Abstract
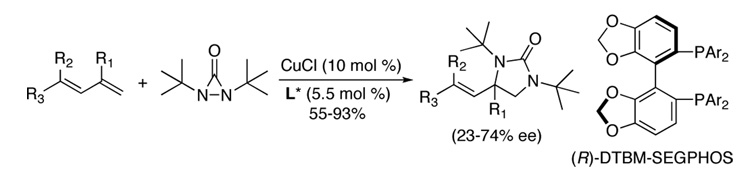
A Cu(I)-catalyzed asymmetric diamination for a variety of conjugated dienes and a triene with encouraging ee’s has been effectively achieved using (R)-DTBM-SEGPHOS as chiral ligand and di-tert-butyldiaziridinone as nitrogen source.
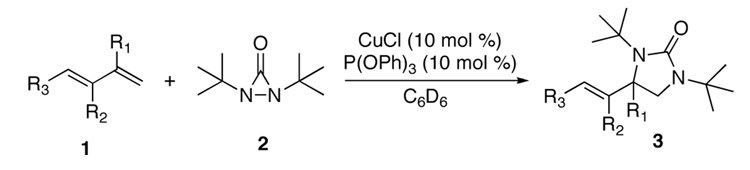
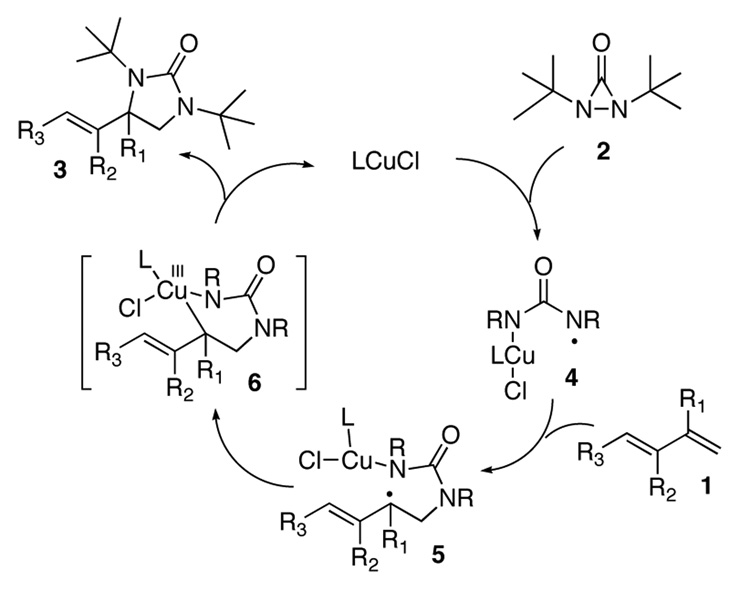
Direct diamination of olefins provides an efficient approach to synthesis of vicinal diamines, which are biologically and chemically important functional moieties.1 While various metal-mediated and catalyzed diamination has been achieved,2–8 catalytic asymmetric diamination of olefins has been less well developed and still remains a challenge in organic synthesis. Earlier, Muñiz and coworkers reported chiral auxiliary based9a and chiral Lewis acid-catalyzed9b asymmetric diamination using bisimidoosmium as reagent. Recently, we reported Pd(0)-catalyzed asymmetric diamination of conjugated dienes10a,b and asymmetric allylic and homoallylic C-H diamination of terminal olefins.10c Previously, we have shown that Cu(I)-catalyzed diamination of conjugated dienes and triene using di-tert-butyldiaziridinone (2)11,12 as nitrogen source occurs regioselectively at the terminal double bond under mild reaction conditions (Scheme 1)8a, which provides complementary regioselectivity to the Pd(0)-catalyzed diamination.7 It is highly desirable develop an asymmetric version of this diamination process to enhance its synthetic utility. However, the Cu(I)-catalyzed diamination likely proceeds via a radical mechanism (Figure 1).8,13–15 The involvement of radical intermediates presents a challenge for asymmetric control.
Scheme 1.
Figure 1.
A Proposed Catalytic Cycle for Cu(I)-catalyzed Diamination
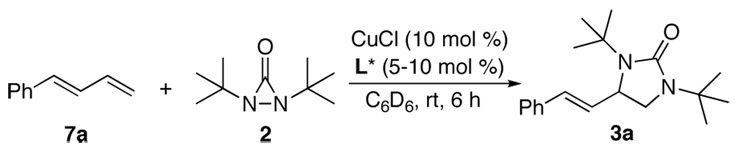
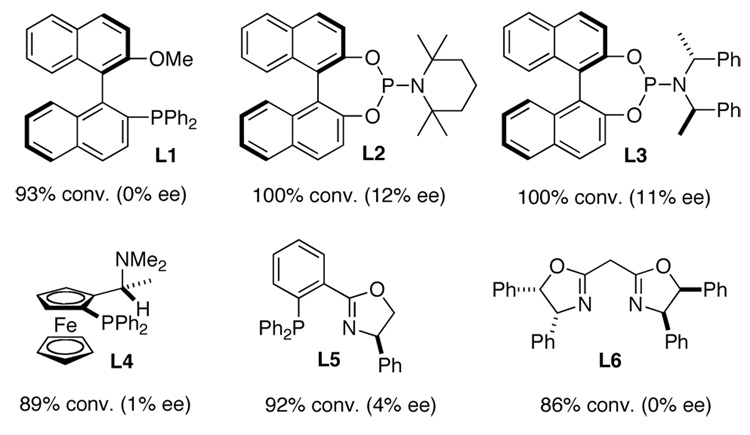
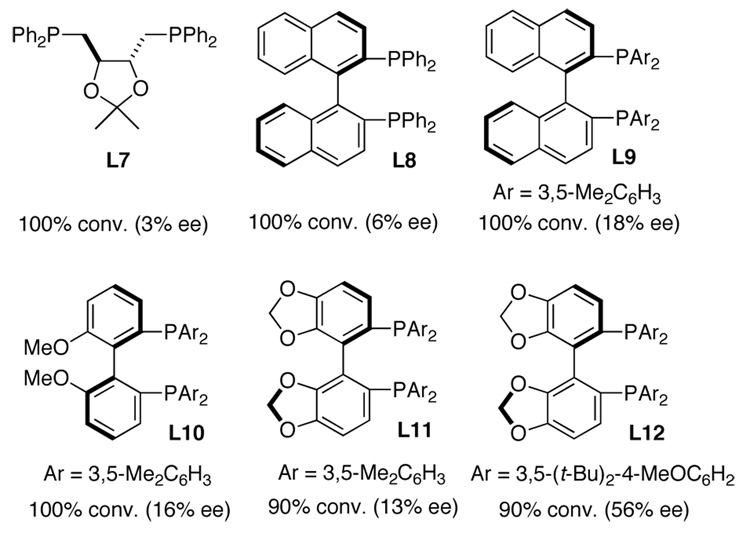
Asymmetric diamination was initially investigated using 10 mol % of CuCl with various commercially available or easily prepared chiral monophosphorus and nitrogen-containing ligands16 and trans-1-phenylbutadiene (7a) as test substrate at room temperature for 6 h (Scheme 2). As shown in Figure 2, all the reactions went smoothly to give diamination product 3a with high conversions, but with 0 to 12% ee. To search for more promising ligands, a series of commercially available chiral bisphosphine ligands17 were subsequently studied for the diamination of trans-1-phenylbutadiene (7a) with a 2/1 ratio of CuCl and ligand. It was found that steric bulkiness on the phosphine atoms had a large impact on the enantioselectivities (Figure 3). Encouragingly, the diamination of 7a with (R)-DTBM-SEGPHOS (L12)17f gave 90% conversion and 56% ee (Figure 3).
Scheme 2.
Figure 2.
Asymmetric Diamination of Diene 7a with Selected Monophosphorus and Nitrogen-containing Ligands 18
Figure 3.
Asymmetric Diamination of Diene 7a with Selected Bisphosphine Ligands 19
To improve the enantioselectivity, the reaction conditions, including solvent, temperature, and the ratio of ligand and CuCl were further investigated. It was found that solvent has a significant effect on both reactivity and enantioselectivity (Table 1, entries 1–7), benzene-d6 proved to be the best solvent. The ratio of ligand and CuCl was also very important, only 24% conversion was obtained with slightly higher ee when 1:1 CuCl and L12 was used (Table 1, entry 8). Lowering temperature improved the enantioselectivity (Table 1, entries 9–13), increasing to 65% ee at 0 °C (small amount of toluene was added to avoid solidification of benzene-d6). Overall, the best reaction conditions involve 10 mol % CuCl and 5.5 mol % L12 in benzene-d6 with a small amount of toluene at 0 °C (Table 1, entry 10).
Table 1.
Studies on Reaction Conditions for Asymmetric Diamination of 7aa
| entry | CuCl (mol %) |
L12 (mol %) |
solvent | conv. (%)b |
ee (%)c |
|---|---|---|---|---|---|
| 1 | 10 | 5 | DME | 63 | 44 |
| 2 | 10 | 5 | Et2O | 43 | 42 |
| 3 | 10 | 5 | CDCl3 | 78 | 19 |
| 4 | 10 | 5 | CD2Cl2 | 100 | 35 |
| 5 | 10 | 5 | THF | 21 | 33 |
| 6 | 10 | 5 | Toluene-d8 | 77 | 53 |
| 7 | 10 | 5 | C6D6 | 90 | 56 |
| 8 | 10 | 10 | C6D6 | 24 | 58 |
| 9 | 10 | 5 | C6D6/PhCH3 (11/1, v/v) |
73 | 63 |
| 10 | 10 | 5.5 |
C6D6/PhCH3 (11/1, v/v) |
72 | 65 |
| 11 | 12 | 5 | C6D6/PhCH3 (11/1, v/v) |
74 | 61 |
| 12 | 15 | 5 | C6D6/PhCH3 (11/1, v/v) |
89 | 56 |
| 13 | 10 | 5.5 | PhCH3 | 30 | 65 |
All the reactions were carried out with trans-1-phenylbutadiene (7a) (0.20 mmol), di-tert-butyldiaziridinone (2) (0.30 mmol) and solvent (0.60 mL). For entries 1–8, the reactions were carried out at rt for 6 h. For entries 9–13, the reactions were carried out at 0 °C for 20 h.
The conversion was determined by crude 1H NMR.
The ee was determined by chiral HPLC (Chiralpak AD-H column).
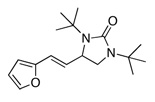
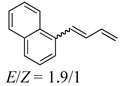
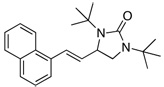
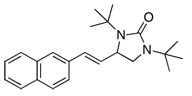
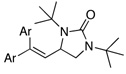
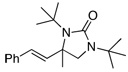
Under the optimized conditions, a variety of conjugated dienes can be regio- and enantioseletively diaminated at the terminal double bond in 59–93% yield with 62–74% ee’s (Table 2, entries 1–10). The ee could be further improved after recrystallization (Table 2, entry 2). For cis-1-phenylbutadiene, isomerization of the cis double bond occurred during the reaction and mainly gave E-isomer product in 70% ee which was a little higher than direct diamination of trans-1-phenylbutadiene (Table 2, entry 1 vs 5). When a mixture of trans- and cis-dienes was subjected to the reaction conditions, the diamination product of E isomer was formed predominately with only a trace amount of Z isomer (Table 2, entries 6 and 7). Asymmetric diamination of 1,1-disubstituted butadienes gave slightly higher ee’s (Table 2, entries 8–10). When a triene was diaminated at room temperature in benzene-d6, the diamination product was obtained in 58% ee (Table 2, entry 11). Asymmetric diamination of trans-1-phenyl-3-methylbutadiene led to 90% yield with only 23% ee (Table 2, entry 12), suggesting that the steric effect and radical stability are important factors for the enantioselectivity.
Table 2.
Catalytic Asymmetric Diamination of Dienes and Triene a
| entry | substrate | product | yield (%)b | ee (%)f |
|---|---|---|---|---|
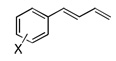 |
||||
| 1 | X = H | 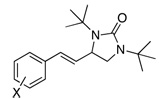 |
69 | 65 |
| 2 | X = 4-OMe | 76 | 67 (>99)g |
|
| 3 | X = 2-OMe | 70 | 67 | |
| 4 |
|
|
80 | 62 |
| 5 | 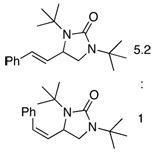 |
60c | 70 | |
| ndh | ||||
| 6 |
|
|
65d | 66 |
| 7 |
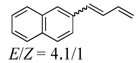
|
|
78d | 66 |
 |
||||
| 8 | Ar=Ph | 93 | 72 | |
| 9 | Ar = p-tolyl | 59 | 74 | |
| 10 | Ar = 2-thienyl | 79 | 66 | |
| 11 | 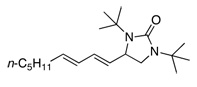 |
55e | 58 | |
| 12 |
|
|
90 | 23 |
All the reactions were carried out with olefin (0.20 mmol), di-tert-butyldiaziridinone (2) (0.30 mmol), CuCl (0.02 mmol), L12 (0.011 mmol), benzene-d6 (0.55 mL) and toluene (0.050 mL) at 0 °C under argon for 20 h unless otherwise stated.
Isolated yield based on diene or triene.
The ratio was determined by 1H NMR.
Only trace amount of isomer was observed by 1H NMR.
The reaction was carried out in benzene-d6 at rt for 20 h.
The ee was determined by chiral HPLC (Chiralpak AD-H column).
The ee after recrystallization from hexanes.
Not determined.
In summary, catalytic asymmetric diamination for a variety of conjugated dienes and a triene with encouraging ee’s has been achieved using CuCl/L12 as the catalyst and di-tert-butyldiaziridinone as the nitrogen source. These results show that Cu(I)-catalyzed asymmetric diamination is feasible despite the fact that the diamination likely involves radical intermediates. Ligand (R)-DTBM-SEGPHOS (L12) provides a very promising lead for further improvement. Searches for more effective chiral ligands, studies of different nitrogen sources, and expansion of the substrate scope will be carried out.
Supplementary Material
The asymmetric diamination procedure, the characterization of the diamination products, and data for the determination of enantiomeric excess of diamination products along with the NMR spectra of diamination products (36 pages).
Acknowledgement
We are grateful to the generous financial support from the General Medical Sciences of the National Institutes of Health (GM083944-01).
References
- 1.For leading reviews, see: Lucet D, Gall TL, Mioskowski C. Angew. Chem., Int. Ed. 1998;37:2580. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. Mortensen MS, O’Doherty GA. Chemtracts: Organic Chemistry. 2005;18:555. Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug. Des. 2006;67:101. doi: 10.1111/j.1747-0285.2006.00347.x.
- 2.For examples of metal-mediated diaminations, see: Co: Becker PN, White MA, Bergman RG. J. Am. Chem. Soc. 1980;102:5676. Hg: Barluenga J, Alonso-Cires L, Asensio G. Synthesis. 1979;962:962. Mn: Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J. Org. Chem. 1985;50:3647. Os: Chong AO, Oshima K, Sharpless KB. J. Am. Chem. Soc. 1977;99:3420. Muñiz K. Eur. J. Org. Chem. 2004:2243. Pd: Bäckvall J-E. Tetrahedron Lett. 1978:163. Tl: Aranda VG, Barluenga J, Aznar F. Synthesis. 1974:504.
- 3.For recent Cu(II)-mediated intramolecular diamination, see: Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250. doi: 10.1021/ja053335v. Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035. doi: 10.1021/ol0706713.
- 4.For Rh(II)-, Fe(III)-catalyzed diamination with TsNCl2, see: Li G, Wei H-X, Kim SH, Carducci MD. Angew. Chem., Int. Ed. 2001;40:4277. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I. Wei H-X, Kim SH, Li G. J. Org. Chem. 2002;67:4777. doi: 10.1021/jo0200769.
- 5.For a recent Pd(II)-catalyzed intermolecular diamination of conjugated dienes, see: Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308. doi: 10.1021/ja051181d.
- 6.For recent Pd(II)- and Ni-catalyzed intramolecular diamination of olefins, see: Streuff J, Hövelmann CH, Nieger M, Muñiz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y. Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew. Chem., Int. Ed. 2007;46:7125. doi: 10.1002/anie.200702160. Muñiz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f. Muñiz K, Hövelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763. doi: 10.1021/ja075041a.
- 7.For Pd(0)-catalyzed intermolecular diamination, see: Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762. doi: 10.1021/ja0680562. Xu L, Du H, Shi Y. J. Org. Chem. 2007;72:7038. doi: 10.1021/jo0709394. Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:7496. doi: 10.1021/ja072080d.
- 8.For Cu(I)-catalyzed intermolecular diamination, see: Yuan W, Du H, Zhao B, Shi Y. Org. Lett. 2007;9:2589. doi: 10.1021/ol071105a. Zhao B, Yuan W, Du H, Shi Y. Org. Lett. 2007;9:4943. doi: 10.1021/ol702061s. Zhao B, Du H, Shi Y. Org. Lett. 2008;10:1087. doi: 10.1021/ol702974s.
- 9.For leading references on asymmetric diamination using bisimidoosmium as reagent, see: Muñiz K, Nieger M. Synlett. 2003:211. Muñiz K, Nieger M. Chem. Commun. 2005:2729. doi: 10.1039/b502150b.
- 10.(a) Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688. doi: 10.1021/ja074698t. [DOI] [PubMed] [Google Scholar]; (b) Xu L, Shi Y. J. Org. Chem. 2008;73:749. doi: 10.1021/jo702167u. [DOI] [PubMed] [Google Scholar]; (c) Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene FD, Stowell JC, Bergmark WR. J. Org. Chem. 1969;34:2254. [Google Scholar]
- 12.For a leading review on diaziridinone, see: Heine HW. In: The Chemistry of Heterocyclic Compounds. Hassner A, editor. Hoboken, NJ: John Wiley & Sons, Inc; 1983. p. 547.
- 13.For a leading review on metal-promoted radical reactions, see: Iqbal J, Bhatia B, Nayyar NK. Chem. Rev. 1994;94:519.
- 14.For leading reviews on CuX-catalyzed atom transfer reactions see: Patten TE, Matyjaszewski K. Acc. Chem. Res. 1999;32:895. Clark AJ. Chem. Soc. Rev. 2002;31:1. doi: 10.1039/b107811a.
- 15.For a leading review on nitrogen-centered radicals, see: Stella L. In: Radicals in Organic Synthesis. Renaud P, Sibi MP, editors. Vol. 2. Weinheim, Germany: Wiley-VCH; 2001. p. 407.
- 16.For leading references on L1–L6, see: Hattori T, Shijo M, Kumagai S, Miyano S. Chem. Express. 1991;6:335. (b) ref. 10a. Sewald N, Wendisch V. Tetrahedron: Asymmetry. 1998;9:1341. Hayashi T, Yamamoto K, Kumada M. Tetrahedron Lett. 1974;15:4405. von Matt P, Pfaltz A. Angew. Chem., Int. Ed. Engl. 1993;32:566. Evans DA, Woerpel KA, Hinman MM, Faul MM. J. Am. Chem. Soc. 1991;113:726. Lowenthal RE, Masamune S. Tetrahedron Lett. 1991;32:7373.
- 17.For leading references on L7–L12, see: Kagan HB, Dang T-P. J. Am. Chem. Soc. 1972;94:6429. ; (b) Miyashita A, Yasuda A, Takaya H, Toriumi K, Ito T, Souchi T, Noyori R. J. Am. Chem. Soc. 1980;102:7932. [Google Scholar]; (c) Mashima K, Matsumura Y-i, Kusano K-h, Kumobayashi H, Sayo N, Hori Y, Ishizaki T, Akutagawa S, Takaya H. J. Chem. Soc., Chem. Commun. 1991:609. [Google Scholar]; (d) Lipshutz BH, Noson K, Chrisman W. J. Am. Chem. Soc. 2001;123:12917. doi: 10.1021/ja011529e. [DOI] [PubMed] [Google Scholar]; (e) Hatano M, Terada M, Mikami K. Angew. Chem., Int. Ed. 2001;40:249. [PubMed] [Google Scholar]; (f) Lipshutz BH, Lower A, Noson K. Org. Lett. 2002;4:4045. doi: 10.1021/ol026755n. [DOI] [PubMed] [Google Scholar]
- 18.The reactions were carried out with trans-1-phenylbutadiene (0.20 mmol), di-tert-butyldiaziridinone (2) (0.30 mmol), CuCl (0.02 mmol), ligand L1–L5 (0.02 mmol), except for L6 (0.01 mmol) in benzene-d6 (0.60 mL) at room temperature for 6 h. The conversions were determined by crude 1H NMR, and the ee’s were determined by chiral HPLC.
- 19.The same conditions as ref. 18 except for L7–L12 (0.01 mmol).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The asymmetric diamination procedure, the characterization of the diamination products, and data for the determination of enantiomeric excess of diamination products along with the NMR spectra of diamination products (36 pages).