Abstract
Adoptive dendritic cell (DC) immunotherapy provides a useful experimental tool to evaluate immunoregulation in vivo, and has previously been used successfully to enhance host resistance in a variety of experimental models of leishmaniasis. Here, we used this approach to identify IL-6 and IL-12 as critical cytokines that cooperate to mediate host protection to Leishmania donovani but act independently to regulate expansion of IL-10+ CD4+ T cells, shown here for the first time to be associated with this infection. Adoptive transfer of LPS-activated bone marrow-derived DC (BMDC) from wild-type mice was therapeutically beneficial and led to enhanced resistance as measured by spleen parasite burden. In contrast, IL-6- or IL-12p40-deficient BMDC had no protective benefit, indicating that production of both cytokines was essential for the therapeutic efficacy of DC. IL-10 production by CD25 FoxP3-IL-10+ CD4+ T cells is a strong correlate of disease progression, and BMDC from wild-type mice inhibited expansion of these cells. Strikingly, IL-12-deficient BMDC could also inhibit the expansion of this T cell population, whereas IL-6-deficient BMDC could not, indicating that IL-6 played a key role in this aspect of DC function in vivo. Breadth of cytokine production is thus an important factor when considering strategies for DC-based interventions.
Keywords: Leishmania, dendritic cells, immunotherapy, cytokines
Introduction
Dendritic cells (DC5) have the task of linking innate and adaptive immunity [1, 2]. DC respond to pathogen-derived and environmental cues using a diverse repertoire of pattern recognition molecules [3, 4], and such recognition in turn regulates the capacity of DC to process and present antigens, the expression of essential costimulatory ligands and diversity of cytokine production [5-7]. This process of maturation empowers DC with a unique capacity to prime naïve CD4+ T cells and to a large extent dictate the differentiation pathway they follow. Although a general consensus is yet to emerge on the molecular basis for specialization of function in DC, a variety of experimental evidence clearly demonstrates that DC either at various stages of differentiation and / or maturation and belonging to distinct lineages may preferentially induce CD4+ T cells with distinct cytokine profiles or function [8-11]. Not surprisingly, therefore, DC are viewed as critical to determining the outcome of infectious diseases. Although DC function in infectious disease models has been most commonly studied at the onset of infection, DC are also likely to play an important role in regulating immunity at later stages of the disease process, as evident from the success of DC-based immunotherapy in experimental and clinical studies [12-17].
Infection with Leishmania donovani, the causative agent of visceral leishmaniasis, results in high levels of systemic IL-10 in both human [18-20] and in experimental models [21, 22]. Experimental studies indicate that IL-10 has pleiotropic effects in leishmaniasis which may all contribute to its potent immunosuppressive activity, including suppression of macrophage activation [23], dendritic cell migration [22], and protective Th1 responses [24]. In spite of the importance of IL-10 in this disease, and the clear indications that direct or indirect targeting of IL-10 production may have benefits for the management of human disease [21] the extent to which CD4+ T cells contribute to IL-10 production in visceral leishmaniasis (VL) is unclear, and the potential of DC-based therapy to modulate the expansion or function of such CD4+ cells is untested.
In this report, we have made use of the exquisite sensitivity of the L. donovani model of VL to DC-based therapy to demonstrate that effective DC therapy requires the production of both IL-6 and IL-12p40 to influence the outcome of infection. In addition, we have for the first time identified that the majority of the IL-10-producing CD4+ T cells that appear during the chronic phase of L. donovani infection lack CD25 and FoxP3 expression. Expansion of these IL-10+ CD4+ T cells is also inhibited by DC transfer but in a distinct manner, requiring IL-6 production but not IL-12p40. These data indicate a complex interplay between these two cytokines for regulating host resistance and CD4+ T cell function.
Results
DC therapy reduces the frequency of IL-10-secreting CD4+ T cells
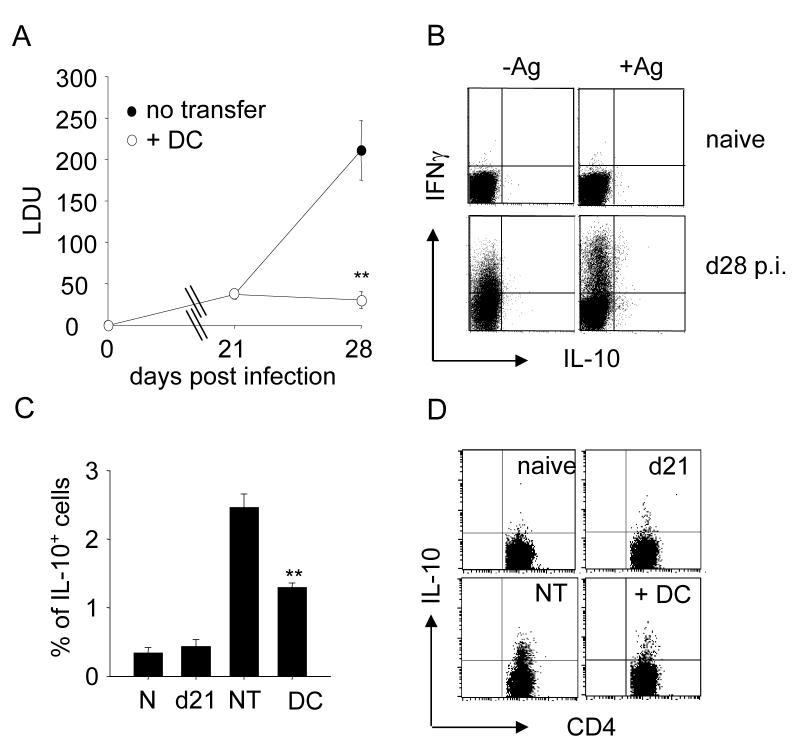
In B6 mice infected with L. donovani, parasite burden in the spleen remains low over the first 1-2 weeks post infection (p.i.), but increases significantly from weeks 3-4 onwards, eventually resulting in a chronic state of infection in this organ [22]. This increase in parasite burden can be largely curtailed by adoptive transfer of LPS-activated bone marrow derived dendritic cells (BMDC) (Fig. 1A and [22] ). In addition to examining the impact of DC transfer of disease outcome, we also wished to examine its effect on one of the major correlates of disease progression, namely IL-10 production. IL-10 plays a major role in governing host resistance to L. donovani infection, as demonstrated by infection of IL-10-/- mice and by IL-10 receptor blockade [21, 22, 24]. Although IL-10 mRNA is associated with a range of cell types during infection, including CD4+ and CD8+ T cells, B cells, NK cells, CD11chi DC and F4/80+ macrophages, on a cell per cell basis CD4+ T cells contain IL-10 mRNA in greatest abundance (Maroof et. al. in preparation). Given the well-documented ability of LPS-activated DC to direct CD4+ T cell differentiation [25], we hypothesized that DC transfer may be responsible for redirecting CD4+ T cell differentiation away from IL-10 production. Direct ex vivo analysis of CD4+ T cells was insufficiently sensitive to identify any IL-10 producing T cells. Therefore to test this hypothesis, we performed intracellular cytokine staining on CD4+ T cells that had been restimulated 6 hours previously with L. donovani amastigote-pulsed BMDC. The antigen specificity of this assay is demonstrated in Fig. 1B. CD4+ T cells from naïve mice with or without in vitro addition of antigen did not produce detectable levels of either IL-10 or IFNγ. As previously reported [26], IFNγ could be readily detected ex vivo in cells isolated from infected mice, though the frequency and magnitude of the IFNγ response was considerably enhanced by antigen re-stimulation in vitro. In contrast, IL-10 was only demonstrable after antigen restimulation. We then determined the frequency of antigen-specific IL-10-producing CD4+ T cells in the presence or absence of DC transfer. As shown in Fig. 1 C, D., IL-10+ CD4+ T cells did not increase in frequency over the first 21 days p.i. In contrast, between day 21 and day 28 p.i, the frequency of IL-10-producing CD4+ T cells increased 4-5-fold (Fig. 1C, D). Adoptive immunotherapy with LPS-activated BMDC was accompanied by a significant reduction in the frequency of CD4+ IL-10+ T cells at day 28 p.i. compared to that seen in mice not receiving BMDC (Fig. 1B, C). These data demonstrate for the first time in this model that CD4+ T cells are an increasing source of IL-10 during the development of chronic infection and that successful DC-based therapy is associated with curtailed expansion of IL-10-producing CD4+ T cells.
Figure 1. BMDC therapy reduces parasite burden and blocks the expansion of IL-10-secreting CD4+ T cells.
(A) Day 21 infected B6 mice were adoptively transferred with 106 LPS- activated BMDC i.v. (open circle) or received no treatment (closed circle). Graph shows splenic parasite burden before and after therapy. (B) Spleen cells from naïve or d28 infected mice were restimulated in vitro for 3h with BMDC (-Ag) or BMDC pre-pulsed with L. donovani antigen (+Ag). After a further 3h in BFA, cells were stained for intracellular IL-10 and IFNγ. (C) Splenocytes from naïve (N) mice, mice at d21 p.i., and mice at d28 p.i. that received LPS-activated BMDC (DC) or not transfer (NT) were restimulated for 6h with Ag-pulsed BMDC and then stained with anti-CD3, anti-CD4 and anti-IL-10 antibodies. Data represent mean of % ± SE of IL-10-secreting CD4+ T-cells (n=5; one of 3 experiments). (D) Representative plots show staining for IL-10 and CD4 on CD3+CD4+ gated cells. ** p<0.01 vs. no transfer at d28
IL-10- secreting CD4+ T cells in experimental VL
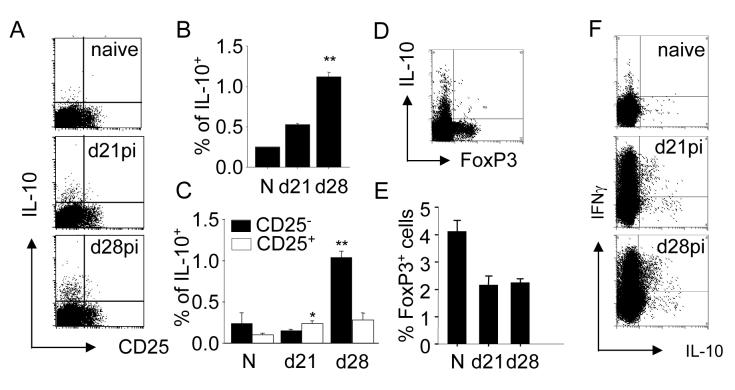
As IL-10-producing CD4+ T cells have not previously been characterized during L. donovani infection, we performed further phenotypic analysis to identify the nature of these cells more precisely. As shown in Fig. 2A-D, the vast majority of L. donovani-specific IL-10-producing T cells, responsible for the increased frequency of IL-10 producing cells observed at day 28 p.i., did not express CD25. Although there was a slight but significant increase in the frequency of IL-10+ CD25+ CD4+ T cells at day 21 p.i. compared to naïve mice (Fig. 2A, D), the frequency of such cells did not subsequently increase. Furthermore at day 28 p.i., almost all IL-10+ CD4+ T cells were negative for expression of the transcription factor FoxP3 (Fig. 2E). Furthermore, the frequency of FoxP3+CD4+cells decreased as infection progressed suggesting that they were effectively diluted out by the expansion of FoxP3-CD4+ T cells (Fig. 2F). These data strongly suggest that transition into a state of chronic infection is accompanied by an increase in frequency of IL-10-secreting CD4+ CD25- T cells rather than an increase in frequency of natural CD4+ CD25+ FoxP3+ Treg cells [27].
Figure 2. L. donovani infection preferentially expands IL-10+IFNγ-CD25- and IL-10+ IFNγ+CD25- CD4+ cells.
(A) Splenocytes from naïve (N), d21 and d28 infected C57BL/6 mice were restimulated for 6h and stained with fluorochrome-conjugated anti-CD4, anti-CD25, and anti-IL-10 antibodies. Representative plots show staining for CD25 and IL-10 of CD4+ gated cells. (B) Mean of % ± SE of total IL10+ producing CD4+ T cells at different time points during infection. (C) Mean of % ± SE of IL-10+ CD25- CD4+ T cells (black bar) and IL-10+ CD25+ CD4+ T cells (open bars) (D) Splenocytes from d28 infected mice were stained with fluorochrome-conjugated anti-IL-10 and anti-FoxP3 antibodies. (E) Mean of % ± SE of FoxP3+CD4+ T cells during infection. (F) Splenocytes as in (A) were stained with fluorochrome-conjugated anti-IL10 and anti-IFNγ. Representative plots show staining of gated CD4+ T cells. (B), (C), (D): n=5, data represent one of two experiments. * p<0.05, ** p<0.01
Some IL-10-producing CD4+ T cells have been described to secrete IFNγ, whereas IFNγ secretion has not been reported for CD4+CD25+ Treg cells [8]. As shown in Fig. 2G, and consistent with previous clinical and experimental data, infection with L. donovani was associated with a significant expansion in the frequency of CD4+ IFNγ+ IL-10- T cells, indicating that conventional Th1 responses are generated in L. donovani infected mice. Of the IL-10-secreting CD4+ T cells observed at day 28 p.i., 39.3 ± 2.86% co-expressed IFNγ. In contrast, we could not detect CD4+ T cells co-expressing IL-10 and IL-4 (data not shown). Collectively, these data indicate that the predominant IL-10-producing CD4+ T cell subsets that expand in the spleen at a time corresponding to increased parasite burden are a mixture of antigen-specific IL-10-producing and antigen-specific IL-10 and IFNγ co-producing CD4+ T cells.
IL-6 and IL-12 play critical but distinct roles in the function of DC
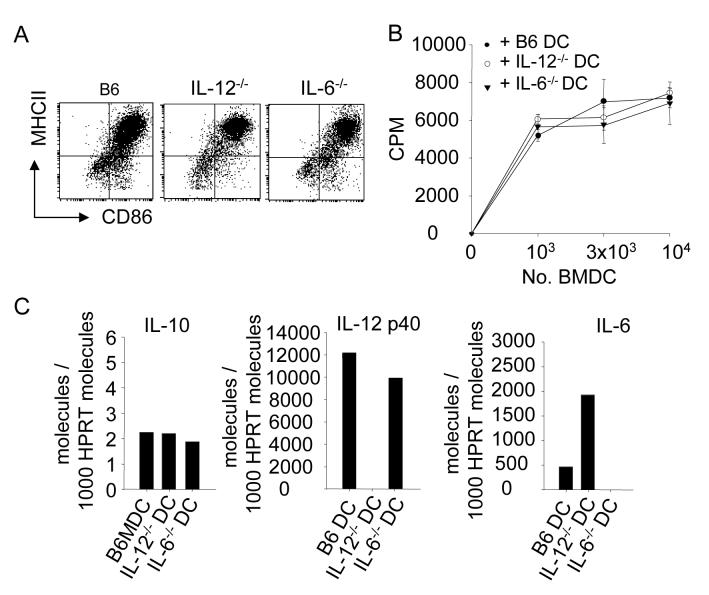
The IL-12p40 subunit of IL-12 and IL-23 has been shown in vitro and in vivo to be critical for many functions of activated DC [28, 29]. More recently, in vitro studies have implicated IL-6 as a key cytokine in regulating the development of CD4+ CD25+ Treg cells [30, 31]. In order to establish whether these cytokines played a part in the in vivo function of DC described here, we generated BMDC from IL-12p40-/- and IL-6-/- mice for use in adoptive DC therapy. LPS-activated BMDC from these mice had the same activation status as BMDC from wild-type mice, as judged by the expression of MHC II molecules and CD86 (Fig. 3A) and they had equivalent capacity to stimulate primary allogeneic MLR (Fig. 3B). Similarly, as revealed by real time RT-PCR, wild-type, IL-12p40-/- and IL-6-/-BMDC accumulated identical amounts of IL-10 mRNA (Fig. 3C) and had IL-12p40 and IL-6 mRNA accumulation as expected from their genotype (Fig. 3C).
Figure 3. Characterization of LPS-activated BMDC from wild type, IL-12-/-, and IL-6-/- mice.
(A) BMDC from wild type, IL-12-/-, IL-6-/- mice were activated with 1μg/ml LPS overnight and stained with anti-CD11c, anti-MHCII, and anti-CD86 antibodies. Plots show the expression of CD86 and MHCII for the three different BMDC. (B) The capacity of various wild type and knock-out BMDC to mount a mixed lymphocyte reaction was tested. Data show mean 3HTdR incorporation ± SE of triplicates for each group. (C) IL-10, IL12p40, and IL-6 mRNA expression by LPS-activated BMDC was measured by real-time PCR. Data show the number of molecules for each cytokine/ 1000 HPRT molecules. One of three experiments is shown. ** p <0.01
As shown in Fig. 4A, both IL-6 and IL-12p40 appeared to be essential and non-redundant cytokines critical for effective DC therapy in this model. This represents the first demonstration of a therapeutic role for IL-6 in this infectious disease model. Furthermore IL-6, but surprisingly not IL-12p40, appeared to play a critical role in the ability of DC to regulate the development of antigen-specific IL-10-producing CD4+ T cells in vivo. Thus, whereas transfer of either wild-type or IL-12p40-/- DC was accompanied by a significant and equivalent reduction in the frequency of CD4+ IL-10+ T cells, BMDC derived from IL-6-/- mice were totally without activity (Fig. 4B). This requirement for IL-6 production by BMDC in order for them to be able to inhibit the expansion of IL-10-producing CD4+ T cells was equally as striking for both the IL-10+ IFNγ+ (Fig. 4C) and the IL-10+ IFNγ- (Fig. 4D) CD4+ T cell populations. Thus, the capacity of BMDC to produce IL-6 and not IL-12p40 appears critical to their capacity to influence the expansion of IL-10-producing CD4+ T cell populations in this infection model. Collectively, our data indicate that in the absence of either IL-6 or IL-12p40, BMDC fail to exert a host protective effect and furthermore indicate that host protection is not strictly correlated with alterations in the frequency of IL-10-producing CD4+ T cells.
Figure 4. IL-6 production by BMDC is essential for inhibition of IL-10+ CD4+ T cells, but both IL-6 and IL-12p40 are essential to promote host resistance.
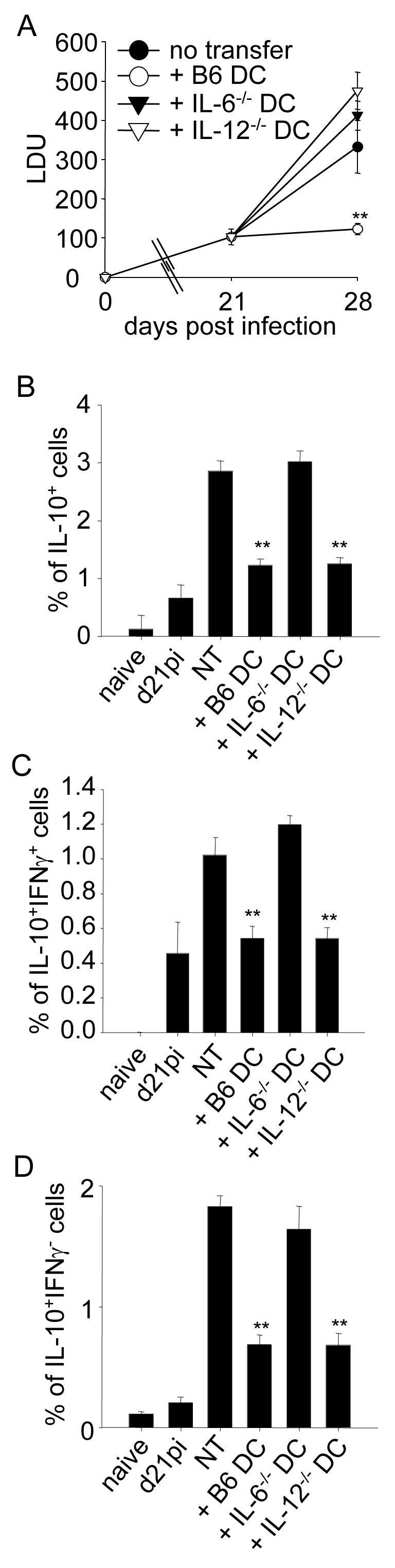
(A) Splenic parasite burden before or after adoptive transfer of LPS-activated BMDC from C57BL/6, B6.IL-12-/- and B6.IL-6-/- mice. (B) Splenocytes from naïve control and infected mice were restimulated in vitro for 6h, and stained with anti-CD3, anti-CD4, anti-IFNγ and anti-IL-10. Graph shows mean of % ± SE of total IL-10 producing CD4+ T cells. (C) As in (B); graph shows mean of % ± SE of IL-10+ IFNγ+ CD4+ T cells. (D) As in (B); graph shows mean of % ± SE of IL-10+ IFNγ - CD4+ T cells. (A), (B), (C): n=5, data show one of two experiments. * p<0.05, ** p < 0.001.
Discussion
The results presented in this manuscript illustrate a previously unrecognized role for IL-6 in protection against L . donovani infection induced by DC therapy. Furthermore, we show that whereas IL-10-producing CD4+ T cells increase in frequency during the critical time period associated with the development of chronic disease, IL-6-dependent inhibition of this response, mediated by DC transfer, is not in itself sufficient to account for the altered level of host resistance.
We and others have previously reported that adoptive immunotherapy with BMDC provides a potent means of increasing host resistance to L. donovani infection [12, 14, 22, 32] with in our hands, transfer of LPS-activated BMDC at day 21 p.i completely inhibiting the normal increase in splenic parasite burden that occurs by day 28 p.i. (Fig. 2 A and [22]). The requirements for cytokine production by DC in these transfer studies has only occasionally been formally examined. We now demonstrate that for therapeutic benefit, transferred BMDC must produce both IL-6 and IL-12p40, with deficiency in either cytokine being sufficient to totally abrogate the function of LPS-activated BMDC in this model. In contrast to the numerous reports on the role of IL-12p40 in host resistance to this infection [4, 12, 13, 33, 34], these data are to our knowledge the first to implicate IL-6 as a important mediator of DC function in L. donovani infection. Wilson and colleagues [32] reported the ability of BMDC from C57BL/6 and B6.IL-12p40-/- mice to transfer host protection into IL-12p40-sufficient or IL-12p40-deficient recipients infected with L. chagasi [32]. BMDC from wild type mice could readily reconstitute host protection in IL-12p40-deficient mice, indicating that DC alone could provide sufficient IL-12p40 in the correct cellular context for the induction of appropriate effector function. Strikingly, these investigators also observed that transfer of IL-12p40-deficient BMDC into wild type mice led to disease exacerbation, suggesting for the first time that cytokine-deficient DC might play a negative regulatory role in vivo. Although during multiple experiments, we have consistently observed that IL-12p40-deficient BMDC have no host protective role, we have not observed the exacerbatory effect reported by Wilson [32]. Nevertheless there are significant differences in the protocols used that may account for this discrepancy, including timing, route, dose and activation status of the BMDC between the two studies.
Administration of antigen-pulsed BMDC plus IL-12 has also recently been shown to have therapeutic benefit in a model of chronic L. amazonenesis infection [35]. Effective therapy was associated with the enhanced expression of T-bet, IL-12Rβ2 and IFNγ, indicative of an increased Th1 response. In spite of this increased Th1 response, however, antigen-pulsed BMDC together with IL-12 had no impact on parasite burden in the tissues of treated mice. The frequency of IL-10+ CD4+ T cells pre and post intervention was not documented in this study, however. Further subtleties in DC function are also likely to exist. For example, in the studies of Moll and colleagues, IL-12p40 production by Langerhans cells was essential for their capacity to vaccinate mice [13], though this was not the case for BMDC activated by CpG [36]. Although CpG oligodeoxynucleotides could stimulate BMDC to allow effective vaccination, other maturation stimuli such as TNFα and anti-CD40 could not. Our studies presented here and recent reports linking IL-10 induction to vaccine efficacy in the L. major infection model [37] suggest that it might be of value to determine whether BMDC matured with these different stimuli have similar capacity to produce IL-6. Indeed, it is tempting to speculate that the extreme potency of CpG oligodeoxynucleotides for therapy [38] and as a vaccine adjuvant [4] in murine leishmaniasis lies not just in their potency at inducing IL-12p40, but also in their ability to activate DC for IL-6 production [39] and hence to inhibit expansion of IL-10-producing CD4+ T cells.
An important corollary of the current study is the formal identification of the subsets of CD4+ T cells that produce IL-10 during the course of L. donovani infection. CD25+CD4+ Treg cells that develop in the thymus under the control of the fork-winged helix family transcription factor Foxp3, have been shown to be a central factor in the maintenance of chronic infection by Leishmania major, operating via IL-10 production [26, 40] and to contribute to immune suppression during malaria infections [41]. Although CD25+CD4+ Treg producing IL-10 could be observed in L. donovani infected mice (Fig. 2), the subset of IL-10-producing CD4+ T cells in which changes in frequency are most closely associated with the transition of the spleen into a state of chronic infection with L. donovani are clearly CD25-FoxP3-. What remains to be determined and is the focus of current investigation is whether these cells have active regulatory function or function by inhibiting host resistance via more direct means e.g. via IL-10-dependent inhibition of macrophage activation. However, in the absence of experimental tools to selectively deplete this subset of CD4+ T cells in vivo or to selectively block IL-10 production by them, we cannot formally rule out at the possibility that their expansion is a consequence rather than a cause of chronic L. donovani infection. Indeed, the lack of association between the therapeutic efficacy of BMDC and their capacity to inhibit the expansion of IL-10+ CD4+ T cells indicates that reducing the frequency of IL-10+ CD4+ T cells in the absence of other, as yet unidentified consequences of BMDC transfer is not in itself sufficient to enhance host protection. TGFβ has been associated with immunosuppression in visceral leishmaniasis [42, 43], though at least for infection with L. donovani, its impact on parasite burden and IFNγ-dependent host resistance are marginal compared to IL-10 [44,45] and we have observed no differences in tissue TGFβ levels following adoptive transfer of BMDC (Stager, unpublished). Further studies will nevertheless be required to dissect the full in vivo consequences of BMDC transfer including the longevity of their effect and their potential impact on additional cell populations that may produce a broader variety of immunosuppressive cytokines.
In summary, therefore, our data implicates DC-derived IL-6 as a key cytokine regulating the therapeutic efficacy of adoptively transferred DC against L. donovani infection, and as a gatekeeper cytokine for controlling the expansion of CD25-FoxP3- IL-10+ CD4+ T cells in vivo.
Material and Methods
Mice and Parasites
6-8 weeks old C57BL/6 (B6) and BALB/c were obtained from Charles River (Margate, UK); B6 background IL-12p40-deficient mice (B6.IL-12p40-/-) were bred under specific-pathogen-free conditions at the London School of Hygiene and Tropical Medicine. Bone marrow from B6 background IL-6 deficient mice (B6.IL-6-/) was a gift from Dr. Manfred Kopf. Mice were killed by cervical dislocation, and splenic parasite burdens were determined from Giemsa stained impression smears. Data are presented as Leishman Donovan Units (LDU) [22]. All animal procedures were approved by the LSHTM Animal Procedures Ethics Committee and performed under UK Home Office license.
Adoptive transfer of bone marrow —derived dendritic cells (BMDC)
BMDC were generated as described elsewhere [22]. To induce maturation, 1μg/ml LPS was added at day 9 for the last 24h of culture. DC maturation was assessed based on MHCII and CD86 expression. Prior to injection, BMDC were enriched by magnetic cell sorting (MACS) using anti-CD11c microbeads (Miltenyi Biotech, Bergisch Galbach, Germany), following manufacturers’ instructions. Purity ranged between 80-85%. 106 LPS-activated BMDC were injected i.v. and splenic parasite loads determined 1 week later.
Mixed lymphocytes reaction (MLR)
CD4+ T cells from BALB/c mice were purified by MACS using anti-CD4 microbeads (Miltenyi Biotech, Bergisch Galbach, Germany) following manufacturers’ instructions. Purified CD4+ T cells were then plated in flat bottom 96 well plates at a concentration of 3×105 cells per well and co-cultured with LPS-activated BMDC from B6 and BALB/c respectively for 96h at the indicated ratios. BMDC were previously irradiated with 2000 rad. 0.5μCi 3H-thymidine was added to each well for the last 8 hours of culture. Cells were then harvested, and 3H-thymidine incorporation measured.
Flow cytometry
LPS-activated BMDC were labeled with allophycocyanin(APC)-conjugated anti-CD11c, phycoerythrin(PE)-conjugated anti-I-A/I-E (M5/117), and fluorescein isothiocyanate (FITC)-conjugated anti-CD86. Mice were killed a week after adoptive transfer. Splenocytes were restimulated in vitro for 3 hours at 37°C in the presence of BMDC or BMDC previously pulsed with 106 paraformaldehyde-fixed amastigotes. Brefeldin A (BFA; 10 µg/ml) was then added for a further 3 hours. Cells were then stained with biotinylated anti-CD8, APC-Cy7-conjugated anti CD8, biotinylated anti-CD4, PerCP-conjugated anti-CD4, Pacific Blue-conjugated anti-CD3, FITC-conjugated anti-CD25, FITC-conjugated anti-FoxP3, APC-conjugated anti-IFNγ, PE-conjugated anti-IL-10, APC-conjugated anti-IL-4. APC- and PE-labeled isotype controls were used to set statistical markers. Samples were analyzed using a FACScan (Becton Dickinson, Mountain View, CA) and Cell Quest software. 250,000 cells were analyzed for each individual mouse (n=5 per group).
Real-time reverse-transcription PCR
RNA was isolated from LPS-activated BMDC with an RNeasy Kit with on-column DNase digestion (Qiagen, Crawley, UK), according to manufacturers’ instructions. RNA was reverse transcribed into cDNA as previously described [22]. Oligonucleotides (5′→ 3′) used for the specific amplification of IL-10, IL-12p40 and HPRT were as described in [22]. For IL-6, the sequences were: ACAACCACGGCCTTCCCTACTT (sense) and CACGATTTCCCAGAGAACATGTG (antisense) [46]. The number of cytokines and HPRT cDNAs in each sample was calculated by real-time PCR with a QuantiTect SYBR green master mix (Qiagen) and a LightCycler (Roche, Penzberg, Germany), according to the manufacturers’ instruction. Standard curves were defined with known amounts of IL-10, IL-6, IL-12, and HPRT cDNA. Number of molecules for each cytokine per 1000 HPRT molecules were calculated.
Statistics
Statistical analysis was performed using a paired Student t test. p < 0.05 was considered significant.
Acknowledgements
The authors thank the staff of the Biological Services Facility for excellent animal husbandry.
Abbreviations used
- BMDC
bone marrow derived dendritic cell
- DC
dendritic cell
- p.i.
post-infection
- VL
visceral leishmaniasis
References
- [1].Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004;16:21–25. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [2].Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- [4].Shah JA, Darrah PA, Ambrozak DR, Turon TN, Mendez S, Kirman J, Wu CY, Glaichenhaus N, Seder RA. Dendritic cells are responsible for the capacity of CpG oligodeoxynucleotides to act as an adjuvant for protective vaccine immunity against Leishmania major in mice. J Exp Med. 2003;198:281–291. doi: 10.1084/jem.20030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- [7].Edwards AD, Manickasingham SP, Sporri R, Diebold SS, Schulz O, Sher A, Kaisho T, Akira S, Reis e Sousa C. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- [8].Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–83. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- [9].Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–816. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [10].Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- [11].Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- [12].Ahuja SS, Reddick RL, Sato N, Montalbo E, Kostecki V, Zhao W, Dolan MJ, Melby PC, Ahuja SK. Dendritic cell (DC)-based anti-infective strategies: DCs engineered to secrete IL-12 are a potent vaccine in a murine model of an intracellular infection. J Immunol. 1999;163:3890–3897. [PubMed] [Google Scholar]
- [13].Berberich C, Ramirez-Pineda JR, Hambrecht C, Alber G, Skeiky YA, Moll H. Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J Immunol. 2003;170:3171–3179. doi: 10.4049/jimmunol.170.6.3171. [DOI] [PubMed] [Google Scholar]
- [14].Ghosh M, Pal C, Ray M, Maitra S, Mandal L, Bandyopadhyay S. Dendritic cell-based immunotherapy combined with antimony-based chemotherapy cures established murine visceral leishmaniasis. J Immunol. 2003;170:5625–5629. doi: 10.4049/jimmunol.170.11.5625. [DOI] [PubMed] [Google Scholar]
- [15].Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, Kunkel SL. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 2005;105:3588–3595. doi: 10.1182/blood-2004-08-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- [17].Wallis RS. Reconsidering adjuvant immunotherapy for tuberculosis. Clin Infect Dis. 2005;41:201–208. doi: 10.1086/430914. [DOI] [PubMed] [Google Scholar]
- [18].Ghalib HW, Piuvezam MR, Skeiky YA, Siddig M, Hashim FA, el-Hassan AM, Russo DM, Reed SG. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Karp CL, el-Safi SH, Wynn TA, Satti MM, Kordofani AM, Hashim FA, Hag-Ali M, Neva FA, Nutman TB, Sacks DL. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sundar S, Reed SG, Sharma S, Mehrotra A, Murray HW. Circulating T helper 1 (Th1) cell- and Th2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am J Trop Med Hyg. 1997;56:522–525. doi: 10.4269/ajtmh.1997.56.522. [DOI] [PubMed] [Google Scholar]
- [21].Murray HW, Lu CM, Mauze S, Freeman S, Moreira AL, Kaplan G, Coffman RL. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect Immun. 2002;70:6284–6293. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ato M, Stager S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol. 2002;3:1185–1191. doi: 10.1038/ni861. [DOI] [PubMed] [Google Scholar]
- [23].Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–2856. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [25].Castro AG, Neighbors M, Hurst SD, Zonin F, Silva RA, Murphy E, Liu YJ, O’Garra A. Anti-interleukin 10 receptor monoclonal antibody is an adjuvant for T helper cell type 1 responses to soluble antigen only in the presence of lipopolysaccharide. J Exp Med. 2000;192:1529–1534. doi: 10.1084/jem.192.10.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Murphy ML, Cotterell SE, Gorak PM, Engwerda CR, Kaye PM. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen, Leishmania donovani. J Immunol. 1998;161:4153–4160. [PubMed] [Google Scholar]
- [27].Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- [28].Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24:207–212. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- [30].Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad el B, Schmitt E, Bopp T, Kallen KJ, Herz U, Schmitt S, Luft C, Hecht O, Hohlfeld JM, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Renz H, Neurath MF, Galle PR, Finotto S. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- [32].Wilson ME, Recker TJ, Rodriguez NE, Young BM, Burnell KK, Streit JA, Kline JN. The TGF-beta response to Leishmania chagasi in the absence of IL-12. Eur J Immunol. 2002;32:3556–3565. doi: 10.1002/1521-4141(200212)32:12<3556::AID-IMMU3556>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [33].Engwerda CR, Ato M, Kaye PM. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol. 2004;20:524–530. doi: 10.1016/j.pt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- [34].Wilson ME, Jeronimo SM, Pearson RD. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb Pathog. 2005;38:147–160. doi: 10.1016/j.micpath.2004.11.002. [DOI] [PubMed] [Google Scholar]
- [35].Vanloubbeeck YF, Ramer AE, Jie F, Jones DE. CD4+ Th1 cells induced by dendritic cell-based immunotherapy in mice chronically infected with Leishmania amazonensis do not promote healing. Infect Immun. 2004;72:4455–4463. doi: 10.1128/IAI.72.8.4455-4463.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ramirez-Pineda JR, Frohlich A, Berberich C, Moll H. Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J Immunol. 2004;172:6281–6289. doi: 10.4049/jimmunol.172.10.6281. [DOI] [PubMed] [Google Scholar]
- [37].Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. IL-10 from Regulatory T Cells Determines Vaccine Efficacy in Murine Leishmania major Infection. J Immunol. 2005;175:2517–2524. doi: 10.4049/jimmunol.175.4.2517. [DOI] [PubMed] [Google Scholar]
- [38].Datta N, Mukherjee S, Das L, Das PK. Targeting of immunostimulatory DNA cures experimental visceral leishmaniasis through nitric oxide up-regulation and T cell activation. Eur J Immunol. 2003;33:1508–1518. doi: 10.1002/eji.200323671. [DOI] [PubMed] [Google Scholar]
- [39].Sparwasser T, Koch ES, Vabulas RM, Heeg K, Lipford GB, Ellwart JW, Wagner H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- [40].Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature Immunology. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- [41].Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- [42].Wilson ME, Young BM, Davidson BL, Mente KA, McGowan SE. The importance of TGF-beta in murine visceral leishmaniasis. J Immunol. 1998;161:6148–6155. [PubMed] [Google Scholar]
- [43].Gomes NA, Gattass CR, Barreto-De-Souza V, Wilson ME, DosReis GA. TGF-beta mediates CTLA-4 suppression of cellular immunity in murine kalaazar. J Immunol. 2000;164:2001–2008. doi: 10.4049/jimmunol.164.4.2001. [DOI] [PubMed] [Google Scholar]
- [44].Zubairi S, Sanos SL, Hill S, Kaye PM. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. Eur J Immunol. 2004;34:1433–1440. doi: 10.1002/eji.200324021. [DOI] [PubMed] [Google Scholar]
- [45].Murray HW, Flanders KC, Donaldson DD, Sypek JP, Gotwals PJ, Liu J, Ma X. Antagonizing deactivating cytokines to enhance host defense and chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2005;73:3903–3911. doi: 10.1128/IAI.73.7.3903-3911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kagari T, Doi H, Shimozato T. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002;169:1459–1466. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]