Abstract
Human exposure to manganese has been associated with a variety of cognitive deficits including learning and memory deficits. However, results from epidemiological studies have been inconsistent in describing the nature of such cognitive deficits. The present study was conducted to evaluate the effects of chronic Mn exposure on memory functioning in non-human primates and to correlate behavioral outcome with brain Mn levels in an attempt to explain outcome variability seen in prior studies. Cynomolgus macaque monkeys were trained to perform memory-related tasks (spatial working memory, non-spatial working memory, reference memory) and exposed to manganese sulfate (15–20 mg/kg/week) over an exposure period lasting 227.5 ± 17.3 days. Blood manganese levels were in the upper range of levels reported for human environmental, medical or occupational exposures. By the end of the manganese exposure period, animals developed mild deficits in spatial working memory, more significant deficits in non-spatial working memory and no deficits in reference memory. Linear regression analyses showed that for most brain regions sampled, there was a significant inverse relationship between working memory task performance and brain Mn concentration. These results suggest that chronic exposure to levels of manganese achieved in this study may have detrimental effects on working memory and that Mn levels achieved in several brain regions are inversely related to working memory performance.
Keywords: Manganese, Monkey, Cognition, Working Memory
1. Introduction
Manganese (Mn) is an essential metal that is necessary for a variety of physiological processes including amino acid, lipid, protein and carbohydrate metabolism (Erikson et al., 2005). In the brain, Mn has a variety of functions including serving as a cofactor for a number of enzymes, including the anti-oxidant enzyme superoxide dismutase (Hurley and Keen, 1987) and several enzymes involved in neurotransmitter synthesis and metabolism (Golub et al., 2005). Although small amounts of Mn are a nutritional necessity for normal brain functioning, Mn can be neurotoxic when exposure increases or when homeostatic mechanisms fail to prevent excess absorption and accumulation (Hauser et al., 1996; Bouchard et al., 2007).
The clinical neurological manifestations of Mn toxicity have mostly been studied in exposed workers in a variety of occupations such as miners (Rodier, 1955), welders (Bowler et al., 2006), and ferroalloy workers (Lucchini et al., 1999), although neurological effects from environmental exposures have also been documented (Kondakis et al., 1989; Lucchini et al., 2007, Rodriguez-Agudelo, et al., 2006; Finkelstein and Jerrett, 2007). While many of the neurobehavioral descriptions of Mn toxicity have emphasized the development of Parkinson-like symptoms including postural instability, bradykinesia, rigidity, micrographia, masked facies, speech disturbances and tremor (Iregren, 1999), Mergler et al. (1999) suggested that Mn neurotoxicity is manifest as a dose-related continuum of dysfunction ranging from more subtle motor, behavioral and cognitive dysfunction to more severe Parkinson-like disease. However, human exposure-effect relationships have been difficult to define across occupational and environmental exposures (Smith et al., 2007) and it is unclear at this time at what level of chronic Mn exposure deleterious clinical effects can occur (Mergler and Baldwin, 1997; Mergler et al., 1999).
Despite numerous investigations of cognitive functioning in patients with exposures to Mn, there are inconsistencies in the results from such studies. Cognitive effects associated with low to moderate Mn exposure have been reported less consistently than motor effects (Iregren, 1999; Bouchard et al., 2007). Some studies have shown little or no dose-effect relationships on several measures of motor and cognitive functioning (Zoni et al., 2007) while others have reported findings such as poor hand steadiness, difficulty in performing fast alternating movements, muscle rigidity, postural instability, poor memory, slow reaction time and decreased cognitive flexibility after moderate (< 1 mg/m3) but long-term Mn exposure (Iregren, 1999). The reasons for such inconsistent results are not entirely clear but may be related at least in part to differences in methodology and choice of neuropsychological assessments across studies as well as the uncertainties surrounding estimation of Mn dose and exposure duration, the presence of confounding health issues, exposure to other potentially toxic agents, and the timing of the cognitive evaluation in relation to the actual exposure (Bouchard et al., 2007).
In order to overcome some of these issues inherent in human epidemiological studies and to increase our understanding of the effects of chronic Mn exposure on neurological functioning, we have investigated the cognitive, motor and behavioral sequelae of chronic Mn exposure in non-human primates. Previously, we showed that monkeys that received Mn sulfate (MnSO4, administered once/week intravenously) at 10 – 15 mg/kg/week over an exposure period lasting 272 ± 17 days, developed subtle but inconsistent deficits in spatial working memory, developed some stereotypic behaviors and had modest decreases in spontaneous activity and manual dexterity by the end of the Mn exposure period (Schneider et al., 2006). Individual animals developed different degrees of cognitive impairment at different times post manganese exposure and the reasons for this variability are not clear, although may relate to different levels of brain Mn accumulation in different animals. The present study was conducted to further evaluate the effects of chronic Mn exposure on cognitive functioning in non-human primates and to attempt to correlate behavioral outcome with brain Mn levels.
2. Results
2.1 Manganese administration and blood levels
The mean ± s.e.m. cumulative Mn dose administered was 185.3 ± 32.8 mg Mn/kg body weight (570.0 ± 100.9 mg MnSO4/kg). The average time from the initiation of Mn administration to the end of the study was 227.5 ± 17.3 days. Blood Mn levels were 10.8 ± 5.0 μg/l at baseline (range 4.2 to 35.8), 89.9 ± 10.5 μg/l at after approximately 14 weeks of Mn administration (range 63.5 to 134.1) and 70.8 ± 15.6 μg/l after 28 weeks of Mn administration (range 33.3 to 118.8).
Effects of Chronic Mn Exposure on Variable Delayed Response (VDR), Delayed Matching to Sample (DMTS) and Visual Pattern Discrimination (VD) Performance
Six animals were successfully trained to perform VDR and VD; five animals also performed DMTS. Both control animals performed VDR, DMTS and VD tasks. One Mn-exposed animal developed some motor impairment after approximately 21 weeks of Mn administration and further cognitive testing was not performed with that animal. None of the other Mn-exposed animals developed any motor impairments that would have influenced their ability to perform tasks.
Before initiation of Mn exposure, the animals had a delay-dependent decrement in performance on the VDR task. That is, there was a significant effect of delay such that performance on short delay trials differed significantly from performance on trials with longer delays [F(5,4) = 44.04, p < 0.001]. At baseline, short duration delay trials (2–5 s or 5–8 s delays) were performed almost without error (96.8 % ± 1.2 and 94.8% ± 1.9 correct responses, respectively). Performance declined on trials with longer duration delays (10 s delay, 86.8% ± 6.6; 20–30 s delay, 75.0% ± 12.9; 30–45 s delays, 57.7% ± 10.8).
As a group, mean overall VDR task performance declined over the Mn exposure period from 82.2 ± 2.7 percent correct responses at baseline to 67.8 ± 7.3 correct responses at week 28 (p < 0.05). However, the effect of Mn exposure on VDR performance, when examining performance at individual delays, varied somewhat from animal to animal resulting in high between animal variances particularly towards the end of the study period. Due to this variability, only performance of trials with a 5s delay showed a statistically significant difference from baseline performance, and this was apparent at week 12 through week 28 of Mn administration (Table 1).
Table 1.
Variable Delayed Response Performance: Mn-Treated Group (n = 6)
| Condition | Delay | Mean Percent Correct Responses | Std. Error |
|---|---|---|---|
| Baseline | D1 | 96.8 | 1.2 |
| D2 | 94.8 | 1.9 | |
| D3 | 86.8 | 2.7 | |
| D4 | 75.0 | 5.3 | |
| D5 | 57.7 | 4.4 | |
| Week 4 | D1 | 91.8 | 4.1 |
| D2 | 83.5 | 6.1 | |
| D3 | 94.0 | 2.7 | |
| D4 | 69.3 | 5.1 | |
| D5 | 61.8 | 3.0 | |
| Week 8 | D1 | 89.7 | 5.2 |
| D2 | 82.5 | 5.2 | |
| D3 | 75.2 | 9.2 | |
| D4 | 64.0 | 2.4 | |
| D5 | 67.2 | 2.1 | |
| Week 12 | D1 | 72.2 | 9.8 |
| D2 | 78.2* | 7.9 | |
| D3 | 73.2 | 6.2 | |
| D4 | 62.8 | 6.2 | |
| D5 | 68.0 | 5.0 | |
| Week 16 | D1 | 84.5 | 6.4 |
| D2 | 75.2* | 9.6 | |
| D3 | 71.2 | 11.4 | |
| D4 | 68.0 | 6.7 | |
| D5 | 53.8 | 2.7 | |
| Week 20 | D1 | 83.5 | 5.9 |
| D2 | 71.0* | 10.1 | |
| D3 | 79.5 | 9.0 | |
| D4 | 75.3 | 8.5 | |
| D5 | 48.7 | 4.9 | |
| Week 24 | D1 | 77.8 | 12.0 |
| D2 | 80.2* | 12.8 | |
| D3 | 72.8 | 7.1 | |
| D4 | 54.2 | 12.8 | |
| D5 | 62.0 | 3.2 | |
| Week 28 | D1 | 81.4 | 12.9 |
| D2 | 75.2* | 10.9 | |
| D3 | 70.4 | 10.8 | |
| D4 | 59.2 | 4.9 | |
| D5 | 52.8 | 4.7 |
Delays: D1 = 2–5 s; D2 = 5–8 s; D3 = 10 s; D4 = 20–30 s; D5 = 30–45 s
p < 0.05 vs. D2 at baseline
In contrast to the Mn administration group, vehicle only treated animals showed improved task performance over the course of the 28 week study period. These animals also initially had a delay-dependent decrement in performance on the VDR task (main effect of delay [F(1,4) = 13.9, p = 0.013]). Performance on short delay trials differed significantly from performance on trials with longer delays. At baseline, short to intermediate duration delay trials (2, 5, or 10s delays) were performed well (99.0 % ± 1.4, 96.5% ± 2.1, and 94.0% ± 1.4 correct responses, respectively) and performance declined on trials with longer duration delays (17 – 30s delay, 68.0% ± 7.1; 30 – 60s delay, 56.0% ± 12.7). However, by week 28 of the study, these animals performed trials at 2, 5, and 10 s delays flawlessly (100% ± 0 correct at each delay) and performance at long delay trials improved significantly over baseline performance levels (Table 2). At week 28, comparison of performance between Mn-treated and vehicle-treated monkeys showed significant differences (p < 0.05) in performance of the groups on medium to long duration delay trials (Figure 1).
Table 2.
Variable Delayed Response Performance: Vehicle-Treated Group (n = 2)
| Condition | Delay | Mean Percent Correct Responses | Std. Error |
|---|---|---|---|
| Baseline | D1 | 99.0 | 1.0 |
| D2 | 96.5 | 1.5 | |
| D3 | 94.0 | 1.0 | |
| D4 | 68.0 | 5.0 | |
| D5 | 56.0 | 9.0 | |
| Week 4 | D1 | 100.0 | 0.0 |
| D2 | 100.0 | 0.0 | |
| D3 | 100.0 | 0.0 | |
| D4 | 87.5 | 12.5 | |
| D5 | 63.0 | 13.0 | |
| Week 8 | D1 | 100.0 | 0.0 |
| D2 | 100.0 | 0.0 | |
| D3 | 85.0 | 10.0 | |
| D4 | 87.5 | 12.5 | |
| D5 | 78.5 | 3.5 | |
| Week 12 | D1 | 100.0 | 0.0 |
| D2 | 100.0 | 0.0 | |
| D3 | 100.0 | 0.0 | |
| D4 | 81.5 | 6.5 | |
| D5 | 81.5 | 6.5 | |
| Week 16 | D1 | 100.0 | 0.0 |
| D2 | 100.0 | 0.0 | |
| D3 | 100.0 | 0.0 | |
| D4 | 94.0 | 6.0 | |
| D5 | 87.5 | 12.5 | |
| Week 20 | D1 | 100.0 | 0.0 |
| D2 | 100.0 | 0.0 | |
| D3 | 94.9 | 5.1 | |
| D4 | 94.0 | 6.0 | |
| D5 | 81.5 | 6.5 | |
| Week 24 | D1 | 100.0 | 0.0 |
| D2 | 100.0 | 0.0 | |
| D3 | 94.0 | 6.0 | |
| D4 | 100.0 | 0.0 | |
| D5 | 94.0 | 6.0 | |
| Week 28 | D1 | 100.0 | 0.0 |
| D2 | 100.0 | 0.0 | |
| D3 | 100.0 | 0.0 | |
| D4 | 88.0* | 0.0 | |
| D5 | 88.0* | 0.0 |
Delays: D1 = 2 s; D2 = 5 s; D3 = 10 s; D4 = 17–30 s; D5 = 30–60 s
p < 0.05 vs. D4/D5 at baseline
Figure 1.
Variable delayed response (VDR) performance of Mn-exposed and vehicle-treated animals at end of study (week 28). The performance of the Mn-exposed group (black bars) on this task was significantly impaired at intermediate to long delay trials (i.e., delays 3, 4 and 5 (10s, 20–30s, and 30–45s for Mn group, respectively and 10s, 17–30s and 30–60s for vehicle group, respectively) compared to the vehicle control group (hatched bars) (*p < 0.05).
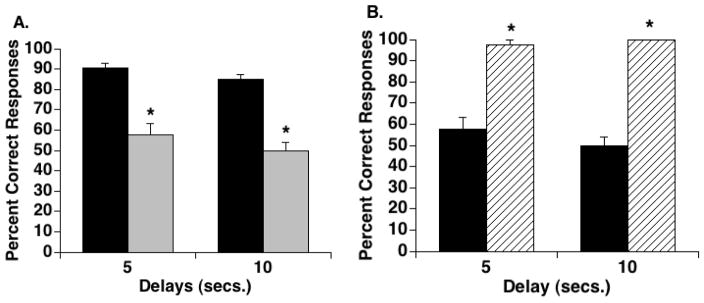
Prior to Mn exposure, the DMTS task was performed at a level of 90.6% ± 2.4 correct responses on trials with a 5s delay and 84.8% ± 2.7 correct responses on trials with a 10s delay. By week 8 of Mn exposure, performance on trials with a 5s delay differed significantly (p < 0.05) from baseline and performance continued to be significantly worse than baseline through week 28 (Table 3). On 10s delay duration trials, performance significantly deteriorated from baseline (p < 0.05) by week 12 of Mn exposure and remained impaired through the end of the study (Table 3, Figure 2). Performance of the vehicle control group on either 5 or 10s delay trials did not significantly differ from baseline through the end of the study (Table 3, Figure 2). A comparison between the Mn- and vehicle-treated groups at week 28 of study on the DMTS task with a 5 s delay showed the groups’ performance to be significantly different (57.5 ± 5.9 percent correct responses and 97.5 ± 2.5 percent correct responses, respectively, p < 0.01). Similarly, the groups significantly differed at week 28 on performance of the 10 s delay trials (Mn: 50.0 ± 2.0 percent correct responses and vehicle: 100.0 ± 0.0 percent correct responses, p < 0.001).
Table 3.
Delayed Matching-To-Sample Performance in Mn- and Vehicle-Treated Groups
| Condition | N | Delay (s) | Mean Percent Correct Responses | Std. Error |
|---|---|---|---|---|
| Mn Baseline | 5 | 5 | 90.6 | 2.4 |
| 10 | 84.8 | 2.7 | ||
| Mn Week 4 | 5 | 5 | 73.0 | 10.3 |
| 10 | 78.0 | 3.4 | ||
| Mn Week 8 | 5 | 5 | 69.0* | 5.8 |
| 10 | 73.0 | 4.6 | ||
| Mn Week 12 | 5 | 5 | 65.0* | 5.7 |
| 10 | 53.0^ | 3.4 | ||
| Mn Week 16 | 5 | 5 | 61.0* | 10.3 |
| 10 | 56.0^ | 4.3 | ||
| Mn Week 20 | 5 | 5 | 59.0* | 4.0 |
| 10 | 48.0^ | 3.7 | ||
| Mn Week 24 | 5 | 5 | 72.5* | 7.5 |
| 10 | 61.3^ | 5.2 | ||
| Mn Week 28 | 5 | 5 | 57.5* | 6.0 |
| 10 | 50.0^ | 2.0 | ||
|
| ||||
| Vehicle Baseline | 2 | 5 | 87.5 | 7.5 |
| 10 | 83.0 | 5.0 | ||
| Vehicle Week 4 | 2 | 5 | 85.0 | 15.0 |
| 10 | 80.0 | 10.0 | ||
| Vehicle Week 8 | 2 | 5 | 95.0 | 5.0 |
| 10 | 80.0 | 10.0 | ||
| Vehicle Week 12 | 2 | 5 | 85.0 | 5.0 |
| 10 | 70.0 | 10.0 | ||
| Vehicle Week 16 | 2 | 5 | 95.0 | 5.0 |
| 10 | 85.0 | 5.0 | ||
| Vehicle Week 20 | 2 | 5 | 95.0 | 5.0 |
| 10 | 90.0 | 0.0 | ||
| Vehicle Week 24 | 2 | 5 | 100.0 | 0.0 |
| 10 | 95.0 | 5.0 | ||
| Vehicle Week 28 | 2 | 5 | 97.5 | 2.5 |
| 10 | 100.0 | 0.0 | ||
p < 0.05 vs. baseline 5 s delay;
p < 0.05 vs. baseline 10 s delay
Figure 2.
Delayed matching-to-sample (DMTS) performance of Mn-exposed and vehicle-treated animals. A: Task performance was significantly affected at both delays at 28 weeks of Mn exposure (black bars denote performance at baseline; gray bars denote performance after 28 weeks of Mn exposure) (*p < 0.05). B: There was a significant difference in performance of the Mn-treated group (black bars) compared to the vehicle control group (hatched bars) on the same DMTS task assessed at the 28 week time point (*p < 0.01). In contrast to the Mn-exposed group, the vehicle group’s performance (which was comparable to the Mn group at baseline) remained stable or slightly improved by week 28 of the study.
Before receiving any Mn, visual pattern discrimination was performed at a 97.2% ± 2.2% correct level, and task performance remained at this level at week 28 of Mn exposure (97.0 ± 4.5% correct responses). The vehicle-treated group performed similarly (baseline = 97.5% ± 0.7; week 28 = 96.5% ± 5.0), with no significant difference between baseline and the end of the study (week 28).
2.2 Correlations Between Brain Mn Levels and Cognitive Outcome
Manganese content of selected brain regions (i.e. dorsolateral frontal cortex, frontal white matter, caudate, putamen and globus pallidus) are shown in Table 4. Mn levels were significantly increased (p < 0.05) in all brain regions surveyed in Mn-exposed monkeys compared to vehicle controls. However, on an animal by animal basis, regional Mn concentrations varied considerably, particularly in the caudate, globus pallidus and frontal white matter. Brain content of other metals (i.e., copper, iron, zinc) was also examined in these samples and there were no significant differences between control animals and Mn-exposed animals in levels of these other metals (data not shown).
Table 4.
Brain Manganese Concentrations (μg/g tissue)
| Animal | Frontal Cortex | Frontal White Matter | Caudate | Putamen | Globus Pallidus |
|---|---|---|---|---|---|
| Control 1 | 0.25 | 0.17 | 0.34 | 0.29 | 0.42 |
| Control 2 | 0.12 | 0.16 | 0.39 | 0.36 | 0.38 |
|
| |||||
| Mn 1 | 0.41 | 0.91 | 1.76 | 1.67 | 2.17 |
| Mn 2 | 0.34 | 0.55 | 1.49 | 1.62 | 3.00 |
| Mn 3 | 0.38 | 0.36 | 0.75 | 1.54 | 2.42 |
| Mn 4 | 0.55 | 1.15 | 2.03 | 2.19 | 4.02 |
| Mn 5 | 0.33 | 1.08 | 2.18 | 2.90 | 1.99 |
Brain Mn levels from 1 animal that did not complete the study are not included
Since the most consistent cognitive effects of Mn exposure were observed on performance of the DMTS task, correlation analyses were performed using DMTS performance and brain Mn content of each region noted above. Correlation analyses showed significant relationships between the change in DMTS performance between baseline and the end of the study and Mn content in all brain regions examined (i.e., frontal white matter (r = −0.93, p = 0.007), caudate (r = −0.93, p = 0.007), putamen (r = −0.92, p = 0.01) and globus pallidus (r = −0.85, p = 0.03)) except for the dorsolateral frontal cortex (r = −0.74, p = 0.09) (Figure 3). In general, animals with higher brain Mn concentrations had worse overall performance on the DMTS task at the end of the study period.
Figure 3.
Performance on the delayed matching-to-sample (DMTS) task was inversely correlated with Mn content in several brain regions. Correlation analyses showed significant (p < 0.05) inverse relationships between percent change in performance from baseline to week 28 and Mn content of all brain regions examined except dorsolateral frontal cortex. Data are from 4 Mn-exposed animals trained to perform the DMTS task and 2 vehicle control animals. Brain Mn levels were not available for the one Mn-exposed animal that developed motor impairment.
Similar analyses were performed using overall VDR performance and brain Mn concentrations. Again, correlation analyses showed significant relationships between the change in overall VDR performance between baseline and the end of the study and Mn content in frontal white matter (r = −0.85, p = 0.01), caudate (r = −0.87, p = 0.01) and putamen (r = -0.98, p < 0.001) but not globus pallidus (r = -0.61, p = 0.15) or dorsolateral frontal cortex (r = −0.58, p = 0.17) (Figure 4).
Figure 4.
Overall performance on the variable delayed response (VDR) task was inversely correlated with Mn content in several brain regions. Correlation analyses showed significant (p < 0.05) inverse relationships between percent change in performance from baseline to week 28 and Mn content of all brain regions examined except globus pallidus and dorsolateral frontal cortex. Data are from 5 Mn-exposed animals trained to perform the VDR task and 2 vehicle control animals. Brain Mn levels were not available for the one Mn-exposed animal that developed motor impairment.
3. Discussion
In the present study, we found significant effects on working memory task performance resulting from chronic Mn exposure in non-human primates. Previously, with lower level exposure, we found subtle and inconsistent effects on cognitive functioning (Schneider et al., 2006). Whole blood Mn concentrations in that study ranged from a mean of 55.7 – 67.1 μg/l and were within the range reported in children and adults receiving environmental, medical or occupational exposures (Gulson et al., 2006; Jiang et al., 2007; Takser et al., 2003). In the present study, mean whole blood Mn concentrations were 89.9 μg/l after approximately 14 weeks of Mn administration and 70.8 μg/l after 28 weeks of Mn administration. These levels are within the upper range of those reported in children and adults receiving environmental, medical and occupational exposures (Santos-Burgoa et al., 2001; Takser et al., 2003) and therefore are within a range relevant to contemporary exposures in specific human populations. For example, Takser et al. (2003) reported mean cord/newborn blood Mn levels ranging from 14.9–92.9 ug/L and Santos-Burgoa et al. (2001) reported blood Mn levels in the general population from central Mexico ranging from 7.5 to 88.0 ug/L.
Although some inter-animal variability in the nature of the cognitive deficit produced by the Mn exposure was observed, the deficits produced with the current level of exposure were more robust and consistent than observed previously at a lower level of exposure (Schneider et al., 2006). Interestingly, non-spatial working memory, as assessed by DMTS performance was more affected that spatial working memory, as assessed by VDR performance. These results are consistent with reports in which working memory deficits have been reported in Mn-exposed populations (Bowler et al., 2006; Lucchini et al., 1999; Martin, 2006; Mergler et al., 1999). Several studies have also reported significant correlations between blood Mn levels and cumulative exposure index and performance on memory tests such as digit span (Lucchini et al, 1995; Lucchini et al., 1999). In addition, the current finding of a greater effect on DMTS performance than on VDR performance might suggest a greater dysfunction in ventrolateral prefrontal cortices (associated with performance of the DMTS task) than in dorsolateral prefrontal cortex (primarily associated with performance of delayed response tasks) (Bachevalier and Mishkin, 1986; Elliott and Dolan, 1999). Previously, we reported an increase in certain stereotypic or compulsive- like behaviors in monkeys with lower level Mn exposure than the current cohort (Schneider et al., 2006). We suggested that the stereotypic and compulsive-like behaviors observed in Mn-intoxicated monkeys may have been related to dysfunction of ventral frontostriatal circuits, since ventral prefrontal cortical regions are involved in response inhibition processes (Rosvold and Mishkin, 1961; Diamond, 1990). These previous findings together with the current results lend support to the hypothesis that ventral prefrontal cortical circuits may be particularly vulnerable to disruption from Mn exposure.
Current data show that although all animals received a similar exposure to Mn over a similar period of time, brain levels of Mn in some regions differed considerably from animal to animal. This may help to explain some of the inter-animal variability in cognitive outcome measures observed currently as well as in our previous study (Schneider et al., 2006). For the most part, linear regression analyses showed significant negative correlation between the change in cognitive performance from baseline to the end of the study (on a per animal basis) and regional brain Mn content. However, performance on neither the DMTS task nor VDR task correlated with Mn concentration in the dorsolateral frontal cortex. This could in part reflect a sampling problem, since DMTS performance is more dependent upon the ventrolateral prefrontal cortex rather than the dorsolateral prefrontal cortex and the former, in particular, was not sampled for brain Mn levels in this study. The lack of significant correlation between VDR performance and Mn content in the dorsolateral frontal cortex was surprising. However, significant effects were found between VDR performance and caudate Mn content, suggesting that Mn-related alterations in performance of the VDR task may be mediated in these animals more at the level of the striatum than at the cortical level. It is well known that delayed response task performance can be disrupted at either the cortical level or at the striatal projection level (Divac et al., 1967). The significant correlations between behavior and frontal subcortical frontal white matter were also surprising, considering the lack of significant findings correlated with frontal cortex Mn content, and may reflect the propensity for subcortical white matter to accumulate Mn (Guilarte et al., 2006).
In addition to a variety of effects on cognition and behavior, studies of Mn-exposed humans have also detected problems in various aspects of learning (Bowler et al., 2006a; Josephs et al., 2005; Martin, 2006; Mergler et al., 1999), including verbal and non-verbal learning. Our current findings also suggest the possibility of a basic learning defect in Mn-exposed monkeys. Vehicle-treated monkeys tested as frequently as Mn-exposed monkeys significantly improved in VDR and DMTS task performance over the course of the 28 week study while Mn-exposed monkeys showed no such improvements over time. A similar effect was demonstrated in our previous study of Mn-exposed animals (Schneider et al., 2006). These data suggest the possibility of impaired learning capacity following various levels of Mn exposure that will need to be examined more directly in future studies.
In summary, chronic manganese exposure in macaque monkeys at the levels described led to impairments in performance of working memory tasks and in particular, a DMTS task that assesses non-spatial working memory. Results suggest that in addition, Mn-exposed animals may have impaired learning capacity. Future studies will be directed at more in depth analyses of potentially at-risk behavioral/cognitive functions and relationships to Mn-induced cortical and sub-cortical pathology.
4. Experimental Procedure
4.1 General Methods
Eight adult male M. fascicularis macaques, 5–6 years old at the start of the study, were used; six received Mn exposure and two served as control animals that were treated exactly the same as the Mn-exposed animals except received only vehicle injections. All animal studies were reviewed and approved by the Animal Care and Use Committees at Thomas Jefferson University and at Johns Hopkins University.
Following quarantine, animals were trained to perform cognitive tasks and their baseline level of functioning was assessed. Once animals achieved a stable performance baseline, they were temporarily transferred to Johns Hopkins University for in vivo imaging studies (note: animals were transported again to Johns Hopkins University for additional imaging studies performed approximately midway through the manganese exposure period and again at the end of the exposure period, results to be reported elsewhere). Upon their return, a stable level of behavioral performance was confirmed and Mn exposure was initiated. Animals received intravenous injections of MnSO4 monohydrate (Sigma-Aldrich, St. Louis, Mo., USA; 15 mg/kg/week for 5 weeks and then 20 mg/kg/week for the remainder of the study period: divided into two doses/week (N = 5) or given as a single weekly dose (N = 1)) into the saphenous vein under light isofluorane anesthesia. Two animals received twice weekly vehicle control injections under the same conditions. Manganese sulfate was prepared fresh for each injection (50 mg/ml in sterile saline), filtered and warmed to 37 °C prior to use. A needle and catheter were inserted into the vein and flushed with sterile saline. MnSO4 was then administered at a rate of 0.5 ml/min over an approximately 4- to 6-minute period, depending upon the total volume to be injected. Vital signs were monitored during the manganese administration. At the end of the MnSO4 infusion, at least 1.0 ml of warm sterile saline was slowly pushed through the catheter. Animals were then returned to their home cage and observed for any possible adverse events.
4.2 Behavioral Training and Testing
Animals were trained to perform cognitive tasks while seated in a restraining chair placed inside a Wisconsin General Test Apparatus (WGTA). When inside the WGTA, the monkey was positioned behind an opaque screen which when raised allowed the animal access to a sliding tray containing two food wells with sliding Plexiglas covers that served as stimulus plaques. The animals were trained to displace the Plexiglas covers in order to obtain rewards from the food wells. All animals were food restricted overnight prior to testing.
Variable Delayed Response (VDR)
In this task, which has both attentional and spatial working memory components (Schneider et al., 1999), animals were trained to retrieve a food reward after observing the experimenter bait one of two wells. Right and left wells were baited in a balanced order. Five different delay lengths (ranging from 2 to 60 s) were randomly distributed in blocks of 8 trials over the 40 trials that made up a daily testing session. The delay conditions were selected to yield performance ranging from approximately 90% correct performance at the shortest delay and approximately chance performance at the longest delay.
Visual Pattern Discrimination
This test, which assesses reference memory, requires animals to discriminate between two black patterns (a plus sign and a minus sign) on a white background. One stimulus (plus sign) was designated as the positive stimulus and this positive stimulus randomly appeared over the right or left food well on any particular trial. The animal was rewarded for choosing the positive stimulus regardless of its spatial location. Animals were trained to perform 20 trials per day to approximately a 90% criterion level.
Delayed Matching To Sample (DMTS)
On this task, which assesses non-spatial working memory, animals were presented with a sample stimulus (orange, gray or blue stimulus plaque) located in the center of the response panel and after a delay of either 5 or 10 seconds the animal was presented with 2 response choices and had to choose the stimulus that matched the sample (and ignore a competing stimulus of a different color) in order to receive reward. A daily session was made up of 20 trials (10 trials per delay) and trials were counterbalanced for side and color. Animals were trained to approximately an 85% criterion level.
4.3 Metal Analysis of Blood and Brain Tissue Using High-Resolution Inductively-Coupled Plasma Mass Spectrometry (HR-ICP-MS)
Whole blood samples were obtained under fasting conditions at baseline, midpoint of the Mn exposure period and at the end of the manganese exposure period. Blood Mn levels measured at mid-point and end of exposure period were taken within 3 weeks after the last manganese dosing. Concentrated nitric acid (HNO3) (Suprapur, Merck) was added to dried whole blood and brain samples. Blood samples were placed at room temperature for 24 hours and digested on a heat block for 1 hr at 70 °C, 1 hr at 100 °C and 1 hr at 110 °C. Brain samples were placed at room temperature for 24 hr and digested either on a heat block (QBT4, Grant) for 3 hrs at 70 °C or using a microwave oven (Multiwave 3000, Anton Paar) using ramp 200W for 10 min and then held for 10 min. Samples were then diluted with 0.6 M HNO3 with 18.2 MΩ water. Blood and brain samples were analyzed for metal content by HR-ICP-MS using a Thermo (Finnigan) model Element 2 instrument (Bremen, Germany), according to a published protocol (Erikson et al., 2005).
4.4 Data Analyses
Means and standard deviations were calculated for various cognitive measures at baseline (i.e., 3 weeks prior to the first Mn exposure) and at weeks 4, 8, 12, 16, 20, 24 and 28 after the first Mn exposure. Task performance prior to and following Mn exposure was compared by repeated measures analysis of variance for each task and for subcomponents of various tasks where appropriate. Animals served as their own controls. Pairwise post-hoc comparisons (Newman-Kuhls t test; Dunnetts post hoc t test) were performed to assess changes between conditions (vehicle and Mn-exposed) and between baseline and each post Mn observation period. In instances where there were significantly different group variances, unpaired t test with Welch correction was employed. Statistical significance was defined at p < 0.05. For correlation analyses, Mn content per brain region of interest was correlated with change from baseline task performance (Pearson correlation coefficient).
Acknowledgments
This work was supported by NIH grant ES010975.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachevalier J, Mishkin M. Visual recognition impairment following ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Bowler R, Roels HA. Neurobehavioral functioning after cessation of manganese exposure: A follow-up after 14 years. Am J Ind Med. 2007;50:831–840. doi: 10.1002/ajim.20407. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Koller W, Schulz PE. Parkinsonism due to manganism in a welder: neurological and neuropsychological sequelae. Neurotoxicol. 2006;27:327–32. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicol. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Diamond A. Developmental progression in human infants and infant monkeys, and the neural bases of inhibitory control of reaching. In: Diamond A, editor. The Development and Neural Bases of Higher Cognitive Functions. New York Academy of Science Press; New York, NY: 1990. pp. 267–317. [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. Differential neural responses during performance of matching and nonmatching to sample tasks at two delay intervals. J Neurosci. 1999;19:5066–5073. doi: 10.1523/JNEUROSCI.19-12-05066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Aschner JL, Aschner M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ Toxicol and Pharmacol. 2005;19:415–421. doi: 10.1016/j.etap.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007;104:420–432. doi: 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaoankar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: A 1H-MRS and MRI study. Toxicol Sci. 2006;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow’s milk formula, sow formula or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Gulson B, Mizon K, Taylor A, Korsch M, Stauber J, Davis M, Louie H, Wu M, Swan H. Changes in manganese and lead in the environment and young children associated with the introduction of methylcyclopentadienyl manganese tricarbonyl in gasoline. Environ Res. 2006;100:100–114. doi: 10.1016/j.envres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Zesiewicz TA, Martinez C, Rosemurgy AS, Olanow CW. Blood manganese correlates with brain magnetic resonance imaging changes in patients with liver disease. Can J Neurol Sci. 1996;23:95–98. doi: 10.1017/s0317167100038786. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Manganese intoxication and chronic liver failure. Ann Neurol. 1994;36:871–875. doi: 10.1002/ana.410360611. [DOI] [PubMed] [Google Scholar]
- Hurley LS, Keen CL. Manganese. In: Underwood E, Mertz W, editors. Trace Elements in Human Health and Animal Nutrition. Academic Press; New York, NY: 1987. pp. 185–225. [Google Scholar]
- Iregren A. Manganese neurotoxicity in industrial exposures: Proof of effects, critical exposure level and sensitive tests. Neurotoxicol. 1999;20:315–323. [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T. Possible health effects of high manganese concentration in drinking water. Arch Environ Health. 1989;44:175–178. doi: 10.1080/00039896.1989.9935883. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parinello G, Garattini S, Resola S, Alessio L. High prevalence of parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med. 2007;50:788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, Mergler D, Sassine MP, Palmi S, Alessio L. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicol. 1999;20:287–297. [PubMed] [Google Scholar]
- Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, Iregren A, Alessio L. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Sacnd J Work Environ Health. 1995;21:143–149. doi: 10.5271/sjweh.1369. [DOI] [PubMed] [Google Scholar]
- Martin CJ. Manganese neurotoxicity: connecting the dots along the continuum of dysfunction. Neurotoxicol. 2006;27:347–349. doi: 10.1016/j.neuro.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, Panisset M, Edwards R, de Geoffroy A, Sassine MP, Hudnell K. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicol. 1999;20:327–342. [PubMed] [Google Scholar]
- Rodier J. Manganese poisoning in Moroccan miners. Br J Ind Med. 1995;12:21–35. doi: 10.1136/oem.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Agudelo Y, Riojas-Rodriguez H, Rios C, Rosas I, Sabido Pedraza E, Miranda J, Siebe C, Texcalac JL, Santos-Burgoa C. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci Total Environ. 2006;368:542–556. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mishkin M. Non-sensory effects of frontal lesions on discrimination learning and performance. In: Delafresnaye JF, editor. Brain Mechanisms and Learning. Blackwell; Oxford: 1961. pp. 555–576. [Google Scholar]
- Santos-Burgoa C, Rios C, Mercado LA, Arechiga-Serrano R, Cano-Valle J, Eden-Wynter RA, Texcalac-Sangrador JP, Rodriguez-Agudelo Y, Montes S. Exposure to manganese: Health effects on the general population, a pilot study in central Mexico. Environ Res. 2001;85:90–104. doi: 10.1006/enrs.2000.4108. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Kovelowski CJ. Chronic exposure to low doses of MPTP. I Cognitive deficits in motor asymptomatic monkeys. Brain Res. 1990;519:122–128. doi: 10.1016/0006-8993(90)90069-n. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Tinker JP, Van Velson M, Menzaghi F, Lloyd GK. The nicotinic acetylcholine receptor agonist SIB-1508Y improves cognitive functioning in chronic low dose MPTP-treated monkeys. J Pharmacol Exp Ther. 1999;290:731–739. [PubMed] [Google Scholar]
- Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, Lucchini R. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50:801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicol. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zheng W, Long L, Zhao W, Li X, Mo X, Lu J, Fu X, Li W, Liu S, Long Q, Huang J, Pira E. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure. Neurotoxicol. 2007;28:126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoni S, Albini E, Lucchini R. Neuropsychological testing for the assessment of manganese neurotoxicity: A review and a proposal. Am J Ind Med. 2007;50:812–830. doi: 10.1002/ajim.20518. [DOI] [PubMed] [Google Scholar]