Abstract
Aims:
Drug withdrawal is suggested to play a role in precipitating mood disorders in individuals with familial predisposition. Age-related differences in affective responses to withdrawal might explain the increased risk of mental illnesses when drug use begins during adolescence. Since there is a lack of animal research examining the effects of opioid withdrawal during adolescence, the present study examined whether there are age-related differences in affective responses to opioid withdrawal.
Main methods:
Adolescent and adult mice were injected with two different morphine regimens, namely low and high, which differed in the dosage. Three and nine days following discontinuation of morphine administration, immobility time in the forced swim test (FST) and locomotion (total distance traveled) were evaluated.
Key findings:
On withdrawal day 3 (WD3), adolescent mice exhibited a decrease in immobility as compared to controls. No significant differences in immobility were observed on withdrawal day 9 (WD9). This effect on FST behaviors was not due to changes in overall motor activity, since no differences in locomotion were observed on either WD3 or WD9 in adolescent mice. In adults, no differences in either FST or locomotor behaviors were observed on WD3. As expected, on WD9, adult mice exhibited an increase in immobility and a decrease in locomotion.
Significance:
This study demonstrates age-dependent differences in both FST scores and locomotor behaviors during opioid withdrawal. FST behaviors are classically used to evaluate mood in rodents, thus this study suggests that opioid withdrawal might affect mood differentially across age.
Keywords: Morphine, Dependence, Age-related differences, Forced swim test (FST), Locomotion, Drug addiction
INTRODUCTION
There is a high rate of comorbidity of mood disorders in drug addicts, even after years of abstinence (reviewed in Maremmani et al., 2006). Moreover, this comorbidity was demonstrated to increase when drug use began at a young age (Gfroerer et al., 2002; Caspi et al., 2005; Tucker et al., 2006; Mathers et al., 2006; Degenhardt et al., 2007). However, the underlying biological mechanisms for this comorbidity are still elusive. It has been suggested that the withdrawal from drug use, specifically opioids, might precipitate mood disorders in predisposed individuals (Shobe and Brion, 1971; Handelsman et al., 1992; White, 2004; Schürks et al., 2005; Janiri et al., 2005). Accordingly, age-related differences in affective responses to withdrawal might explain the increased risk of mental illnesses when drug use begins during adolescence.
Adolescence is not a uniquely human trait and is also observed in other mammalian species, including rodents. Rodent and human adolescents share some familiar characteristics (reviewed in Spear, 2000). Like humans, the distinctive nature of rodent adolescent behaviors is, at least in part, the result of altered levels of anxiety, sensitivity to stress, and perception of reward and aversion (Spear, 2000). There is considerable literature demonstrating differences between adolescents' and adults' withdrawal from nicotine and alcohol. Adolescent rodents exhibit milder affective signs, such as aversion and anxiety, during nicotine and alcohol withdrawal as compared to adults (Wilmouth and Spear, 2006; O'Dell et al., 2006, 2007; Doremus-Fitzwater and Spear, 2007; Kota et al, 2007; Abreu-Villaça et al, 2007). Reduced negative affects during abstinence from both tobacco/nicotine (Smith et al., 2008) and cannabis/marijuana (Vandrey et al., 2005; Budney et al., 1999) were also reported in adolescent humans as compared to adults. The diminished negative consequences seen in adolescents were suggested, at least in part, to contribute to the development of a compulsive drug abuse pattern in adolescents (O'Dell et al., 2006).
Studying the consequences of opioid withdrawal in adolescents is clearly important, given the recent surge in prescription medication abuse among adolescents, especially pain killers such as Oxycodone (National Survey, 2006). Additionally, in adults, many of the effects of nicotine and alcohol are mediated by the opioid system. Specifically, the mu opioid receptor was demonstrated to be involved in smoking initiation and alcohol consumption (Hall et al., 2001; Zhang et al., 2006). The mu opioid receptor also mediates the rewarding properties of, and dependence to, both alcohol and nicotine (Corrigall et al., 2000; Hall et al., 2001; Berrendero et al., 2002; Walters et al., 2005; Ghozland et al., 2005). Similarly, the kappa opioid receptor was demonstrated to be involved in alcohol consumption and dependence (Kovacs et al., 2005; Walker and Koob, 2008). Unfortunately, there is limited animal research examining the effects of opioid exposure during adolescence and lack of studies on the effect of withdrawal. This is somewhat surprising given the role that the opioid system plays in the development of dependence for nicotine and alcohol, and the differences between adolescents and adults during withdrawal from these drugs.
Age-related differences in sensitivity to acute and repeated morphine administration seem to depend on the specific behavioral response measured. When examining morphine's antinociception properties, one study failed to find differences in morphine-induced antinociception between adolescents and young adults using the foot-shock technique (Nozaki et al., 1975). Another study found that adolescent rodents are more sensitive to morphine-induced antinociception in the hot-plate paradigm (Ingram et al., 2007). Yet a third study confirmed the enhanced sensitivity to morphine-induced antinociception in the hot-plate, but found that adolescent rodents are less sensitive to the antinociception properties of morphine using the tail-flick test. However, there seems to be agreement that adolescent rodents exhibit a more rapid onset of tolerance with repeated doses (Nozaki et al., 1975; Ingram et al., 2007). Regarding morphine-induced hyperactivity, adolescents are more sensitive to acute morphine-induced locomotion (Spear et al., 1982; White et al., 2008). Likewise, animals treated during adolescence exhibited a higher locomotor response to morphine during adulthood, thus it appears that adolescent's opioid system is more sensitive to locomotor sensitization (White et al., 2008). There is also controversy regarding age-related differences in morphine reward properties. An earlier study showed a lack of morphine reward in adolescent mice (Bolanos et al., 1996), while two subsequent studies reported no differences between adolescents and adults (Campbell et al. 2000; Zheng et al., 2003). However, as mentioned above, there remains a lack of studies on the effect of opioid withdrawal during adolescence in mice.
Similar to humans, opioid withdrawal in rodents elicits numerous somatic and affective signs. Negative affects observed in adults include aversion and dysphoria (Schaefer and Michael, 1983; Hand et al., 1988; Schulteis et al., 1994; Grasing and Ghosh, 1998). Dysphoria (or depressive-like behavior) during withdrawal was examined in adult rodents using, among others, the forced swim test (FST) paradigm (Molina et al, 1994; Grasing and Ghosh, 1998; Zurita and Molina, 1999; Anraku et al., 2001). However, the affective responses to opioid withdrawal in adolescent rodents, including mice, are yet to be characterized. Thus, in this study, using the FST paradigm, we examined the effects of withdrawal on mood across age. The FST is a behavioral despair model wherein rodents are placed in a small apparatus filled with enough water so that their tails cannot touch the bottom. Escape from this apparatus is impossible. A period of extreme motor activity is initially observed during which the animal will actively attempt to escape. However, once unsuccessful, they will make fewer and fewer escape attempts - which manifests through longer periods of time in which they present a characteristic immobile posture. Mood is evaluated based on the length of time spent mobile or immobile. For the FST, we used Porsolt's modified version for mice which consists of one exposure to the apparatus (Porsolt et al., 1977).
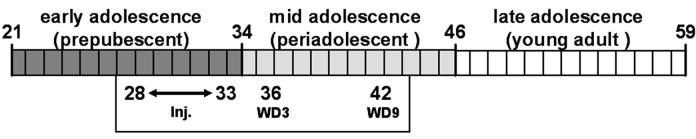
In this study, we examine the effects of morphine withdrawal on both FST and locomotor behaviors across age. Two regimens of morphine of differing dosages were used. For both regimens, morphine was administrated repeatedly for 6 days. Behaviors were examined 3 and 9 days following discontinuation of morphine administration, i.e. on withdrawal day 3 (WD3) and withdrawal day 9 (WD9). Studies in adult rats undergoing opioid withdrawal demonstrated that the dysphoric effect (i.e. increased FST immobility) started around withdrawal day 3 (Molina et al, 1994, Anraku et al., 2001) and lasted well into the second and third week of withdrawal (Grasing and Ghosh, 1998). Therefore, in this study we chose to examine the effects of withdrawal in adolescents on WD3 and WD9. We did not examine the response in the third week of withdrawal, given that adolescence in mice only lasts a short period of time, thus by the third week of withdrawal the mice are already considered young adults (Fig 1).
Fig. 1. Schematic representation of the timing of injections and tests in relation to the developmental period of the mice.
Numbers represent age in days; Inj - 6 days of injections. WD3 – withdrawal day 3; WD9 – withdrawal day 9. The developmental periods are based on studies by Spear and colleagues (reviewed in Spear, 2000).
MATERIALS AND METHODS
Animals and drugs
All procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee. Male C57BL/6 mice, purchased from Harlan Lab (Houston, TX), were housed 4-5 per cage with food and water ad lib. They were acclimated to the temperature-controlled vivarium with a 12 h/12 h light/dark cycle (light on at 07:00) for at least one week prior to treatment. Mice treated with morphine and saline were housed in separate cages. Separate animal groups were used for each behavioral test (FST, locomotion). In a preliminary experiment, we used mice that were bred in our vivarium (breeders were purchased from Harlan Lab, Houston, TX) and their behavioral responses were compared to the responses observed in mice purchased from Harlan Lab (Houston, TX) and acclimated to the vivarium for at least one week. Our behavioral tests did not indicate any response differences based on breeding history. Thus, in subsequent studies, we used mice that where purchased from Harlan Lab and acclimated to the vivarium for at least one week. Morphine sulfate was purchased from Sigma (St. Louis, MO).
Adult and adolescent mice were examined in this study. The choice for the age of the adolescent mice was based on studies by Spear and colleagues (reviewed in Spear, 2000) which demonstrated three developmental stages for rodents from weaning to adulthood. Since the adolescent period in rodents is quite short, in this study we chose to conduct behavioral testing during what is considered to be their mid-adolescence/periadolescent period (Fig. 1). Accordingly, mice were purchased at postnatal day 22 (PND 22). They were acclimated for the vivarium until PND 28, when morphine injections began, and behavioral testing was performed on PND 38 (WD3) and PND 44 (WD9). Thus, in this study, mice were injected during what is considered the late phase of their prepubescent period, and they were tested during mid-adolescence/periadolescent period (Fig. 1). We refer to this group as adolescents. In contrast, adult mice were injected during PND 63-68, and tested at PND 71 (WD3) and PND 77 (WD9).
Morphine regimen
Adult (PND 63) and adolescent (PND 28) mice were injected twice daily (9 a.m. and 5 p.m.) for 6 consecutive days for a total of 12 injections. We used two morphine regimens which we refer to as high and low morphine regimens. For the high morphine regimen, mice were injected with increasing doses of morphine (10-40 mg/kg, s.c.). Specifically, on days 1 and 2, the mice were injected with 10 mg/kg morphine. On days 3 and 4, they were injected with 20 mg/kg morphine. On days 5 and 6, they were injected with 40 mg/kg morphine. The high morphine regimen dosage was selected based on our previous studies demonstrating that such levels induce a significant antinociceptive tolerance (Eitan et al., 2003). Additionally, a PubMed search verified that physical dependence on morphine was produced using a similar regimen, namely twice daily injections of escalating morphine doses (el-Kadi and Sharif 1994; Spanagel et al., 1994; Matthes et al., 1996; Kest et al., 2001; Contet et al., 2008). For the low morphine regimen, mice were injected with a constant 10 mg/kg morphine dose (s.c.) for all 12 injections. The ‘low morphine’ regimen is similar to the regimen used in previous studies which reported an increase in immobility in adults undergoing withdrawal (Molina et al., 1994, Zurita and Molina, 1999) – 10 mg/kg morphine for 6 days. Note that drug naïve mice can easily tolerate a 10 mg/kg morphine dosage, thus such a dose can be administered from the outset (i.e. starting from day 1). However, the high morphine regimen is provided using increasing doses since injecting 40 mg/kg morphine to drug naïve mice straight away is considered a very harsh treatment. Thus, when higher doses of morphine are used, it is very common to do it via an increasing dose regimen. Indeed, this provides us with two different regimens that differ in more than one manner. For ease of reference, we call these two regimens low and high morphine. Control mice received 12 injections of saline (s.c.).
Forced swim test (FST)
Adult (n=8-14) and adolescent (n=10-18) mice were subjected to the FST test 3 and 9 days after the last injection. Note that the sample size of the adolescents is larger than for adults. This is because (as will be demonstrated below) we observed an unexpected result on WD3 for this age group, an observation that we repeated multiple times in order to be certain beyond any doubt that it is correct. Since prior FST exposure is known to alter the outcome in subsequent tests, different mice were used for each time period (Porsolt et al., 2001). The FST was performed in the second half of the light phase, between 3 pm to 6 pm. Mice were habituated to the room for at least 30 minutes prior to testing. One mouse at a time was examined in the testing room.
At the time of testing, the body weights of the adolescent mice were approximately 70% of adults. The size of the apparatus relative to body mass might have an effect on FST behaviors. Thus, we used different apparatus sizes for the adults and adolescents to account for body mass differences. A literature search revealed that an apparatus of at least 10 cm diameter and water level of at least 10 cm deep is most commonly used for measuring FST behaviors in mice (also reviewed by Jacobson and Cryan, 2006). Thus, for the adolescent mice we used an apparatus of 10 cm in diameter, 18.3 cm deep, and a 10 cm water level. Accordingly, for the adults, we used an apparatus of 13.8 cm in diameter, 24.5 cm deep, and a 13.5 cm water level. Water levels for both adolescents and adults were sufficient to ensure that they could not touch the bottom of the test chamber. The water temperature was set to 24.5-25°C, and the water was replaced after each mouse. Mice were placed in the apparatus and videotaped for 10 minutes. Digital video output was analyzed using EthoVision 3.1 Video Tracking System software (Noldus Information Technology). As described by Crowley et al. (2004), a 2 cm/sec cutoff was applied; less than 2 cm of movement per second was deemed immobile. Immobility during each 1 minute interval of the test and for the entire 10 minute period was calculated. FST behaviors were also scored using the sampling method by an observer blind to the treatment (data not shown). Both scoring methods yielded the same results with a high and significant correlation of 0.91 (Bartlett's chi-square: 144.315, df=1, p<0.0000000001).
Locomotion
Mice (n=10-18) were injected twice daily for 6 consecutive days with saline, the low morphine regimen, or the high morphine regimen. Their locomotion was recorded on WD3 and WD9. Similar to the FST, different groups of mice were examined in each time period. Locomotion was recorded in the second part of the light phase, between 3 pm to 6 pm. Mice were habituated to the room for at least 30 minutes prior to testing. Mice were placed in an upright cylindrical container (261 mm in diameter and 355 mm high) and recorded for 60 minutes by an overhead camera. The apparatus was cleaned thoroughly with water and completely dried between tests. Total distance traveled (locomotion) was scored using EthoVision 3.1 (Noldus Information Technology).
Data analyses
Separate analyses of variance were computed for each of the dependent measures of these studies (immobility time in sec; distance traveled in cm). The overall design of the analyses was a factorial consisting of between-group factors of age (adolescent versus adult), testing day (WD3 versus WD9) and drug treatment (saline, low morphine, and high morphine). Separate analyses were computed for each level of age and testing day using total immobility scores (summated over 10 min) or total distance traveled scores (summated over either minutes 1-10 or 1-60) or a within-group factor of time (FST: 1-10 minutes; locomotion: 1-10 min or 1-60 minutes). Differences less than 0.05 were deemed statistically significant. Additional post-hoc contrasts between each treatment group were computed using Tukey's HSD test; this procedure adjusts the critical t value upward to account for multiple comparisons (Kirk, 1982).
RESULTS
FST and locomotor behaviors in adolescents during morphine withdrawal
Withdrawal day 3 (WD3)
Adolescent mice exhibited a decrease in immobility time on the third day following discontinuation of morphine, as compared to saline treated controls. One-way ANOVA of the total immobility scores (summed across the entire 10 minute test; Fig. 2A) revealed a significant effect of treatment (F2, 45=5.602, p=0.007). Tukey's HSD post-hoc contrasts revealed a significant decrease in immobility time in mice withdrawing from the low morphine regimen, as compared to saline injected controls (p=0.007). A decrease in total immobility time was also noted in mice withdrawing from the high morphine regimen, but this did not reach statistical significance (p=0.064). An additional analysis was computed using immobility time during each minute of the 10 minute test period. As expected, in all treatment groups, immobility levels increased with time (Fig. 2B). However, mice withdrawing from the low morphine regimen exhibited lower immobility levels at each one-minute interval as compared to controls, with the exception of the first minute (Fig. 2B). Here also, the decrease in immobility was less pronounced in mice withdrawing from the high morphine regimen. These mice exhibited a significant decrease in immobility levels, as compared to controls, in just some of the 10 one-minute intervals.
Fig. 2. FST behaviors of adolescent mice during morphine withdrawal.
(A) and (B) Total immobility score during the entire 10 minute test on WD3 and WD9, respectively. (C) and (D) Immobility scores for each one-minute interval on WD3 and WD9, respectively. Results are presented as mean ± SEM. (**) indicates a significant difference from controls (p<0.001); (#) indicates a significant difference from controls (p<0.01); and (*) indicates a significant difference from controls (p<0.05). White bars and ○ - saline treated control mice; gray bars and  - mice withdrawing from low morphine regimen; and black bars and ■- mice withdrawing from high morphine regimen.
- mice withdrawing from low morphine regimen; and black bars and ■- mice withdrawing from high morphine regimen.
In a separate experiment, we measured locomotor behavior in the adolescent mice on WD3 to confirm that the FST results are not due to an effect of morphine withdrawal on overall motor activity. One-way ANOVA of both the total distance traveled during the 60 minute test (Fig. 3A) and the total distance traveled during the first 10 minutes of the test revealed no significant effect of treatment. Similarly, split-plot analysis of total distance traveled for the one-minute intervals (Fig. 3B) revealed no significant effect of treatment at any time interval.
Fig. 3. Locomotion of adolescent mice during morphine withdrawal.
(A) and (B) Total distance traveled (cm) during the entire 60 minute test on WD3 and WD9, respectively. (C) and (D) Total distance traveled (cm) in each one-minute interval on WD3 and WD9, respectively. Results are presented as mean ± SEM. White bars and ○ saline treated control mice; gray bars and  - mice withdrawing from low morphine regimen; and black bars and ■- mice withdrawing from high morphine regimen.
- mice withdrawing from low morphine regimen; and black bars and ■- mice withdrawing from high morphine regimen.
Withdrawal day 9 (WD9)
In contrast to the FST behaviors of adolescents on WD3, on withdrawal day 9 (WD9) one-way ANOVA of the immobility scores during the entire 10 minutes test revealed no significant effect of treatment (Fig. 2C) and split-plot ANOVA revealed no significant differences in any one-minute interval (Fig. 2D) between the treatment groups.
Additionally, no significant differences in locomotor behaviors between the withdrawal and control groups were observed for adolescents on WD9. One-way ANOVA of both the total distance traveled during the 60 minute test (Fig. 3C) and the total distance traveled during the first 10 minutes of the test revealed no significant effect of treatment. Similarly, split-plot analysis of total distance traveled for the one-minute intervals (Fig. 3D) revealed no significant effect of treatment at any time interval.
Analysis across days
Two-way ANOVA of the FST behaviors for adolescents across days revealed a significant effect of day (F1, 79=22.143, p<0.0001) and a significant interaction between treatment and day (F2, 79=5.756, p=0.0005). Two-way ANOVA of the locomotor behaviors for adolescents across days revealed no significant effects.
FST and locomotor behaviors in adults during morphine withdrawal
Our results for adolescents are somewhat surprising given that adult rats withdrawing from morphine were demonstrated to exhibit increased immobility in the FST (Grasing and Ghosh, 1998) and a decrease in locomotion (Schulteis et al., 1994). However, since FST behaviors of adult mice during morphine withdrawal have yet to be tested, we next examined the effect of morphine withdrawal on FST and locomotor behaviors in adult mice to determine whether our results are due to age or species differences.
Withdrawal day 3 (WD3)
One-way ANOVA of the total immobility scores for adult mice on WD3 revealed no significant effect of treatment (Fig. 4A). Similarly, no significant differences were observed in the split-plot ANOVA between groups at any of the one-minute intervals (Fig. 4B). Likewise, for the locomotor behavior, no significant effect of treatment was observed in the one-way ANOVA of the locomotion during both the 60 minute test (Fig. 5A) and when analyzing the first 10 minutes of the test. Also, no significant differences were revealed in the split-plot analysis at any of the one-minute intervals (Fig. 5B).
Fig. 4. FST behaviors of adult mice during morphine withdrawal.
(A) and (B) Total immobility score during the entire 10 minute test on WD3 and WD9, respectively. (C) and (D) Immobility scores for each one-minute interval on WD3 and WD9, respectively. Results are presented as mean ± SEM. (*) indicates a significant difference from controls (p<0.05). White bars and ○ - saline treated control mice; gray bars and  - mice withdrawing from low morphine regimen; and black bars and ■- mice withdrawing from high morphine regimen.
- mice withdrawing from low morphine regimen; and black bars and ■- mice withdrawing from high morphine regimen.
Fig. 5. Locomotion of adult mice during morphine withdrawal.
(A) and (B) Total distance traveled (cm) during the entire 60 minute test on WD3 and WD9, respectively. (C) and (D) Total distance traveled (cm) in each one-minute interval on WD3 and WD9, respectively. Results are presented as mean ± SEM. (*) indicates a significant difference from controls (p<0.05). White bars and ○ - saline treated control mice; gray bars and  - mice withdrawing from low morphine regimen; and black bars and ■- mice withdrawing from high morphine regimen.
- mice withdrawing from low morphine regimen; and black bars and ■- mice withdrawing from high morphine regimen.
Withdrawal day 9 (WD9)
On WD9, as expected, one-way ANOVA of the total immobility scores (Fig. 4C) revealed a significant effect of treatment (F2, 29=3.468, p=0.045). Tukey's HSD post-hoc contrasts revealed a significant increase in immobility in mice withdrawing from the low morphine regimen as compared to saline injected controls (p=0.036). No significant differences were observed between the mice withdrawing from the high morphine regimen as compared to saline injected controls. In the split-plot analysis, no significant differences were observed at any particular one-minute interval (Fig. 4D).
Additionally, for the adults on WD9, a significant decrease in locomotion was observed for both the high and low morphine regimens. One-way ANOVA of the total locomotion during the 60 minute test (Fig. 5C) revealed a significant effect of treatment (F2, 37=5.051, p=0.011). Tukey's HSD post hoc contrasts revealed a significant decrease in locomotion in mice withdrawing from both the low morphine (p=0.020) and high morphine (p=0.036) regimens as compared to saline injected controls. Likewise, a significant effect was also revealed in the one-way ANOVA of total distance traveled during the first 10 minutes (F2, 37=3.358, p=0.046). As seen in Fig. 5D, the decrease in locomotion as compared to controls is more pronounced during some of the one-minute intervals.
Analysis across days
No significant effects were revealed in the two-way ANOVA of the FST behaviors for adults across days. Two-way ANOVA of the locomotor behaviors for adults across days revealed a significant effect of treatment (F2, 72=6.427, p=0.003).
Age-dependent differences in FST and locomotor behaviors during morphine withdrawal
Analysis revealed significant differences in FST behaviors between adolescent and adult mice during morphine withdrawal. A three-way ANOVA (age × day × treatment) revealed a significant effect of age (F1, 135=20.716, p<0.0001), a significant effect of day (F1, 135=19.024, p<0.0001), a significant interaction between age and day (F1, 135=4.934, p=0.028), a significant interaction between treatment and day (F2, 135=6.071, p=0.003), and a significant interaction between treatment and age (F2, 135=3.580, p=0.031). Additionally, analysis revealed significant differences in locomotion between adolescent and adult mice during morphine withdrawal. Three-way ANOVA of the locomotor results revealed a significant interaction between treatment and age (F2, 130=3.741, p=0.026).
DISCUSSION
In this study, we observed age-dependent differences in FST and locomotor behaviors in mice during opioid withdrawal. For adults, no differences in FST behaviors were observed on WD3, as compared to saline-treated controls. However, an increase in immobility was observed on WD9. Note that also a decrease in locomotion was observed on WD9 which might be a contributing factor for the increase in immobility in adults. However, it appears that the locomotion results could not solely explain the observed FST behaviors in adults. This is because the adult mice withdrawing from the high morphine regimen did not exhibit a similar increase in immobility as compared to the mice withdrawing from the low morphine regimen, although both groups did exhibit similar decreases in locomotion. The results obtained in this study for the adult mice are consistent with previous studies on rats. During opioid withdrawal, adult rats were reported to exhibit increased immobility (Grasing and Ghosh, 1998), elevated intracranial self-stimulation (ICSS) thresholds (Schaefer and Michael, 1983; Schulteis et al., 1994), and decreased locomotion (Schulteis et al., 1994) - a combination that is classically understood to represent a dysphoric mood.
In contrast, adolescent mice exhibited a decrease in immobility on WD3. This was more pronounced in the mice withdrawing from the low morphine regimen. Such a decrease was not observed in adults. Additionally, no significant increase in immobility was observed on WD9 as compared to controls. Moreover, unlike the adults, no significant differences in locomotion were observed on either WD3 or WD9. Thus, the FST outcomes were not a result of changes in overall locomotor activity.
The interpretation of the FST behavioral results, namely the extrapolation from motor activity to mood, should be performed with caution, especially when extrapolating from a measure of rodent response to the possible mental state of humans during opioid withdrawal. Furthermore, additional tests such as tail suspension test, sucrose consumption/preference, and intracranial self-stimulation should be conducted to further support any conclusion. Nonetheless, when extrapolating using the traditional understanding of FST results, we conclude that opioid withdrawal might precipitate different mood responses in adolescents and adults. In this regard, the response of adolescents on WD3 (i.e. decreased immobility) is interpreted to represent mood elation while the response of adults on WD9 (i.e. increased immobility) is a dysphoric response.
Multiple studies demonstrate a reduced intensity of negative affective signs in adolescents during nicotine and alcohol withdrawal as compared to adults (Wilmouth and Spear, 2006; O'Dell et al., 2006, 2007; Kota et al., 2007; Doremus-Fitzwater and Spear, 2007). Moreover, the diminished negative consequences seen in adolescents were suggested, at least in part, to contribute to the development of a compulsive abuse pattern in adolescents (O'Dell et al., 2006). Similarly, in this study, we demonstrate that opioid withdrawal in adolescents does not precipitate the expected dysphoric mood that is observed in adults. Specifically, on WD3, a decrease in immobility was observed in the FST test. In fact, though intuitively unexpected, our results could be interpreted to mean that adolescents are not only experiencing reduced negative affects, but actually exhibit a mood elation (i.e. positive affect) in response to withdrawal. Thus, further studies are required to clarify the nature of the adolescents' emotional experience of opioid withdrawal and how they differ from adults.
In adult rodents, the opioid system is known to be involved in modulating emotional states. Depressive-like behaviors can be induced by agonism at the kappa opioid receptors, while kappa receptor antagonists act as antidepressants (Mague et al., 2003; Todtenkopf et al., 2004; Carlezon et al., 2006). Such depressive-like activity is at least partially mediated by activation of the dynorphin/kappa opioid receptor system in the limbic system (Shirayama et al., 2004). In contrast to the kappa receptor, antagonism at the delta opioid receptors produce depressive-like behaviors, while agonists act as antidepressants (Jutkiewicz et al., 2003; Torregrossa et al., 2006; Vergura et al., 2008). Delta receptors' antidepressant-like effect seems to be mediated by enkephalin (Baamonde et al., 1992; Nieto et al., 2005). The delta opioid receptor was also shown to be involved in emotionality during aging (Narita et al., 2006) and during withdrawal from cocaine (Perrine et al., 2008). Thus, the observed age-related differences in this study might be explained by differences in the expression and functionality of the opioid system at different stages during development.
Studies on the opioid system's expression and functionality during adolescence are limited. Some literature suggests that the opioid system is still immature during adolescence. For instance, different expression levels of opioid receptors are observed across lifespan (Ueno et al., 1988; Gazyakan et al., 2000; Carretero et al., 2004; Kivell et al., 2004). Also, there are changes in the cellular localization and functionality of the opioid system throughout adolescence (Wang et al., 2003a, b). As mentioned above, FST behaviors are modulated differently by different opioid receptors. Thus, age-dependent differences in expression levels and/or the availability of different opioid receptors could result in different FST behavioral responses to withdrawal. An alternative explanation for these age-related differences during withdrawal is that morphine exposure or withdrawal might modulate the expression of opioid receptors differently in adolescents and adults. Indeed, in at least one instance, chronic morphine exposure during puberty was shown to result in long-term alterations to the mu and kappa opioid receptors (Byrnes, 2008). Additionally, in adults, repeated morphine exposure and withdrawal induces changes in the expression of endogenous opioid ligands in different brain areas (Uhl et al., 1988; Yukhananov et al., 1993; Nylander et al., 1995a, b; Fukunaga et al., 1998; Van Bockstaele et al., 2000). Thus, age-dependent differential modulation of dynorphin and/or enkephalin could also result in the observed age-dependent differences in FST behavior during withdrawal. In adults, a decrease in FST immobility was observed 24 hours following the cessation of morphine administration (i.e. on withdrawal day 1; Anraku et al., 2001). This was suggested to be due to enhanced activation of the endogenous opioid systems by uncontrollable stress in the sensitized animals. Thus, yet another explanation could be that age-related differences in expression/sensitization of the endogenous opioid system during withdrawal could lead to differences in the activation of the endogenous opioid system by the uncontrollable stress.
Age-related differences in morphine pharmacokinetics might also provide a possible explanation for the age-dependent differences in FST behaviors during withdrawal. There are a limited number of studies on the pharmacokinetics of morphine in mice, especially during adolescence. We are aware of only one study, Diaz et al., 2007, on the pharmacokinetic of morphine in adolescent mice. That study used only one dose of morphine and, unfortunately, did not directly compare morphine effects in adolescents and adults. However, the reported half life of morphine in that study for adolescents (24 minutes) is within the range reported earlier for adult mice (Handal et al., 2002). Thus, it appears that there are no major age-dependent differences in morphine pharmacokinetics. Additionally, a study in rats compared the time at which morphine-induced antinociception reached one-half its maximal value in adolescents and adults. That study did find that morphine acted faster in the adolescents when using the tail-flick test, however no differences in the response time to morphine were found when examined in the hot plate test (White et al., 2008). However, species-dependent differences are possible. Given the limited number of studies currently available, we cannot exclude the possibility that a subtle difference in morphine pharmacokinetics might exist between adolescents and adults. Thus, it is possible that differences in pharmacokinetics (even subtle) might be a contributing factor to the behavioral differences observed.
The FST behaviors while mobile are used in some instances to provide information regarding which neural substrates are involved in the decreased immobility. This is based on observations that rodents treated with different antidepressants that enhance predominantly the serotonergic, noradrenergic or dopaminergic systems exhibit different enhancements in swimming or climbing behaviors (reviewed in Cryan et al., 2005; see also Perona et al., 2008). Increased swimming behavior is generally considered a serotonergic effect, while enhanced climbing is seen as a noradrenergic or dopaminergic effect. In this study we observed a significant increase in swimming behavior in adolescents on WD3 but no effect on climbing (data not shown). However, it is important to note that our saline treated mice naturally presented very little climbing behavior. Thus, given the low baseline of climbing behavior, it is possible that an underlying age-dependent modulation in noradrenergic and/or dopaminergic tone during withdrawal is undetectable in our experiments. Future studies will examine the receptor mechanisms involved, as well as other brain pathways and signaling cascades that are important in the manifestation of age-related differences during drug withdrawal.
CONCLUSION
This study demonstrates the existence of age-related differences in affective responses to opioid withdrawal. We focused on FST and locomotor behaviors, given the high comorbidity of mood disorders in drug addicts. Thus, this study raises several key questions: Do human addicts undergoing opioid withdrawal also exhibit differential mood responses across age? And if so, is it possible that, in addition to familial predisposition, age of exposure to drugs of abuse may also be a contributing factor to the precipitation of differential mood disorders in addicts? Specifically, is age of drug exposure an important factor for the observed comorbidity of hypomania in heroin addicts (reviewed in Maremmani et al. 2006)? This study may provide some insights into the role of withdrawal from drugs of abuse in precipitating future mental illnesses, and will potentially have clinical implications on treatment approaches for drug addiction during adolescence.
ACKNOWLEDGMENTS
These studies were supported by NIDA (DA022402). We also would like to thank Mr. Kris Roberts for his assistance with the EthoVision analysis and Mr. Menachum M Slodowitz for his editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/conflict of interest: The authors have no financial interests to disclose.
REFERENCES
- Abreu-Villaça Y, Medeiros AH, Lima CS, Faria FP, Filgueiras CC, Manhães AC. Combined exposure to nicotine and ethanol in adolescent mice differentially affects memory and learning during exposure and withdrawal. Behavioural Brain Research. 2007;181(1):136–146. doi: 10.1016/j.bbr.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berl) 2001;157(2):217–220. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- Baamonde A, Daugé V, Ruiz-Gayo M, Fulga IG, Turcaud S, Fournié-Zaluski MC, Roques BP. Antidepressant-type effects of endogenous enkephalins protected by systemic RB 101 are mediated by opioid delta and dopamine D1 receptor stimulation. The European Journal of Pharmacology. 1992;216(2):157–166. doi: 10.1016/0014-2999(92)90356-9. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. The journal of neuroscience. 2002;22(24):10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. European journal of pharmacology. 1996;317(1):1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy P, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94(9):1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Chronic morphine exposure during puberty induces long-lasting changes in opioid-related mRNA expression in the mediobasal hypothalamus. Brain Research. 2008;1190:186–192. doi: 10.1016/j.brainres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiology and behavior. 2000;68(4):487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. The Journal of Pharmacology and Experimental Therapeutics. 2006;316(1):440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carretero J, Bodego P, Rodriguez RE, Rubio M, Blanco E, Burks DJ. Expression of the mu-opioid receptor in the anterior pituitary gland is influenced by age and sex. Neuropeptides. 2004;38(23):63–68. doi: 10.1016/j.npep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biological Psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Contet C, Filliol D, Matifas A, Kieffer BL. Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of micro opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology. 2008;54(3):475–486. doi: 10.1016/j.neuropharm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology (Berl) 2000;149(2):107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Jones MD, O'Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacology, biochemistry, and behavior. 2004;78(2):269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience and biobehavioral reviews. 2005;29(45):547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Coffey C, Moran P, Carlin JB, Patton GC. The predictors and consequences of adolescent amphetamine use: findings from the Victoria Adolescent Health Cohort Study. Addiction. 2007;102(7):1076–1084. doi: 10.1111/j.1360-0443.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcoholism, clinical and experimental research. 2007;31(9):1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, Polakiewicz R, Evans CJ. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. Journal of Neuroscience 2003. 2003;23(23):8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Kadi AO, Sharif SI. The influence of various experimental conditions on the expression of naloxone-induced withdrawal symptoms in mice. General pharmacology. 1994;25(7):1505–1510. doi: 10.1016/0306-3623(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Fukunaga Y, Nishida S, Inoue N, Miyamoto M, Kishioka S, Yamamoto H. Time course of morphine withdrawal and preproenkephalin gene expression in the periaqueductal gray of rats. Brain research. Molecular brain research. 1998;55(2):221–231. doi: 10.1016/s0169-328x(97)00374-4. [DOI] [PubMed] [Google Scholar]
- Gazyakan E, Disko U, Haaf A, Heimrich B, Jackisch R. Postnatal development of opioid receptors modulating acetylcholine release in hippocampus and septum of the rat. Brain research. Developmental brain research. 2000;123(2):135–141. doi: 10.1016/s0165-3806(00)00091-2. [DOI] [PubMed] [Google Scholar]
- Gfroerer JC, Wu LT, Penne MA. Initiation of marijuana use: Trends, patterns, and implications. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2002. (DHHS Publication No. SMA 02–3711, Analytic Series A–17). [Google Scholar]
- Ghozland S, Chu K, Kieffer BL, Roberts AJ. Lack of stimulant and anxiolytic-like effects of ethanol and accelerated development of ethanol dependence in mu-opioid receptor knockout mice. Neuropharmacology. 2005;49(4):493–501. doi: 10.1016/j.neuropharm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Grasing K, Ghosh S. Selegiline prevents long-term changes in dopamine efflux and stress immobility during the second and third weeks of abstinence following opiate withdrawal. Neuropharmacology. 1998;37(8):1007–1017. doi: 10.1016/s0028-3908(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl) 2001;154(1):43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- Hand TH, Koob GF, Stinus L, Le Moal M. Aversive properties of opiate receptor blockade: evidence for exclusively central mediation in naive and morphine-dependent rats. Brain Research. 1988;474(2):364–368. doi: 10.1016/0006-8993(88)90452-0. [DOI] [PubMed] [Google Scholar]
- Handal M, Grung M, Skurtveit S, Ripel A, Mørland J. Pharmacokinetic differences of morphine and morphine-glucuronides are reflected in locomotor activity. Pharmacology, biochemistry, and behavior. 2002;73(4):883–892. doi: 10.1016/s0091-3057(02)00925-5. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Kanof PD. The dysphoria of heroin addiction. The American Journal of Drug and Alcohol Abuse. 1992;18(3):275–287. doi: 10.3109/00952999209026067. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology. 2007;32(3):600–606. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Dario T, Reina D, Paparello F, Pozzi G, Addolorato G, Di Giannantonio M, De Risio S. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology. 2005;52(1):37–44. doi: 10.1159/000086176. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Woods JH, Winsauer PJ. Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behavioural pharmacology. 2003;14(7):509–516. doi: 10.1097/00008877-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacology, biochemistry, and behavior. 2001;70(1):149–156. doi: 10.1016/s0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. second ed. Wadsworth; Belmont California: 1982. p. 83. [Google Scholar]
- Kivell BM, Day DJ, McDonald FJ, Miller JH. Developmental expression of mu and delta opioid receptors in the rat brainstem: evidence for a postnatal switch in mu isoform expression. Brain research. Developmental brain research. 2004;148(2):185–196. doi: 10.1016/j.devbrainres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. The Journal of pharmacology and experimental therapeutics. 2007;322(1):399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Kovacs KM, Szakall I, O'Brien D, Wang R, Vinod KY, Saito M, Simonin F, Kieffer BL, Vadasz C. Decreased oral self-administration of alcohol in kappa-opioid receptor knockout mice. Alcoholism, clinical and experimental research. 2005;29(5):730–738. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. The Journal of pharmacology and experimental therapeutics. 2003;305(1):323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Perugi G, Pacini M, Akiskal HS. Toward a unitary perspective on the bipolar spectrum and substance abuse: opiate addiction as a paradigm. Journal of affective disorders. 2006;93(13):1–12. doi: 10.1016/j.jad.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Mathers M, Toumbourou JW, Catalano RF, Williams J, Patton GC. Consequences of youth tobacco use: a review of prospective behavioural studies. Addiction. 2006;101(7):948–958. doi: 10.1111/j.1360-0443.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383(6603):819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology (Berl) 1994;114(3):433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Narita M, Kaneko C, Tamai E, Khotib J, Miyatake M, Shindo K, Nagumo Y, Tanaka S, Suzuki T. Age-related emotionality is associated with cortical delta-opioid receptor dysfunction-dependent astrogliosis. Neuroscience. 2006;137(4):1359–1367. doi: 10.1016/j.neuroscience.2005.10.067. [DOI] [PubMed] [Google Scholar]
- National Survey . Results from the 2005 National Survey on Drug Use & Health: National Findings. Office of Applied Studies, Substance Abuse & Mental Health Services Administration (SAMHSA), US department of Health and Human Services; Rockville, Maryland, USA: 2006. [Google Scholar]
- Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135(2):305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Akera T, Lee CY, Brody TM. The effects of age on the development of tolerance to and physical dependence on morphine in rats. The journal of pharmacology and experimental therapeutics. 1975;192(3):506–512. [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain research. 1995a;683(1):25–35. doi: 10.1016/0006-8993(95)00279-y. [DOI] [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. The effects of morphine treatment and morphine withdrawal on the dynorphin and enkephalin systems in Sprague-Dawley rats. Psychopharmacology (Berl) 1995b;118(4):391–400. doi: 10.1007/BF02245939. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186(4):612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicology and teratology. 2007;29(1):17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona MT, Waters S, Hall FS, Sora I, Lesch KP, Murphy DL, Caron M, Uhl GR. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behavioural pharmacology. 2008;19(56):566–574. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54(2):355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Archives internationales de pharmacodynamie et de thérapie. 1977;229(2):327–336. [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Current protocols in neuroscience. 2001:10A. doi: 10.1002/0471142301.ns0810as14. Chapter 8, Unit 8. [DOI] [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. Morphine withdrawal produces differential effects on the rate of lever-pressing for brain self-stimulation in the hypothalamus and midbrain in rats. Pharmacology, biochemistry, and behavior. 1983;18(4):571–577. doi: 10.1016/0091-3057(83)90283-6. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. The Journal of pharmacology and experimental therapeutics. 1994;271(3):1391–1398. [PubMed] [Google Scholar]
- Schürks M, Overlack M, Bonnet U. Naltrexone treatment of combined alcohol and opioid dependence: deterioration of co-morbid major depression. Pharmacopsychiatry. 2005;38(2):100–102. doi: 10.1055/s-2005-837812. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. Journal of neurochemistry. 2004;90(5):1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Shobe FO, Brion P. Long-term prognosis in manic-depressive illness. Archives of general psychiatry. 1971;24(4):334–337. doi: 10.1001/archpsyc.1971.01750100044006. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl.) 1994;115(12):121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behavioural brain research. 1982;4(3):279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172(4):463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Research. 2006;1069(1):172–181. doi: 10.1016/j.brainres.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Collins RL, Klein DJ. Does solitary substance use increase adolescents' risk for poor psychosocial and behavioral outcomes? A 9-year longitudinal study comparing solitary and social users. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2006;20(4):363–372. doi: 10.1037/0893-164X.20.4.363. [DOI] [PubMed] [Google Scholar]
- Ueno E, Liu DD, Ho IK, Hoskins B. Opiate receptor characteristics in brains from young, mature and aged mice. Neurobiology of aging. 1988;9(3):279–283. doi: 10.1016/s0197-4580(88)80066-6. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Ryan JP, Schwartz JP. Morphine alters preproenkephalin gene expression. Brain research. 1988;459(2):391–397. doi: 10.1016/0006-8993(88)90658-0. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Menko AS, McHugh K, Drolet G. Decreases in endogenous opioid peptides in the rat medullo-coerulear pathway after chronic morphine treatment. The journal of neuroscience. 2000;20(23):8659–8666. doi: 10.1523/JNEUROSCI.20-23-08659.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug and alcohol dependence. 2005;78(2):205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus LH, Regoli D, Guerrini R, Salvadori S, Caló G. Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH(2)-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides. 2008;29(1):93–103. doi: 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33(3):643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46(6):933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Cuzon VC, Pickel VM. Postnatal development of mu-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation. Neuroscience. 2003a;118(3):695–708. doi: 10.1016/s0306-4522(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Cuzon VC, Pickel VM. Ultrastructural localization of delta-opioid receptors in the rat caudate-putamen nucleus during postnatal development: relation to synaptogenesis. The Journal of comparative neurology. 2003b;467(3):343–353. doi: 10.1002/cne.10920. [DOI] [PubMed] [Google Scholar]
- White DA, Michaels CC, Holtzman SG. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacology, biochemistry, and behavior. 2008;89(2):188–199. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM. Pleasure into pain: the consequences of long-term opioid use. Addictive behaviors. 2004;29(7):1311–1324. doi: 10.1016/j.addbeh.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacology, biochemistry, and behavior. 2006;85(3):648–657. doi: 10.1016/j.pbb.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukhananov RYu, Zhai QZ, Persson S, Post C, Nyberg F. Chronic administration of morphine decreases level of dynorphin A in the rat nucleus accumbens. Neuropharmacology. 1993;32(7):703–709. doi: 10.1016/0028-3908(93)90084-g. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kendler KS, Chen X. The mu-opioid receptor gene and smoking initiation and nicotine dependence. Behavioral and brain functions. 2006;2:28. doi: 10.1186/1744-9081-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Ke X, Tan B, Luo X, Xu W, Yang X, Sui N. Susceptibility to morphine place conditioning: relationship with stress-induced locomotion and novelty-seeking behavior in juvenile and adult rats. Pharmacology, biochemistry, and behavior. 2003;75(4):929–935. doi: 10.1016/s0091-3057(03)00172-2. [DOI] [PubMed] [Google Scholar]
- Zurita A, Molina V. Prior morphine facilitates the occurrence of immobility and anhedonia following stress. Physiology and behavior. 1999;65(45):833–837. doi: 10.1016/s0031-9384(98)00247-9. [DOI] [PubMed] [Google Scholar]