Abstract
There is considerable interest in novel cell imaging tools that avoid the use of fluorescent proteins. One widely used class of such reagents are “pro-fluorescent” biarsenical dyes such as FlAsH, ReAsH, CrAsH, and Cy3As. Despite their utility, biarsenicals are plagued by high background labeling and cytotoxicity, and are challenging to apply in oxidizing cellular locale. Here we demonstrate that [(3-oxospiro[isobenzofuran-1(3H),9′-[9H]xanthene]-3′,6′-diyl)bis(iminomethylene-2,1-phenylene)]bis-(9CI), a rhodamine-derived bisboronic acid (RhoBo) described initially as a monosaccharide sensor, functions as a cell-permeable, turn-on fluorescent sensor for a tetraserine-motifs in recombinant proteins. RhoBo binds peptides or proteins containing Ser-Ser-Pro-Gly-Ser-Ser with affinities in the nanomolar concentration range, and prefers this sequence to simple monosaccharides by >10,000-fold. RhoBo fails to form fluorescent complexes with constituents of the mammalian cell surface, as judged by epifluorescent, confocal, and TIRF microscopy, but fluoresces brightly within the Ser-Ser-Pro-Gly-Ser-Ser-rich cell interior. These results suggest that current efforts to identify optimal serine-rich sequences for RhoBo will allow it to function effectively as a selective small-molecule label for appropriately tagged proteins either upon or within living cells.
There is considerable interest in novel biomolecule imaging tools that avoid the use of fluorescent proteins.1–3 One widely used class of such reagents are “pro-fluorescent” biarsenical dyes such as FlAsH,4 ReAsH,5 CrAsH,6 and Cy3As.7 These cell-permeable molecules selectively label recombinant proteins containing a linear4 or split8 tetracysteine motif via thiol-arsenic exchange reactions that convert the non-fluorescent 1,2-ethanedithiol (EDT)-bound forms of these dyes into highly fluorescent protein-bound complexes. Despite their utility, however, biarsenicals are plagued by high background signals, cytotoxicity,9,10 and can be challenging to apply in oxidizing cellular locales.11,12 Non-toxic, redox-insensitive alternatives that combine the convenience and selectivity of a biarsenical with the brightness of a fluorescent protein would be a valuable addition to the cell biologist’s “fluorescent toolbox”.2 Here we report that [(3-oxospiro[isobenzofuran-1(3H),9′-[9H]xanthene]-3′,6′-diyl)bis (iminomethylene-2,1-phenylene)]bis-(9CI), a rhodamine-derived bisboronic acid (RhoBo) described initially as a monosaccharide sensor,13 can function as a cell-permeable, turn-on fluorescent sensor for tetraserine-motifs in engineered proteins.
It has been known since 1953 that phenyl boronic acid condenses with polyols to form boronate esters,14,15 and since 1994 that the fluorescence of certain mono- and bis-boronic acid dyes increases upon esterification with simple sugars.16,17 The equilibrium stabilities of these complexes are low, however, with Kd values in the mM concentration range. We hypothesized that bis-boronic acid dyes would form higher affinity complexes with proteins containing a linear tetraserine motif. RhoBo was chosen as the ideal molecule with which to investigate this hypothesis, as it benefits from a simple synthesis and low monosaccharide affinity, and forms boronate esters that emit at wavelengths > 500 nm,13 a useful range for experiments in live cells.
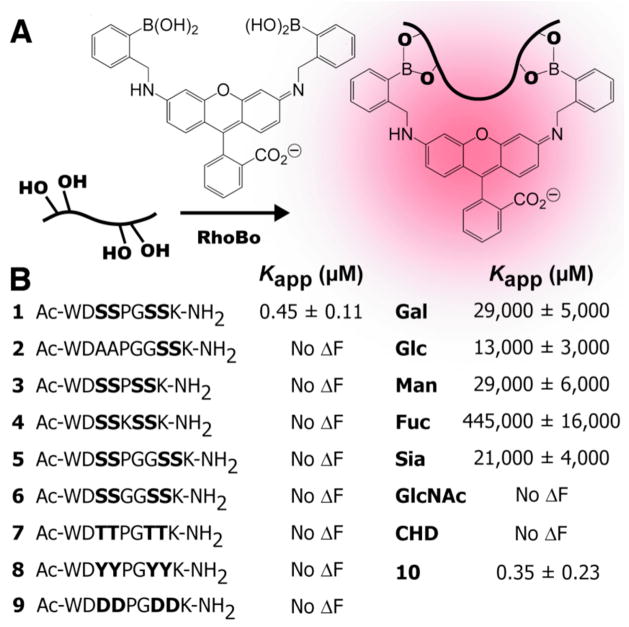
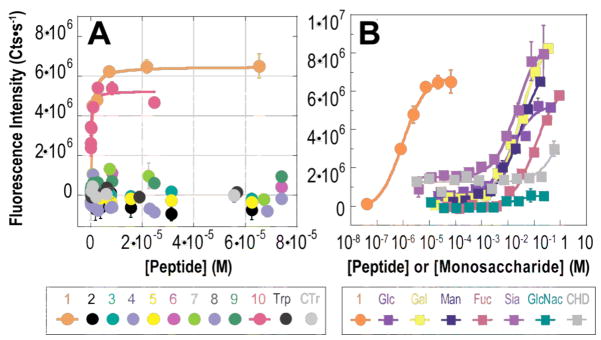
First, we asked whether RhoBo would form fluorescent complexes with peptides containing 2–4 serine residues separated by a variety of intervening sequences (Figures 1 and 2). Each peptide was incubated with RhoBo (17.1 μM) in buffer at 37 °C and the fluorescence emission at 580 nm was monitored as a function of peptide concentration. Under these conditions, peptide 1, containing the sequence Ser-Ser-Pro-Gly-Ser-Ser, formed the highest affinity complex with RhoBo (Kapp = 452 ± 106 nM). Titrations with peptides containing two serines rather than four (2) or shorter (3, 4) or longer (5) intervening sequences, or aspartate residues in place of serine (9) led to no detectable fluorescence change. Minimal fluorescence changes were observed with peptides containing threonine (7) or tyrosine (8) in place of serine. No fluorescence change was observed when RhoBo was incubated with the serine proteases trypsin or chymotrypsin. Notably, the equilibrium stability and brightness of the RhoBo•1 complex (3955.5 M−1cm−1) compares favorably with the complex formed between ReAsH-EDT2 and the optimized tetracysteine sequence FLNCCPGCCMEP.18 RhoBo also formed a high affinity complex with a small well-folded protein, a derivative of the 36-aa pancreatic fold polypeptide aPP containing an N-terminal SSPGSS tag (10) (Kd = 347 ± 234 nM (Figure 2A).
Figure 1.
(A) Scheme illustrating a likely mode of condensation between RhoBo and a compound containing four hydroxyl groups. (B) Apparent equilibrium dissociation constant (Kapp) of complexes between RhoBo and the peptides and monosaccharides shown.
Figure 2.
Changes in the fluorescence emission of RhoBo (17.1 μM) in the presence of (A) peptides 1–10 and (B) indicated monosaccharides. Reactions were incubated in 100 mM phosphate buffer (pH 7.4) supplemented with 10% DMSO (37 °C, 60 min) and the emission monitored at 580 nm. Each point represents the average of three or more independent trials ± the standard error.
Next we examined the affinity of RhoBo for simple monosaccharides, including those prevalent in mammalian oligosaccharide frameworks,19 to evaluate the extent to which these hydroxyl-rich functionalities might compete with protein tetraserine motifs. Although significant fluorescent changes were observed upon incubation of RhoBo with galactose (Gal), glucose (Glc), fucose (Fuc), mannose (Man), and sialic acid (Sia), these changes occurred at concentrations at least 10,000 times higher than required for peptide 1 (Figure 2B). Almost no fluorescence change was observed with N-acetylglucosamine (GlcNac) or with cis-1,2-cyclohexane diol (CHD). We also examined whether RhoBo would form fluorescent complexes with components of a 377-member mammalian glycan microarray (v.3.1; http://www.functionalglycomics.org/). None of the glycans on this array exhibited higher fluorescence than galactose when incubated with RhoBo (84 μM) (Table S-3).
To determine whether the RhoBo•1 complex possessed the expected 1:1 stoichiometry, we performed a preparative-scale reaction at a concentration of each reactant that significantly exceeded Kapp (140 μM), separated the products by HPLC, and determined their masses using MALDI-TOF mass spectrometry. Under these conditions, an equimolar mixture of 1 and RhoBo was converted into a single major product (71% yield) that possessed the expected 1:1 complex mass (expected m/z = 1696.18, found m/z = 1695.2; Table S-1). An isomeric product possessing the identical mass (16% yield) was also formed. These isomers possess identical quantum yields (ϕ) (0.89 ± 0.01) whose value compares well with the quantum yield of fluorescein (0.95) 20 and rhodamine 110 (0.91),21 and exceeds that of peptide-bound FlAsH (0.5)5 and RhoBo alone (0.3 ± 0.03).
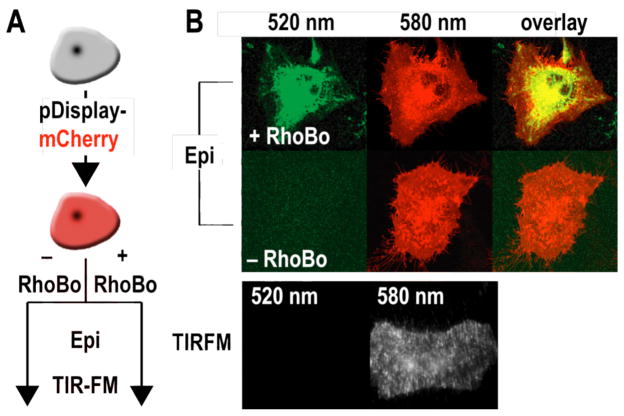
Finally we evaluated whether RhoBo could circumnavigate the complex, saccharide-rich cell surface to image tetraserine-containing proteins in the cytosol. In contrast to the tetracysteine motif recognized by biarsenicals, which is absent from the human proteome,22 more than 100 human proteins, including the highly abundant myosin heavy chain, contain an SSPGSS sequence. HeLa cells were first transfected with pDisplay-mCherry, to fluorescently label the plasma membrane, and then treated for 30 min with either 0 or 1 μM RhoBo before imaging using epifluorescence and total internal reflection fluorescence microscopy (TIRFM) (Figure 3A). The epifluorescence images show significant signal at the outer plasma membrane at the mCherry emission maximum (580 nm), irrespective of RhoBo treatment. Only those cells treated with RhoBo, however, show significant signal when RhoBo was excited specifically using 514 nm light (emission was monitored at 520 nm). In this case fluorescence is observed throughout the cell interior with maximal intensity in perinuclear regions and minimal intensity in the nucleus and outer plasma membrane (Figure 3B). RhoBo-treated cells were then imaged using TIRFM, which revealed significant signal at the mCherry emission maximum (580 nm) but not at the RhoBo emission maximum (520 nm). These images confirm the absence of even low levels of RhoBo emission within the outer 200 nm of the plasma membrane. It is of course possible that RhoBo is associated with constituents of the plasma membrane, but if so, then the complexes formed are not bright. The surprising observation that RhoBo does not fluoresce on the cell surface, coupled with its low toxicity (Figure S5), suggests that current efforts to identify more highly preferred tetraserine motifs will reveal RhoBo as a selective small-molecule tag for proteins on and within living cells.
Figure 3.
(A) Experimental strategy to evaluate the extent of cell surface labeling by RhoBo. HeLa cells were transfected with pDisplay-mCherry (emission maximum 580 nm), incubated in the presence or absence of RhoBo (1 μM) (emission maximum 520 nm), and (B) imaged using epifluorescent and TIRF microscopy.
Supplementary Material
Synthesis and spectroscopic characterization of RhoBO and RhOBO•1 and protocols for cell culture and fluorescence microscopy. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We are grateful to the Yale Center for Genomics and Proteomics and the National Institutes of Health (GM 83257) for their support of this work, and to Professor Robert Strongin (Portland State) for providing a sample of RhoBo. JA and DMB were both supported by NIH MSTP TG 5T32GM07205.
References
- 1.Soh N. Sensors. 2008;8:1004–1024. doi: 10.3390/s8021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 3.Chen I, Ting AY. Curr Opin Biotechnol. 2005;16:35–40. doi: 10.1016/j.copbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Griffin BA, Adams SR, Tsien RY. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 5.Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. J Am Chem Soc. 2002;124:6063–76. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 6.Cao H, Chen B, Squier TC, Mayer MU. Chem Comm. 2006:2601–3. doi: 10.1039/b602699k. [DOI] [PubMed] [Google Scholar]
- 7.Cao H, Xiong Y, Wang T, Chen B, Squier TC, Mayer MU. J Am Chem Soc. 2007;129:8672–8673. doi: 10.1021/ja070003c. [DOI] [PubMed] [Google Scholar]
- 8.Luedtke NW, Dexter RJ, Fried DB, Schepartz A. Nat Chem Bio. 2007;3:779–84. doi: 10.1038/nchembio.2007.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroffekova K, Proenza C, Beam KG. Pflugers Archiv-European Journal of Physiology. 2001;442:859–866. doi: 10.1007/s004240100619. [DOI] [PubMed] [Google Scholar]
- 10.Langhorst MF, Genisyuerek S, Stuermer CA. Histochem Cell Biol. 2006;125:743–7. doi: 10.1007/s00418-005-0136-3. [DOI] [PubMed] [Google Scholar]
- 11.Griffin BA, Adams SR, Jones J, Tsien RY. Methods Enzymol. 2000;327:565–78. doi: 10.1016/s0076-6879(00)27302-3. [DOI] [PubMed] [Google Scholar]
- 12.Gaietta GM, Giepmans BN, Deerinck TJ, Smith WB, Ngan L, Llopis J, Adams SR, Tsien RY, Ellisman MH. Proc Natl Acad Sci U S A. 2006;103:17777–82. doi: 10.1073/pnas.0608509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KK, Escobedo JO, StLuce NN, Rusin O, Wong D, Strongin RM. Org Lett. 2003;5:5007–5010. doi: 10.1021/ol035978q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuivila HG, Keough AH, Soboczenski EJ. J Org Chem. 1954;19:780–783. [Google Scholar]
- 15.Lorand JP, Edwards JO. J Org Chem. 1959;24:769–774. [Google Scholar]
- 16.James TD, Sandanayake K, Shinkai S. Chem Commun. 1994:477–478. [Google Scholar]
- 17.James TD, Shinkai S. Host-Guest Chemistry. Vol. 218. 2002. Artificial receptors as chemosensors for carbohydrates; pp. 159–200. [Google Scholar]
- 18.Martin BR, Giepmans BNG, Adams SR, Tsien RY. Nat Biotechnol. 2005;23:1308–1314. doi: 10.1038/nbt1136. [DOI] [PubMed] [Google Scholar]
- 19.Werz DB, Ranzinger R, Herget S, Adibekian A, von der Lieth CW, Seeberger PH. ACS Chem Biol. 2007;2:685–691. doi: 10.1021/cb700178s. [DOI] [PubMed] [Google Scholar]
- 20.Lakowicz JR. Principles of Fluorescence Spectroscopy. 2. Kluwer Acadamic; New York: 1999. [Google Scholar]
- 21.Leytus SP, Melhado LL, Mangel WF. Biochem J. 1983;209:299–307. doi: 10.1042/bj2090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin MZ, Wang L. Physiology. 2008;23:131–141. doi: 10.1152/physiol.00007.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthesis and spectroscopic characterization of RhoBO and RhOBO•1 and protocols for cell culture and fluorescence microscopy. This material is available free of charge via the Internet at http://pubs.acs.org.