Abstract
The polymerase chain reaction (PCR) is a new diagnostic technique for the detection of enteroviral infection; however, it currently provides only qualitative results. The aim of this study was to adapt PCR for the accurate quantitation of enteroviral RNA in clinical specimens. For this purpose, we designed a standard RNA which was homologous to sequences at the 5' end of the coxsackie B3 enterovirus genome but contained a single-base-pair mutation which created a novel internal restriction site. Serial dilutions of this standard template RNA were mixed with a fixed concentration of coxsackie B3 enterovirus RNA. The viral and standard templates were reversed transcribed to cDNA and coamplified by PCR, and a comparison of the radioactive PCR products was made. Since the templates were both present in a single reaction tube and competed for the same primers, the ratio of products remained proportional throughout the amplification process. By this approach, a fourfold-difference in viral titer was clearly distinguishable. Moreover, we were able to accurately quantitate as few as 15 50% tissue culture infectious doses, which reflects common clinical viral titers. This study lays the foundation for quantitation of enteroviral RNA in clinical specimens and establishes a technique that can readily be applied to the diagnosis of enteroviral infection.
Full text
PDF


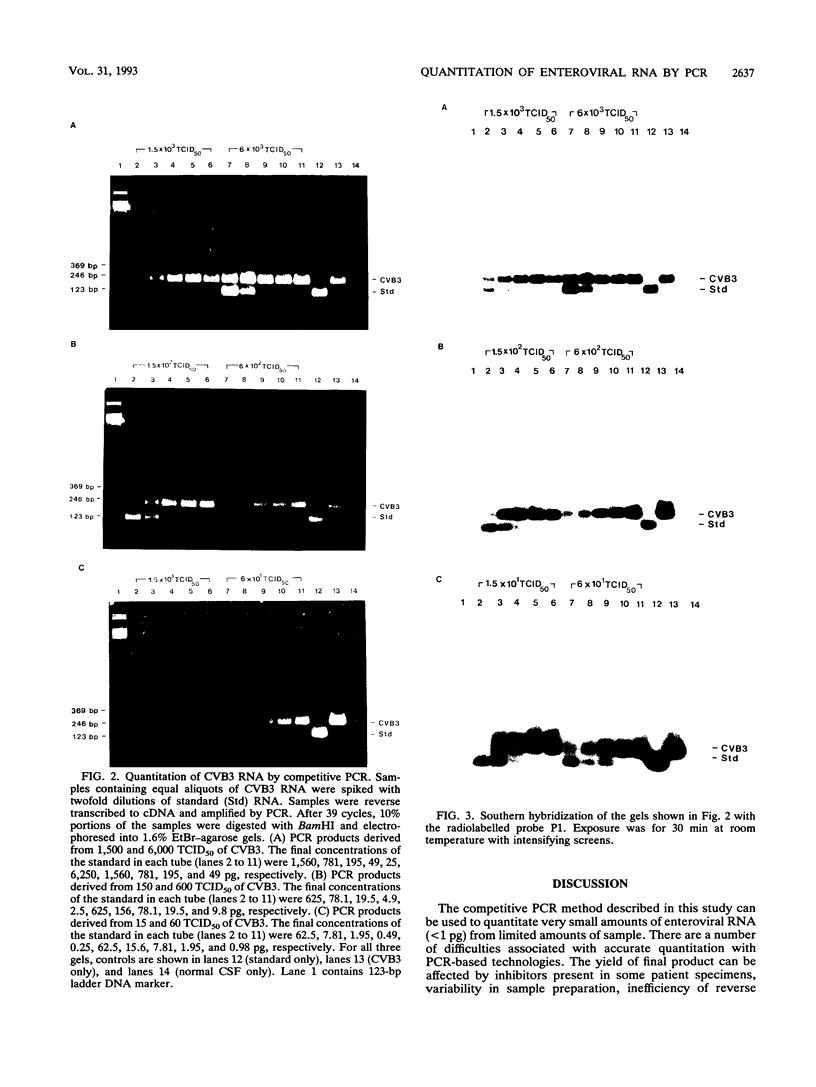
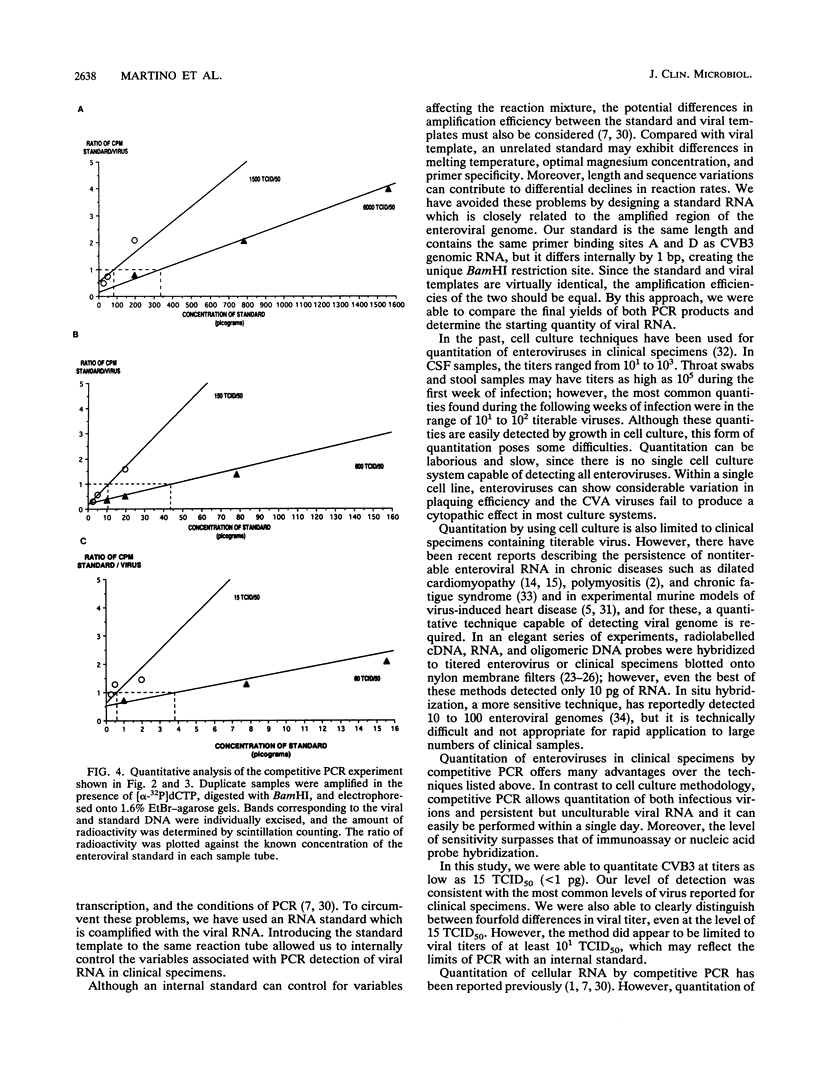


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles N. E., Dubowitz V., Sewry C. A., Archard L. C. Dermatomyositis, polymyositis, and Coxsackie-B-virus infection. Lancet. 1987 May 2;1(8540):1004–1007. doi: 10.1016/s0140-6736(87)92271-9. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Tracy S., Gauntt C. J., Fortmueller U. Molecular detection and identification of enteroviruses using enzymatic amplification and nucleic acid hybridization. J Clin Microbiol. 1990 May;28(5):843–850. doi: 10.1128/jcm.28.5.843-850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cronin M. E., Love L. A., Miller F. W., McClintock P. R., Plotz P. H. The natural history of encephalomyocarditis virus-induced myositis and myocarditis in mice. Viral persistence demonstrated by in situ hybridization. J Exp Med. 1988 Nov 1;168(5):1639–1648. doi: 10.1084/jem.168.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R., Liu P., Wee L., Butany J., Sole M. J. Verapamil ameliorates the clinical and pathological course of murine myocarditis. J Clin Invest. 1992 Nov;90(5):2022–2030. doi: 10.1172/JCI116082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Auvinen P., Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989 Dec;70(Pt 12):3261–3268. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Horsnell C., Maaronen M., Khan M., Kalkkinen N., Auvinen P., Kinnunen L., Stanway G. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8847–8851. doi: 10.1073/pnas.89.18.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka N., Kuge S., Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987 Jan;156(1):64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Jenkins O., Booth J. D., Minor P. D., Almond J. W. The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the Picornaviridae. J Gen Virol. 1987 Jul;68(Pt 7):1835–1848. doi: 10.1099/0022-1317-68-7-1835. [DOI] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- La Monica N., Meriam C., Racaniello V. R. Mapping of sequences required for mouse neurovirulence of poliovirus type 2 Lansing. J Virol. 1986 Feb;57(2):515–525. doi: 10.1128/jvi.57.2.515-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. M., Stålhandske P. O., Pettersson U. Genome of coxsackievirus B3. Virology. 1987 Jan;156(1):50–63. doi: 10.1016/0042-6822(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A., Abzug M. J., Levin M. J. Development and application of RNA probes for the study of picornaviruses. Mol Cell Probes. 1988 Mar;2(1):65–73. doi: 10.1016/0890-8508(88)90045-x. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A., Eastman P. S., Ruth J. L., Hirata K. K., Levin M. J. Nonisotopic oligomeric probes for the human enteroviruses. J Clin Microbiol. 1988 Dec;26(12):2669–2671. doi: 10.1128/jcm.26.12.2669-2671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A., Levin M. J., Villarreal L. P., Tracy S. M., Semler B. L., Wimmer E. Factors affecting the detection of enteroviruses in cerebrospinal fluid with coxsackievirus B3 and poliovirus 1 cDNA probes. J Clin Microbiol. 1985 Aug;22(2):220–224. doi: 10.1128/jcm.22.2.220-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A., Levin M. J., Villarreal L. P. Use of subgenomic poliovirus DNA hybridization probes to detect the major subgroups of enteroviruses. J Clin Microbiol. 1984 Dec;20(6):1105–1108. doi: 10.1128/jcm.20.6.1105-1108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Stanway G., Hughes P. J., Mountford R. C., Reeve P., Minor P. D., Schild G. C., Almond J. W. Comparison of the complete nucleotide sequences of the genomes of the neurovirulent poliovirus P3/Leon/37 and its attenuated Sabin vaccine derivative P3/Leon 12a1b. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1539–1543. doi: 10.1073/pnas.81.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee L., Liu P., Penn L., Butany J. W., McLaughlin P. R., Sole M. J., Liew C. C. Persistence of viral genome into late stages of murine myocarditis detected by polymerase chain reaction. Circulation. 1992 Nov;86(5):1605–1614. doi: 10.1161/01.cir.86.5.1605. [DOI] [PubMed] [Google Scholar]
- Yousef G. E., Bell E. J., Mann G. F., Murugesan V., Smith D. G., McCartney R. A., Mowbray J. F. Chronic enterovirus infection in patients with postviral fatigue syndrome. Lancet. 1988 Jan 23;1(8578):146–150. doi: 10.1016/s0140-6736(88)92722-5. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Yousef G. E., Bowles N. E., Archard L. C., Mann G. F., Mowbray J. F. Detection of enterovirus RNA in experimentally infected mice by molecular hybridisation: specificity of subgenomic probes in quantitative slot blot and in situ hybridisation. J Med Virol. 1988 Dec;26(4):375–386. doi: 10.1002/jmv.1890260405. [DOI] [PubMed] [Google Scholar]
- Zoll G. J., Melchers W. J., Kopecka H., Jambroes G., van der Poel H. J., Galama J. M. General primer-mediated polymerase chain reaction for detection of enteroviruses: application for diagnostic routine and persistent infections. J Clin Microbiol. 1992 Jan;30(1):160–165. doi: 10.1128/jcm.30.1.160-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]