Abstract
Rationale and Objectives
To retrospectively investigate the prevalence of tracheal collapse in an emphysema cohort. The occurrence of a large degree of tracheal collapse might have important implications for the clinical management of respiratory symptoms and air trapping in emphysema patients.
Material and Methods
Paired full-inspiratory and end-expiratory thin-section volumetric CT scans were available for 1071 long term smokers with clinically and physiologically confirmed emphysema. The percent reduction in the cross-sectional tracheal lumen area from full inspiration to end-expiration was automatically computed at 2.5 mm intervals along the centerline of the trachea using customized software.
Results
The maximum tracheal collapse did not follow a normal distribution in the emphysema cohort (P<0.0001, skewness/kurtosis tests for normality); the median collapse was 18%, intraquartile range 11% to 30%. Statistically significant differences were found in the distribution of maximal collapse by gender (P<0.005, Wilcoxon rank-sum test). Overall, 10.5% of males and 17.1% of females showed evidence of tracheomalacia based on the criteria of a ≥50% reduction in the cross-sectional tracheal lumen area at end-expiration.
Conclusion
Our study offers insights into the prevalence of tracheal collapse in a cohort of emphysema patients; future work is needed to determine the possible relationship between tracheal collapse and air trapping in emphysema subjects.
Keywords: Tracheal Collapse, Tracheomalacia, Prevalence, Emphysema
1. INTRODUCTION
A high degree of tracheal collapse is associated with tracheomalacia, which can be diagnosed in both children and adults [1]. Tracheomalacia can be congenital or acquired; associated symptoms include dyspnea, wheeze, cough, sputum production, and hemoptysis. Investigators have suggested a correlation of tracheobronchomalacia with chronic inflammation and irritants, such as cigarette smoke [2,3]. Due to its non-specific symptoms, it may be an underdiagnosed condition.
Bronchoscopy is considered the gold standard for diagnosing tracheobronchomalacia [1]; a reduction in the cross-sectional lumen area of the trachea greater than 50% is considered indicative of tracheomalacia. The reported prevalence of the disease has ranged from 1% to 13% for all subjects referred for bronchoscopic evaluation [3–5] and as high as 23% for subjects with a history of chronic bronchitis [2]. The condition is commonly associated with increased age and chronic obstructive pulmonary disease (COPD) [3].
Recent studies have investigated the use of CT imaging as a less invasive means for the diagnosis of tracheomalacia [6–16]. Scanning a subject during a dynamic expiratory maneuver has been shown to be better at eliciting tracheal collapse than a scan at end-expiration [7,9,12]; however, in clinical practice end-expiratory scans may be more commonly acquired.
This study focuses specifically on subjects in an emphysema cohort; emphysema is a widely spread disease and affects around 1.8% of the global population [17]. Patients with emphysema typically exhibit a progressive increase in dyspnea; determining the underlying causes of their symptoms is essential for effective clinical care of these patients. Zhang et al. [16] observed a higher frequency and greater severity of air trapping in patients with tracheobronchomalacia compared to a controlled group. Investigating tracheal collapse in emphysema subjects may provide insight into a cause contributing to the air trapping associated with the disease.
The purpose of this research was to investigate tracheal collapse as seen at end-expiration in a large cohort of emphysema subjects, with a specific interest in determining what percentage of subjects showed evidence of tracheomalacia. Further investigation into the relationship with age and gender is also presented.
2. METHODS
2.1 Patients
Image data for this study was selected retrospectively from a database comprised of anonymized image data from multiple centers. Information regarding the clinical history of each subject was not available; however, the subjects were known to be long term smokers with clinically and physiologically confirmed emphysema. Complete (i.e., no missing slices) thin section image data at both a full inspiration (TLC) and forced end-expiration (RV) were the inclusion criteria for this study. Overall, image data from 1071 subjects meet the inclusion criteria. Information regarding age and gender was available for only 431 of the subjects; of whom 267 were known males (median age 64 years, range 41 to 76) and 164 were known females (median age 62 years, range 41 to 76). The use of the anonymous image datasets from an ongoing clinical trial was approved by our local institutional review board.
2.2 CT Scan Protocol
Image datasets from subjects in an emphysema-related clinical trial in which CT scans were obtained at total lung capacity (TLC) and at residual volume (RV) were used; no participants were recruited exclusively for this study. This convenience dataset was chosen because it provided two image series obtained in the same setting, at different levels of suspended respiration. For the image series at TLC, subjects were instructed to suspend breathhold after twice breathing deeply, and then inhaling as deeply as possible, at which point the imaging would commence. The RV series were obtained after instructing participants to breathe deeply twice, then exhaling as completely as possible before breathholding, at which point the imaging would commence.
Subjects were imaged on one of twelve scanner models from four commercial vendors: General Electric (GE Medical Systems; Waukesha, WI); Philips (Philips Healthcare, Andover, MA); Siemens (Siemens Medical Solutions, Forcheim, Germany); and Toshiba (Toshiba America Medical Systems, Tustin, CA). Image acquisition protocols were standardized across all models to maintain comparable image quality; scanning centers were instructed to use a standardized scan protocol of 120 kVp, 150 to 300 mA, and a pitch of 1.375 to 1.5 [18]. Thin section series were reconstructed from the image data for each breathhold; the slice thickness of the thin series was dependent on the capability of each scanner model, and ranged from 0.6 mm to 3.2 mm. Images were reconstructed with a display field of view containing the widest cross-section of the lungs, using non-enhancing reconstruction algorithms (e.g., GE Bone algorithm, Philips D, Siemens B50, or Toshiba FC51).
2.3 Measurement of Tracheal Collapse
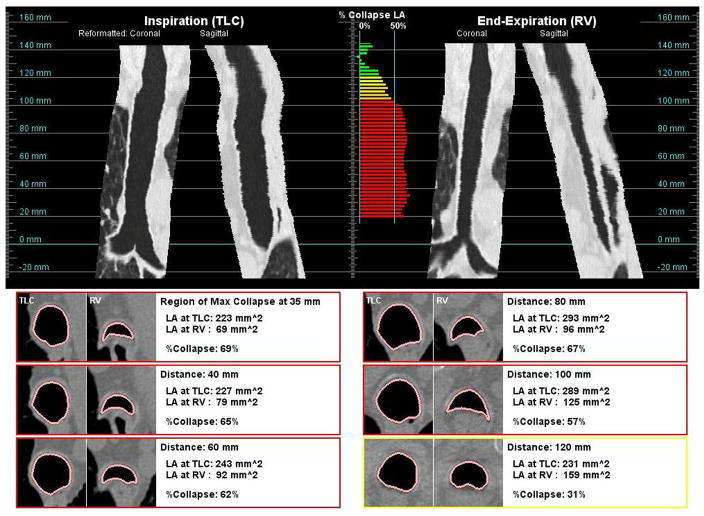
The image data was transferred to a noncommercial image analysis workstation and database for evaluation. Customized software was developed in-house to measure the collapse of the trachea and generate a report of the analysis to facilitate review (Figure 1). The report shows reformatted coronal and sagittal views of the trachea at inspiration and expiration, the percent collapse along the trachea, and cross-sectional images at selected intervals along the trachea including the location of maximal collapse. The percent collapse, shown in the middle of the report, is color-coded by the degree of collapse at each measurement location: < 30% collapse (green), 30 to 40% (yellow), 40 to 50% (orange), and ≥50% (red).
Figure 1.
Example of an analysis report; reformatted coronal and sagittal views along the trachea are shown at both inspiration (top-left) and end-expiration (top-right) breathhold; the color-coded degree of collapse is shown at each measurement point along the trachea (top-middle); examples cross-sectional views of the trachea at both inspiration levels are shown side-by-side at selected locations (bottom).
The centerline of the trachea was automatically extracted from the inspiration and expiration series of all subjects, using imaging analysis techniques similar to that of 3D region growing [19,20]. The results were reviewed by a research associate with expertise in the field of airway segmentation. When necessary, minimal manual editing to correct the tracheal centerline was performed. Using the extracted centerline, cross-sectional images of the trachea were reconstructed perpendicular to the orientation of the trachea at 2.5 mm intervals. On each cross-sectional image, a contour of the inner lumen and the tracheal lumen area were automatically computed [21]. The measurement technique was evaluated using a tube phantom consisting of tubes (3 to 17 mm in diameter) embedded in synthetic lung parenchyma; the overall accuracy of the lumen area measurement was within 5% for all tubes.
At each measurement point, the lumen area was computed at both full inspiration (LATLC) and end-expiration (LARV); in addition, the percent change in cross-sectional area between TLC and RV was also computed (ΔLA) using the following formula: 100%*(LATLC − LARV)/LATLC. To minimize effects from the closing of the epiglottis near the superior region of the trachea, analysis of the trachea was restricted to the lesser of the following: the inferior 100 mm of trachea (as measured from the carina) or 90% of the extracted trachea length; the average collapse (ΔLAavg) and maximum collapse (ΔLAmax) were automatically determined in this region of analysis.
2.4 Statistical Analysis
Statistical analysis was performed using Stata 8.0 (Stata Corporation, College Station, Texas). Non-parametric analysis was performed as neither age, LATLC, ΔLAavg, nor ΔLAmax followed a normal distribution (P<0.001, skewness/kurtosis tests for normality). The correlation between age and maximal collapse was evaluated with Spearman’s correlation coefficient. Two-sample Wilcoxon rank-sum (Mann-Whitney) tests were performed to evaluate if the distribution of values for each measurement differed significantly by gender.
3. RESULTS
For all subjects, the distribution of the maximum tracheal collapse at end-expiration was heavily skewed as the median was at 18% and the intraquartile range was between 11% and 30%. The distribution of the maximal tracheal collapse was significantly different by gender (P<0.003). For men, the median was 16% (intraquartile range 11% to 25%); for women, the median was 20% (intraquartile range 13% to 37%) (Figure 2).
Figure 2.
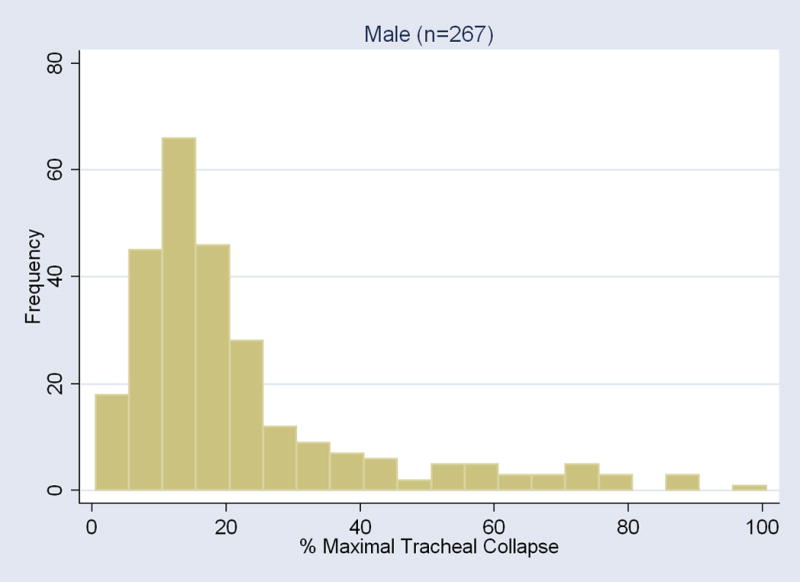
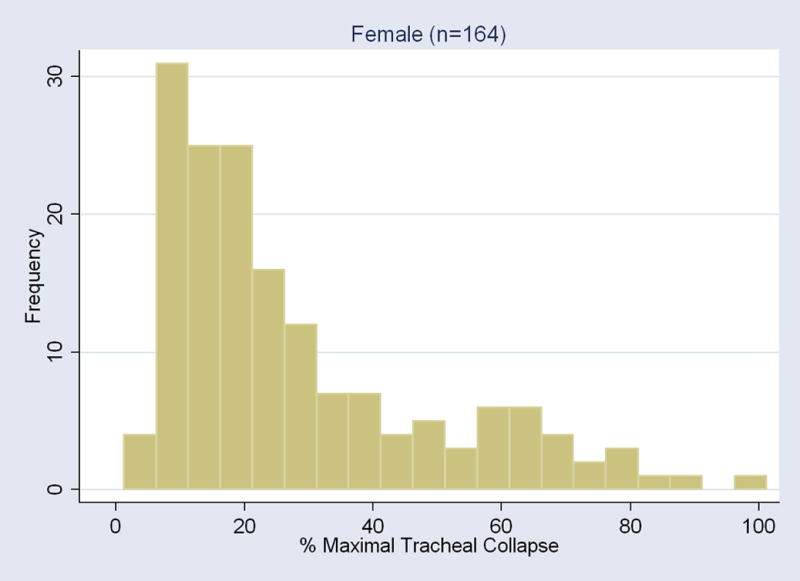
Histogram of the maximal collapse of the tracheal lumen area elicited with the end-expiration CT for each gender.
Table 1 provides a summary of the tracheal measurements. For males, the mean (± standard deviation) cross-sectional lumen area of the trachea at TLC was 296 ± 60 mm2, which was significantly larger (P<0.001) than that of females 220 ± 38 mm2. The average cross-sectional lumen area was also significantly larger at end-expiration for males (P<0.001).
Table 1.
Summary of the measurements; significant differences were found between the male and female subjects. The mean ± SD are shown for measurements.
| All (n=1071)* | Male (n=267) | Female (n=164) | P-value** | |
|---|---|---|---|---|
| Age (years) | - | 64 ± 7 | 62 ± 8 | P < 0.005 |
| Measurement | ||||
| LATLC (mm2) | 268 ± 64 | 296 ± 60 | 221 ± 38 | P < 0.001 |
| LARV (mm2) | 224 ± 74 | 253 ± 70 | 179 ± 54 | P < 0.001 |
| ΔLAavg (%) | 16 ± 19 | 14 ± 17 | 19 ± 20 | P < 0.03 |
| ΔLAmax (%) | 25 ± 20 | 22 ± 19 | 28 ± 21 | P < 0.003 |
| Number of Subjects with Maximal Trachea Collapse | ||||
| ≥28% | 27.7% (n=297) | 21.3% (n=57) | 35.4% (n=58) | |
| ≥50% | 13.4% (n=143) | 10.5% (n=28) | 17.1% (n=28) | |
| ≥70% | 5.1% (n=55) | 4.9% (n=13) | 6.7% (n=11) |
Age and gender information was available for only 431 of the 1071 subjects in the emphysema cohort.
Two-sample Wilcoxon rank-sum (Mann-Whitney) test between male and female
The age distribution was significantly different between genders, with the male cohort being an average of two years older than the female cohort (P<0.005). There was a slight correlation between age and maximal collapse for each gender; for males, the Spearman correlation coefficient was 0.15 (P<0.03); for females, the Spearman correlation coefficient was 0.26 (P<0.001) (Figure 3).
Figure 3.
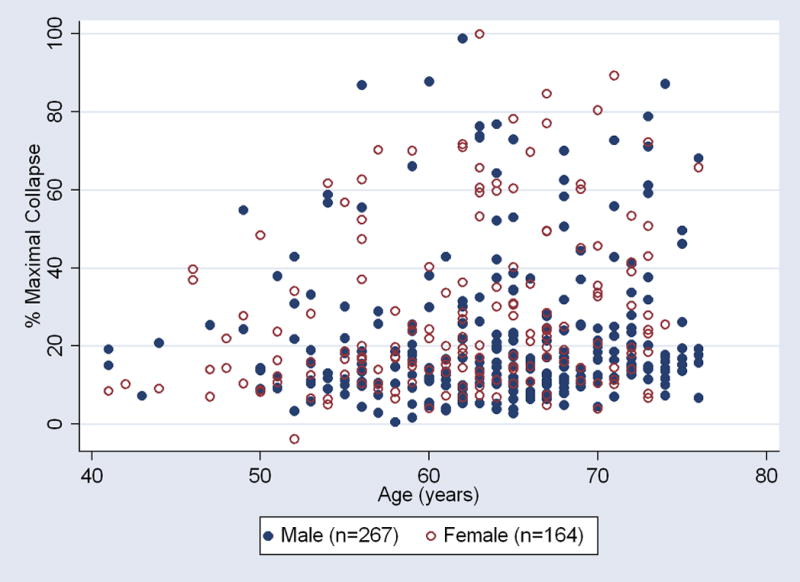
Scatter plot of the relationship between age and maximal trachea collapse. There was a slight relationship between the degree of trachea collapse and age for both genders in the emphysema cohort.
Overall, 13.4% (143 of 1071) of the subjects showed evidence of tracheomalacia based on the criteria of a ≥50% reduction in the cross-sectional trachea lumen area at end expiration. By gender, 10.5% (28 of 267) of known males and 17.1% (28 of 164) of known females showed evidence of tracheomalacia based on the 50% criteria. Other authors have suggested alternative lower or higher thresholds [6,14]. If the threshold was lowered to 28%, the prevalence would be 27.7% (297 of 1071) for all, 21.3% (57 of 267) for males, and 35.4% (58 of 164) for females. If the threshold was raised to 70%, the prevalence would be 5.1% (55 of 1071) for all, 4.9% (13 of 267) for males, and 6.7% (11 of 164) for females.
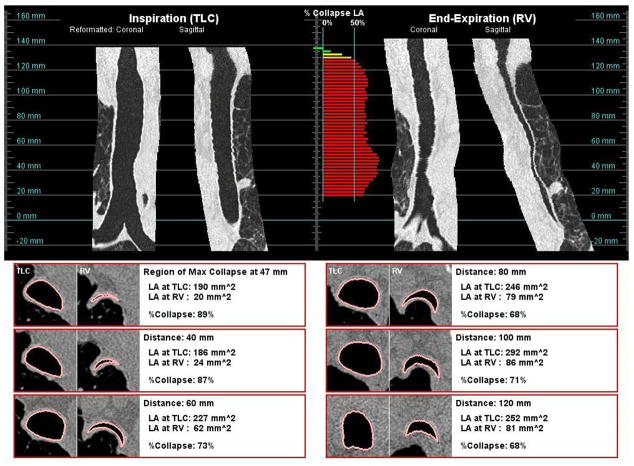
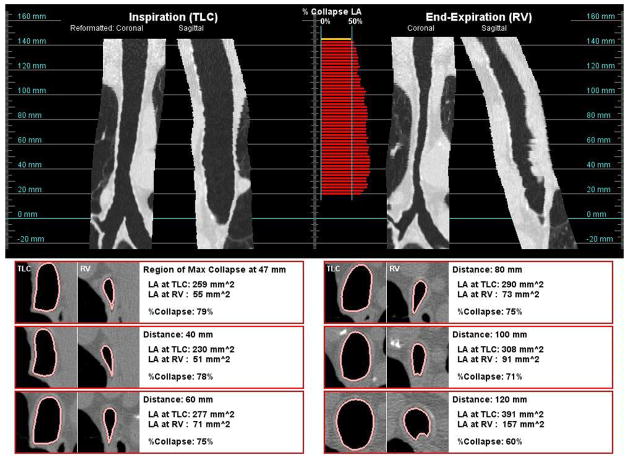
Figure 4 presents examples of two subjects whose trachea collapsed by more than 50% during expiration. For both subjects, the tracheal collapse was not restricted to one portion of the trachea, but was along the entire length of the trachea. These figures also illustrate how the shape may differ by subject; one subject had a greater anterior posterior collapse, while the other subject had a greater lateral collapse of the trachea.
Figure 4.
The shape of the collapse of the trachea may take different forms. Subject (a), a 71 year old female, had a greater collapse of the posterior membrane. Subject (B), a 73 year old male, showed a greater collapse in the lateral axis.
4. DISCUSSION
In this study, we observed a large degree of tracheal collapse ≥50% at end-expiration in 13.4% of patients in an emphysema cohort. Female gender and increased age appeared to correspond with a higher degree of collapse. This might have important implications on the respiratory symptoms and air trapping in emphysema patients, which warrants further study. Previous studies have suggested that tracheomalacia may be an underdiagnosed condition [1,2,8,11,22]; this is possibly due to its unspecific symptoms, which may be associated with other diseases.
A number of studies have investigated the prevalence of tracheomalacia using bronchoscopy. In a study of patients with various respiratory symptoms, Herzog et al. [4] reported that only 1% (16 of 1500) had tracheomalacia. In patients with pulmonary disease, Ikeda et al. [5] reported that 12.7% (542 of 4283) had some form of airway collapse > 50%. In a study of subjects with a history of chronic bronchitis, Jokinen et al. [2] reported that 23% (50 of 214) had tracheomalacia.
In a large study of patients with various symptoms, Jokinen et al. [3] reported that 4.4% (94 of 2150) had some form of airway malacia. The age of these 94 subjects with malacia ranged from 46 to 78 years, and 82% were male and 18% were female. It has been suggested that the higher reported occurrence in males may be reflective of smoking being more common among males at the time of this study [1]. However, the demographics of the entire study population were not presented; thus, the gender differences may also be reflective of a higher number of males in the study.
The potential of CT for less invasive evaluation of tracheomalacia is exemplified by a recent study that retrospectively evaluated the sensitivity of dynamic expiration scanning (i.e., a volumetric image acquisition during expiration) using the results of bronchoscopy as the gold standard. For 29 subjects, the dynamic imaging showed 98% sensitivity for the diagnosis of tracheomalacia using a maximum collapse of the cross-sectional trachea lumen area ≥50% as the diagnostic threshold [13].
In a study similar to this one, Hasegawa et al. [11] investigated the frequency of tracheomalacia incidentally detected on CT pulmonary angiography of 163 patients (73 male, 90 female) with suspected pulmonary embolism. 10% (16 of 163) of subjects showed evidence of tracheomalacia based on the 50% collapse threshold; of these 16 subjects, 7 were male and 9 were female. Their ages ranged from 41 to 95 years, with a mean age of 72 years; 15 had a known cause of tracheomalacia, such as prior tube intubation (n=12) and a history of asthma or COPD (n=5). A difference of their study was that they used only expiration scans; the degree of collapse was estimated by comparing a “collapsed” region with respect to a nearby “un-collapsed” region of the trachea, as compared to this study where the collapse is relative to the size of the trachea at the same location at inspiration.
Both end-expiratory and dynamic expiratory CT imaging protocols have been investigated for evaluation of tracheomalacia. Aquino et al. [6] studied trachea collapse in 23 normal and 10 subjects with acquired tracheomalacia using paired inspiration and end-expiratory CT. ROC analysis showed a threshold of 28% collapse of the lumen area of the midtrachea would have a positive predictive value of 89–100% and a negative predictive value of 95–100%. However, recent studies have demonstrated that paired inspiration and dynamic expiratory CT is better for eliciting tracheal collapse than end-expiratory imaging [7,9,12]. For 14 subjects with confirmed tracheomalacia, Baroni et al. [7] demonstrated that the dynamic CT elicited an average of 53.9% collapse of the trachea lumen area at the level of the aortic arch, whereas the end-expiration scan elicited only an average of 35.7% collapse at the same location.
If the standard criteria of 50% collapse of the tracheal lumen area were alone to be used for a radiological diagnosis of tracheomalacia, then the prevalence would be 13.4% (143 of 1071) for our cohort of long term smokers with clinically and physiologically confirmed emphysema. However, the results of the study by Baroni et al. [7] would suggest that our estimate of the prevalence of tracheomalacia using a 50% threshold level might be low as a result of the end-expiratory scan protocol. If the suggested threshold of 28% from the Aquino et al. [6] study were used on our data, then the prevalence of tracheomalacia would be 27.7% (297 of 1071) for our emphysema cohort. Since we do not have bronchoscopic confirmation, we cannot say with certainty which threshold most closely matches the true prevalence.
Care should be taken before making a diagnosis or estimating the prevalence of tracheomalacia based on a single threshold, which may have been developed using only a limited numbers of subjects. In a study of 10 young, nonsmoking adult males, Stern et al. [14] found their average (± SD) collapse of the trachea lumen area to be 38 ± 18% (range 11% to 61%), yet four of the healthy subjects had a collapse greater than 50% during a forced inspiration and expiration maneuver. The authors suggested a collapse greater than 70% should be used as a threshold for tracheomalacia. Since an argument could be made for multiple levels, Table 1 reports the estimated prevalence at 28%, 50%, and 70%; although, it is recognized that the 50% threshold level has been most commonly applied in previous studies.
There were limitations in our study. First, the emphysema cohort may have confounded the results. Emphysema restricts lung motion, and likely alters the dynamics of breathing and tracheal collapse when compared to normal subjects. The differences in the degree of collapse may also simply be reflective of individual differences in respiratory effort and ability. The relationship between change in lung volume and collapse, as well as the reproducibility of the collapse is an area of further investigation. Although the image data was from multiple centers, our investigations into quality assurance have shown good compliance with the standardized imaging protocol [18].
Second, dynamic expiratory scans were not used; although, they have been shown to be better at eliciting tracheal collapse than end-expiratory imaging [7,9,12]. Unfortunately, neither dynamic expiratory nor bronchoscopic findings were available for our cohort of subjects. The imaging protocol for the clinical trial in which the subjects were enrolled did not require dynamic scans. By retrospectively using the available TLC and RV volumetric scans, our study did not increase the radiation exposure of subjects above what was required for the clinical trial. It should be noted though that a low-dose dynamic expiratory protocol for analysis of the central airways has been proposed to help minimize the radiation exposure [15].
Third, clinical histories for our subjects were not available, which limits our understanding of clinical factors that may influence the tracheal collapse. However, our study may offer insights into the prevalence of the tracheal collapse seen in a cohort of emphysema subjects as well as possible gender differences.
Finally, a complete validation of the software was not performed. However, steps were taken in order to ensure the validity of the results. The measurement technique has been previously evaluated [21], and the report allows a visual confirmation of the analysis. Although untested, we would expect the accuracy and reproducibility of the software to be similar, if not better, than a manual approach on axial images, which requires the following user steps: 1) visually locating the area of maximal collapse at end-expiration, 2) finding the corresponding location on the inspiration scan, and 3) manually contouring the trachea on both scans for analysis.
Understanding the effect tracheal collapse has on patient symptoms is important considering its suggested underdiagnosis in everyday clinical practice. In the future, exploring physiological, pathological, and technical factors that may influence the measured collapse will be essential for obtaining reproducible measurements and better understanding the disease.
5. CONCLUSION
In our study of long term smokers with clinically and physiologically confirmed emphysema, we found that 13.4% of the subjects showed a collapse of the trachea ≥50% at end-expiration. This observation might have important implications of possible effect of tracheal collapse on the respiratory symptoms, air trapping and therapy evaluation in emphysema patients. Further studies are needed to investigate this postulate further; however, our current work might offer an important insight into the prevalence of tracheal collapse in patients with emphysema.
Acknowledgments
UC Discovery Grant IT106-10158.
Grant Support provided by University of California Discovery Grant IT106-10158 in collaboration with Broncus Technologies, Inc. and Emphasys Medical Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert A. Ochs, Department of Radiological Sciences, David Geffen School of Medicine, UCLA.
Iva Petkovska, Department of Radiological Sciences, UCLA.
Hyun J. Kim, Department of Radiological Sciences, UCLA.
Fereidoun Abtin, Department of Radiological Sciences, UCLA.
Matthew S Brown, Department of Radiological Sciences, UCLA.
Jonathan G Goldin, Department of Radiological Sciences, UCLA.
References
- 1.Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127(3):984–1005. doi: 10.1378/chest.127.3.984. [DOI] [PubMed] [Google Scholar]
- 2.Jokinen K, Palva T, Nuutinen J. Chronic bronchitis: a bronchologic evaluation. ORL J Otorhinolaryngol Relat Spec. 1976;38:178–186. doi: 10.1159/000275273. [DOI] [PubMed] [Google Scholar]
- 3.Jokinen K, Palva T, Sutinen S, Nuutinen J. Acquired tracheobronchomalacia. Ann Clin Res. 1977;9:52–57. [PubMed] [Google Scholar]
- 4.Herzog H. Expiratory stenosis of the trachea and great bronchi by loosening of the membraneous portion; plastic chip repair. Thoraxchirurgie. 1958;5:281–391. [PubMed] [Google Scholar]
- 5.Ikeda S, Hanawa T, Konishi T, et al. Diagnosis, incidence, clinicopathology and surgical treatment of acquired tracheobronchomalacia. Nihon Kyobu Shikkan Gakkai Zasshi. 1992;30:1028–1035. [PubMed] [Google Scholar]
- 6.Aquino SL, Shepard JO, Ginns LC, Moore RH, Halpern E, Grillo HC, McLoud TC. Acquired Tracheomalacia: Detection by Expiratory CT Scan. Journal of Computer Assisted Tomography. 2001;25(3):394–399. doi: 10.1097/00004728-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Baroni RH, Feller-Kopman D, Nishino M, Hatabu H, Loring SH, Ernst A, Boiselle PM. Tracheobronchomalacia: comparison between end-expiratory and dynamic expiratory CT for evaluation of central airway collapse. Radiology. 2005;235(2):635–41. doi: 10.1148/radiol.2352040309. [DOI] [PubMed] [Google Scholar]
- 8.Boiselle PM, Lee KS, Ernst A. Multidetector CT of the Central Airways. Journal of Thoracic Imaging. 2005;20(3):186–195. doi: 10.1097/01.rti.0000171624.84951.f2. [DOI] [PubMed] [Google Scholar]
- 9.Ferretti GR, Jankowski A, Perrin MA, Chouri N, Arnol N, Aubauda L, Pepin JL. Multi-detector CT evaluation in patients suspected of tracheobronchomalacia: Comparison of end-expiratory with dynamic expiratory volumetric acquisitions. Eur J Radiology. 2008 doi: 10.1016/j.ejrad.2007.08.029. in press. [DOI] [PubMed] [Google Scholar]
- 10.Gilkeson RC, Ciancibello LM, Hejal RB, Montenegro HD, Lange P. Tracheobronchomalacia: dynamic airway evaluation with multidetector CT. AJR Am J Roentgenol. 2001;176(1):205–10. doi: 10.2214/ajr.176.1.1760205. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa I, Boiselle PM, Raptopoulos V, Hatabu H. Tracheomalacia incidentally detected on CT pulmonary angiography of patients with suspected pulmonary embolism. AJR Am J Roentgenol. 2003;181(6):1505–9. doi: 10.2214/ajr.181.6.1811505. [DOI] [PubMed] [Google Scholar]
- 12.Heussel C, Hafner B, Lill J, Schreiber W, Thelen M, Kauczor HU. Paired inspiratory/expiratory spiral CT and continuous respiration cine CT in the diagnosis of tracheal instability. European Radiology. 2001;11(6):982–989. doi: 10.1007/s003300100818. [DOI] [PubMed] [Google Scholar]
- 13.Lee KS, Sun MRM, Ernst A, Feller-Kopman D, Majid A, Boiselle PM. Comparison of Dynamic Expiratory CT With Bronchoscopy for Diagnosing Airway Malacia: A Pilot Evaluation. Chest. 2007;131:758–764. doi: 10.1378/chest.06-2164. [DOI] [PubMed] [Google Scholar]
- 14.Stern EJ, Graham CM, Webb WR, Gamsu G. Normal trachea during forced expiration: dynamic CT measurements. Radiology. 1993;187(1):27–31. doi: 10.1148/radiology.187.1.8451427. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Hasegawa I, Feller-Kopman D, Boiselle PM. Dynamic Expiratory Volumetric CT Imaging of the Central Airways: Comparison of Standard-Dose and Low-Dose Techniques. Academic Radiology. 2003;10(7):719–724. doi: 10.1016/s1076-6332(03)80117-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Hasegawa I, Hatabu H, Feller-Kopman D, Boiselle PM. Frequency and severity of air trapping at dynamic expiratory CT in patients with tracheobronchomalacia. Am J Roentgenol. 2004;182:81–85. doi: 10.2214/ajr.182.1.1820081. [DOI] [PubMed] [Google Scholar]
- 17.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 18.Goldin JG, Brown MS, Abtin F, Tam S, Kim HJ, Angel E, McNitt-Gray MF, Pais R, da Costa I, Ahmad S, Gjertson D. Baseline CT Characteristics and Image Quality of the Multicenter VENT Study. Proc American Thoracic Society. 2007 [Google Scholar]
- 19.Paik DS, Beaulieu CF, Jeffrey RB, Rubin GD, Napel S. Automated flight path planning for virtual endoscopy. Med Phys. 1998;25:629–637. doi: 10.1118/1.598244. [DOI] [PubMed] [Google Scholar]
- 20.Wood SA, Zerhouni EA, Hoford JD, Hoffman EA, Mitzner W. Measurement of three-dimensional lung tree structures by using computed tomography. J Appl Physiol. 1995;79:1687–1697. doi: 10.1152/jappl.1995.79.5.1687. [DOI] [PubMed] [Google Scholar]
- 21.Ochs R, Kim HJ, Goldin J, McNitt-Gray M, Brown M. Evaluation of airway measurements in phantom parenchyma and soft tissue regions. Proc SPIE Medical Imaging. 2008:6916–74. [Google Scholar]
- 22.Palombini BC, Villanova CA, Araújo E, Gastal OL, Alt DC, Stolz DP, Palombini CO. A pathogenic triad in chronic cough: asthma, postnasal drip syndrome, and gastroesophageal reflux disease. Chest. 1999;116:279–284. doi: 10.1378/chest.116.2.279. [DOI] [PubMed] [Google Scholar]