Abstract
Formation of the medial epithelial seam (MES) by palatal shelf fusion is a crucial step of palate development. Complete disintegration of the MES is the final essential phase of palatal confluency with surrounding mesenchymal cells. In general, the mechanisms of palatal seam disintegration are not overwhelmingly complex, but given the large number of interacting constituents; their complicated circuitry involving feedforward, feedback, and crosstalk; and the fact that the kinetics of interaction matter, this otherwise simple mechanism can be quite difficult to interpret. As a result of this complexity, apparently simple but highly important questions remain unanswered. One such question pertains to the fate of the palatal seam. Such questions may be answered by detailed and extensive quantitative experimentation of basic biological studies (cellular, structural) and the newest molecular biological determinants (genetic/dye cell lineage, gene activity, kinase/enzyme activity), as well as animal model (knockouts, transgenic) approaches. System biology and cellular kinetics play a crucial role in cellular MES function; omissions of such critical contributors may lead to inaccurate understanding of the fate of MES. Excellent progress has been made relevant to elucidation of the mechanism(s) of palatal seam disintegration. Current understanding of palatal seam disintegration suggests epithelial–mesenchymal transition and/or programmed cell death as two most common mechanisms of MES disintegration. In this review, I discuss those two mechanisms and the differences between them.
Keywords: palate, medial epithelial seam, EMT, programmed cell death
INTRODUCTION
Palatogenesis is an important event during the craniofacial development of the group of higher vertebrates known as amniotes. The stages of palatal development traditionally have been defined by the position of the palatal shelves in the oral cavity and the opposing palatal shelves’ level of union at the midline (Ferguson, 1988). The palatal shelves develop from the maxillary prominence of the first branchial arch and initially grow vertically along the lateral sides of the developing tongue. At a precise stage of embryonic development, the palatal shelves are remodeled to become reoriented to a horizontal position above the tongue, and the medial edges epithelial cells of the two palatal shelves meet at the midline. In alligators, rodents, and humans, the medial edge epithelia (MEE) of the two opposite palatal shelves that arise from the maxillary processes join to form a two-layered medial epithelial seam (MES; Ferguson, 1988). Then, the epithelial seam disappears and the palate becomes confluent. These steps are tightly regulated; failure of palatal shelf growth, elevation, adhesion, and fusion or failure of mesenchymal differentiation can cause cleft palate (Gritli-Linde, 2007), the most common craniofacial deformity (1 in every 1,000 births).
During Prof. Elizabeth D. Hay’s renowned work on embryogenesis, her study of palate development–especially the formation and disappearance of the MES–occurred near the end of her research career (1988 to 2007). Accordingly, I shall limit this review to the aspects of palatogenesis relevant to palatal fusion, in particular the current understanding of the mechanisms responsible for MES disintegration into mesenchyme, which initiates palatal confluence.
The cellular mechanism underlying seam degeneration and the fate of MES cells have long been a major focus of the field; however, several controversies still surround these topics. For the past 50 years, scientists have used the latest, most sophisticated methods available to them, ranging from basic ultrastructural cell biology to cell labeling using genetic lineage markers. Two major models have been proposed for seam degeneration: Epithelial–Mesenchymal Transition or EMT (Shuler et al., 1992; Hay, 1995; Kaartinen et al., 1997; Lavrin and Hay, 2000; Sun et al., 2000a; Sun et al., 2000b; Nawshad and Hay, 2003; Nawshad et al., 2004a,b; Takahara et al., 2004; Takigawa and Shiota, 2004; Kang and Svoboda, 2005; Lagamba et al., 2005; Pungchanchaikul et al., 2005; Jin and Ding, 2006) and Programmed Cell Death or PCD (Glucksmann, 1965; Saunders, 1966; DeAngelis and Nalbandian, 1968; Smiley and Dixon, 1968; Farbman, 1969; Hayward, 1969; Shapiro and Sweney, 1969; Mato et al., 1972; Hudson and Shapiro, 1973; Pratt and Martin, 1975; Greene and Pratt, 1976; Greene, 1989; Taniguchi et al., 1995; Cuervo and Covarrubias, 2004; Vaziri Sani et al., 2005; Dudas et al., 2006a, 2007; Xu et al., 2006; Lee et al., 2008). Additionally, migration of MES cells along the midline toward the nasal and oral epithelial also has been suggested as an alternative method of MES disintegration (Carette and Ferguson, 1992). This confusion was compounded by the fact that transforming growth factor-beta3 (TGFβ3), which is essential for palatal seam disintegration, is capable of all of these cellular phenotypical changes (Nawshad et al., 2004a).
While palate development requires seam disintegration during the final stage of palatogenesis, well coordinated orientation of palatal shelve size and shape and proper growth, elevation, and adhesion are equally important. This review focuses on palatal seam disintegration, the final phase of palate development. Here, I discuss two of the three mechanisms that manifest during palatal seam disintegration (EMT and PCD), and I illuminate the causes of different–and often contradictory–findings. Because the concept of migration (Carette and Ferguson, 1992) was ultimately contradicted by the same group Martinez-Alvarez et al. (2000b), I do not separately elaborate upon migration seam disintegration mechanism; rather I include it within my discussion of EMT.
PHASES OF PALATE FUSION
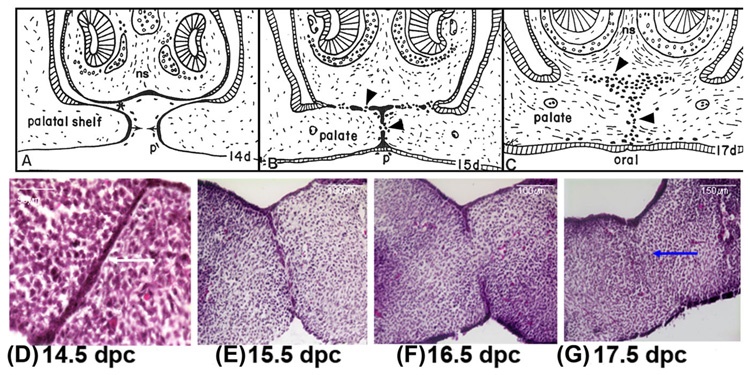
MEE cells promote the adherence of the epithelial cell covering to the opposing palatal shelves, which accomplishes the primary palatal closure (Waterman et al., 1973; Greene and Pratt, 1976). The process of palatogenesis is remarkably similar among vertebrates. In mice, the palatal shelves grow out bilaterally from the internal surfaces of the maxillary processes; they elongate on either side of the tongue and then become horizontal above the tongue as it descends (Fig. 1A). When the opposing shelves approach each other (Fig. 1B), the cells of the outer layer (periderm) of the opposed MEE may undergo PCD and slough off, exposing the lateral surfaces of the underlying basal MEE cells for close contact with each other, promoting formation of the midline and nasopalatine seams (arrowheads, Fig. 1C). The epithelial cells from the opposing MEE are joined by desmosomal junctions that result in the secure union of the two palatal shelves to each other (DeAngelis and Nalbandian, 1968; Chaudhry and Shah, 1973). As the head grows, the MES thins to a single layer of cells (Fig. 1D). The palatal epithelial seam subsequently undergoes complete disintegration (Fig. 1E) of the epithelial cells, resulting in mesenchymal portion of the two palatal shelves to become continuous (Fig. 1C, F). Eventually, the epithelial cells are absent from the midline (Fig. 1F) and fusion is completed by connective tissue confluence (Fig. 1C,F). Ultimately, the palatal mesenchyme becomes the site for palatal bone development.
Fig. 1.
Camera lucida drawings of paraffin sections of the developing rodent palate. The anterior palate (shown) of the mouse, fuses with the nasal septum (ns), but the posterior palate does not because there is no nasal septum posteriorly. A: Between 13 and 14 days post coitum (dpc), palatal shelves move horizontally (arrows) across the mouth above the tongue. At this time, the periderm (outer layer of the oral epithelium) sloughs off the epithelium along the medial palatal edge (p) and ventral nasal septum (*). B: The palatal processes do not fuse in vivo until periderm sloughs; therefore, there are few, if any, peridermal cells left to be trapped in vivo in the MEE and nasal septum seams. These epithelial seams transform to mesenchyme (arrowheads, B and C) between 15 and 17 dpc. C: The palate shelves fuse together and with the nasal septum by transforming the adherent epithelial seams to mesenchyme to become confluent. The dark cells (arrowheads) show diagrammatically the relative contribution to mesenchyme made by these epithelial seams. D–G: Hematoxylin and eosin-stained sections of rodent palates were fixed at different stages of palatogenesis in vitro showing chronological disintegration of the palatal seam (white arrow, D) to reach complete confluence (blue arrow, G) of the mesenchyme by 17.5 dpc. A–C from Griffith and Hay (2003), © Development, The Company of Biologist Ltd.
Current understanding of palatal seam disintegration indicates EMT and PCD are the most common mechanisms of MES disintegration. Here, I discuss those two mechanisms and how they differ.
EPITHELIAL MESENCHYMAL TRANSITION OR EMT
EMT is the phenotypic transition of a cell that is integrated into a coherent sheet with apical–basal polarity to an association with a less coherent, more individually motile group of cells without apical–basal polarity (Savagner, 2001). EMT may be considered as the entire series of events involved in the transition from an epithelial to a mesenchymal phenotype (Thiery, 2003). The epithelial state of organization may vary, and the specifics of developmental EMTs differ from case to case, depending largely on whether the starting state is an epithelial sheet or a sheet of cells with epithelial properties (Hay, 2005). However, experimental perturbations suggest that developmental EMTs do not necessarily follow a standard series of phenotypic changes that are linked or ordered. Some EMTs involve more stringent requirements than others for the maintenance of an intact epithelium, which may influence the order of events–and even their necessity.
Taken one step at a time, a primary EMT (those that occur in the primary embryonic epithelium) is more complex process than initially thought (Trelstad et al., 1967). Necessarily, at some point, a primary EMT must involve loss of epithelial phenotype, or de-epithelialization, which would by itself leave nonepithelial, or nominal “mesenchymal” cells, in place of what was an epithelium, and also leave a surrounding epithelium with free edges where the process of de-epithelialization had stopped. The first, most important EMT in the embryo of higher vertebrates produces the mesenchyme that condenses to form definitive mesoderm (the middle layer of the embryo) and endoderm (the inner layer of the embryo). This process is called gastrulation (Hay, 1995). All of the mesenchymal cells that form the connective tissue of the body have to arise from epithelia (Hay, 1968). Gastrulation in the lower chordates is a totally epithelial event. The initial transformation, from epithelial to mesenchymal, occurs in higher vertebrates during the invagination of epiblast derived cells through the primitive streak to form mesoderm (Hay, 1968). Primary developmental EMTs are one of the morphogenic mechanisms driving germ layer reorganization of the initial primary embryonic epithelium during gastrulation, neurulation, and neural crest formation (Thiery, 2003).
Developmental EMT involves cells that have secondarily adopted an epithelial organization and then undergo an EMT during organogenesis. These include ventral somite de-epithelialization to form the sclerotome (Brand-Saberi et al., 1996), EMT of cells in the endocardial endothelium to form the endocardial cushions in the atrioventricular canal of the heart (Potts et al., 1991), and EMT of border cells in the ovarian follicles (Abdelilah-Seyfried et al., 2003). Trelstad et al. (1982) presented early transmission electron microscope (TEM) evidence that disappearance of the Mullerian duct in male rat embryos occurs by conversion of epithelial cells to mesenchyme, rather than cell death. Subsequently, similar TEM evidence indicated that disappearance of the cervical sinus in chick embryos involves EMT (Hay, 1995). There also are many mesenchymal-to-epithelial transitions (METs; Barasch, 2001) that play an important role in organogenesis, such as notochord, kidney development (Thesleff and Ekblom, 1984), and formation of the caudal or secondary neural tube as well as somites. (Griffith et al., 1992).
However, considerable amounts of craniofacial crest mesenchyme form connective tissue that contributes to the head and face in vertebrae (Noden, 1986). Portions of the skull derive from primitive streak mesenchyme moving anteriorly into the head. To form its superficial features, the face of higher vertebrates is ingenious in its use of local EMT to remove unwanted epithelium–an amazing evolution of the functions of EMT. The nose forms a nostril by invagination of the outside epithelium inward. How could EMT contribute to such a process? In the case of the nose and lip, the maxillary process fuses its medial nasal edge epithelium with the epithelium of the intermaxillary segment after sloughing of the periderm (outer keratinized layer) to produce an epithelial seam that undergoes EMT to achieve mesenchymal confluency while keeping the lip intact (Sun et al., 2000a).
The cytological events of overt EMT in the palatal and lip seams are quite similar. They are orderly and controlled in such a manner as to lead to gradual replacement of the epithelium by mesenchyme while maintaining effective contact between the craniofacial primordia until complete confluence is achieved (Savagner et al., 1997). The transforming epithelial cells first extend delicate filopodia through breaks in the surrounding basement membrane, then larger pseudopodia, and finally elongated mesenchymal cells emerge that migrate across the area once occupied by epithelial seam. Events that must be regulated include production of metalloproteinases to remove the basement membrane through which filopodia will extend, formation by epithelial cells of new front ends expressing filopodia and appropriate ECM receptors, and the turning on in these cells of appropriate actin-myosin–mediated motility and signal transduction mechanisms for motility (Sun et al., 2000a).
A careful reexamination of palatal fusion in the rat embryo by Fitchett and Hay (1989) produced definitive TEM evidence for phenotypic transformation of the MES into mesenchymal cells to achieve mesenchymal confluence across the palate. In addition, the seam was shown to turn on vimentin, generally a mesenchymal intermediate filament (Hay, 1990) before the transformation and to lose the epithelial determinant, Syndecan (Fitchett and Hay, 1989). While these unltra-structural studies demonstrated EMT during palatal seam disintegration, cell linage studies tracing seam cells with incorporation of dyes to confirm the definitive concept of EMT did not occur until 1991 (Shuler et al., 1991, 1992). Using a fluorescent dye, DiI (l, l′-dioctadecyl-3, 3, 3′, 3′-tetramethylindo-carbocyanine perchlorate), as a cell lineage tracer, Shuler et al. (1991, 1992) confirmed the ultrastructural findings and showed EMT does take place during seam disintegration. The use of DiI to trace MES cells was a remarkable achievement. DiI remains hydrophobic, presumably within plasma lemma, and does not stand up to fixation; its presence can only be detected in frozen sections. These dyes have the enormous advantage of labeling external epithelia specifically, without the necessity of transplanting the labeled cells. The dye diffuses across the membrane, where intracellular esterases cleave off the acetates to release the fluorophore as a water-soluble compound that cannot diffuse out of the labeled cells. DiI also has been used to trace the cell lineage of another cell type that undergoes an EMT: the neural crest cells (Serbedzija et al., 1989, 1991, 1992). The approach used by Serbedzija and colleagues was adapted for the in vitro and in vivo model of palatal fusion to characterize MEE cell fate (Shuler et al., 1991, 1992).
This ground-breaking study was immediately challenged by Carette and Ferguson (1992) using confocal laser scanning fluorescence microscopy (CLSFM) coupled with the use of the DiI as a lineage marker (as used by Shuler et al., 1991). They showed that instead of EMT, palatal medial edge epithelial seam cells migrate and adopt a conserved migratory phenotype. These cells migrate orally and nasally to be recruited into and to constitute the epithelial triangles on the oral and nasal aspects of the palate. They subsequently become incorporated into the surface epithelia of the nasal and oral aspects of the palate of origin with little or no cross-migration.
The authors Carette and Ferguson (1992) disputed the ultrastructural findings of Fitchett and Hay (1989) as a result of findings facilitated by the use of confocal microscopy, which was a relatively new optical microscopic technique. At the time, CLSFM offered improved resolution, enhanced contrast and a reduction of out-of-focus interference over conventional microscopy techniques. Most importantly, the ability of the confocal microscope to optically section living specimens obviates the necessity of chemical fixation, reducing artifacts caused by tissue processing. In addition, CLSFM proved less damaging to living cells than conventional epifluorescence microscopy and provides the ability to study temporal phenomena by repetitive observation during timecourse studies. Carette and Ferguson (1992) did not address the findings of Shuler et al. (1991, 1992), who used similar techniques, the same species, and the same cell tracing dye, but who also reached a different conclusion in support of EMT, perhaps because the papers were simultaneously in press.
Shortly thereafter, Griffith and Hay (1992) used a dichloro-substituted derivative of carboxydichlorofluorescein diacetate succinimidyl ester (CCFSE) as a tracer for transforming MEE cells in vitro and in vivo, and agreed with the findings of Shuler et al. (1991, 1992). The novelty of their findings stemmed from the use of CCFSE, which resists bleaching better than CFSE. Unlike DiI, which does not stand up to fixation, CCFSE-labeled tissue may be handled with ease. The advantages of the cytoplasmic CCFSE product are that it is a stable cytoplasmic marker and it stands up to formaldehyde fixation and paraffin embedding. Such products may be fixed in formaldehyde and embedded in paraffin or plastic. The dye is rendered lipid insoluble once it enters the epithelium and enters basal cells by means of gap junctions. Once within cells, CCFSE becomes packaged into intracellular parcels. This packaging has the obvious advantage to the cells of compartmentalizing materials with possible toxic effects and the unexpected advantage for researchers of providing an identifiable TEM marker. The presence of membrane-bound CCFSE does not harm the cells, as judged by their very healthy, euchromatic nuclei and participation in palate development.
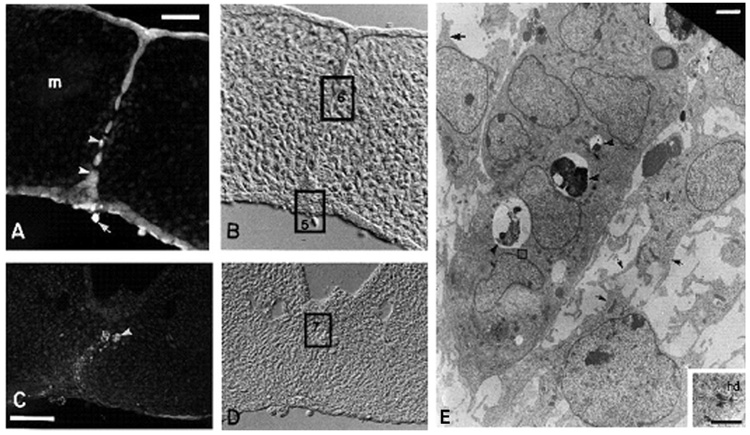
Griffith and Hay (1992) showed that CCFSE is superior to DiI for cytology and it also may be identified by both light and electron microscopic observation. They found that labeling living epithelium of the prefusion palate could be accomplished by in vitro and in vivo exposure to CCFSE, a lipid soluble dye that diffuses into periderm, becomes lipid insoluble, and enters basal cells by means of gap junctions. In this study, Griffith and Hay (1992) demonstrated the fate of the basal layers of the medial edge epithelia of mouse palatal shelves after they fuse to form the midline epithelial seam. They were able to demonstrate with high resolution that EMT of cells is a method for removal of epithelia during palatogenesis (Fig. 2). In areas where the midline seam was intact, TEM demonstrated isolation bodies within the seam cells (arrowheads, Fig. 2A), but not within the surrounding mesenchymal cells, confirming that the carboxyfluorescein had indeed been confined to the epithelial cells and did not pass (Fig. 2A).
Fig. 2.
Demonstration of epithelial–mesenchymal transition (EMT) by tracing palatal medial edge seam (MES) cell with carboxydichlorofluorescein diacetate succinimidyl ester (CCFSE). A–D: One day after in vitro labeling, CCFSE labeling is present in the cells of the midline seam (A, B) and in the mesenchyme-like cells deriving from epithelium in the region of the seam (C, D). These labeled mesenchyme-like cells are indistinguishable from others in the mesoderm after attainment of palatal confluence, as shown in D (photographed with Nomarski optics). The CCFSE, originally diffuse in distribution, is condensing into one or more fluorescent spots per cell (arrowheads, A and C), which represent dye packaged within isolation bodies. Mesenchymal cells situated outside the midline seam (m, A) have no isolation bodies showing that the CCFSE absorbed through the periderm was confined to the epithelial cells and did not pass through the basal plasmalemma into the mesenchyme. Periderm staining is brighter than that of the basal epithelial cells. A sloughed periderm cell is labeled (arrow, A). Palate C was cultured in a medium (Abbott) that promoted faster development and it already has sloughed most of the CCFSE-containing surface epithelial cells. In the region shown here, the nasal septum epithelium does not fuse with the palate. E: This epithelial island is a remnant of the disappearing seam (as at the square labeled 6). Isolation bodies are present (arrowheads, E). Mesenchymal cells are distinguished morphologically by their shape and well-developed pseudopodia and filopodia (arrows, E). The epithelial cells in the island are joined by desmosomes, one of which (square, E) is enlarged in the inset. Scale bars = 25 mm in A, 50 mm in C, 2 µm in E; 0.5 µm in inset. A–E from Griffith and Hay (2003), © Development, The Company of Biologist Ltd.
After 24 hr in culture, CCFSE labeling was present in the cells of the midline epithelial seam–in the epithelial islands (Fig. 2A) and in mesenchymal cells located at the midline (Fig. 2C). Brightly labeled spots, which TEM identified as isolation bodies, could be distinguished within the cytoplasm of CCFSE-labeled cells (arrowheads, Fig. 2A). Sloughed periderm could still be seen (arrow, Fig. 2A). Mesodermal confluence (Fig. 2D) was achieved by transformation of the epithelial seam to mesenchyme (Fig. 2C). In the disappearing seam, isolation bodies were present in epithelial islands (arrowheads, Fig. 2E). In the mouse (inset, Fig. 2E), desmosomes and newly forming half desmosomes were present in the seam and the islands. And newly transformed mesenchymal cells of the midline (arrowhead, rectangle 7, Fig. 2D) contained isolation bodies. Classification of the dye-containing cells as mesenchymal was based on several established criteria (Hay, 1990): (1) bipolar or stellate shape, (2) presence of pseudopodia and/or filopodia, and (3) lack of epithelial cell junctions, such as desmosomes.
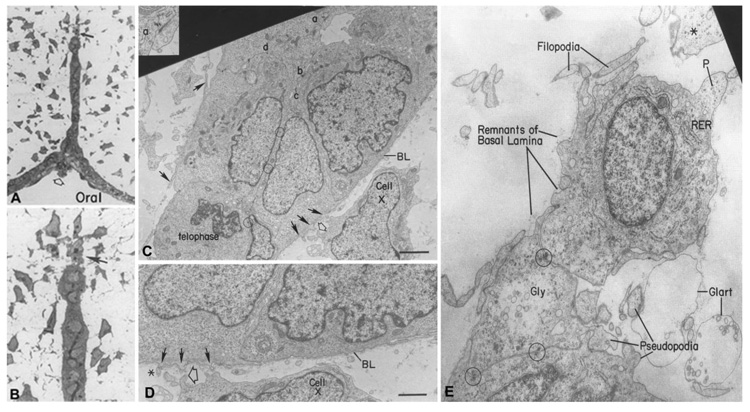
Fitchett and Hay (1989), in their ultrastructural study, showed that basal epithelial cells undergo profound changes in cell shape as the midline epithelial seam disappears. In the two-cell-thick epithelial seam, the basal cells were at first cuboidal and then they elongated in the plane of the seam and the basal surface was irregular. As the seam stretched, the cells slid past each other to become a monolayer that began to break up. At the tips of broken seams, single elongate or stellate-shaped cells could be seen by light microscopy leaving the epithelium (arrows, Fig. 3A,B). At the TEM level, the seam showed intact but thinning basal lamina (BL, Fig. 3C,D), into which filopodia (solid arrows, Fig. 3C,D) that were forming on the cells protruded. Formerly smooth basal surface became covered with the appearance of filopodial processes. Beginning as small protuberances under the basal lamina (solid arrows, Fig. 3C,D), these structures over time became quite impressive as they probed into the mesenchymal space (Filopodia, Fig. 3E).
Fig. 3.
Role of epithelial–mesenchymal transition (EMT) in palate epithelial seam disintegration. A, B: Light micrograph of a palate seam breaking up into mesenchyme (A), magnified further in (B) to show the transforming cell (arrow) at the tip of the breaking seam. Electron micrograph of the two-cell-thick midline epithelial seam in vivo near the nasal surface. C: Enlargement of a portion of the epithelial seam at C (a, inset) shows a desmosome, many of which appear in the major figures C (b–d). Circles indicate location of additional desmosomes between opposed epithelial cell layers. The seam shows the typical excellent health of the MEE in vivo. A telophase is present indicating mitosis is still occurring. Cell X is the same mesenchymal cell in C and D. The basal lamina (BL) is still mainly intact, but filopodia (closed arrows) are being extended through or along it. Open arrows in C and D are on the same cell process of this seam in surrounding mesenchyme. Asterisk in D is a pre-existing mesenchymal cell process in close contact with epithelial filopodium. Anterior palate (rodent). E: Electron micrograph of an elongating cell breaking away from the tip of a disappearing epithelial seam. The basal lamina is almost completely gone and the cell at the tip of the seam is extending numerous filopodia and pseudopodia typical of mesenchymal cells. Circles identify desmosomes still present in the seam. These persist until the cell undergoing EMT breaks away and the one linking the top cell to the bottom seam indicates its origin from the epithelium. Gly, glycogen; Glart, glutaraldehyde artifact; P, leading pseudopodium. Scale bars = 3 µm in C, 1.5 µm in D, 100 nm in E. From Fitchett and Hay (1989), © Developmental Biology, Academic Press Inc.
Griffith and Hay (1992) also suggested the presence of living epithelial cells joined by desmosomes was paramount for the formation of a seam that holds the two fusing palatal shelves together. Once the connection was established, the epithelial cells were “removed” by EMT and integrated into the mesenchymal compartment of the palate, where they remain and can be identified later in palatal development, functioning as fibroblasts and playing yet-to-be-identified roles in morphogenesis.
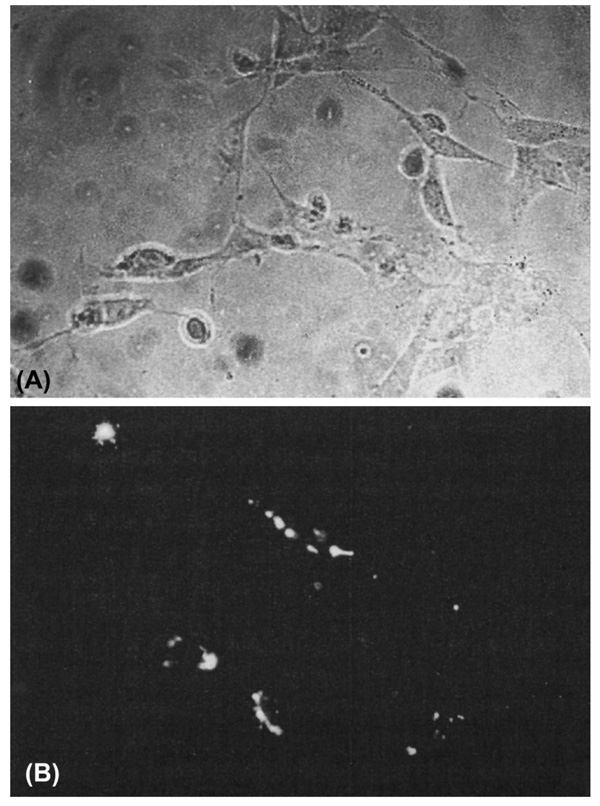
Studies using cell lineage analysis of MEE were combined with immunohistochemistry for phenotypic markers and ultrastructural examinations to characterize the morphology of the MEE at different stages in the process of palatal fusion. The results of the cell lineage studies have led to two conclusions regarding the fate of the MEE: (1) EMT (Fitchett and Hay, 1989; Shuler et al., 1991, 1992; Griffith and Hay, 1992), and (2) migration to the oral and nasal surface epithelia (Carette and Ferguson, 1992). However, as part of both of these MEE morphology options, the cells do not undergo cell death but remain viable. Moreover, Shuler (1995) showed that after breakdown of the basement membrane and the discontinuity of the midline epithelium, cells with a mesenchymal phenotype containing the cell lineage marker are identified (Fig. 4).
Fig. 4.
Labeled Palatal cells in culture maintain mesenchymal morphology. Cells isolated from the midline position of a palatal shelf after the completion of palatal fusion and placed in organ culture retain the marker for cell lineage and have a fibroblastic morphology. A: Phase-contrast microscopy of palatal mesenchyme cells in culture. B: DiI (l, l′-dioctadecyl-3, 3, 3′, 3′-tetramethylindo-carbo-cyanine perchlorate) fluorescence of the same field of cultured cells. From Shuler, CF (1995), © Crit Rev Oral Biol Med, International and American Associations for Dental Research.
As mentioned earlier, Carette and Ferguson (1992) reported that the disappearance of the midline seam in palatal fusion in vitro was due to MEE cells becoming transiently motile and migrating into the nasal and oral epithelia of the palate, where they become morphologically indistinguishable from the surrounding cells in “epithelial triangles.” However, Griffith and Hay (1992) demonstrated ultrastructurally that the CCFSE-labeled epithelial seam cells of the MEE undergo EMT. CCFSE-containing cells in the midline area after palatal confluence are clearly fibroblastic in morphology. The interpretation that these fibroblasts subsequently reenter epithelium at their final destination (Carette and Ferguson, 1992) was not evidence-based. The major criticism of this work by Griffith and Hay (1992) was that no cytology was presented, making the fluorescent images impossible to interpret. Several palates were exposed to DiI and frozen sections were examined at 24 and 48 hr with no fixation. The label was distributed in a hit or miss fashion, some in the seam and some in the oral epithelium, and it was not clear that enough label was present at 0 time and 24 hr to follow at 48 hr. Confocal images were then presented showing single living palates at 24, 48, and 120 hr, photographed as whole mounts at different levels into the tissue. As such, it would not have been possible from these images to say whether or not label was present in mesenchymal cells. Thus, the conclusion that labeled cells from the seam migrate back into the surface epithelium was not supported. Moreover, the so-called triangles of epithelium near the midline seam, which Carette and Ferguson (1992) reported accumulate such cells, were not a reproducible feature in Griffith and Hay (1992) cultures. Griffith and Hay (1992) traced labeled mesenchymal cells originating in the midline seam through their differentiation into fibroblasts, as judged by their ultrastructure. These fibroblasts were still present in abundance at just before birth (E18), while the label in the epithelium was greatly diminished. Interestingly, the same group that proposed migration theory failed to conclusively confirm seam cell migration using replication defective retroviral vector, CXL, which carries the E. coli LacZ gene, analyzed by β-galactoside activity (Martinez-Alvarez et al., 2000b). And, in fact, they agreed and showed that although most of the seam cells undergo PCD, some MES cells do undergo EMT (Martinez-Alvarez et al., 2000b).
The EMT concept was further confirmed by Kaartinen et al. (1995, 1997), who for the first time implicated a role of TGFβ3 in palatal seam EMT. These revolutionary studies confirmed an essential function for TGFβ3 in normal palatal morphogenesis and directly implicated TGFβ3 in the mechanisms of palatal EMT. Since then, numerous studies showed activation of TGFβ3 signaling pathways and its downstream molecules in palatal EMT (Kang and Svoboda, 2002; Cui et al., 2003, 2005; Nawshad and Hay, 2003; Nawshad et al., 2004b, 2007; Lagamba et al., 2005). However, these studies do not rule out the possibility of PCD during complete seam disintegration.
Based on the studies described in this section, EMT is obviously the candidate of choice to create palatal confluence. Death of the MEE seam would disrupt mesenchyme continuity across the palate that may result in cleft palate. Defects in the connective tissue component and mesenchymal dynamics of the palate would hardly be tolerated during oral activities in prenatal embryos.
PROGRAMMED CELL DEATH OR PCD
Programmed cell death (PCD) is synonymous to apoptosis. PCD is an important mechanism in development and homeostasis in adult tissues for the removal of either superfluous, infected, transformed, or damaged cells by activation of an intrinsic suicide program. Apoptosis, a form of PCD, is a program of cellular suicide critical for proper embryonic development and homeostasis of adult tissues (Danial and Korsmeyer, 2004), which is characterized by maintenance of intact cell membranes during the suicide process so as to allow adjacent cells to engulf the dying cell. This preventive measure is important so that dying cells do not release their contents and trigger a local inflammatory reaction. The apoptotic program is executed by a family of highly conserved proteases known as caspases, which dismantle the cell in an orderly manner by cleaving a large number of cellular substrates. Aberrant caspase regulation has been unequivocally linked to the pathogenesis of a variety of diseases, including various neurological disorders and cancers (Riedl and Shi, 2004). While pathological connotation of cell death is more closely linked with apoptosis, the physiological relevance of cell death remains elusive. Eminent “cell death” authorities like the late Prof. Stan Korsmeyer are reticent when using the term apoptosis in the context of normal physiological condition. Therefore, I chose to use the term pro-grammed cell death (PCD) in this review.
While growth (increase in mass) and proliferation (increase in cell number) seems to contribute more during the formation of multi cellular organisms during embryogenesis and organogenesis, PCD is present in mammalian blastocysts, and its normal pattern is crucial for further development. Both sections of the blastocyst (inner cell mass and trophoectoderm) undergo PCD during normal development (Hardy, 1997). Distortions of PCD in the blastocyst result in compromise of future maturation and may lead to either early embryonic death or the formation of anomalies in the fetus (Brison and Schultz, 1997). At the later stages of normal embryo development, PCD plays a key role in the formation of the extraembryonic structures and the embryo itself. One example of the role of PCD can be seen in the hand plate when cells that develop between the fingers are eliminated through PCD. The digits themselves begin to form as condensations of initial mesenchymal tissue. These condensations are the primary signs of future digit location (Mori et al., 1995).
While palatal seam disintegration by EMT received ample attention, it is noteworthy that PCD as a method of seam regression was reported as early as 1951 by Glucksmann, and subsequently by many others (Glucksmann, 1965; Pourtois, 1966; Saunders, 1966; DeAngelis and Nalbandian, 1968; Farbman, 1968, 1969; Smiley and Dixon, 1968; Shapiro and Sweney, 1969). For close to two decades programmed cell death remained accepted virtually without challenge as the operative mechanism for removal of the epithelial barrier produced when opposed palatal shelves become adherent. There were a handful of subsequent reports suggesting PCD as method of choice for palatal seam disintegration (Greene and Pratt, 1976; Pratt and Greene, 1976; Mori et al., 1994; Taniguchi et al., 1995; Martinez-Alvarez et al., 2000a,b; Cuervo and Covarrubias, 2004). Thus, for nearly 50 years, outstanding results were reported in support of either EMT or PCD.
It is true that the concept of EMT as a means of palatal seam disintegration was at its peak during the 1990s. But the hypothesis of PCD that was well documented by mostly ultra-structural studies since 1951 by Glucksmann have reappeared using sophisticated techniques. Martinez-Alvarez et al. (2000b) confirmed seam cell PCD using replication defective retroviral vector, CXL, which carries the E. coli LacZ gene, analyzed by β-galactoside activity. But they also showed substantial EMT was functional during palatal seam disintegration and conclude that probably PCD and EMT occur during seam disintegration. These findings were contradicted with results from Cuervo and Covarrubias (2004), who used a technique similar to that of Martinez-Alvarez et al. (2000b) to label MEE genetically with an adenovirus carrying LacZ gene; the former found that almost all medial edge epithelial seam cells underwent PCD. In addition to genetically labeling with LacZ, Cuervo and Covarrubias (2004) used a CCFSE-labeling method similar to that used by Griffith and Hay (1992), and concluded PCD is a means of seam disintegration, challenging the findings of the latter. Cuervo and Covarrabias (2004) argued that the lack of quantitative data analyses and inadequate follow through of so-called “transformed” cells to establish whether these transformed cells were dying or whether they were phagocytes containing dying cells. Indeed, it was an excellent point that the authors raised based on the work of Martinez-Alvarez et al. (2000b).
Discrimination between living and dead cells is an essential requirement for appropriate clearance of dying cells. Removal of dying cells by phagocytes plays an important role in many biological processes, including embryological development and tissue remodeling. Phagocytosis of dying cells is clearly an important physiological mechanism to remove effete cells before they disgorge their contents of potential histotoxic products. To show that palatal epithelial seam cells die and are phagocytosed, Martinez-Alvarez et al. (2000b) showed the presence of macrophages near the seam cell and dead cells being phagocytosed. Interestingly, Fitchett and Hay (1989), in their first report of palatal EMT, did observed phagocytic activity in the seam in vivo according to the position along the sagittal palatal axis. However, they concluded that those phagocytic activities were restricted to the midpalatal raphe and posterior palate and probably involve removal of degenerating peridermal cells caught in the seam due to early midline contact in these regions. But Griffith and Hay (1992) did address this issue by ultrastructural findings and demonstrated that the isolation bodies were not lysosomes within a dying midline seam by staining palates with the Gomori stain for acid phosphatase, a major lysosomal enzyme marker. They found no correlation between the presence of CCFSE label and the presence of acid phosphatase. Furthermore, evidence that the isolation bodies were not phagosomes was provided by TEM observations that the isolation bodies in the epithelia of labeled palates did not appear until approximately 12 hr after exposure to the dye. They confirmed by electron microscopy that the dye diffused into the epithelial cells and was not taken up by the cells by phagocytosis (as indicated by the lack of phagosomes in the fluorescent tissues up to 12 hr after labeling). The isolation bodies that appear at 24 hr might be called autophagosomes except they lack acid phosphatase. The dye did not leave the cells again after passing from the periderm to basal epithelial layer. This finding was confirmed by TEM observations that CCFSE isolation bodies are only seen within the cells of the midline epithelial seam and newly transformed mesenchyme. Preexisting mesenchymal cells in the palate did not contain isolation bodies. However, these speculations by Griffith and Hay (1992) were addressed and overruled by the presence of macrophages near the seam cell and the dead cells were being phagocytosed (Martinez-Alvarez et al., 2000b). They showed clearly that terminal deoxynucleotidyl transferase-labeled dUTP nick end labeling, TUNEL-positive dying cells were engulfed by macrophages by the presence of F4/80 marker.
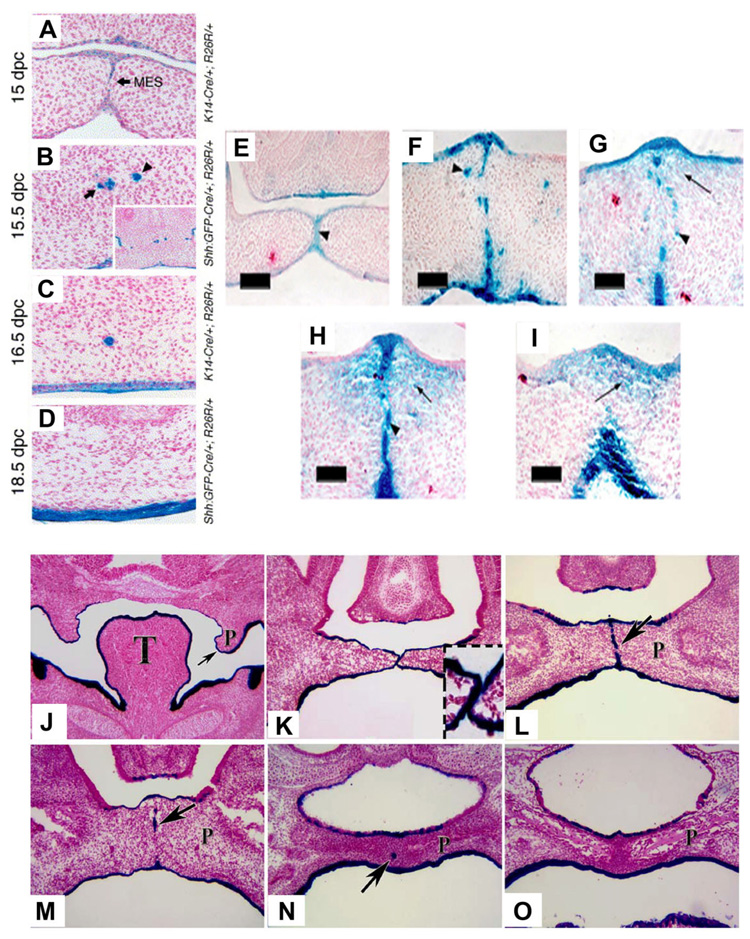
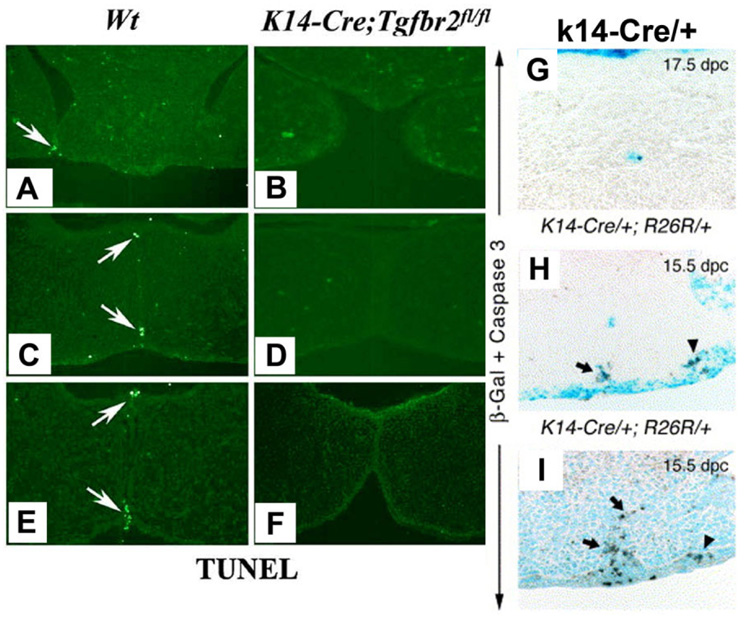
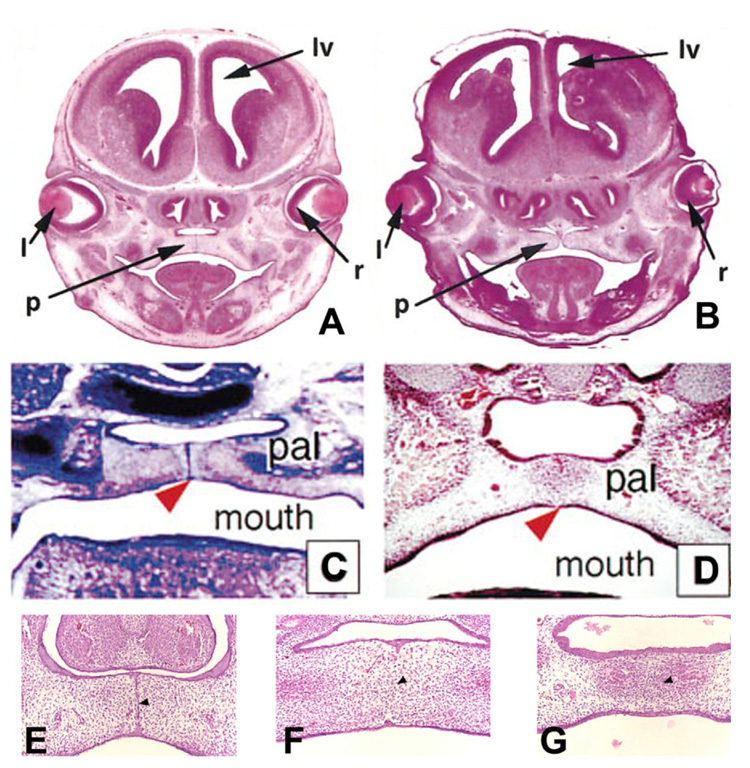
These studies during the early part of the current decade were just the beginning of PCD hypotheses for palatal seam disintegration. Very recently, convincing results (Fig. 5A–D, J–O) obtained by using the Cre-Loxp-based genetic labeling system (in which the expression of Cre recombinase is driven by a cytokeratin 14 (K14) promoter (Vasioukhin and Fuchs, 2001) and R26R reporter locus is specifically activated and irreversibly labeled in the MES epithelium) ruled outEMTor migration to have any role in palatal seam disintegration (Vaziri Sani et al., 2005; Xu et al., 2006). While they used genetic labeling to overwhelmingly disprove EMT, they used TUNEL (Fig. 6A–F) and/or cleaved Caspase-3 protein expression (Fig. 6G–I) to suggest PCD as the only mechanism of MES disintegration. Although Gritli-Linde (2007) recently disputed the reliability of the fate mapping of the MES cells and the regulation to preserve seam morphology used by Xu et al. (2006) when compared with Vaziri Sani et al. (2005), both concluded very similar outcome supporting PCD. In support of PCD, Gritli-Linde (2007) stated the importance Apoptotic Protease Activating Factor (Apaf)-1 in palatogenesis. Indeed, earlier findings (Fig. 7A,B) by Honarpour et al. (2000) showed a lack of palatal shelves adherence and Cecconi et al. (1998) showed (Fig. 7C,D) absence of MES disintegration in Apaf-1 knockout mice (Honarpour et al., 2000), confirming the essential role of Apaf-1 in palatogenesis.
Fig. 5.
Fate Mapping with different genetic labeling method during palatal seam disintegration. A–D: Frontal sections from 15 days post coitum (dpc) K14-Cre/+; R26R/+ (A), 15.5 dpc ShhGFPCre/+; R26R/+ (B), 16.5 dpc K14-Cre/+; R26R/+ (C), and 18.5 dpc ShhGFPCre/+; R26R/+ (D) embryos at the anterior segments of the palate stained for β-galactosidase. MES, midline epithelial seam (arrows in panels A). Inset in B is a low-magnification view of the micrograph. At 18.5 dpc, the palate is virtually cleared from lacZ-positive epithelial islands (D). Mesenchymal cells are totally devoid of β-galactosidase activity. A, B, C, and D are from Vaziri Sani et al. (2005), © Developmental Biology, Elsevier Inc. β-gal staining pattern in (K14-Cre; R26R) embryos demonstrates the occurrence of EMT during and after seam degeneration. E: β-gal staining in the anterior region of the palate at early 14.5 dpc, where the two shelves have just made contact. Note that the MEE cells were strongly labeled with β-gal (arrowhead), but no signal was present in mesenchymal cells. F: β-gal staining in the middle region of the palate at early 14.5 dpc, where seam degeneration has just been initiated. Some epithelial-like β-gal-positive cells have dissociated from the midline and migrated into the mesenchymal region (arrowhead). G, H: β-gal staining in the middle region of the palate at late 14.5 dpc, when seam degeneration is advanced. Both clump-like blue cells (arrow-head in G, H) and typical mesenchymal-looking blue cells (arrow in G, H) were observed in the mesenchymal region of the palate. I: β-gal staining in the fully fused palate at 15.5 dpc, showing that a high portion of the mesenchymal cells were β-gal positive (arrow). Scale bars = 100 µm in E, 50µm F–I. E, F, G, H, I are from Jin and Ding (2006), © Development, The Company of Biologist Ltd. β-Galactosidase staining of frontal sections from K14-Cre; R26R embryos. J: At 13.5 dpc, the secondary palate shelf projects toward the midline, palatal epithelium cells are β-galactosidase positive. K: At 14 dpc, the opposite secondary palate shelves contact each other, and the medial edge epithelial (MEE) cells are all β-galactosidase positive (insert). L: At 14.5 dpc, the palatal shelves have fused, and the midline epithelial seam (MES) is β-galactosidase positive (arrow), no β-galactosidase positive cells can be found in the palatal mesenchyme. M: At E15.5, most of the MES (arrow) has disappeared. N: At 16.5 dpc, few β-galactosidase–positive cells remain in the midline (arrow) and no β-galactosidase positive cells can be found in the palatal mesenchyme. O: At 17.5 dpc, no β-galactosidase positive cells can be found in the palatal mesenchyme. P, palatal shelf; T, tongue. Figs. J, K, L, M, N, and O are from Xu et al. (2006), © Developmental Biology, Elsevier Inc.
Fig. 6.
Detection of PCD in palatal seam disintegration. A–F: The fate of the medial edge epithelia (MEE) is changed in the K14-Cre; Tgfbr2fl/fl mutant palate. At 14.5 days post coitum (dpc), wild-type MEE cells show positive TUNEL staining, a marker for cell death (arrow) from anterior to posterior part of the palate. A, C, E: No cell death can be detected in the K14-Cre; Tgfbr2fl/fl mutant palate. B, D, F: At 15.5 dpc, palatal fusion process has reached the end, most of the wild-type MEE cells have disappeared. G, H, I: Immunohistochemistry with anti-activated caspase-3. Palatal sections palate from a 17.5 dpc K14-Cre/+; R26R/+ embryo showing activated-caspase-3 immunostaining (brown color) in a lacZ positive epithelial island (G). 15.5 dpc K14-Cre/+; R26R/+ embryo showing anti-activated caspase-3 immunostaining in the regressing lacZ-positive MES (arrow) and in the lateral epithelium (arrowhead; H). Palatal section from a 15.5 dpc K14-Cre/+ embryo (no R26R allele) showing cells positive for activated caspase-3, showing pre-PCD in both the regressing MES (arrow) and the epithelium at the junction of fusion between the maxillary and intermaxillary processes (arrowhead; I). A–F are from Xu et al. (2006), © Developmental Biology, Elsevier Inc. and G–I are from Vaziri Sani et al. (2005), © Developmental Biology, Elsevier Inc.
Fig. 7.
Palate development in Apaf-1 mutant mice. Cranial sections from wild-type and Apaf-1 mutant mice cut coronally and stained with hematoxylin and eosin are shown at ×50 magnification. A, B: Wild-type at 15.5 days post coitum (dpc; A) and Apaf-1 mutant mice at gestational ages equivalent to wild-type embryos (B). In A and B, “l”, “de”, “tv”, “v”, “lv”, “p”, “ge”, and “r” show the positions of the lens, diencephalon, telencephalic vesicle, third ventricle, lateral ventricle, palate, ganglionic eminence, and retina, respectively. A and B are from Honapour et al. (2000), © Developmental Biology, Academic Press. C: Transversal section through the caudal third of the palate of an 14.5 dpc homozygous embryo. The palatal shelves meet in the midline (arrowhead) but do not fuse. pal, secondary palate. D: Transversal section through the caudal third of the palate of an 14.5 dpc wild-type embryo, showing complete fusion of the palatal shelves in the midline (arrowhead). pal, secondary palate; C and D are from Cecconi et al. (1998), © Cell, Cell Press. E: The MEE seam (arrowhead) forms normally in Apaf-1 mutant embryos at 14.5 dpc. F: The MEE seam undergoes degeneration in Apaf-1 mutant embryos at 15.5 dpc to establish the mesenchyme confluence cross the midline (arrowheads). G: At 16.5 dpc, both wild-type and Apaf-1 mutant embryos form a continuous palate with no sign of seam cells in the midline area (arrowhead). E, F, and G are from Jin and Ding (2006) © Development, The Company of Biologist Ltd.
But these overwhelmingly convincing results (Vaziri Sani et al., 2005; Xu et al., 2006) also were challenged through the use of the same genetic labeling technique (Jin and Ding, 2006). They reported contradictory results, showing EMT as the only way for MES disintegration (Fig. 5E–I). Finally, to find out whether PCD is functionally required for seam degeneration in vivo, Jin and Ding (2006) examined MES cell PCD and palatal fusion in Apaf-1–deficient mice, in which caspases 9 and 3 (key caspase effectors) are absent and showed they were not activated. These findings show normal palatogenesis (Fig. 7E–G), thus completely ruling out Apaf-1–dependent PCD in MES disintegration.
PCD has largely been attributed to the activation of caspases, which cleave many substrates to produce the characteristics of a dying cell (Fischer et al., 2003). However, developmental PCD is often unaffected by many caspase knockouts, and other changes may be important that are caspase-independent (Hague and Paraskeva, 2004). Part of this discrepancy may stem from dual pathways for PCD, one being Caspase dependent by Apaf-1 and the other being Caspase-independent (Hansen and Nagley, 2003). Nevertheless, PCD can still take place by means of an Apaf-1 independent manner (Susin et al., 2000) as the cells deficient in these molecules can still die. Vaux and Korsmeyer (1999) argue that the pathways for physiological program cell deaths occur during development may not necessarily the same those in pathological conditions and well could be independent of caspases. Moreover, mutations of caspase 9 and 3 caused perinatal lethality and the only organ shown to be abnormal as a result was the brain (Chipuk and Green, 2005). It raises the possibility that during palate development PCD still may occur independent of caspases (Ahmed et al., 2007). One of the candidate genes, Apoptosis Inducing Factor (AIF), causes PCD in a caspase-independent manner (Hansen and Nagley, 2003). Arnoult et al. (2002) showed that inhibition of caspases can still activate AIF to cause PCD. Interestingly, AIF is expressed in most of the murine developing organs (Joza et al., 2001) concomitant with the timing of palatogenesis. Moreover, TGFβ, which is essential for palatal seam disintegration, is capable of activating both AIF (Dormann and Bauer, 1998) and several caspases by Smad-dependent and -independent pathways (Schuster and Krieglstein, 2002).
Although studying the role of genes in the development of transgenic knockouts or conditional knockouts of selective genes on specific organ is extremely convincing, it is noteworthy that some of the knockout models can demonstrate a surprisingly high degree of phenotypic variability among individual mouse lines and penetrance of the phenotype in a mixed-background colony could well be due to the presence of additional modifier loci (Doetschman, 1999). Often the genetic background of mice is a major factor in many of the observed phenotypes.
OR BOTH: EMT AND PCD
In view of these conflicting results, either between EMT (Shuler et al., 1992; Hay, 1995; Kaartinen et al., 1997; Lavrin and Hay, 2000; Sun et al., 2000a,b; Nawshad and Hay, 2003; Nawshad et al., 2004a,b; Takahara et al., 2004; Takigawa and Shiota, 2004; Kang and Svoboda, 2005; Lagamba et al., 2005; Pungchanchaikul et al., 2005; Jin and Ding, 2006) and PCD (Glucksmann, 1965; Saunders, 1966; DeAngelis and Nalbandian, 1968; Smiley and Dixon, 1968; Farbman, 1969; Hayward, 1969; Shapiro and Sweney, 1969; Mato et al., 1972; Hudson and Shapiro, 1973; Pratt and Martin, 1975; Greene and Pratt, 1976; Greene, 1989; Taniguchi et al., 1995; Cuervo and Covarrubias, 2004; Vaziri Sani et al., 2005; Dudas et al., 2006a, 2007; Xu et al., 2006; Lee et al., 2008) or even within the same proponents (Fitchett and Hay, 1989; Shuler et al., 1991; Carette and Ferguson, 1992; Griffith and Hay, 1992; Shuler et al., 1992; Martinez-Alvarez et al., 2000b; Cuervo and Covarrubias, 2004; Vaziri Sani et al., 2005; Jin and Ding, 2006; Xu et al., 2006), two studies propose a new concept, where both EMT and PCD play important role in seam disintegration (Martinez-Alvarez et al., 2000b) and are required for complete seam disintegration (Ahmed et al., 2007). Ahmed et al. (2007) showed a connection between complex TGFβ3 signaling and the chronology of events that occur as MES cells disappear, including both EMT and PCD. Their study, which was undertaken in primary MEE in culture to manipulate, dissect, and interpret complex TGFβ3 signaling pathways in palatogenesis, showed that the sedentary seam epithelial cells underwent significant phenotypical changes from cell cycle arrest, migration and PCD chronologically in time dependent manner during palatogenesis. Ahmed et al. (2007) demonstrated that TGFβ3 signal by means of multiple pathways to activate many transcription factors to generate several cellular outcomes, such as cell cycle arrest, migration, and PCD in chronological order by the presence of high molecular weight by Clamped Electric Field Electrophoresis and low molecular weight DNA fragmentation by conventional gel electrophoresis. Their results clearly demonstrated that PCD was functional during palatal seam disintegration, but so were cell cycle arrest and EMT. These findings are currently being confirmed in the in vivo model.
CONCLUSION
PCD and EMT are highly conserved, fundamental processes that govern morphogenesis in development. Evidence suggests they both contribute to palatogenesis, especially during palatal seam disintegration. I do not question the importance of PCD nor do I dispute EMT as means of palatal seam disintegration, but I think it is worth investigating an ideal technique to resolve “the cellular decisions” with respect to division, differentiation, and PCD in palatal seam disintegration.
In general, the mechanisms of palatal seam disintegration are not overwhelmingly complex but because of the large numbers of interacting constituents, their complicated circuitry involving feedforward, feedback, and crosstalk, and the fact that the kinetics of interaction matter–this otherwise simple mechanism can be rightfully quite difficult to interpret. As a result of this complexity, apparently simple but highly important questions remain unanswered, one such question is the fate of palatal seam. Such question may be answered by detailed and extensive quantitative experimentation of both basic biological (cellular, structural) and newest molecular biological (genetic/dye cell lineage, gene activity, kinase/enzyme activity) as well as animal model (knockouts, transgenic) approaches. System biology and cellular kinetics play a crucial role in cellular MES function; omissions of such a critical contributor may lead to inaccurate understanding of the fate of MES.
To date, most ultrastructural studies, dye labeling, viral LacZ labeling, or genetically labeling with Cre recombinase demonstrate the presence of EMT or disprove it. But when it comes to studies of PCD as an alternative method, most experiments are based purely on TUNEL-positive cells, unlike overwhelmingly convincing labeling of epithelial cells with Cre-Loxp-based genetic labeling. There is no genetic labeling evidence that PCD alone is necessary or sufficient to cause palatal seam disintegration. In vivo, apoptotic cells appear rare, even in tissues such as the thymus in which extensive PCD is a normal feature during regression, presence of TUNEL-positive cells is not common (Abraham and Shaham, 2004). The percentage of apoptotic cells determined by TUNEL method has their own limitations. Despite electron microscopy being the gold standard for PCD detection in embryogenesis, many laboratories use in situ TUNEL assay, which stains fragmented DNA strands in situ and is a quantifiable method. A major disadvantage of the TUNEL assay is that it often overestimates apoptotic nuclei, as it labels not only fragmented DNA but also RNA or DNA in the process of repair as well as some cells undergoing necrosis (Kovacevic et al., 2007). Therefore, concluding PCD as method of seam disintegration based only on TUNEL experiment may be inadequate, particularly when establishing PCD over EMT as disproving EMT was done on outstanding Cre-Loxp-based genetic labeling. Features associated with PCD, including permeability to the vital dye acridine orange, phosphatidylserine exposure on the outer leaflet of the plasma membrane, and DNA fragmentation (Susin et al., 2000). During earlier studies, these experiments were surprisingly missing when PCD was being confirmed for palatal seam disintegration.
Although, each proposition in favor of either EMT or PCD has extremely convincing supporting data, the frustrating conclusion seems to result from the firm standing on either EMT or PCD as only mechanism of seam disintegration and the proponents of one hypothesis not agreeing with the others. The key to understanding EMT and PCD lies not only in the instructions the MES cells carry with them, but also within the characteristics of the landscape that determine the way cells behave during development. Therefore, biological, genetic, and structural behaviors of the palatal MES cells must be incorporated into relevant investigations to mitigate the controversy. Systematic aggregation and proper evaluation of all these findings (not one model alone) will shed light on the accurate interpretation of the exact mechanism of palatal seam disintegration.
References
- Abdelilah-Seyfried S, Cox DN, Jan YN. Bazooka is a permissive factor for the invasive behavior of discs large tumor cells in Drosophila ovarian follicular epithelia. Development. 2003;130:1927–1935. doi: 10.1242/dev.00420. [DOI] [PubMed] [Google Scholar]
- Abraham MC, Shaham S. Death without caspases, caspases without death. Trends Cell Biol. 2004;14:184–193. doi: 10.1016/j.tcb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Liu CC, Nawshad A. Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3. Dev Biol. 2007;309:193–207. doi: 10.1016/j.ydbio.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol. 2002;159:923–929. doi: 10.1083/jcb.200207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J. Genes and proteins involved in mesenchymal to epithelial transition. Curr Opin Nephrol Hypertens. 2001;10:429–436. doi: 10.1097/00041552-200105000-00021. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Wilting J, Ebensperger C, Christ B. The formation of somite compartments in the avian embryo. Int J Dev Biol. 1996;40:411–420. [PubMed] [Google Scholar]
- Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod. 1997;56:1088–1096. doi: 10.1095/biolreprod56.5.1088. [DOI] [PubMed] [Google Scholar]
- Carette MJ, Ferguson MW. The fate of medial edge epithelial cells during palatal fusion in vitro: an analysis by DiI labelling and confocal microscopy. Development. 1992;114:379–388. doi: 10.1242/dev.114.2.379. [DOI] [PubMed] [Google Scholar]
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- Chaudhry AP, Shah RM. Palatogenesis in hamster. II. Ultrastructural observations on the closure of palate. J Morphol. 1973;139:329–350. doi: 10.1002/jmor.1051390304. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. Do inducers of apoptosis trigger caspase-independent cell death? Nat Rev Mol Cell Biol. 2005;6:268–275. doi: 10.1038/nrm1573. [DOI] [PubMed] [Google Scholar]
- Cuervo R, Covarrubias L. Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogenesis. Development. 2004;131:15–24. doi: 10.1242/dev.00907. [DOI] [PubMed] [Google Scholar]
- Cui XM, Chai Y, Chen J, Yamamoto T, Ito Y, Bringas P, Shuler CF. TGF-beta3-dependent SMAD2 phosphorylation and inhibition ofMEEproliferation during palatal fusion. Dev Dyn. 2003;227:387–394. doi: 10.1002/dvdy.10326. [DOI] [PubMed] [Google Scholar]
- Cui XM, Shiomi N, Chen J, Saito T, Yamamoto T, Ito Y, Bringas P, Chai Y, Shuler CF. Overexpression of Smad2 in Tgf-beta3-null mutant mice rescues cleft palate. Dev Biol. 2005;278:193–202. doi: 10.1016/j.ydbio.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- DeAngelis V, Nalbandian J. Ultra-structure of mouse and rat palatal processes prior to and during secondary palate formation. Arch Oral Biol. 1968;13:601–608. doi: 10.1016/0003-9969(68)90138-6. [DOI] [PubMed] [Google Scholar]
- Doetschman T. Interpretation of phenotype in genetically engineered mice. Lab Anim Sci. 1999;49:137–143. [PubMed] [Google Scholar]
- Dormann S, Bauer G. TGF-beta and FGF-trigger intercellular induction of apoptosis: analogous activity on non-transformed but differential activity on transformed cells. Int J Oncol. 1998;13:1247–1252. doi: 10.3892/ijo.13.6.1247. [DOI] [PubMed] [Google Scholar]
- Dudas M, Kim J, Li WY, Nagy A, Larsson J, Karlsson S, Chai Y, Kaartinen V. Epithelial and ectomesenchymal role of the type I TGF-beta receptor ALK5 during facial morphogenesis and palatal fusion. Dev Biol. 2006a;296:298–314. doi: 10.1016/j.ydbio.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas M, Li WY, Kim J, Yang A, Kaartinen V. Palatal fusion - where do the midline cells go? A review on cleft palate, a major human birth defect. Acta Histochem. 2007;109:1–14. doi: 10.1016/j.acthis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Electron microscope study of palate fusion in mouse embryos. Dev Biol. 1968;18:93–116. doi: 10.1016/0012-1606(68)90038-9. [DOI] [PubMed] [Google Scholar]
- Farbman AI. The epithelium-connective tissue interface during closure of the secondary palate in rodent embryos. J Dent Res. 1969;48:617–624. doi: 10.1177/00220345690480050201. [DOI] [PubMed] [Google Scholar]
- Ferguson MW. Palate development. Development. 1988;103 suppl:41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchett JE, Hay ED. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol. 1989;131:455–474. doi: 10.1016/s0012-1606(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Glucksmann A. Cell death in normal development. Arch Biol (Liege) 1965;76:419–437. [PubMed] [Google Scholar]
- Greene RM. Signal transduction during craniofacial development. Crit Rev Toxicol. 1989;20:137–152. doi: 10.3109/10408448909017907. [DOI] [PubMed] [Google Scholar]
- Greene RM, Pratt RM. Developmental aspects of secondary palate formation. J Embryol Exp Morphol. 1976;36:225–245. [PubMed] [Google Scholar]
- Griffith CM, Hay ED. Epithelial-mesenchymal transformation during palatal fusion: carboxyfluorescein traces cells at light and electron microscopic levels. Development. 1992;116:1087–1099. doi: 10.1242/dev.116.4.1087. [DOI] [PubMed] [Google Scholar]
- Griffith CM, Wiley MJ, Sanders EJ. The vertebrate tail bud: three germ layers from one tissue. Anat Embryol (Berl) 1992;185:101–113. doi: 10.1007/BF00185911. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A. Molecular control of secondary palate development. Dev Biol. 2007;301:309–326. doi: 10.1016/j.ydbio.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Hague A, Paraskeva C. Apoptosis and disease: a matter of cell fate. Cell Death Differ. 2004;11:1366–1372. doi: 10.1038/sj.cdd.4401497. [DOI] [PubMed] [Google Scholar]
- Hansen TM, Nagley P. AIF: a multifunctional cog in the life and death machine. Sci STKE. 2003;2003:PE31. doi: 10.1126/stke.2003.193.pe31. [DOI] [PubMed] [Google Scholar]
- Hardy K. Cell death in the mammalian blastocyst. Mol Hum Reprod. 1997;3:919–925. doi: 10.1093/molehr/3.10.919. [DOI] [PubMed] [Google Scholar]
- Hay ED. Organization and fine structure of epithelium and mesenchyme in the developing chick embryo. In: Billingham RF, editor. Epithelial-mesenchymal interactions. Baltimore: Williams and Wilkins Co; 1968. pp. 31–55. [Google Scholar]
- Hay ED. Role of cell-matrix contacts in cell migration and epithelial-mesenchymal transformation. Cell Differ Dev. 1990;32:367–375. doi: 10.1016/0922-3371(90)90052-x. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Hayward AF. Ultrastructural changes in the epithelium during fusion of the palatal processes in rats. Arch Oral Biol. 1969;14:661–678. doi: 10.1016/0003-9969(69)90188-5. [DOI] [PubMed] [Google Scholar]
- Honarpour N, Du C, Richardson JA, Hammer RE, Wang X, Herz J. Adult Apaf-1-deficient mice exhibit male infertility. Dev Biol. 2000;218:248–258. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- Hudson CD, Shapiro BL. A radioautographic study of deoxyribonucleic acid synthesis in embryonic rat palatal shelf epithelium with reference to the concept of programmed cell death. Arch Oral Biol. 1973;18:77–84. doi: 10.1016/0003-9969(73)90022-8. [DOI] [PubMed] [Google Scholar]
- Jin JZ, Ding J. Analysis of cell migration, transdifferentiation and apoptosis during mouse secondary palate fusion. Development. 2006;133:3341–3347. doi: 10.1242/dev.02520. [DOI] [PubMed] [Google Scholar]
- Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Cui XM, Heisterkamp N, Groffen J, Shuler CF. Transforming growth factor-beta3 regulates trans-differentiation of medial edge epithelium during palatal fusion and associated degradation of the basement membrane. Dev Dyn. 1997;209:255–260. doi: 10.1002/(SICI)1097-0177(199707)209:3<255::AID-AJA1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kang P, Svoboda KK. PI-3 kinase activity is required for epithelial-mesenchymal transformation during palate fusion. Dev Dyn. 2002;225:316–321. doi: 10.1002/dvdy.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Svoboda KK. Epithelial-Mesenchymal Transformation during Craniofacial Development. J Dent Res. 2005;84:678–690. doi: 10.1177/154405910508400801. [DOI] [PubMed] [Google Scholar]
- Kovacevic M, Simic O, Jonjic N, Stifter S. Apoptosis and cardiopulmonary bypass. J Card Surg. 2007;22:129–134. doi: 10.1111/j.1540-8191.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- Lagamba D, Nawshad A, Hay ED. Microarray analysis of gene expression during epithelial-mesenchymal transformation. Dev Dyn. 2005;234:132–142. doi: 10.1002/dvdy.20489. [DOI] [PubMed] [Google Scholar]
- Lavrin IG, Hay ED. Re: epithelial-mesenchymal transformation, palatogenesis and cleft palate. Angle Orthod. 2000;70:181–182. doi: 10.1043/0003-3219(2000)070<0181:LFOR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lee J-M, Kim J-Y, Cho K-W, Lee M-J, Cho S-W, Kwak S, Cai J, Jung H-S. Wnt11/Fgfr1b cross-talk modulates the fate of cells in palate development. Dev Biol. 2008;314:341–350. doi: 10.1016/j.ydbio.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Martinez-Alvarez C, Bonelli R, Tudela C, Gato A, Mena J, O’Kane S, Ferguson MW. Bulging medial edge epithelial cells and palatal fusion. Int J Dev Biol. 2000a;44:331–335. [PubMed] [Google Scholar]
- Martinez-Alvarez C, Tudela C, Perez-Miguelsanz J, O’Kane S, Puerta J, Ferguson MW. Medial edge epithelial cell fate during palatal fusion. Dev Biol. 2000b;220:343–357. doi: 10.1006/dbio.2000.9644. [DOI] [PubMed] [Google Scholar]
- Mato M, Smiley GR, Dixon AD. Epithelial changes in the presumptive regions of fusion during secondary palate formation. J Dent Res. 1972;51:1451–1456. doi: 10.1177/00220345720510053301. [DOI] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Okamoto Y, Osawa M, Shiota K. Cytochemical identification of programmed cell death in the fusing fetal mouse palate by specific labeling of DNA fragmentation. Anat Embryol (Berl) 1994;190:21–28. doi: 10.1007/BF00185843. [DOI] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Kimura S, Irie H, Takigawa T, Shiota K. Programmed cell death in the interdigital tissue of the fetal mouse limb is apoptosis with DNA fragmentation. Anat Rec. 1995;242:103–110. doi: 10.1002/ar.1092420114. [DOI] [PubMed] [Google Scholar]
- Nawshad A, Hay ED. TGF{beta}3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J Cell Biol. 2003;163:1291–1301. doi: 10.1083/jcb.200306024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawshad A, LaGamba D, Hay ED. Transforming growth factor beta (TGF-beta) signalling in palatal growth, apoptosis and epithelial mesenchymal transformation (EMT) Arch Oral Biol. 2004a;49:675–689. doi: 10.1016/j.archoralbio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Nawshad A, LaGamba D, Olsen BR, Hay ED. Laser capture microdissection (LCM) for analysis of gene expression in specific tissues during embryonic epithelial-mesenchymal transformation. Dev Dyn. 2004b;230:529–534. doi: 10.1002/dvdy.20064. [DOI] [PubMed] [Google Scholar]
- Nawshad A, Medici D, Liu CC, Hay ED. TGFbeta3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J Cell Sci. 2007;120:1646–1653. doi: 10.1242/jcs.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. Patterning of avian craniofacial muscles. Dev Biol. 1986;116:347–356. doi: 10.1016/0012-1606(86)90138-7. [DOI] [PubMed] [Google Scholar]
- Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991;88:1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois M. Onset of the acquired potentiality for fusion in the palatal shelves of rats. J Embryol Exp Morphol. 1966;16:171–182. [PubMed] [Google Scholar]
- Pratt RM, Greene RM. Inhibition of palatal epithelial cell death by altered protein synthesis. Dev Biol. 1976;54:135–145. doi: 10.1016/0012-1606(76)90292-x. [DOI] [PubMed] [Google Scholar]
- Pratt RM, Martin GR. Epithelial cell death and cyclic AMP increase during palatal development. Proc Natl Acad Sci U S A. 1975;72:874–877. doi: 10.1073/pnas.72.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pungchanchaikul P, Gelbier M, Ferretti P, Bloch-Zupan A. Gene expression during palate fusion in vivo and in vitro. J Dent Res. 2005;84:526–531. doi: 10.1177/154405910508400608. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Saunders JW., Jr Death in embryonic systems. Science. 1966;154:604–612. doi: 10.1126/science.154.3749.604. [DOI] [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Burgan S, Fraser SE, Bronner-Fraser M. Vital dye labeling demonstrates a sacral neural crest contribution to the enteric nervous system of chick and mouse embryos. Development. 1991;111:857–866. doi: 10.1242/dev.111.4.857. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Shapiro BL, Sweney L. Electron microscopic and histochemical examination of oral epithelial-mesenchymal interaction (programmed cell death) J Dent Res. 1969;48:652–660. doi: 10.1177/00220345690480050801. [DOI] [PubMed] [Google Scholar]
- Shuler CF. Programmed cell death and cell transformation in craniofacial development. Crit Rev Oral Biol Med. 1995;6:202–217. doi: 10.1177/10454411950060030301. [DOI] [PubMed] [Google Scholar]
- Shuler CF, Guo Y, Majumder A, Luo RY. Molecular and morphologic changes during the epithelial-mesenchymal transformation of palatal shelf medial edge epithelium in vitro. Int J Dev Biol. 1991;35:463–472. [PubMed] [Google Scholar]
- Shuler CF, Halpern DE, Guo Y, Sank AC. Medial edge epithelium fate traced by cell lineage analysis during epithelial-mesenchymal transformation in vivo. Dev Biol. 1992;154:318–330. doi: 10.1016/0012-1606(92)90071-n. [DOI] [PubMed] [Google Scholar]
- Smiley GR, Dixon AD. Fine structure of midline epithelium in the developing palate of the mouse. Anat Rec. 1968;161:293–310. doi: 10.1002/ar.1091610303. [DOI] [PubMed] [Google Scholar]
- Sun D, Baur S, Hay ED. Epithelial-mesenchymal transformation is the mechanism for fusion of the craniofacial primordia involved in morphogenesis of the chicken lip. Dev Biol. 2000a;228:337–349. doi: 10.1006/dbio.2000.9946. [DOI] [PubMed] [Google Scholar]
- Sun D, Griffith CM, Hay ED. Carboxyfluorescein as a marker at both light and electron microscope levels to follow cell lineage in the embryo. Methods Mol Biol. 2000b;135:357–363. doi: 10.1385/1-59259-685-1:357. [DOI] [PubMed] [Google Scholar]
- Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prevost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC, Kroemer G. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000;192:571–580. doi: 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara S, Takigawa T, Shiota K. Programmed cell death is not a necessary prerequisite for fusion of the fetal mouse palate. Int J Dev Biol. 2004;48:39–46. doi: 10.1387/ijdb.15005573. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Shiota K. Terminal differentiation of palatal medial edge epithelial cells in vitro is not necessarily dependent on palatal shelf contact and midline epithelial seam formation. Int J Dev Biol. 2004;48:307–317. doi: 10.1387/ijdb.041840tt. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Sato N, Uchiyama Y. Apoptosis and heterophagy of medial edge epithelial cells of the secondary palatine shelves during fusion. Arch Histol Cytol. 1995;58:191–203. doi: 10.1679/aohc.58.191. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Ekblom P. Role of transferring in branching morphogenesis, growth and differentiation of the embryonic kidney. J Embryol Exp Morphol. 1984;82:147–161. [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Hay ED, Revel JD. Cell contact during early morphogenesis in the chick embryo. Dev Biol. 1967;16:78–106. doi: 10.1016/0012-1606(67)90018-8. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Hayashi A, Hayashi K, Donahoe PK. The epithelial-mesenchymal interface of the male rate Mullerian duct: loss of basement membrane integrity and ductal regression. Dev Biol. 1982;92:27–40. doi: 10.1016/0012-1606(82)90147-6. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vaziri Sani F, Hallberg K, Harfe BD, Mc-Mahon AP, Linde A, Gritli-Linde A. Fate-mapping of the epithelial seam during palatal fusion rules out epithelial-mesenchymal transformation. Dev Biol. 2005;285:490–495. doi: 10.1016/j.ydbio.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Waterman RE, Ross LM, Meller SM. Alterations in the epithelial surface of A-Jax mouse palatal shelves prior to and during palatal fusion: a scanning electron microscopic study. Anat Rec. 1973;176:361–375. doi: 10.1002/ar.1091760311. [DOI] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Jr, Urata MM, Chai Y. Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol. 2006;297:238–248. doi: 10.1016/j.ydbio.2006.05.014. [DOI] [PubMed] [Google Scholar]