Abstract
Background
Plasma carotenoids are considered a valid biological marker for fruit and vegetable dietary intake. Recent studies show that low carotenoid levels are associated with a high risk of inflammation, cancer, and cardiovascular disease.
Aim of the study
To determine whether low plasma carotenoids are associated with increased mortality among older adults.
Methods
Longitudinal study among 1,043 adults, 65 years and older, in the InCHIANTI study, a population-based cohort of adults living in the community in the Tuscany region, Italy.
Results
Mean total carotenoid concentration was 1.80 μmol/l. During eight years of follow-up, 310 (29.7%) of participants died. Eight-year survival was lower in the lowest compared with the highest tertile of total serum carotenoids (P < 0.0001 by Mantel-Haenszel chi-square). In a multivariate Cox proportional hazards model adjusted for age, education, smoking, body mass index, energy intake, and chronic diseases, adults in the highest tertile of plasma carotenoids at enrollment had lower mortality compared to those in the lowest tertile (Hazards Ratio obtained by considering carotenoids level as an ordinal variable 0.81, 95%; CI 0.65–0.99; P for trend = 0.046).
Conclusions
Low plasma carotenoids are an independent risk factor for mortality among older adults living in the community.
Keywords: aging, carotenoids, fruits, mortality, vegetables
Introduction
Epidemiologic studies suggest that a diet high in fruit and vegetable intake is protective against cardiovascular disease [19], stroke [15], and cancer [30]. Fruits and vegetables are high in bioactive compounds such as carotenoids, flavonoids, and other plant polyphenols. Carotenoids are powerful antioxidants and have been shown to protect against damage caused by oxidative stress. The reduction in oxidative damage is related to the decreased risk of all-cause, cancer and cardiovascular disease mortality [12]. Carotenoids act as free radical scavengers, modulate immune responses, and play an important role in the redox regulation involved in inflammation [25]. Flavonoids account for about two-thirds of polyphenols, the most abundant group of dietary antioxidants. Polyphenols reduce oxidative stress and inflammation and appear to be vasoprotective [24]. Carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene) occur in a wide variety of fruits and vegetables. Plasma carotenoids can be reliable quantified and are considered a valid biological marker for vegetable and fruit intake [10].
The relationship between plasma carotenoids and mortality has not been well characterized. Low total serum carotenoids were an independent predictor of mortality among older women, aged 70–79 years, living in the community in Baltimore, Maryland [29]. Low plasma alpha- and β-carotene concentrations were associated with a higher risk of death among men and women, aged 70–75 years, in a multicenter study in Europe [2].
We hypothesized that low plasma carotenoid concentrations were associated with increased mortality in older adults. In order to address this hypothesis, we examined the relationship between plasma carotenoid levels and mortality in the InCHIANTI study, a population-based cohort of older adults living in the community in Tuscany, Italy.
Methods
Study population
The study participants consisted of men and women, aged 65 and older, who participated in the Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, conducted in two small towns in Tuscany, Italy. The rationale, design, and data collection have been described elsewhere, and the main outcome of this longitudinal study is mobility disability [6]. Briefly, in August 1998, 1,270 people aged 65 years and older were randomly selected from the population registry of Greve in Chianti (pop. 11,709) and Bagno a Ripoli (pop. 4,704), and of 1,256 eligible subjects, 1,155 (90.1%) agreed to participate. Of the 1,155 participants, 1,043 (90.3%) participated in the blood drawing. During the eight-years of follow-up study, 310 participants died, 22 refused to participated to the study, and eight moved away from the area. Participants received an extensive description of the study and participated after written, informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethical Committee. Participants were evaluated again for a three-year follow-up visit from 2001–2003 (n = 926) and six-year follow-up visit from 2004–2006 (n = 844) at which time they underwent repeated phlebotomy and laboratory testing and assessment of physical performance. At the end of the field data collection, we collected data on mortality of the original InCHIANTI cohort, using data from the Mortality General Registry maintained by the Tuscany Region and the death certificates that are deposited immediately after the death at the Registry office of the Municipality of residence.
Demographic information and information on smoking and medication use were collected using standardized questionnaires. Smoking history was determined from self-report and dichotomized in the analysis as “current smoking” versus “ever smoked” and “never smoked”. Education was recorder as years of school. Average daily intakes of energy (kcal), was estimated using the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire, validated in the InCHIANTI population [27]. All participants were examined by a trained geriatrician, and diseases were ascertained according to standard, pre-established criteria and algorithms based upon those used in the Women’s Health and Aging Study for coronary heart disease, chronic heart failure, stroke and cancer [14]. Weight was measured using a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Mini-mental status examination (MMSE) was administered at enrollment [9].
Laboratory analyses
Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were immediately obtained and stored at −80°. Carotenoids have been shown to be stable for at least 15 years under these conditions [3]. Aliquots of plasma were shipped on dry ice to Dr. Semba’s laboratory for measurements of plasma carotenoids. The laboratory protocol was originated by Schleicher at the Centers for Disease Control in Atlanta and is described in better detail in the paper by Dorgan [5]. Carotenoids were measured using high performance liquid chromatography (HPLC) [22]. Total carotenoids were calculated as the sum of α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene in μmol/l. Within-run and between-run coefficients of variation, respectively, were 7.3 and 9.6% for α-carotene, 4.5 and 5.4% for β-carotene, 2.7 and 3.5% for β-cryptoxanthin, 2.6 and 7.1% for lutein, 6.2 and 6.8% for zeaxanthin, and 7.5 and 7.8% for lycopene.
Statistical analysis
Variables are reported as means (standard deviations) for normally distributed parameters or as percentages. Characteristics of subjects according to their vital status were compared using t test for continuous variables and chi-square tests for categorical variables. Plasma carotenoids was analyzed both as a continuous variable and as tertiles, where the tertiles for carotenoids were defined as 1.466, 1.467–1.971 > 1.971 μmol/l. Survival analysis (Kaplan Meier) and Cox proportional hazards models adjusted for age, sex, education, BMI, total energy intake, coronary heart disease, chronic heart failure, stroke and cancer were used to examine the relationship between plasma carotenoids and mortality (Table 3, model 1 and 2). Covariates for adjustment where selected by using the univariate Cox proportional hazards models as those that were associated with mortality with a P-value < 0.10 (Table 2). Kaplan Meier survival curves were compared using log-rank test. All analyses were performed using SAS (v. 8.2, SAS Institute, Inc., Cary, NC) with a statistical significance level set at P < 0.05.
Table 3.
Age- and sex-adjusted and multivariate relationship between carotenoids and other risk factors with all-cause mortality in older adults
| Characteristic | HR | 95% CI | P |
|---|---|---|---|
| Model 1 | |||
| Plasma carotenoids (μmol/l) | Pa for trend | 0.0005 | |
| Tertile 1 | 1.00 | – | – |
| Tertile 2 | 0.881 | 0.681–1.140 | 0.34 |
| Tertile 3 | 0.627 | 0.470–0.836 | 0.002 |
| Age (years) | 1.146 | 1.130–1.163 | <0.001 |
| Sex (female) | 0.648 | 0.517–0.812 | 0.0002 |
| Model 2 | |||
| Plasma carotenoids (μmol/l) | Pa for trend | 0.04 | |
| Tertile 1 | 1.00 | – | – |
| Tertile 2 | 0.985 | 0.731–1.327 | 0.92 |
| Tertile 3 | 0.716 | 0.514–0.995 | 0.04 |
| Age (years) | 1.152 | 1.129–1.175 | <0.001 |
| Sex (female) | 0.589 | 0.446–0.779 | 0.0002 |
| Education (years) | 1.152 | 1.129–1.175 | 0.18 |
| Body mass index (kg/m2) | 1.152 | 1.129–1.175 | 0.91 |
| Total energy intake (kcal/day) | 1.004 | 0.977–1.032 | 0.77 |
| Congestive heart failure | 1.310 | 1.137–1.510 | 0.0002 |
| Coronary heart disease | 1.072 | 0.643–1.787 | 0.79 |
| Cancer | 1.748 | 1.099–2.779 | 0.02 |
| Stroke | 1.561 | 1.318–1.850 | <0.0001 |
| Current smoking | 1.500 | 1.020–2.205 | 0.04 |
P for trend was obtained by considering carotenoids level as an ordinal variable
Table 2.
Univariate relationship between carotenoids and other risk factors with all-cause mortality in older adults
| Characteristic | HR | 95% CI | P |
|---|---|---|---|
| Plasma carotenoids (μmol/l) | Pa for trend | <0.0001 | |
| Tertile 1 | 1.00 | – | – |
| Tertile 2 | 0.712 | 0.520–0.977 | 0.04 |
| Tertile 3 | 0.445 | 0.318–0.623 | <0.0001 |
| Age (years) | 1.206 | 1.177–1.236 | <0.001 |
| Sex (female) | 0.747 | 0.572–0.976 | 0.03 |
| Education (years) | 0.867 | 0.822–0.915 | <0.001 |
| Body mass index (kg/m2) | 0.951 | 0.917–0.987 | 0.008 |
| Total energy intake (kcal/day) | 0.956 | 0.932–0.980 | 0.0004 |
| Current smoking | 1.098 | 0.747–1.612 | 0.64 |
| Congestive heart failure | 1.628 | 1.389–1.908 | <0.001 |
| Coronary heart disease | 1.409 | 0.762–2.603 | 0.27 |
| Cancer | 1.416 | 0.840–2.388 | 0.19 |
| Stroke | 2.311 | 1.726–3.095 | <0.0001 |
P for trend was obtained by considering selenium quartile level as an ordinal variable
Results
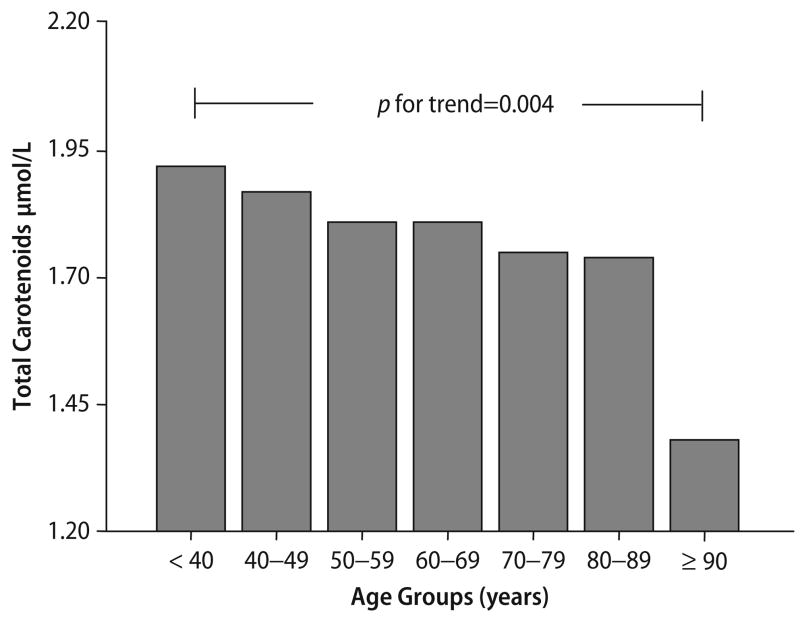
Mean total carotenoid concentration was 1.80 (0.69) μmol/l. The mean plasma level of total carotenoids with aging is significantly lower with aging (P-for trend = 0.0004) (Fig. 1). During the eight years of follow-up, 310 (29.7%) of participants died. The demographic and disease characteristics of the participants who were alive versus those who died during the eight years of follow up are shown in Table 1. Adults who died were older, more likely to be men, more likely to had a lower plasma total carotenoids and had higher baseline prevalence of congestive heart failure and stroke compared to those who survived over eight years of follow-up.
Fig. 1.
Distribution of the mean total carotenoids (μmol/l) across age groups
Table 1.
Characteristics the study population at enrollment and at eight-years follow-up
| Characteristic | All Participants (n = 1,043) | Survived (n = 733) | Died (n = 310) | Pb |
|---|---|---|---|---|
| Age (years) | 75.53 (7.42) | 72.92 (5.58) | 81.71 (7.58) | <0.001 |
| Sex (% female) | 591 (56.66) | 431 (58.80) | 160 (51.61) | 0.03 |
| Education (years)a | 5.32 (3.39) | 5.67 (3.28) | 4.47 (3.15) | 0.59 |
| Current smokers (%) | 140 (13.42) | 96 (13.10) | 94 (14.19) | 0.64 |
| Body mass index (kg/m2) | 27.47 (4.09) | 27.68 (4.06) | 26.88 (4.10) | 0.66 |
| Plasma carotenoids (μmol/l)a | 1.80 (0.69) | 1.88 (0.68) | 1.61 (0.69) | <0.001 |
| Total energy intake (kcal/day)a | 1,905 (567) | 1,946 (579) | 1,807 (523) | 0.14 |
| Coronary heart disease (%) | 46 (4.41) | 29 (3.96) | 17 (5.48) | 0.27 |
| Congestive heart failure (%) | 56 (5.37) | 16 (2.18) | 40 (12.90) | <0.0001 |
| Cancer (%) | 65 (6.23) | 41 (5.59) | 24 (7.74) | 0.19 |
| Stroke | 55 (5.27) | 19 (2.59) | 36 (11.61) | <0.0001 |
Mean (SD) for continuous variables or percentages as noted
Age-adjusted ANCOVA
Univariate Cox proportional hazards models were used to examine the relationship between plasma carotenoids concentrations at baseline and mortality. Age, sex, lower education, higher BMI, lower total energy, current smoking, congestive heart failure, and stroke, were significant risk factors for mortality (Table 2).
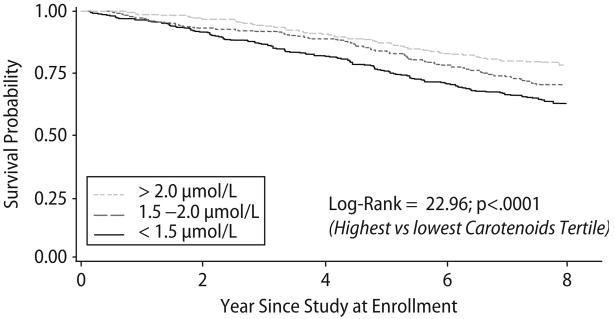
Eight year survival was lower in the lowest tertile compared with the highest tertile of total carotenoids (P < 0.0001 by Mantel-Haenszel chi-square) (Fig. 2). From the highest to the lowest tertile of total carotenoids 74 (21.2%), 105 (30.2%) and 131 (37.8%) participants died after 8 years of follow-up, respectively.
Fig. 2.
Kaplan Meier survival curves of older adults by tertiles of plasma carotenoids at enrollment
In Cox hazards models adjusted for age and sex, participants with the highest tertile of plasma carotenoids at enrollment had lower mortality compared to those in the lowest tertile (Hazards Ratio 0.63, 95%; CI 0.47–0.84; P = 0.002) (Table 3, Model 1). After adjusting for age, sex, education, smoking, body mass index, energy intake, and chronic diseases, adults in the highest tertile of plasma carotenoids at enrollment had lower mortality compared to those in the lowest tertile although the association was slightly attenuated (Hazards Ratio obtained by considering carotenoids level as an ordinal variable 0.81, 95%; CI 0.65–0.99; P for trend = 0.046) (Table 3, Model 2).
When IL-6 (log) was included in the model as covariates, in addition to the covariates already in model 2, adults in the highest tertile of plasma carotenoids at enrollment had lower mortality compared to those in the lowest tertile (Hazards Ratio obtained by considering carotenoids level as an ordinal variable 0.73, 95%; CI 0.52–0.98; P for trend = 0.042).
Discussion
The present study suggests that low total plasma carotenoids, a marker for fruit and vegetable intake, are an independent predictor of 8 year, all-cause mortality among older persons. These results are consistent with some previous findings. It was recently shown in the women’s health and aging study (WHAS) that total carotenoids are a strong predictor of mortality among older women living in the community [29]. A study of Dutch elderly men and women living in the city of Arnhem also showed that there was an inverse association between all-cause mortality and total serum carotenoids [32]. Recently, low plasma carotenoid levels were significantly associated with all-cause mortality in men but not in women in the EVA study [1]. We reported the results that total plasma carotenoid levels are significantly lower with aging, although our mean plasma levels were similar to those reported by other authors [26, 28].
The underlying biological mechanism by which carotenoids (or other substances found in fruits and vegetables) could contribute to an increased risk of mortality may be related to increased oxidative stress and inflammation. A low-grade inflammatory state is common among older adults and is characterized by increased concentrations of cytokines and acute phase proteins [7]. The age-related pro-inflammatory state is associated with the metabolic syndrome, atherosclerosis, cardiovascular disease, and cerebrovascular disease. Recent studies suggest that a high intake of fruits and vegetables reduces biomarkers of inflammation such as C-reactive protein, IL-6, and IL-18 and protects against cardiovascular disease [23]. The findings from the present study are also consistent with recent observations that the Mediterranean diet, characterized by a higher intake of fruits, vegetables, and nuts, is associated with decreased mortality in older men and women [21, 31].
An earlier study relating low serum β-carotene and 7-year all cause morality found that serum β-carotene was inversely related to markers of inflammation in men only [16]. However, more recent studies suggest that specific antioxidant nutrients mechanistically decrease levels of hydrogen peroxide and lipid peroxides to suppress up-regulation of IL-6 in disabled older women [33]. The present study showed a relationship between plasma total carotenoids and mortality in older persons in this cohort, even after adjustment for IL-6 at enrollment. This is consistent with the present findings that an increased level of plasma total carotenoids is protective against mortality independent of inflammatory markers.
In our study, the prevalence of smoking and alcohol use were much higher among men than among women. Plasma carotenoid levels are lower in smokers, as carotenoids are involved in the quenching of free radicals that are produced by smoking [4]. Alcohol consumption has been associated with lower serum β-carotene [11] and lutein/zeaxanthin concentrations [13].
A limitation of the present study is that the specific causes of death among the 310 participants who died have not yet been ascertained, and that this study reports only all-cause mortality. Among the 89 women who died in WHAS, the major causes of death were heart disease, cancer, stroke, infection, and chronic obstructive pulmonary disease [29]. Even if specific causes of death were known in the InCHIANTI study, currently the power to detect a relationship between plasma carotenoid levels and specific causes of mortality may be limited because of the sample size of deaths within specific categories. Causality is difficult to prove in an observational study such as this, and there could be unmeasured differences between the various carotenoid quantities that could explain the results.
Previous observational studies have examined the relationship between serum β-carotene, one of the six major dietary carotenoids, and mortality. The relationship between circulating β-carotene concentrations and mortality has not been consistent [8]. A pooled analysis of nine cohorts also found inconclusive evidence of a reduction in coronary heart disease. This was attributed to difficulty in adjusting for nondietary confounding factors [20]. In the Japan Collaborative Cohort Study of 39,140 people, ages 40–79, a lower risk of death from lung cancer was significant or of marginal significance for the highest serum concentrations of α-carotene, β-carotene, β-cryptoxanthin, zeaxanthin/lutein, canthaxanthin, total carotenoids, vitamin E, and total cholesterol, when compared to the lowest concentrations [18]. In a study involving nearly 12 years of follow-up, high levels of alpha-carotene, β-carotene, and lycopene were associated with a significantly decreased risk of cardiovascular mortality [17].
In conclusion, these results suggest that low total plasma carotenoids are an independent predictor of mortality among older adults living in the community. This work further shows the important relationship between antioxidant nutrients and mortality among older persons. Further work is needed to identify the sub-groups in the population which might be helped by interventions that reduce mortality in older populations.
Acknowledgments
This work was supported by National Institute on Aging Contracts N01-AG-916413, N01-AG-821336, N01-AG-5–0002, and NIA Grant R01 AG027012. This research was supported in part by the Intramural Research Program, National Institute on Aging, NIH.
References
- 1.Akbaraly TN, Favier A, Berr C. Total plasma carotenoids and mortality in the elderly: results of the epidemiology of vascular ageing (EVA) study. Br J Nutr. 2008;29:1–7. doi: 10.1017/S0007114508998445. [DOI] [PubMed] [Google Scholar]
- 2.Buijsse B, Feskens EJ, Schlettwein-Gsell D, Ferry M, Kok FJ, Kromhout D, de Groot LC. Plasma carotene and α-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the survey in Europe on nutrition and the elderly, a concerted action. Am J Clin Nutr. 2005;82:879–886. doi: 10.1093/ajcn/82.4.879. [DOI] [PubMed] [Google Scholar]
- 3.Comstock GW, Alberg AJ, Helzlsouer KJ. Reported effects of long-term freezer storage on concentrations of retinol, β-carotene, and α-tocopherol in serum or plasma summarized. Clin Chem. 2004;39:1075–1078. [PubMed] [Google Scholar]
- 4.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, Packer L. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increased γ-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 2003;77:160–166. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 5.Dorgan JF, Boakye NA, Fears TR, Schleicher RL, Helsel W, Anderson C, Robinson J, Guin JD, Lessin S, Ratnasinghe LD, Tangrea JA. Serum carotenoids and α-tocopherol and risk of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1276–1282. [PubMed] [Google Scholar]
- 6.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher AE, Breeze E, Shetty PS. Antioxidant vitamins and mortality in older persons: findings from the nutrition add-on study to the medical research council trial of assessment and management of older people in the community. Am J Clin Nutr. 2003;78:999–1010. doi: 10.1093/ajcn/78.5.999. [DOI] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Food Nutrition Board. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. National Academy of Sciences; Washington, DC: 2000. [Google Scholar]
- 11.Galan P, Viteri FE, Bertrais S, Czernichow S, Faure H, Arnaud J, Ruffieux D, Chenal S, Arnault N, Favier A, Roussel AM, Hercberg S. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. 2005;59:1181–1190. doi: 10.1038/sj.ejcn.1602230. [DOI] [PubMed] [Google Scholar]
- 12.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004;160:1223–1233. doi: 10.1093/aje/kwh339. [DOI] [PubMed] [Google Scholar]
- 13.Gruber M, Chappell R, Millen A, La-Rowe T, Moeller SM, Iannaccone A, Kritchevsky SB, Mares J. Correlations of serum lutein + zeaxanthin: findings from the third national health and nutrition examination survey. J Nutr. 2004;134:2387–2394. doi: 10.1093/jn/134.9.2387. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The women’s health and aging study: health and social characteristics of older women with disability. NIH Publication No. 95–4009. National Institute on Aging; Bethesda: 1995. [Google Scholar]
- 15.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367:320–326. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 16.Hu P, Reuben DB, Crimmins EM, Harris TB, Huang MH, Seeman TE. The effects of serum β-carotene concentration and burden of inflammation on all-cause mortality risk in high-functioning older persons: Mac-Arthur studies of successful aging. J Gerontol. 2004;8(59A):849–854. doi: 10.1093/gerona/59.8.m849. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Kurata M, Suzuki K, Hamajima N, Hishida H, Aoki K. Cardiovascular disease mortality and serum carotenoid levels: a Japanese population-based follow-up study. J Epidemiol. 2006;16:154–160. doi: 10.2188/jea.16.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Wakai K, Suzuki K, Tamakoshi A, Seki N, Ando M, Nishino Y, Kondo T, Watanabe Y, Ozasa K, Ohno Y JACC Study Group. Serum carotenoids and mortality from lung cancer: a case-control study nested in the Japan collaborative cohort study. Cancer Sci. 2003;94:57–63. doi: 10.1111/j.1349-7006.2003.tb01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 20.Knekt P, Ritz J, Pereira MA, O’Reilly EJ, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Rimm EB, Ascherio A. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80:1508–1520. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- 21.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, van Staveren WA. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women. JAMA. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 22.van Lettow M, Harries AD, Kumwenda JJ, Zijlstra EE, Clark TD, Taha TE, Semba RD. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis. 2004;4:61. doi: 10.1186/1471-2334-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 24.Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16:77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 25.McEligot AJ, Yang S, Meyskens FL. Redox regulation by intrinsic species and extrinsic nutrients in normal and cancer cells. Annu Rev Nutr. 2005;25:261–295. doi: 10.1146/annurev.nutr.25.050304.092633. [DOI] [PubMed] [Google Scholar]
- 26.Michelon E, Blaum C, Semba RD, Xue QL, Ricks MO, Fried LP. Vitamin and carotenoid status in older women: associations with the frailty syndrome. J Gerontol A Biol Sci Med Sci. 2006;61:600–607. doi: 10.1093/gerona/61.6.600. [DOI] [PubMed] [Google Scholar]
- 27.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 28.Polidori MC, Stahl W, Eichler O, Niestroj I, Sies H. Profiles of anti-oxidants in human plasma. Free Radic Biol Med. 2001;30:456–462. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 29.Ray AL, Semba RD, Walston J. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women’s health and aging studies. J Nutr. 2006;136:172–176. doi: 10.1093/jn/136.1.172. [DOI] [PubMed] [Google Scholar]
- 30.Riboli E, Norat T. Epidemiologic evidence of the protective effect or fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(suppl):559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 31.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 32.Waart FG, Schouten EG, Stalenhoef AFH, Kok FJ. Serum carotenoids, α-tocopherol and mortality risk in a prospective study among dutch elderly. Int J Epidemiol. 2001;30:136–143. doi: 10.1093/ije/30.1.136. [DOI] [PubMed] [Google Scholar]
- 33.Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, Guralnik J, Fried LP. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]