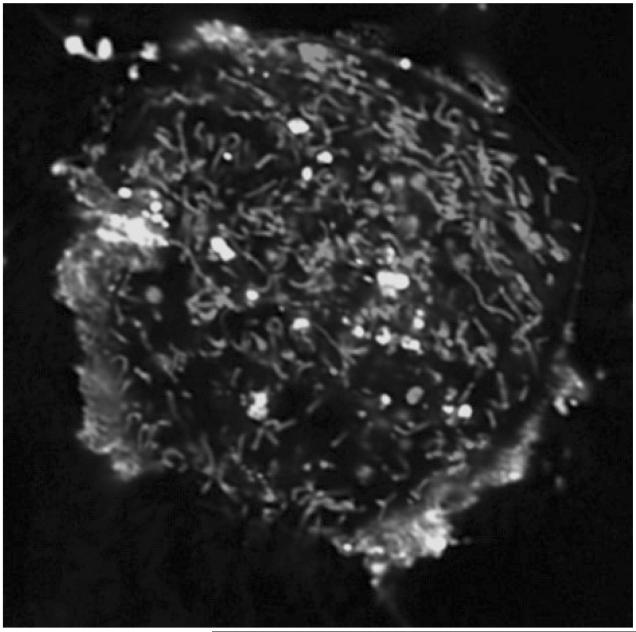
Cardiolipins (CLs) in bacterial membranes still likely play an important role in their capacity to be recognized, penetrate, and invade host cells. Not surprisingly, liposomes containing CL are effectively integrated into cells. As shown in Fig. 1, liposomes with fluorescently labeled CL 7-nitrobenz-2-oxa-1,3-diazole-CL (NBD-CL) are quickly incorporated into mouse embryonic cells. However, these liposomes and CL are isolated from the cell’s content in vacuoles (lysophagosomes). Notably, CL-containing liposomes are not integrated and are not colocalized with mitochondria.
Figure 1.
Confocal fluorescence microphotograph of mouse embryonic cells co-incubated with fluorescently labeled CL (NBD-CL). Mitochondria were stained with MitoTracker® Red CMXRos. NBD-CL was delivered into cell by co-incubation with small unilamellar liposomes containing NBD-CL and 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine (2:1) for 15 min at 37°C in PBS. Note that NBD-CL is not integrated into mitochondria but stays isolated in on the cell surface or sequestered in separate vacuoles. (Please see Supporting Information for an enlarged version of this figure in color.)
The struggle between mitochondrial and off-mitochondrial mechanisms is a critical feature of the cell’s and body’s normal existence. Poorly controlled tumor cells suppress the mitochondrial apoptotic mechanisms to avoid cell death and to achieve unlimited proliferative potential [3, 4]. These very complex interactions between mitochondrial and off-mitochondrial mechanisms and pathways determine the behavior and sensitivity of cells to regulating agents and drugs. Dysregulation of the mitochondrial apoptotic mechanisms is a well-recognized pathway contributing or leading to the transformation of normal cells into cancer cells. Not surprisingly, numerous studies have focused on the mitochondria/cell interface with the goal to use their specific features for drug discovery. Mitochondria targeting of low Mr molecules and development of vehicles for the selective delivery of drugs are emerging as prime pharmacological approaches. This review is centered on a newly discovered early stage of mitochondria-dependent (intrinsic) apoptosis - oxidative lipid signaling involving a mitochondria-specific phospholipid CL. While ancestors of CL played a pivotal role in adaptation of bacteria to changing environments, CL adopted several important roles in mitochondrial organization and function [5, 6]. For example, the polyunsaturation and oxidizability of CL fatty acid residues regulate the release of pro-apoptotic factors [7, 8]. In this review, we describe how cells decode these mechanisms and why elucidation of oxidative lipid signaling are instrumental in the development of anti/pro-apoptotic drugs and their targeting to mitochondria. We will also present evidence that translocation of CL from the inner to the outer mitochondrial membranes facilitates its association with cyt c yielding a new catalytic entity with peroxidase activity selectively directed toward CL oxidation. This CL oxidation process is required for the release of pro-apoptotic factors from mitochondria into the cytosol, i. e., execution of the apoptotic program.
2 Cytochrome c structure and ligands
Cyt c, a 12 kDa protein located in the mitochondrial intermembrane space, participates in the transport of electrons in the respiratory chain by shuttling electrons from complex III (ubiquinol cyt c oxidoreductase) to complex IV (cyt c oxidase) [9]. For many years, this activity was viewed as the only function of cyt c. Recently, this notion of monofunctionality of cyt c has been shaken when the protein attracted new attention due to several additional roles it plays [10, 11]. As an effective acceptor of electrons, cyt c has been suggested to act as a component of the enzymatic antioxidant system of mitochondria [12, 13]. This is based on its ability to accept electrons from superoxide radicals and dump then on complex IV. In this capacity, cyt c has an unlimited ability to prevent superoxide accumulation in mitochondria. To what extent this remarkable antioxidant potential of cyt c is realized during excessive spillage of electrons on complexes I and III under conditions of oxidative/nitrosative stress - still remains to be quantified. It has been documented that extramitochondrial cyt c is an active participant of programmed cell death (apoptosis) whereby it is released from mitochondria and interacts with apoptotic protease activating factor (Apaf-1), causing the formation of apoptosomal complexes and activation of proteolytic caspase cascades [11, 14]. Finally, chronologically the latest function of cyt c has been associated with its ability to be activated and fulfill the task of a peroxidase [8, 15]. As a hemo-protein, cyt c has all the prerequisites to be converted into a peroxidase. Paradoxically, the peroxidase activity of cyt c under normal conditions is negligibly low [15, 16]. However, numerous studies in model systems explored the ability of cyt c‘s conversion into an active peroxidase as a result of post-translational modifications. It has been demonstrated that oxidative or nitrative chemical modifications of the protein lead to the induction of significant peroxidase activity [17-19]. Similarly, interaction of cyt c with hydrophobic anions - detergents or anionic phospholipids - can trigger peroxidase activation [20-22]. However, to date the physiological significance of these “properoxidative” modifications of cyt c has not been defined. Our previous work has identified mitochondrial apoptotic events leading to specific interactions and high affinity binding of cyt c with a mitochondria-specific phospholipid CL. This results in the activation of cyt c into a CL-specific peroxidase with selective catalytic competence toward peroxidation of polyunsaturated molecular species of CL. Ultimately, this activity results in the accumulation of CL oxidation products, mainly CL-OOH and their reduction products, CL-OH [8]. This is significant because oxygenated CL species appear to be essential for mitochondrial membrane permeabilization and release of pro-apoptotic factors (including cyt c itself) into the cytosol [8, 23]. Elucidation of these different modalities through which cyt c exerts its diversified catalytic functions requires a better understanding of its structural organization. In particular, knowledge of phylogenetically conserved segments in cyt c should provide insight regarding their role and evolutionary development.
3 Cyt c interactions with other proteins and ligands
Modifications of cyt c amino acid residues could have functional effects on its ability to transport electrons and modulate apoptosis. It was demonstrated that cyt c uses several lysines (including Lys13, Lys72, and Lys87; here and below residue numeration is for horse heart cyt c) to react with cyt c peroxidase and cyt c oxidase [24]. These same lysine residues are also likely involved in interactions with cyt b5 and c1. Notably, cyt c peroxidase is present only in relatively primitive organisms such a yeast and bacteria. Its function (accepting reducing equivalents from cyt c and reducing H2O2 to water) in higher species might be regulated by cyt c/CL complexes.
McLendon’s group has shown that a large number of cyt c residues interact with Apaf-1, e.g., Lys7, Lys25, Lys39, Lys72, and also sequence 62-65 (Glu-Thr-Leu-Met) [25]. Lys72 is important to cyt c activity (Lys72Ala mutation eliminated its electron transport function). Mutation of Lys7, Lys25, Lys39, and domain 62-65 reduced the activity of cyt c-Apaf-1-caspase-9 complex toward procaspase-3 cleavage. Single site-directed mutations resulted in a 5-10-fold decrease in procaspase-3 cleavage activity, whereas multiple mutations reduced caspase activity by more than 1000-fold [25].
As a polyvalent cation, cyt c has multiple binding sites for a variety of small anions, including phosphate, ADP, ATP, and citrate [26, 27]. Experiments with ATP photoaffinity - labeled cyt c have demonstrated that this site involves the invariant residues Lys72, Lys86, Lys87, and Arg91 [27-29]. ATP binds to this site at physiological concentrations and under physiological ionic strength conditions, which suggests that this site is of biological significance. The occupancy of the site depends on the [ATP]/[ADP] ratios but is independent of the redox state of cyt c. ATP bound to the site diminishes the electron flow in the respiratory chain [28]. Both complex III and IV activities are affected. As the ATP binding site includes Lys residues that are involved in the interaction of cyt c with the reductase and oxidase, the inhibition is likely to be the result of direct steric and electrostatic effects.
Lysine residues are also preferential sites for lipid hydroperoxide-derived modification on cyt c [30]. The major adducts are associated with Lys5, Lys7, Lys8, His33, Lys86, Lys87, Lys99, and Lys100. In addition, Isom et al. [31] have shown that 4-hydroxy-2-nonenal (4HNE), a secondary product of lipid peroxidation, forms conjugates with cyt c through His33, Lys87, and Arg38.
Chemical modification of cyt c by lipid peroxidation products was recently deemed physiologically relevant after it was discovered that cyt c can oxidize CL and phosphatidylserine (PS), in vivo [8, 32]. In complementary experiments, we also demonstrated that the complex of cyt c/CL (i) exhibits peroxidase activity [15], (ii) produces CL hydroperoxides required for release of proapoptotic factors [8], and (iii) potentially utilizes CL hydroperoxides as substrates for further peroxidase reactions [33].
4 Conservancy of cyt c amino acid residues
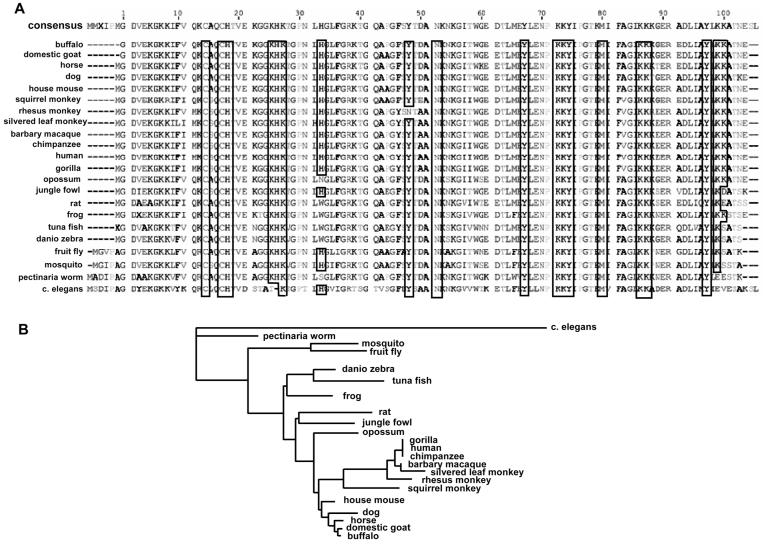
Overall, cyt c is highly conserved among different species (Fig. 2). The level of conservancy of cyt c‘s segments specifically involved in its different functions may provide information on their phylogenetic significance. Therefore, we will briefly consider participation of different domains of cyt c in its functional endeavors.
Figure 2.
(A) Alignment of cyt c protein sequences. Sequence alignment of cyt c from different species was prepared using Geneious Basic software (http://www.geneious.com/, New Zealand) with modifications. Aminoacid residues relevant to the peroxidase function of cyt c/CL complexes (binding of CL, attachment of heme, catalytic function) are shown as “framed.” Note the high conservancy of these residues. (B) Phylogenic tree of cyt c demonstrating the evolutionary relationships between several sequences. (This was generated using Geneious Basic software - http://www.geneious.com/, New Zealand with modifications; the length of tree branches did not reflect actual divergence). (Please see Supporting Information for an enlarged version of this figure in color.)
From an evolutionary standpoint, one of the major functions of cyt c is to transport electrons through the respiratory chain. Cyt c is a typical hemoprotein with the covalent attachment of heme to the polypeptide chain through thioether bonds to the cysteine residues of a Cys-Xxx-Xxx-Cys-His peptide motif. The distal ligand - methionine - is also strictly invariable among all living organisms (with differing numeration, because the length of the polypeptide chain is different). Lysines participating in interaction with cyt c peroxidase and cyt c oxidase (Lys72 and Lys87 [24]) are also invariantly present in all organisms, whereas Lys13 in some cases (Saccharomyces cerevisiae and Caenorhabditis elegans) is substituted for the similarly basic and polar arginine. The ATP binding domain formed by Lys72, Lys86, Lys87, and Arg91 [27-29] is also very conservative, i. e., there are no single substitutes for these amino acid residues among living organisms.
Within the context of this review, it is important that tyrosine residues likely involved in its peroxidase function (but also essential for the electron transport) [34-36] are preserved as well. Horse heart cyt c Tyr48, Tyr67, Tyr74, and Tyr97 are present in all other species (with one exception of Asn for one species, Macaca mulatta). One additional tyrosine is present in primates near Tyr47; two additional tyrosines are located at the N-terminus of cyt c of C. elegans. His26 and His33, which play a putative role in peroxidase reaction of partially unfolded cyt c [37, 38], are also more or less evolutionary stable - correspondingly only substitution of His26 for Thr in C. elegans and His33 for Trp, Asn, or Cys in several other species, occur.
Lysine residues responsible for Apaf-1 interaction and further caspases activation [25] are not as well conserved: Lys7 is present in S. cerevisiae, but in Drosophila melanogaster, Xenopus tropicalis, Danio rerio, and mammals, this Lys is substituted for polar acidic Glu. It is worth noting that neighboring Lys8 is invariant in almost all cases. To the contrary, Lys25 and Lys39 are invariant in all species except S. cerevisiae and C. elegans - correspondingly, there are nonpolar neutral proline and polar basic histidine in yeast and nonpolar neutral alanine and polar neutral threonine in nematodes. It is probable that this diversity reflects an evolutionary new function of cyt c, i.e., participation in apoptosis.
Overall, the segments of the protein essential for the peroxidase function of cyt c seem to preserve conservancy from species to species. All prerequisites for peroxidase activity, namely, heme moiety, its distal and proximal ligands (Met, His), tyrosine residues, and amino acids required for the binding with CL (Lys, Asn, His, see below) are highly conserved.
5 CL binding to cyt c
As the current review is focused on the pro-apoptotic peroxidase function of cyt c, we will further discuss structural requirements essential for the association of cyt c with anionic phospholipids, particularly, CL. There is no single universally accepted opinion on the binding of CL to cyt c. The most commonly used model for cyt c binding to the membrane involves the A-site for electrostatic interaction and the C-site for hydrophobic interaction. Site A consists of basic residues, such as Lys72 and Lys73. Site C is another lipid-binding domain with a high affinity for protonated acidic phospholipids. The invariant Asn52 in horse heart cyt c has been assigned as the amino acid residue that binds to protonated acidic phospholipids via hydrogen bonds. It is believed that a hydrophobic component of the C-site mediated interaction is due to an extended lipid anchorage of one of the fatty acid chains into the hydrophobic channel in cyt c [39-41]. The work of Kostrzewa et al. [42] revealed one more possible site of lipid/cyt c interaction, Lys86, and Lys87 along with Lys72. Using paramagnetically-labeled cyt c, these authors demonstrated that cyt c electrostatically interacts with the membrane and found that cyt c does not penetrate the membrane’s interior. In 2005, Nantes’s group based on low pH (<7.5) modification of cyt c proposed the existence of additional sites of lipid-cyt c interaction: Lys22, Lys25, His26, Lys27, His33, and Lys87 [43]. This collective site (L-site) is on the opposite side of the cyt c molecule compared to sites A and C. It is possible that this site is involved in the attachment of the protein to the membrane lipid bilayer.
One possible approach to study the structural organization of the cyt c molecule and assess the contribution of its domains regarding interactions with anionic phospholipids, is to utilize comparative molecular modeling and crystallographic information available in existing databases. We have analyzed the structure of a number of putative CL-binding proteins. There are 37 known crystal structures of molecules with CL as a ligand in the Protein Data Bank (http://www.pdb.org). They belong to five distinct groups: photosynthetic reaction center from Rhodobacter sphaeroides, yeast cyt bc1 complex, formate dehydrogenase N from Escherichia coli, bovine mitochondrial ADP/ATP carrier, and bovine heart cyt c oxidase.
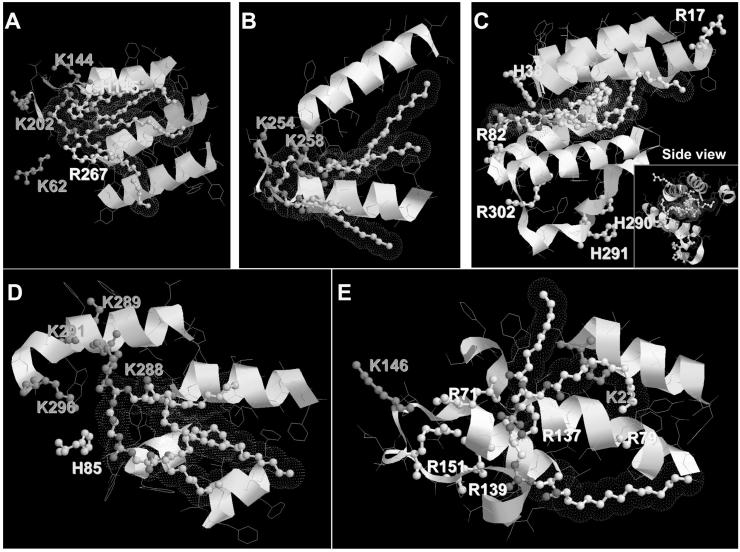
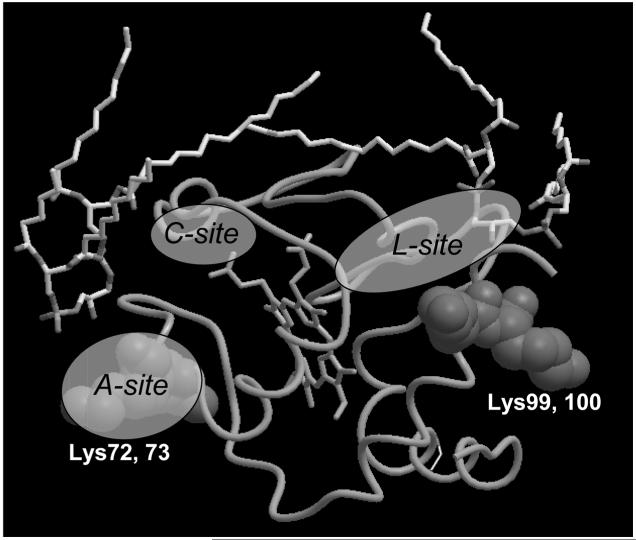
Our strategy to further characterize the nature of CL binding to cyt c was based on the analysis of likely participants in close proximity to the CL-binding domain (within a 5 Å distance) (Fig. 3). We found that Lys, Arg, and His residues are located in close proximity to the polar negatively charged heads of CL. Additionally, hydrophobic α-helixes are neighboring the CL fatty acid chain. The same approach was documented in the review of Palsdottir and Hunte [44] for several negatively charged phospholipids. Tight binding interactions with three residues were observed: Lys-Lys-Yyy, Arg-Lys-Yyy, and His-Arg-Asn. Based on these comparisons, the overall structure Xxx-Xxx-Yyy, where Xxx is a positively charged residue and Yyy a polar residue, can be inferred as a CL-binding motif. These authors pointed out that the suggested motifs are nonlinear, i. e., ligands from different subunits may contribute. Also, several CL-binding domains composed of two amino acid residues or even one (lysine or serine) may exist. After determination of CL-binding domains on known crystal structures, we aligned this CL-binding domain to cyt c (1HRC). We revealed two possible sites for CL binding - one near Lys72 and Lys73 and the other one contiguous to Lys99 and Lys100 (Fig. 4).
Figure 3.
CL-binding domains of (A) photosynthetic reaction center (1QOV), (B) formate dehydrogenase (1KQF), (C) cyt c oxidase polypeptide I (1V54, region of binding with CLD 3269), (D) ubiquinol-cyt c reductase complex core (1KB9), and (E) ADP/ATP translocase 1 (2C3E, region of binding with CLD 800). Polar basic (K, R) and weak basic (H) residues shown with purple, yellow, and blue, respectively. Structures were generated using RasTop Molecular Visualization Software (http://www.geneinfinity.org/rastop). (Please see Supporting Information for an enlarged version of this figure in color.)
Figure 4.
Alignment of CL-binding domain of formate dehydrogenase (1KQF) to cyt c (1HRC). Two critical domains have been identified, Lys72-Lys73 and Lys99-Lys100. Protein alignment (by lysine residues) was performed using ICM-Browser software (http://www.molsoft.com/icm_browser.html). (Please see Supporting Information for an enlarged version of this figure in color.)
Obviously, additional studies based on molecular modeling are needed to further characterize other essential motifs contributing to specific hydrophobic interactions of CL with nonpolar grooves in the protein structure. These studies may be useful for the development of optimized inhibitors capable of disrupting cyt c/CL interactions, and hence the formation of a peroxidase competent complex. This approach may lead to the development of new anti-apoptotic agents.
6 Mitochondria-targeted inhibitors of peroxidase activity of cyt c/CL complexes
Given the importance of mitochondrial events during the initial critical stages of the execution of the apoptotic program, the design of mitochondriotropic agents is believed to be a promising novel strategy in regulating apoptosis. In the context of anticancer therapy, stimulation of pro-apoptotic mitochondrial events in tumor cells, and suppression of mitochondrial stages of apoptosis in surrounding normal cells may represent a promising paradigm for new effective therapies. Different approaches targeting the regulation of components of the mitochondrial antioxidant system such as manganese superoxide dismutase (Mn-SOD) were tested and showed significant antitumor efficiency, particularly in combination therapy [45]. Another opportunity is employment of nanoparticles loaded with cell death signals (such as cyt c) and targeted to cancer cells. We have recently demonstrated that single walled carbon nanotubes can be coated with specific phospholipid recognition signals along with cyt c and directed to and taken-up by phagocytozing cells resulting in triggering of apoptosis (Konduru et al., unpublished data).
The peroxidase reaction of cyt c/CL complexes is driven by H2O2 as a source of oxidizing equivalents. During apoptosis, the latter is provided by disrupted electron transport. Interaction of CL with cyt c yields a complex whose normal redox potential is about minus (-) 400 mV more negative than that of intact cyt c [46]. As a result, cyt c/CL cannot accept electrons from mitochondrial complex III leading to enhanced production of superoxide whose dismutation yields H2O2. Thus, the formation of cyt c/CL complexes in mitochondria is, in principle, sufficient to trigger mitochondrial generation of H2O2 to feed the peroxidase cycle and catalyze CL oxidation. Clearly, prevention or inhibition of H2O2 production should block the supply of oxidizing equivalents and inhibit the peroxidase-catalyzed CL oxidation. Our efforts are focused on several effective approaches to achieve this goal.
Nitroxide radicals are ideal candidates for this role as they can readily accept excessive electrons from damaged mitochondrial electron carriers and hence prevent superoxide production. Moreover, reduction of nitroxides produces hydroxylamines which can further act as radical scavengers and donors of electrons capable of quenching the peroxidase reaction. Provided the concentration of nitroxides is sufficiently high in mitochondria, they could be ideal candidates as inhibitors of mitochondrially driven CL oxidation.
Recently, several strategies have been developed to deliver and concentrate low Mr cargos in mitochondria. Compounds targeting mitochondria (mitochondriotropics) act through several diverse accumulation mechanisms and chemical features [47]. One of them is based on the conjugation of the nitroxides with a hydrophobic cation, triphenylphosphonium (TPP) that is readily electrophoresed into mitochondria at the expense of their membrane potential (MP) [48]. Indeed, TPP-nitroxide was able to inhibit apoptosis in cells by accumulating in their mitochondrial fractions. The disadvantage of this protocol is that TPP reduces mitochondrial MP and induces damage. Additionally, effective scavenging of electrons by nitroxides may be associated not only with the benefit of preventing superoxide and H2O2 generation but also with a risk of energy crisis. Indeed, high concentrations of accumulated nitroxides may completely block electron transport in mitochondria, hence block the ATP production.
Another approach is based on conjugates of nitroxides with fragments of molecules with relatively high affinity to mitochondrial membranes. For example, fragments of gramicidin S (GS) have been successfully used for this purpose and proved to be effective in preventing superoxide production in cells, CL oxidation in mitochondria and protecting cells against different pro-apoptotic triggers such as actinomycin D, radiation, or staurosporine [49-51]. More importantly, these mitochondria-targeted nitroxides were able to protect against apoptosis in vivo by preventing CL oxidation induced by intestinal hemorrhagic shock [52]. Optimization of nitroxide carriers promises to give a new generation of effective anti-apoptotic agents acting at an early mitochondrial stage.
Additional opportunity to inhibit peroxidase activity of cyt c/CL complexes toward CL oxidation is to use alternative sacrificial substrates and thus prevent CL oxidation. Among such potent reductants of peroxidase and its reactive intermediates is nitric oxide (NO6). We have recently developed a prodrug - alkyl-hydroxylamine - whose metabolism by peroxidases converts it into an NO donor. Conjugation of alkyl-hydroxylamine with TPP (TPPO) selectively targeted the conjugate - (2-hydroxyaminovinyl)TPP - into mitochondria whereby the cargo was converted into an NO-donor by a cyt c-dependent metabolism. This resulted in the release of NO, and most importantly, protection against apoptosis (Stoyanovsky et al., unpublished data).
6.1 Imidazole fatty acids
Peroxidase activity of cyt c/CL complexes is central to CL oxidation and the effects of CL-OOH in the mitochondrial membrane permeabilization and release of pro-apoptotic factors. Without CL, cyt c is a very poor peroxidase due to a hexacoordinate occupancy of Fe in the heme [16]. Upon binding of CL, the protein undergoes partial unfolding accompanied by loosening of its Fe coordination by Met80 resulting in enhanced access of the heme catalytic site to small molecules like H2O2. If the availability of the heme can be blocked by a small molecule, the peroxidase activity will be minimized. In this respect, an interesting group of compounds are imidazole derivatives of fatty acids. We have designed and synthesized several derivatives in such a way that the carboxy group interacts with one of the critical Lys residues of the protein, while the imidazole moiety at different positions of the acyl chain - protruding into the hydrophobic pocket - appears to interact with the heme iron to lock the catalytic site and form a high affinity complex. In this complex, H2O2 will have no access to the heme catalytic site. Experiments are now underway to assess the effectiveness of imidazole fatty acids as inhibitors of peroxidase activity of cyt c in model cell culture systems.
6.2 Modified oxidizable derivatives of CL
An interesting opportunity for inhibition of the peroxidase activity toward oxidation of CL may be explored by using CL homologs with readily oxidizable functionalities that will compete with the oxidation of PUFAs of CL. In our preliminary experiments with NBD-CL, we found that NBD groups are the prime target for the peroxidase-catalyzed oxidation. This suggests that via appropriate optimization protocols, CL derivatives may be designed that will be effective at outcompeting natural substrates of in cyt c/CL complexes and preventing accumulation of pro-apoptotic CL-OOH.
6.3 Dietary manipulations of CL molecular species to lower CL oxidizability
Strategies for this objective may include the employment of nonoxidizable natural CLs. Our previous work has established that tetraoleoyl-CL (TOCL) does not undergo oxidation within cyt c/TOCL complexes incubated in the presence of H2O2 [33]. It is tempting to speculate that dietary manipulation resulting in the enrichment of cells with nonoxidizable molecular species of CL may be instrumental in protecting cells against apoptosis. Conversely, utilization of highly PUFAs and loading of CLs with highly oxidizable acyl groups could render targeted cells more vulnerable to oxidative modification and, therefore, apoptotic cell death. This approach may be particularly important for developing antitumor therapies to overcome the common resistance of tumor cells to pro-apoptotic drugs. With this rationale in mind, we set out to determine whether highly oxidizable PUFA naturally found in the diet, e.g., docosahexaenoic acid (DHA), can create a permissive environment for apoptosis in the intestine. We initially demonstrated that dietary DHA is incorporated into rat intestinal (colonic) mitochondrial CL, which was associated with an increase in oxidative stress and apoptosis in vivo [53, 54]. In complimentary experiments, in order to elucidate the subcellular origin of oxidation induced by DHA, immortalized mouse colonocytes were treated with DHA and cytosolic reactive oxygen species (ROS, measured using CMH2-DCFDA), membrane lipid oxidation (PLOOH, measured using diphenyl-1-pyrenylphosphine, DPPP), mitochondrial MP (measured using rhodamine-123), and intracellular Ca2+ (measured using Fluo-4 and Rhod-2) was assayed by live-cell fluorescence microscopy [55, 56]. DHA primed cells exhibited a 151% increase in lipid oxidation, which could be blocked by a mitochondria-specific antioxidant, MitoQ. In addition, under certain conditions, MP dissipation was 21% greater in DHA primed cells. These proapoptotic effects were reversed by preincubation with inhibitors of the mitochondrial permeability transition pore and mitochondrial Ca2+ uniporter. In addition, DHA-induced apoptosis was partially blocked by MitoQ [55]. Collectively, these data indicate that select long chain PUFA potentiate mitochondrial lipid oxidation and the dissipation of MP which contribute to the induction of apoptosis.
6.4 Manipulation of CL levels in target cells using RNA interference
Finally, genetic manipulations may be employed to reduce the absolute level of CL capable of participating in the formation of productive peroxidase complexes with cyt c. Synthesis of CL from its precursor, phosphatidyl glycerol, is catalyzed by CL synthase (CLS) in the mitochondrial matrix [57, 58]. We have recently demonstrated using RNA interference strategies, that the content of synthesized CL can be reduced, resulting in an increased resistance of cells to apoptosis by limiting CL oxidation (Huang et al., unpublished data). It is also well known that CLS is under strong regulation of thyroid hormones [57]. Therefore, it may be possible to therapeutically utilize thyroid hormones to manipulate lipid peroxidation and cell susceptibility to apoptosis.
In summary, the function of cyt c as a CL-specific peroxidase is realized very early during the execution of the apoptotic program. At this stage, the hostile events are still secluded within the mitochondria and do not reach the cytosolic targets. Manipulation of cyt c interactions with CL, inhibition of the peroxidase activity and prevention of CL peroxidation are prime targets for the discovery of anti-apoptotic drugs acting before the “point-of-no-return” in the fulfillment of the cell death program (Fig. 5). Therefore, mitochondria-targeted disruptors and inhibitors of cyt c/CL peroxidase complexes and suppression of CL peroxidation represent new strategies in anti-apoptotic drug discovery.
Figure 5.
CL-activated peroxidase activity of cyt c indicates new drug discovery targets aimed at inhibition of CL oxidation in mitochondria and prevention of apoptosis. Peroxidase activity of Cyt c/CL complexes leads to CL oxidation and accumulation of products required for the release of pro-apoptotic factors from mitochondria. Consequently, agents and factors that inhibit the peroxidase activity and prevent CL oxidation may act as anti-apoptotic agents. Mitochondrial targeting of such agents may lead to discovery of new potent drugs. Several options shown on the schema include conjugates of hemi-GS with nitroxide radicals (TEMPO). Specifically, GS-TEMPO is selectively accumulated in mitochondria where it acts as an electron scavenger capable of preventing superoxide formation and its dismutation into H2O2 that is necessary for CL oxidation. GS-TEMPO acts as an effective anti-apoptotic agent. Imidazole-fatty acids act as inhibitors of peroxidase activity due to the ability to strongly interact heme-iron. Mitochondriatargeted donors of NO6 - such as 2-hydroxyamino-vinyl-triphenyl-phosphonium (HVTP) - activatable by peroxidase activity of cyt c owe their anti-apoptotic potency to the NO6-dependent reduction of reactive intermediates of the peroxidase cycle. Finally, GSH-dependent mechanisms are involved in elimination of H2O2 that feeds the peroxidase cycle and reduction of CL hydroperoxides (phospholipids-dependent GSH peroxidase). (Please see Supporting Information for an enlarged version of this figure in color.)
Acknowledgments
Supported by National Institutes of Health Grants U19 AI068021, CA59034, DK071707, R03 TW007320-01, Centers for Disease Control, National Institute of Occupational Safety and Health Grant OH008282, and The Human Frontier Science Program.
Abbreviations
- Apaf-1
apoptotic protease activating factor
- CL
cardiolipin
- cyt c
cytochrome c
- DHA
docosahexaenoic acid
- GS
gramicidin S
- MP
membrane potential
- NBD-CL
7-nitrobenz-2-oxa-1,3-diazole-CL
- ROS
reactive oxygen species
- TOCL
tetraoleoyl-CL
- TPP
triphenylphosphonium
Footnotes
The authors have declared no conflict of interest.
7 References
- [1].Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- [2].Lorenzo HK, Susin SA, Penninger J, Kroemer G. Apoptosis inducing factor (AIF): A phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 1999;6:516–524. doi: 10.1038/sj.cdd.4400527. [DOI] [PubMed] [Google Scholar]
- [3].Bertram JS. The molecular biology of cancer. Mol. Aspects Med. 2000;21:167–223. doi: 10.1016/s0098-2997(00)00007-8. [DOI] [PubMed] [Google Scholar]
- [4].Igney FH, Krammer PH. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- [5].Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- [6].Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- [7].Nomura K, Imai H, Koumura T, Kobayashi T, et al. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kagan VE, Tyurin VA, Jiang J, Tyurina YY, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- [9].Nelson DL, Cox MM, Lehninger A. In: Lehninger Principles of Biochemistry. 4th Edn. Freeman WH, editor. Palgrave; Basingstoke, New York: 2004. [Google Scholar]
- [10].Liu X, Kim CN, Yang J, Jemmerson R, et al. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- [11].Li P, Nijhawan D, Budihardjo I, Srinivasula SM, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- [12].Korshunov SS, Krasnikov BF, Pereverzev MO, Skulachev VP. The antioxidant functions of cytochrome c. FEBS Lett. 1999;462:192–198. doi: 10.1016/s0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- [13].Pereverzev MO, Vygodina TV, Konstantinov AA, Skulachev VP. Cytochrome c, an ideal antioxidant. Biochem. Soc. Trans. 2003;31:1312–1315. doi: 10.1042/bst0311312. [DOI] [PubMed] [Google Scholar]
- [14].Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- [15].Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, et al. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prasad S, Maiti NC, Mazumdar S, Mitra S. Reaction of hydrogen peroxide and peroxidase activity in carboxymethylated cytochrome c: Spectroscopic and kinetic studies. Biochim. Biophys. Acta. 2002;1596:63–75. doi: 10.1016/s0167-4838(02)00205-4. [DOI] [PubMed] [Google Scholar]
- [17].Chen YR, Deterding LJ, Sturgeon BE, Tomer KB, et al. Protein oxidation of cytochrome c by reactive halogen species enhances its peroxidase activity. J. Biol. Chem. 2002;277:29781–29791. doi: 10.1074/jbc.M200709200. [DOI] [PubMed] [Google Scholar]
- [18].Jang B, Han S. Biochemical properties of cytochrome c nitrated by peroxynitrite. Biochimie. 2006;88:53–58. doi: 10.1016/j.biochi.2005.06.016. [DOI] [PubMed] [Google Scholar]
- [19].Cassina AM, Hodara R, Souza JM, Thomson L, et al. Cytochrome c nitration by peroxynitrite. J. Biol. Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- [20].Pinheiro TJ, Cheng H, Seeholzer SH, Roder H. Direct evidence for the cooperative unfolding of cytochrome c in lipid membranes from H-(2)H exchange kinetics. J. Mol. Biol. 2000;303:617–626. doi: 10.1006/jmbi.2000.4159. [DOI] [PubMed] [Google Scholar]
- [21].Muga A, Mantsch HH, Surewicz WK. Membrane binding induces destabilization of cytochrome c structure. Biochemistry. 1991;30:7219–7224. doi: 10.1021/bi00243a025. [DOI] [PubMed] [Google Scholar]
- [22].Mustonen P, Virtanen JA, Somerharju PJ, Kinnunen PK. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987;26:2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- [23].Belikova NA, Jiang J, Tyurina YY, Zhao Q, et al. Cardiolipin-specific peroxidase reactions of cytochrome c in mitochondria during irradiation-induced apoptosis. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:176–186. doi: 10.1016/j.ijrobp.2007.03.043. [DOI] [PubMed] [Google Scholar]
- [24].Poulos TL, Kraut J. A hypothetical model of the cytochrome c peroxidase. Cytochrome c electron transfer complex. J. Biol. Chem. 1980;255:10322–10330. [PubMed] [Google Scholar]
- [25].Yu T, Wang X, Purring-Koch C, Wei Y, et al. A mutational epitope for cytochrome C binding to the apoptosis protease activation factor-1. J. Biol. Chem. 2001;276:13034–13038. doi: 10.1074/jbc.M009773200. [DOI] [PubMed] [Google Scholar]
- [26].Osheroff N, Brautigan DL, Margoliash E. Mapping of anion binding sites on cytochrome c by differential chemical modification of lysine residues. Proc. Natl. Acad. Sci. USA. 1980;77:4439–4443. doi: 10.1073/pnas.77.8.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Corthesy BE, Wallace CJ. The oxidation-state-dependent ATP-binding site of cytochrome c. A possible physiological significance. Biochem. J. 1986;236:359–364. doi: 10.1042/bj2360359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Craig DB, Wallace CJ. Studies of 8-azido-ATP adducts reveal two mechanisms by which ATP binding to cytochrome c could inhibit respiration. Biochemistry. 1995;34:2686–2693. doi: 10.1021/bi00008a036. [DOI] [PubMed] [Google Scholar]
- [29].McIntosh DB, Parrish JC, Wallace CJ. Definition of a nucleotide binding site on cytochrome c by photoaffinity labeling. J. Biol. Chem. 1996;271:18379–18386. doi: 10.1074/jbc.271.31.18379. [DOI] [PubMed] [Google Scholar]
- [30].Williams MV, Wishnok JS, Tannenbaum SR. Covalent adducts arising from the decomposition products of lipid hydroperoxides in the presence of cytochrome c. Chem. Res. Toxicol. 2007;20:767–775. doi: 10.1021/tx600289r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Isom AL, Barnes S, Wilson L, Kirk M, et al. Modification of Cytochrome c by 4-hydroxy-2-nonenal: Evidence for histidine, lysine, and arginine-aldehyde adducts. J. Am. Soc. Mass Spectrom. 2004;15:1136–1147. doi: 10.1016/j.jasms.2004.03.013. [DOI] [PubMed] [Google Scholar]
- [32].Jiang J, Kini V, Belikova N, Serinkan BF, et al. Cytochrome c release is required for phosphatidylserine peroxidation during Fas-triggered apoptosis in lung epithelial A549 cells. Lipids. 2004;39:1133–1142. doi: 10.1007/s11745-004-1340-1. [DOI] [PubMed] [Google Scholar]
- [33].Tyurina YY, Kini V, Tyurin VA, Vlasova II, et al. Mechanisms of cardiolipin oxidation by cytochrome c: Relevance to pro and antiapoptotic functions of etoposide. Mol. Pharmacol. 2006;70:706–717. doi: 10.1124/mol.106.022731. [DOI] [PubMed] [Google Scholar]
- [34].Harrison JE. A proposed hydrogen transfer function for cytochrome c. Proc. Natl. Acad. Sci. USA. 1974;71:2332–2334. doi: 10.1073/pnas.71.6.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Barr DP, Gunther MR, Deterding LJ, Tomer KB, et al. ESR spin-trapping of a protein-derived tyrosyl radical from the reaction of cytochrome c with hydrogen peroxide. J. Biol. Chem. 1996;271:15498–15503. doi: 10.1074/jbc.271.26.15498. [DOI] [PubMed] [Google Scholar]
- [36].Qian SY, Chen YR, Deterding LJ, Fann YC, et al. Identification of protein-derived tyrosyl radical in the reaction of cytochrome c and hydrogen peroxide: Characterization by ESR spin-trapping, HPLC and MS. Biochem. J. 2002;363:281–288. doi: 10.1042/0264-6021:3630281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Balakrishnan G, Hu Y, Oyerinde OF, Su J, et al. A conformational switch to beta-sheet structure in cytochrome c leads to heme exposure. Implications for cardiolipin peroxidation and apoptosis. J. Am. Chem. Soc. 2007;129:504–505. doi: 10.1021/ja0678727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chevance S, Le Rumeur E, de Certaines JD, Simonneaux G, et al. 1H NMR structural characterization of the cytochrome c modifications in a micellar environment. Biochemistry. 2003;42:15342–15351. doi: 10.1021/bi035044+. [DOI] [PubMed] [Google Scholar]
- [39].Tuominen EK, Wallace CJ, Kinnunen PK. Phospholipid-cytochrome c interaction: Evidence for the extended lipid anchorage. J. Biol. Chem. 2002;277:8822–8826. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- [40].Rytomaa M, Kinnunen PK. Reversibility of the binding of cytochrome c to liposomes. Implications for lipid-protein interactions. J. Biol. Chem. 1995;270:3197–3202. doi: 10.1074/jbc.270.7.3197. [DOI] [PubMed] [Google Scholar]
- [41].Rytomaa M, Mustonen P, Kinnunen PK. Reversible, nonionic, and pH-dependent association of cytochrome c with cardiolipin-phosphatidylcholine liposomes. J. Biol. Chem. 1992;267:22243–22248. [PubMed] [Google Scholar]
- [42].Kostrzewa A, Pali T, Froncisz W, Marsh D. Membrane location of spin-labeled cytochrome c determined by paramagnetic relaxation agents. Biochemistry. 2000;39:6066–6074. doi: 10.1021/bi992559l. [DOI] [PubMed] [Google Scholar]
- [43].Kawai C, Prado FM, Nunes GL, Di Mascio P, et al. pH-Dependent interaction of cytochrome c with mitochondrial mimetic membranes: The role of an array of positively charged amino acids. J. Biol. Chem. 2005;280:34709–34717. doi: 10.1074/jbc.M412532200. [DOI] [PubMed] [Google Scholar]
- [44].Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochim. Biophys. Acta. 2004;1666:2–18. doi: 10.1016/j.bbamem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- [45].Epperly MW, Tyurina YY, Nie S, Niu YY, et al. MnSOD-plasmid liposome gene therapy decreases ionizing irradiation-induced lipid peroxidation of the esophagus. In vivo. 2005;19:997–1004. [PubMed] [Google Scholar]
- [46].Basova LV, Kurnikov IV, Wang L, Ritov VB, et al. Cardiolipin switch in mitochondria: Shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry. 2007;46:3423–3434. doi: 10.1021/bi061854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Horobin RW, Trapp S, Weissig V. Mitochondriotropics: A review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J. Control. Release. 2007;121:125–136. doi: 10.1016/j.jconrel.2007.05.040. [DOI] [PubMed] [Google Scholar]
- [48].Adlam VJ, Harrison JC, Porteous CM, James AM, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- [49].Wipf P, Xiao J, Jiang J, Belikova NA, et al. Mitochondrial targeting of selective electron scavengers: Synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J. Am. Chem. Soc. 2005;127:12460–12461. doi: 10.1021/ja053679l. [DOI] [PubMed] [Google Scholar]
- [50].Jiang J, Kurnikov I, Belikova NA, Xiao J, et al. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J. Pharmacol. Exp. Ther. 2007;320:1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- [51].Fink MP, Macias CA, Xiao J, Tyurina YY, et al. Hemigramicidin-TEMPO conjugates: Novel mitochondria-targeted anti-oxidants. Biochem. Pharmacol. 2007;74:801–809. doi: 10.1016/j.bcp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- [52].Macias CA, Chiao JW, Xiao J, Arora DS, et al. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann. Surg. 2007;245:305–314. doi: 10.1097/01.sla.0000236626.57752.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- [54].Chapkin RS, Hong MY, Fan YY, Davidson LA, et al. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–199. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- [55].Ng Y, Barhoumi R, Tjalkens RB, Fan YY, et al. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–1921. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–5568. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- [57].Hatch GM. Regulation of cardiolipin biosynthesis in the heart. Mol. Cell Biochem. 1996;159:139–148. doi: 10.1007/BF00420916. [DOI] [PubMed] [Google Scholar]
- [58].Schlame M, Hostetler KY. Cardiolipin synthase from mammalian mitochondria. Biochim. Biophys. Acta. 1997;1348:207–213. doi: 10.1016/s0005-2760(97)00119-7. [DOI] [PubMed] [Google Scholar]