Abstract
CD14+ peripheral blood monocytes can differentiate into fibroblast-like cells called fibrocytes, which are associated with and are at least partially responsible for wound healing and fibrosis in multiple organ systems. Signals regulating fibrocyte differentiation are poorly understood. In this study, we find that when added to human PBMCs cultured in serum-free medium, the profibrotic cytokines IL-4 and IL-13 promote fibrocyte differentiation without inducing fibrocyte or fibrocyte precursor proliferation. We also find that the potent, antifibrotic cytokines IFN-γ and IL-12 inhibit fibrocyte differentiation. In our culture system, IL-1β, IL-3, IL-6, IL-7, IL-16, GM-CSF, M-CSF, fetal liver tyrosine kinase 3, insulin growth factor 1, vascular endothelial growth factor, and TNF-α had no significant effect on fibrocyte differentiation. IL-4, IL-13, and IFN-γ act directly on monocytes to regulate fibrocyte differentiation, and IL-12 acts indirectly, possibly through CD16-positive NK cells. We previously identified the plasma protein serum amyloid P (SAP) as a potent inhibitor of fibrocyte differentiation. When added together, the fibrocyte-inhibitory activity of SAP dominates the profibrocyte activities of IL-4 and IL-13. The profibrocyte activities of IL-4 and IL-13 and the fibrocyte-inhibitory activities of IFN-γ and IL-12 counteract each other in a concentration-dependent manner. These results indicate that the complex mix of cytokines and plasma proteins present in inflammatory lesions, wounds, and fibrosis will influence fibrocyte differentiation.
Keywords: fibrosis, inflammation, monocytes, pentraxin, serum amyloid P
INTRODUCTION
Fibrosis involves an aberrant and excessive production of extracellular matrix (ECM) proteins, especially collagen and fibronectin, by fibroblasts and fibroblast-like cells [1]. Unlike orderly wound healing, a persistence of neutrophils, eosinophils, lymphocytes, and monocyte-macrophages leads to the maintenance of cytokine levels and the inhibition of enzymes that degrade ECM proteins [2]. These events, which are thought to be a result of repeated episodes of injury, generate positive-feedback loops that lead to increased ECM protein deposition. Fibrosis leads to dysfunction as a result of the functional constraints placed by the fibrotic lesion on the given tissue or organ, as well as the alteration of the type and number of cells within tissues.
Much remains to be understood about the source of the fibroblast-like cells that are responsible for fibrosis as well as wound healing. The “classical” hypothesis is that local interstitial fibroblasts migrate into inflammatory areas and produce new ECM proteins, ultimately leading to fibrosis or wound healing [3]. One alternative hypothesis suggests that circulating bone marrow-derived precursors present within the blood are attracted to sites of injury, where they differentiate into spindle-shaped, fibroblast-like cells called fibrocytes and at least in part, mediate tissue repair and fibrosis [4–9]. Fibrocytes express markers of hematopoietic cells (CD45, MHC class II, CD34) and stromal cells (collagens I and III and fibronectin) [4, 5, 10–12]. Fibrocyte precursors appear to differentiate from a subpopulation of CD14+ peripheral blood monocytes [5, 10–12]. We have shown that fibrocyte differentiation can be inhibited in vitro and in vivo by the plasma protein serum amyloid P (SAP) and aggregated IgG [11–15].
One of the key features of fibrosis is a distinctive cytokine profile with elevated levels of profibrotic Th-2 cytokines such as IL-4 and IL-13 [16–18]. These profibrotic Th-2 cytokines stimulate collagen production and deposition by fibroblasts and promote the activation and differentiation of these fibroblasts to a more active cell type, the myofibroblast [19]. Many fibrotic lesions are associated with this Th-2 inflammatory environment including asthma, scleroderma (systemic sclerosis), cardiac fibrosis, hepatic fibrosis, early rheumatoid arthritis, thermal injury, hypertrophic scars, and various lung-fibrosing pathologies [16, 20–24]. In all of these cases, elevated levels of one or more profibrotic cytokines (IL-4, IL-10, IL-13, or TGF-β) appear to correlate with disease progression.
The effects of cytokines on cells can be observed by their effects on signal transduction pathways. For example, IL-4 binds to IL-4Rα, whereas IL-13 binds to IL-13Rα1. After binding IL-4, IL-4Rα then associates with the common γ-chain of the IL-2R family or with the IL-13Rα1 chain for signaling [25, 26]. For IL-13 signaling, IL-13Rα1 associates with IL-4Rα to generate a signaling complex. IL-4 or IL-13 signaling induces the phosphorylation and dimerization of the cytoplasmic transcription factor STAT-6 [27, 28].
The profibrotic Th-2 cytokines are regulated in part by the presence of Th-1 cytokines, such as IL-12 and IFN-γ The Th-1 and Th-2 cytokine groups exert reciprocal, inhibitory effects on each other, and the presence of IL-12 or IFN-γ inhibits IL-4 or IL-13 responses and vice versa [16, 29, 30]. In hematopoietic cells, IL-12 signaling leads to the specific activation of STAT-4, whereas IFN-γ activates primarily STAT-1 [27, 28, 31].
As cytokines such as IL-4 and IL-13 are associated with wound healing and fibrosis, and fibrocytes are also associated with wound healing and fibrosis, we examined the effect of cytokines on fibrocyte differentiation [16, 17, 21]. We find that IL-4 and IL-13 promote fibrocyte differentiation without inducing proliferation, whereas IL-12 and IFN-γ inhibit fibrocyte differentiation. We also show that IL-4, IL-13, and IFN-γ act directly on monocytes to regulate fibrocyte differentiation. In competition studies, we find that IL-4 and IL-13 counteract IL-12 and IFN-γ, and vice versa but that the fibrocyte-inhibitory activity of SAP is dominant over IL-4 and IL-13. These data suggest that the cytokine milieu present at sites of wound healing, inflammation, and fibrosis have a profound effect on the ability of monocytes to differentiate into fibrocytes.
MATERIALS AND METHODS
Cell culture and fibrocyte differentiation assay
Blood was collected from healthy adult volunteers in accordance with Rice University’s Institutional Review Board (Houston, TX, USA). PBMCs were isolated from heparinized blood by Ficoll-Paque Plus (GE Healthcare Biosciences, Piscataway, NJ, USA) as described previously [11, 12]. PBMCs were incubated in serum-free medium (SFM), which consists of RPMI 1640 (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10 mM HEPES (Sigma-Aldrich), nonessential amino acids (Sigma-Aldrich), 1 mM sodium pyruvate (Sigma-Aldrich), 2 mM glutamine (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich), and ITS+3 (Sigma-Aldrich). PBMCs were cultured in flat-bottomed, 96-well, tissue-culture plates in 200 μl vol at 2.5 × 105 cells per ml in a humidified incubator containing 5% CO2 at 37°C for the indicated times. Cytokines (all from Peprotech, Rocky Hill, NJ, USA) and SAP (EMD Biosciences, San Diego, CA, USA) were added at the indicated concentrations. Commercial SAP preparations contain 140 mM NaCl, 10 mM Tris, 0.1% azide, and 10 mM EDTA, which may influence many cellular processes. Therefore, we routinely buffer-exchange SAP into 20 mM sodium phosphate, pH 7.4. Briefly, SAP preparations are diluted 1:1 vol:vol with 20 mM sodium phosphate, pH 7.4, and added to 10 kDa cutoff centrifugal filters (Microcon YM-10, Millipore, Billerica, MA, USA) and centrifuged at 10,000 g for 10 min. The upper reservoir is then resuspended with ~400 μl 20 mM phosphate buffer and centrifuged again. This process is repeated four times to exchange the buffer to 20 mM sodium phosphate. Buffer-exchanged preparations were then checked by protein assay, chromatography on a Superose 12 size-exclusion column (GE Healthcare Biosciences), and Coomassie-stained polyacrylamide gels to assess their concentration and purity. We routinely found that the buffer-exchanged SAP was >98% pure and is a 135-kDa complex of 27 kDa monomers. Fibrocytes were counted after culturing cells for 5 days. Cells were air-dried, fixed in methanol, and stained with eosin and methylene blue (Hema 3 Stain, Fisher Scientific, Hampton, NH, USA) [11, 12]. Fibrocytes from duplicate wells were counted in five different 900 μm diameter fields per well, using the criteria of adherent cells with an elongated spindle shape and the presence of an oval nucleus. All cultures were counted by at least two independent observers. THP-1 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI 1640 containing 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich), and 2 mM glutamine (Invitrogen) at 2 × 104 cells per ml and subcultured when cell concentrations reached 5 × 105 cells/ml.
Preparation of monocytes
To determine if cytokines have a direct effect on monocytes, we depleted lymphocytes and dendritic cells using negative selection with magnetic Dynabeads (Dynal-Biotech, Invitrogen), as described previously [11, 32–35]. Briefly, PBMCs were incubated for 30 min on ice in PBS containing 2% BSA (Fraction V, globulin-free, Sigma-Aldrich) with 5 μg/ml primary antibodies against CD2 (clone RPA-2.10, mouse IgG1, BD Biosciences, San Diego, CA, USA), CD3 (UCHT1, mouse IgG1, BD Biosciences), CD19 (LT19, mouse IgG1, Serotec, Oxford, UK), CD56 (B159, mouse IgG1, BD Biosciences), CD94 (DX22, mouse IgG1, BioLegend, San Diego, CA, USA), CD209 (DCN46, mouse IgG2b, BD Biosciences), and TCR-γδ (B1, mouse IgG1, BD Biosciences). PBMCs were then washed and incubated with Dynabeads coated with human anti-mouse IgG for 30 min before removal of antibody-coated cells by magnetic separation, which was repeated four times. A sample of the monocyte preparation was incubated with 5 μg/ml primary antibodies against CD3, CD11b (clone ICRF44, mouse IgG1, BD Biosciences), CD14 (clone MEM-18, mouse IgG1, Serotec), CD16, CD19, pan-CD45 (clone HI30, mouse IgG1, BD Biosciences), CD56, and CD209. Cells were then washed twice in ice-cold PBS and then incubated with 2.5 μg/ml secondary FITC-conjugated F(ab′)2 goat anti-mouse IgG antibodies (cross-adsorbed against human Ig, Southern Biotechnology, Birmingham, AL, USA). Cells were then analyzed by flow cytometry (FACScan, BD Biosciences), as described previously [11, 32–36]. Only negatively selected cells in excess of 98% pure were used. The remaining 2% of cells expressed CD45 but were negative for CD3, CD11b, CD14, CD16, CD19, CD56, and CD209. Control depletions used PBMCs incubated with murine IgG1 and IgG2b control antibodies (BD Biosciences), followed by magnetic bead depletion or PBMCs incubated with Dynabeads, coated with human anti-mouse IgG beads without primary antibodies. Neither of the control depletion protocols affected cytokine or SAP responses (data not shown).
Immunohistochemistry
PBMCs were cultured on eight-well glass microscope slides (Lab-Tek, Nalge Nunc International, Naperville, IL, USA) for 5 days as described previously [11, 12]. Slides were air-dried for at least 60 min before fixation in acetone for 15 min. Nonspecific binding was blocked by incubation in 4% BSA (Fraction V, Sigma-Aldrich) in PBS for 60 min. Slides were incubated with 5 μg/ml primary antibodies in PBS containing 4% BSA for 60 min. Slides were stained with CD14 (clone MEM-18), CD34 (clone QBend10, mouse IgG1, Beckman Coulter, Fullerton, CA, USA), CD43 (clone 1G10, mouse IgG1, BD Biosciences), pan-CD45, or proliferating cell nuclear antigen (PCNA; clone PC10, mouse IgG2a, EMD Biosciences, San Diego, CA, USA). Collagen I was stained with rabbit polyclonal antibody (600-401-103, Rockland Inc., Gilbertsville, PA, USA), and collagen III was stained with rabbit polyclonal 600-401-105 (Rockland Inc.). Isotype-matched, irrelevant mouse mAb (BD Biosciences) or irrelevant rabbit polyclonal antibodies (Jackson ImmunoResearch, West Grove, PA, USA) were used as controls. Slides were then washed in six changes of PBS over 30 min and incubated for 30 min with 2.5 μg/ml biotinylated goat F(ab′)2 anti-mouse IgG or biotinylated goat F(ab′)2 anti-rabbit IgG (both cross-adsorbed against human Ig, Southern Biotechnology). After washing, the biotinylated antibodies were detected by ExtrAvidin alkaline phosphatase (Sigma-Aldrich) in PBS containing 4% BSA. Staining was developed with the Vector Red alkaline phosphatase kit (Vector Laboratories, Burlingame, CA, USA) for 5 min. Sections were then counterstained for 10 s with Gill’s hematoxylin #3, diluted 1:5 with water (Sigma-Aldrich), rinsed in water, and then treated with Scott’s tap water substitute for 2 min. Slides were then dehydrated through 70%, 95%, and 100% ethanol, cleared with xylene, and mounted with Permount (VWR, West Chester, PA, USA). All procedures were performed at room temperature.
Phosphoprotein analysis by flow cytometry
Detection of phosphorylated STAT proteins was performed as described previously [37–39]. Briefly, PBMCs were stimulated for 15 min at 37°C in SFM containing cytokines as indicated and were then washed twice in ice-cold PBS. PBMCs were then incubated with 5 μg/ml CD14-biotin (Serotec) for 30 min on ice. PBMCs were then fixed in 1.6% paraformaldehyde in PBS for 10 min at room temperature and then washed twice in ice-cold PBS. The cell pellet was then resuspended in ice-cold methanol and incubated overnight at −20°C. Cells were then washed three times in ice-cold PBS before staining on ice for 60 min with a 1/50 dilution of Alexa 488-conjugated, antiphosphorylated STAT antibodies or Alexa 488-conjugated, irrelevant antibodies (all from BD Biosciences) in PBS containing 4% BSA. PBMCs were then washed twice in PBS and then incubated with a 1/100 dilution of streptavidin-PerCP (BD Biosciences) in PBS containing 4% BSA for 30 min. PBMCs were washed twice in PBS and then resuspended in PBS containing 2% BSA and analyzed by flow cytometry using a FACScan (BD Biosciences), analyzing ~20,000 events [40]. Data were analyzed using WinMDI software (WinMDI Version 2.8, 2000, written by Joe Trotter, Flow Cytometry Core Facility, The Scripps Institute, La Jolla, CA, USA).
Videomicroscopy
PBMCs were cultured in SFM in the presence or absence of cytokines in eight-well slides (#1 coverglass, Nalge Nunc International) in 400 μl vol at 5× 105 cells per ml in a humidified incubator containing 5% CO2 at 37°C. For videomicroscopy, an Imagepoint charged-coupled device (CCD) camera (Photometrics, Tucson, AZ, USA), mounted on a Nikon TMS inverted microscope with a 10× objective and a blue filter on the illuminator, was placed in the incubator. The CCD used an exposure integration of 4 and a 21-dB gain, and to prevent condensation, the Peltier cooler was disconnected. An external power supply was used to operate the TMS 6V illuminator lamp at 2.3 V/1.8 A. Output from the CCD camera was recorded on S-VHS tape on an AG-6740 time-lapse video recorder (Panasonic, Secaucus, NJ, USA) set at 960 H.
Statistics
Statistical analysis was performed using GraphPad Prism software (Graph-Pad Software, San Diego, CA, USA). Differences between two groups were assessed by Student’s t-test. Differences between multiple groups were assessed by ANOVA using Tukey’s post-test. Significance was defined as P < 0.05. In the figures, * indicates P < 0.05; ** indicates P < 0.01.
RESULTS
IL-4 and IL-13 promote, whereas IL-12 and IFN-γ inhibit, fibrocyte differentiation
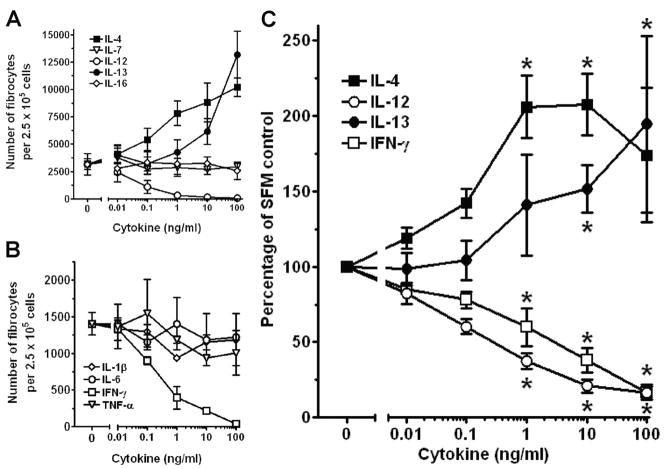
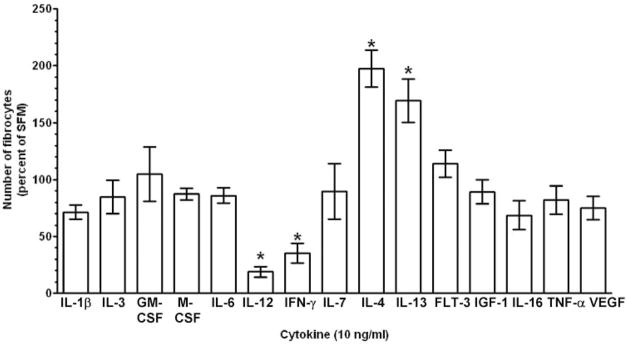
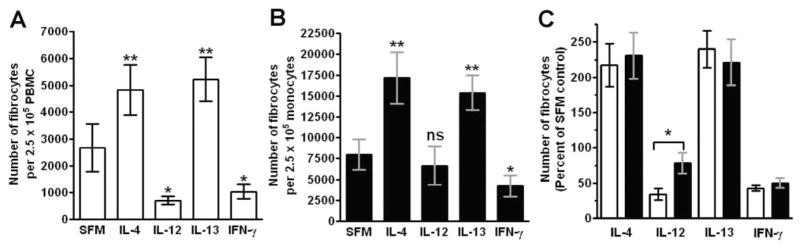
As SAP and aggregated IgG inhibit fibrocyte differentiation, it is possible that other molecules that bind to and activate monocytes could also regulate fibrocyte differentiation [11, 12]. Therefore, we tested if potent monocyte activators, such as the inflammatory cytokines IL-1β, IL-6, IL-12, IL-18, IFN-γ, and TNF-α; differentiation-inducing cytokines, such as IL-3, GM-CSF, and M-CSF; or cytokines associated with fibrosis, such as IL-4 and IL-13, could influence fibrocyte differentiation. The levels of cytokines found in wound fluid, inflammatory lesions, and fibrosis are generally within the 100- to 10,000-pg/ml range [21, 41–43]. PBMCs were cultured for 5 days in SFM with cytokines in a dose range from 0.001 to 100 ng/ml. Of the profibrotic cytokines tested, only in cultures containing IL-4 and IL-13 was there an increase in the number of fibrocytes (Fig. 1, A and C; see below for evidence that these are fibrocytes). The effects of IL-4 and IL-13 were statistically significant at 1 and 10 ng/ml, respectively, whereas a range of other cytokines had no statistically significant effect (Figs. 1C and 2). In contrast, the presence of IFN-γ or IL-12 led to a statistically significant inhibition of fibrocyte differentiation at 1 ng/ml and above (Figs. 1, A–C, and 2). These data suggest that fibrocyte differentiation is enhanced by IL-4 or IL-13 and inhibited by IFN-γ or IL-12 and that IL-1β, IL-3, IL-6, IL-7, IL-16, GM-CSF, M-CSF, FLT-3, IGF-1, VEGF, and TNF-α do not appear to affect the number of fibrocytes after 5 days in our culture conditions (Figs. 1 and 2).
Fig. 1.
Effect of cytokines on fibrocyte differentiation. PBMCs at 2.5 × 105 cells per ml were cultured in SFM for 5 days with the indicated concentrations of cytokines. Cells were then air-dried, fixed, and stained, and fibrocytes were enumerated by morphology. (A) The effects of IL-4, IL-7, IL-12, IL-13, and IL-16 on PBMCs from one representative individual. (B) The effects of IL-1β, IL-6, IFN-γ, and TNF-α on PBMCs from one representative individual. Results are expressed as the mean ± SD of the number of fibrocytes per 2.5 × 105 cells. (C) As the number of fibrocytes differentiating from PBMCs varies between individuals, we analyzed the effect of IL-4, IL-12, IL-13, and IFN-γ on the differentiation of fibrocytes from different donors. For each donor, counts were normalized to the no-cytokine control count to obtain a percent of control. (Control fibrocyte counts were 2716±450 per 2.5×105 cells; mean ± SEM.) Results are expressed as the mean ± SEM of the percent of control (n=6 donors). Statistical significance in comparison with the no-cytokine control (SFM) was determined by ANOVA; *, P < 0.05.
Fig. 2.
Effect of a range of cytokines on fibrocyte differentiation. PBMCs at 2.5 × 105 cells per ml were cultured in SFM for 5 days with 10 ng/ml of the indicated cytokine. Cells were then air-dried, fixed, and stained, and fibrocytes were enumerated by morphology. Results are expressed as the mean ± SEM of the percent of control from at least three separate donors. (Control fibrocyte counts were 3842±475 per 2.5×105 cells; mean±SEM.) Statistical significance in comparison with the no-cytokine control (SFM) was determined by ANOVA; *, P < 0.05. FLT-3, Fetal liver tyrosine kinase 3; IGF-1, insulin growth factor 1; VEGF, vascular endothelial growth factor.
IL-4 and IL-13 promote fibrocyte differentiation without altering morphology or phenotype
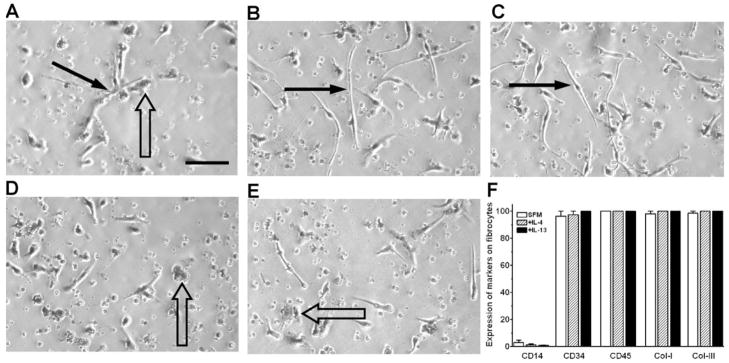
To determine if the presence of IL-4 or IL-13 alters the morphology of fibrocytes, PBMCs were cultured with cytokines for 5 days in SFM and then air-dried and stained with eosin and methylene blue. In cultures without the addition of exogenous cytokines, fibrocytes and monocyte/macrophages were readily detectable (Fig. 3A). When cultured in the presence of IL-4 or IL-13, the elongated, spindle-shaped fibrocytes (Fig. 3, solid arrows) were more numerous, compared with cells cultured without the addition of exogenous cytokines, but there were no discernable effects on the morphology of the fibrocytes (Fig. 3, B and C). When cultured in the presence of IL-12 or IFN-γ, the numbers of fibrocytes were reduced, compared with cells cultured without the addition of exogenous cytokines (Fig. 3, D and E). IL-12 and IFN-γ are classical macrophage-activation agents [31, 44], and as expected, the presence of IL-12 or IFN-γ led to an increase in the number of macrophages (Fig. 3, D and E, open arrows). IL-4 and IL-13 had no obvious effect on the number of macrophages. These data indicate that IL-4 and IL-13 increase the number of spindle-shaped fibrocytes without altering the morphology of these cells.
Fig. 3.
Morphology and expression of molecules by fibrocytes. PBMCs at 2.5 × 105 cells per ml were cultured in SFM for 5 days in the presence or absence of 3 ng/ml cytokines. (A) In SFM, some PBMCs become elongated spindle-shaped fibrocytes (solid arrow), and some cells differentiate into macrophages (open arrow). In the presence of (B) IL-4 or (C) IL-13, the number of elongated, spindle-shaped fibrocytes (solid arrows) increased. In the presence of (D) IL-12 or (E) IFN-γ, the number of elongated, spindle-shaped fibrocytes decreased, and the numbers of macrophages (open arrows) increased. Original bar is 0.1 mm. (F) PBMCs at 2.5 × 105 cells per ml were cultured in eight-well glass slides in SFM for 5 days in the presence or absence of 3 ng/ml IL-4 or IL-13. Cells were then air-dried, fixed in acetone, and stained with antibodies. At least 100 elongated cells with oval nuclei were examined from at least 10 randomly selected fields, and the percentage of elongated cells that were positively stained by antibodies against the indicated markers is expressed as the mean ± SEM (n=3 separate donors).
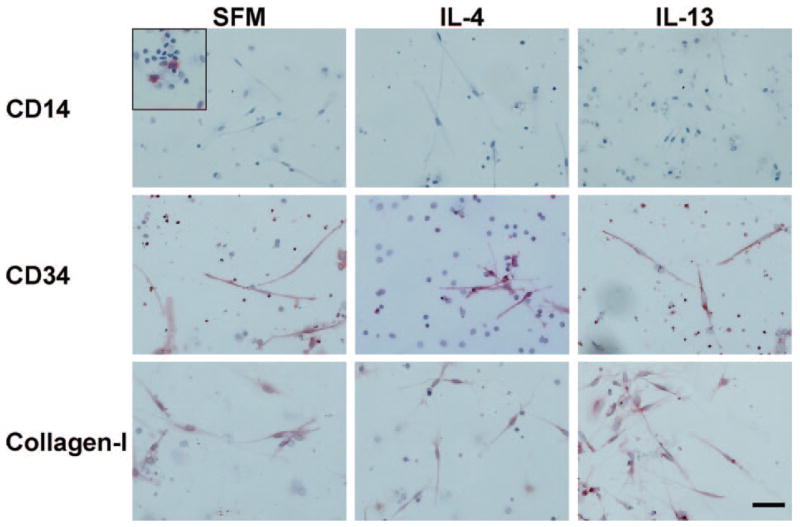
To confirm that the elongated, spindle-shaped cells are fibrocytes and to test if the presence of IL-4 or IL-13 altered their expression of some of the known fibrocyte markers, we stained the cells for markers expressed by fibrocytes. In cultures without the addition of exogenous cytokines, more than 95% of the elongated, spindle-shaped cells expressed CD34, CD43, CD45, and collagen-I and -III (Figs. 3F and 4). When cultured in the presence of IL-4 or IL-13, the elongated cells were likewise positive for CD34, CD43, CD45, and collagen-I (Figs. 3F and 4 and data not shown). These data indicate that IL-4 and IL-13 increase the number of CD34-, CD43-, CD45-, and collagen-I-positive fibrocytes without altering their expression of these markers. As observed previously [45–47], when cultured in the presence of IL-4 or IL-13, the macrophages were positive for CD206 and CD209, whereas in cultures containing IL-12 or IFN-γ, the macrophages were positive for CCR7 (data not shown).
Fig. 4.
Expression of CD14, CD34, and collagen-I by fibrocytes. PBMCs were cultured in eight-well glass slides in SFM for 5 days in the presence or absence of IL-4 or IL-13. Cells were then air-dried, fixed, and stained with antibodies. Cells were counterstained with hematoxylin to identify nuclei. Positive staining was identified by red staining with nuclei counterstained blue. Original bar is 50 μm. The inset for CD14 shows at the same scale positive staining on macrophages from a different area in the same culture.
Direct and indirect effect of cytokines on monocyte-to-fibrocyte differentiation
Fibrocyte precursors appear to differentiate from a subpopulation of CD14+ peripheral blood monocytes [5, 10–12]. To determine whether cytokines regulate fibrocyte differentiation by directly affecting monocytes or affect other cells present in the PBMC population, which then affect monocytes, we incubated purified monocytes with cytokines. To obtain monocytes, PBMCs were depleted of B cells, NK cells, T cells, and dendritic cells by negative selection. This generally produced a population of monocytes that were more than 98% monocytes (data not shown) [11, 12, 32–35], which were then cultured for 5 days in SFM containing IL-4, IL-12, IL-13, or IFN-γ. When cultured in SFM, ~4% of the purified monocytes differentiated into fibrocytes at 5 days. As observed with PBMCs (Figs. 1 and 5A), the addition of IL-4 or IL-13 to purified monocytes increased the numbers of fibrocytes seen at 5 days (Fig. 5B). The addition of IFN-γ to purified monocytes decreased the number of fibrocytes at 5 days (Fig. 5B). Compared with PBMCs, IL-12 did not affect the ability of purified monocytes to differentiate into fibrocytes (Fig. 5B). To more easily compare the response of PBMCs and monocytes to cytokines, we re-analyzed the data from Figure 5, A and B, as the mean ± SEM of the percent of SFM control. This shows that IL-12 only had a significant inhibitory effect on fibrocyte differentiation in whole PBMCs. These data suggest that IL-4, IL-13, and IFN-γ may have a direct action on monocytes, whereas IL-12 does not act directly on monocytes to regulate fibrocyte differentiation.
Fig. 5.
Direct effect of cytokines on monocytes leads to the inhibition of fibrocyte differentiation. Cells were cultured at 2.5 × 105 cells per ml in SFM for 5 days in the presence or absence of 3 ng/ml cytokine. (A) Compared with PBMCs cultured in SFM, IL-4 and IL-13 significantly promoted fibrocyte differentiation (**, P < 0.01), as determined by ANOVA. Compared with PBMCs cultured in SFM, IL-12 and IFN-γ significantly inhibited fibrocyte differentiation (*, P < 0.05), as determined by ANOVA. (B) Compared with monocytes cultured in SFM, IL-4 and IL-13 significantly promoted fibrocyte differentiation (**, P < 0.01), as determined by ANOVA. Compared with monocytes cultured in SFM, IFN-γ significantly inhibited fibrocyte differentiation (*, P < 0.05), as determined by ANOVA. However, in monocyte cultures, IL-12 did not significantly inhibit fibrocyte differentiation [not significant (ns)]. (A and B) Results are expressed as the mean ± SEM of the number of fibrocytes per 2.5 × 105 cells (n=6 separate donors). (C) Data from A and B are represented as the mean ± SEM of the percent of SFM control of fibrocytes from the six individuals. Open bars are data from PBMC cultures, and solid bars are data from monocyte cultures. Only the IL-12 data are statistically different between the PBMC and monocyte cultures (P < 0.05), as determined by Student’s t-test.
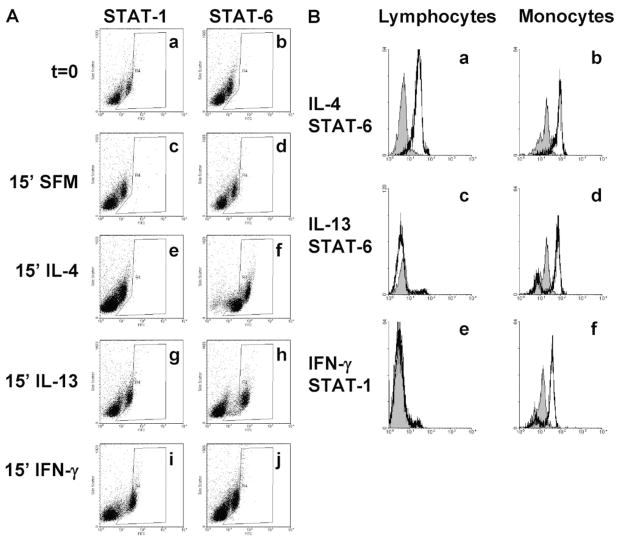
To confirm that IL-4, IL-13, and IFN-γ were having a direct effect on monocytes, we analyzed STAT protein phosphorylation, which is a characteristic for these cytokines [27, 28]. PBMCs were incubated for 15 min, which is the optimal time for STAT activation [37–39, 41], in SFM or SFM containing IL-4, IL-13, or IFN-γ. PBMCs were then fixed and stained for phosphorylated STAT proteins. As expected, PBMCs incubated for 15 min in SFM without exogenous cytokines did not lead to any discernable STAT activation (Fig. 6A, histograms c and d). PBMCs incubated with IL-4 generated a phosphorylated STAT-6 response in lymphocytes and monocytes (Fig. 6, A, histograms e and f, and B, histograms a and b), whereas IL-13 generated a phosphorylated STAT-6 response that was restricted to monocytes (Fig. 6, A, histograms g and h, and B, histograms c and d). PBMCs cultured in the presence of IFN-γ generated a phosphorylated STAT-1 response, which was also restricted to monocytes (Fig. 6, A, histograms i and j, and B, histograms e and f). As a control, we analyzed STAT-3 and STAT-5 activation, which was not significantly phosphorylated by any of the cytokines used (data not shown). We were unfortunately unable to acquire reliable phosphorylated STAT-4 data, so we were unable to analyze IL-12-generated STAT signaling data (data not shown). However, these data do indicate that IL-4, IL-13, and IFN-γ directly act on the monocyte population to generate a signaling cascade, which may influence the differentiation of monocytes into fibrocytes.
Fig. 6.
Analysis of phosphorylation of STAT proteins by cytokines using flow cytometry. PBMCs were incubated in the presence or absence of 3 ng/ml cytokines for 15 min at 37°C. Cells were then washed, fixed, and stained. (A) Histograms show fluorescence intensity (x-axis) and side-scatter characteristics (y-axis) from ~10,000 events. Increased phosphorylated of STAT proteins are indicated by shifts to the right (x-axis) in each plot. The “R4” gate is used to show increased fluorescence intensity compared with the unstimulated control cells. Compared with unstimulated PBMCs, cells incubated with IL-4 show phosphorylated STAT-6 in the small, low, side-scatter (lymphocyte) and the granular, higher side-scatter (monocyte) populations (histogram f). Cells incubated with IL-13 also show phosphorylated STAT-6 but only in the monocyte population (histogram h). Compared with unstimulated PBMCs, cells incubated with IFN-γ show phosphorylated STAT-1 but only in the monocyte population (histogram i). (B) Expression of phosphorylated STAT proteins in lymphocytes and monocytes, gated on forward- and side-scatter characteristics, and CD14 expression. Solid line represents phosphorylated STAT-1 or STAT-6 staining after 15 min of stimulation with the indicated cytokine. Filled areas indicate staining of cells without cytokine stimulation for 15 min. These data indicate that IL-4 induced a phosphorylated STAT-6 response in lymphocytes (a) and monocytes (b), IL-13 induced a phosphorylated STAT-6 response in monocytes (d) but not in lymphocytes (c), and IFN-γ induced a phosphorylated STAT-1 response in monocytes (f) but not in lymphocytes (e).
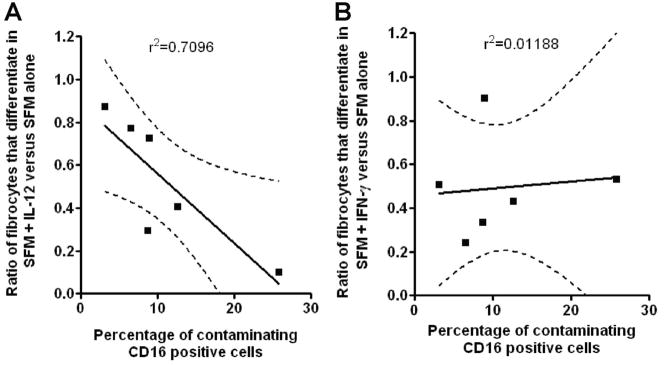
We also analyzed a series of depletion experiments, where we were unable to purify monocytes to greater than 98% purity. In these experiments, we depleted the B cells, T cells, and dendritic cells but were left with a population of contaminating, CD16-positive NK cells, as determined by flow cytometry. To determine whether the number of residual, CD16-positive NK cells in the monocyte preparations influenced the effect of IL-12, the percent of CD16-positive NK cells in the monocyte preparation was correlated with the effect of IL-12 (ratio of fibrocytes that differentiate in SFM with IL-12 vs. SFM alone). We found an inverse correlation (r2=0.7096; n=6) between the number of fibrocytes and the number of contaminating NK cells (Fig. 7A). In comparison, we analyzed the effect of residual, CD16-positive NK cells in the monocyte preparation on the effect of IFN-γ (ratio of fibrocytes that differentiate in SFM with IFN-γ vs. SFM alone). We found no significant correlation (r2=0.0119; n=6) between the number of fibrocytes and the number of contaminating NK cells (Fig. 7B). Together, these data suggest that the inhibitory effect of IL-12 on fibrocyte differentiation in the PBMC population was probably a result of an indirect effect, where IL-12 stimulated NK cells to secrete other factor(s) such as IFN-γ [48–50], which then directly acted on monocytes to inhibit fibrocyte differentiation.
Fig. 7.
Fibrocyte differentiation in monocyte cultures containing increasing numbers of CD16-positive NK cells. Monocyte preparations containing variable numbers of CD16+ NK cells were cultured at 2.5 × 105 cells per ml in SFM for 5 days in the presence or absence of (A) 3 ng/ml IL-12 or (B) 3 ng/ml IFN-γ. Cells were then air-dried, fixed, and stained, and fibrocytes were enumerated by morphology. Results are expressed as a correlation of the ratio of the number of fibrocytes that differentiate in cytokines compared with SFM (y-axis) and the percentage of CD14-negative, CD16-positive NK cells (x-axis). The solid line represents the regression line, and the dotted line shows the 95% confidence limits.
IL-4 and IL-13 promote fibrocyte differentiation without inducing proliferation
There are two possible mechanisms by which IL-4 and IL-13 could increase the number of fibrocytes observed at 5 days. First, IL-4 or IL-13 could induce the proliferation of fibrocyte precursors or fibrocytes. Second, IL-4 or IL-13 could increase the number of fibrocytes that differentiate from a precursor population. To distinguish between these possibilities, we videotaped PBMCs in culture continuously for 8 days, starting when cells were first plated into the culture well. We were unable to detect any PBMCs that divided over this time-frame when cultured in the presence or absence of IL-4 or IL-13, although we could readily observe enhanced fibrocyte differentiation when IL-4 or IL-13 was present compared with no added cytokine (data not shown). Specifically, when we observed fibrocytes backward in time on the videotapes to when they were initially added to the chamber slides, we never saw fibrocytes or their precursors divide, even in the presence of IL-4 or IL-13. To further assess proliferation, PBMCs that were cultured in the presence or absence of IL-4 or IL-13 were stained with PCNA, a marker of proliferating cells [51–53]. We were unable to detect fibrocytes expressing PCNA, although control cultures of THP-1 cells had readily detectable PCNA in the nucleus of dividing cells (data not shown). These data suggest that IL-4 and IL-13 promote an increased number of precursor cells to differentiate into fibrocytes rather than inducing fibrocyte proliferation.
SAP, but not IL-12 or IFN-γ, can inhibit IL-4- or IL-13-induced fibrocyte differentiation
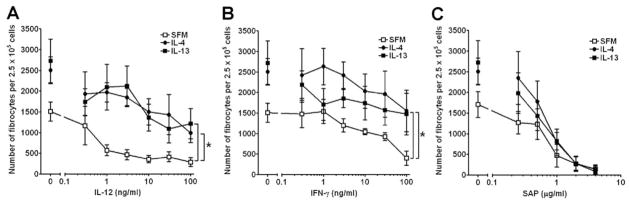
As inflammatory and fibrotic environments rarely, if ever, contain single cytokines and to further understand the effects of cytokines on fibrocyte differentiation, we examined the effects of stimulating PBMCs with combinations of cytokines. PBMCs were cultured in SFM with increasing concentrations of the fibrocyte-inhibiting cytokines IL-12 or IFN-γ in the absence or presence of the fibrocyte-promoting cytokines IL-4 or IL-13 (Fig. 8). As expected, compared with PBMCs cultured without exogenous cytokines, PBMCs cultured in SFM containing increasing concentrations of IL-12 or IFN-γ had significantly lower numbers of fibrocytes, as determined by Student’s t-test (SFM vs. 100 ng/ml IL-12, P=0.0008: SFM vs. 100 ng/ml IFN-γ, P=0.0034; Fig. 8, A and B). However, the presence of an intermediate dose of 3 ng/ml IL-4 or IL-13 could significantly counteract the ability of IL-12 or IFN-γ to decrease the number of fibrocytes (Fig. 8, A and B). These data indicate that IL-4 or IL-13 can counteract IL-12 or IFN-γ and vice versa.
Fig. 8.
SAP inhibits IL-4- or IL-13-induced fibrocyte differentiation. PBMCs were cultured in SFM for 5 days with increasing concentrations of (A) IL-12, (B) IFN-γ, or (C) SAP in the presence or absence of 3 ng/ml IL-4 or IL-13. Compared with PBMCs cultured with increasing concentrations of IL-12 or IFN-γ alone, cells cocultured with IL-4 or IL-13 had increased numbers of fibrocytes. Compared with PBMCs cultured in 100 ng/ml IL-12 or IFN-γ alone, cells cocultured with IL-4 or IL-13 had significantly increased numbers of fibrocytes as determined by ANOVA. However, compared with PBMCs cultured with increasing concentrations of SAP alone, at SAP concentrations above 1 μg/ml, IL-4 or IL-13 had no effect on the numbers of fibrocytes. Results are expressed as the mean ± SEM of the number of fibrocytes per 2.5 × 105 cells (n=6 separate donors); *, P < 0.05.
We previously found that SAP is a potent inhibitor of fibrocyte differentiation [11–13, 15]. To determine if SAP could counteract the profibrotic cytokines IL-4 or IL-13, we cultured PBMCs in SFM with increasing concentrations of SAP in the absence or presence of IL-4 or IL-13 (Fig. 8C). As expected, PBMCs cultured in SFM containing increasing concentrations of SAP had significantly lower numbers of fibrocytes, as determined by Student’s t-test (SFM vs. 4 μg/ml SAP, P=0.0031; Fig. 8C). In the presence of 3 ng/ml IL-4 or IL-13, this inhibitory activity was maintained (Fig. 8C). These data indicate that the fibrocyte-inhibitory activity of SAP has a dominant effect over the fibrocyte-promoting activities of IL-4 and IL-13.
DISCUSSION
In humans, fibrocytes have been detected in tumors, skin wounds, hypertrophic scars, bronchial asthma, pulmonary fibrosis, and nephrogenic fibrosing dermopathy [6, 54–57]. In animal models, fibrocytes are associated with experimental lung, kidney, and liver fibrosis, intimal hyperplasia of the carotid artery, chronic granuloma formation, and skin wounding [6, 58–65]. We have also recently shown that fibrocytes are involved in cardiac fibrosis following ischemia-reperfusion injury in mice and bleomycin-induced lung fibrosis in rats and mice and that the fibrocytes and the fibrosis associated with these processes are inhibited by SAP [13, 15]. These studies clearly indicate that bone marrow-derived fibrocytes are actively involved in many forms of fibrosis.
We found that IL-4 and IL-13 promote fibrocyte differentiation, whereas IL-12 and IFN-γ inhibit fibrocyte differentiation. IL-4, IL-13, and IFN-γ appear to act directly on monocytes, whereas IL-12 acts indirectly, possibly through NK cells. In proinflammatory “Th-1-like” diseases such as uveitis, there are elevated levels of IL-6, IFN-γ, and IL-12 [66–68]. In pulmonary sacroidosis bronchoalveolar lavage (BAL) fluid samples, there are elevated levels of IL-12 and IFN-γ [69, 70]. Also in the BAL of patients with lung infections, the predominant cytokines include IFN-γ and IL-12 [42]. In profibrotic diseases, such as systemic sclerosis, asthma, and idiopathic pulmonary fibrosis, there are elevated levels of IL-4 and IL-13 [43, 69, 71, 72]. Reports measuring multiple cytokines from the same sample support these findings and indicate that it is rare to detect IL-4 or IL-13 without the presence of IL-12 or IFN-γ [21, 73, 74]. These reports and our data suggest that in many chronic inflammatory conditions, the presence of IL-4 or IL-13, even when IL-12 or IFN-γ are present, may promote, or at least maintain, fibrocyte differentiation and fibrosis.
We have shown that 3 ng/ml IL-4 or IL-13 can compete with 100 ng/ml IL-12 and IFN-γ to regulate fibrocyte differentiation. However, the molecular weights of these cytokines are different. Three of the recombinant cytokines have similar molecular weights: IL-4 is 15 kDa; IL-13 is 13 kDa; and IFN-γ is 17 kDa. Therefore, 3 ng/ml IL-4 and IL-13 is ~200 μM, and 3 ng/ml IFN-γ is 176 μM, indicating these cytokines are approximately equimolar. However, IL-12 is a heterodimer of one p35 and one p40 chain and has a corresponding molecular weight of 75 kDa. Therefore, 3 ng/ml IL-12 is 40 μM, approximately one-fifth the concentration of the other three cytokines used. This indicates that IL-12 is a more potent cytokine on a molar basis at inhibiting fibrocyte differentiation.
The fibrocyte-inhibitory activity of SAP was dominant over the fibrocyte-promoting activity of IL-4 and IL-13. This suggests that if SAP is present at an inflammatory site, SAP may well prevent fibrocyte differentiation. Although the levels of SAP have been measured by many groups in the serum, plasma, and urine of healthy individuals and patients with a wide variety of diseases, the concentration of SAP at inflammatory sites is unknown [75–80]. Immunohistochemical staining of SAP has shown that SAP is associated with extracellular tissues, especially reticulin and elastic fibers, found in kidney, skin, and blood vessels, but whether SAP, once bound to ECM, is still biologically active is unclear, and the actual concentration of biologically active SAP present in these sites is unknown [81–86].
How SAP enters the extracellular spaces is unclear. SAP is produced in the liver and like many other large serum proteins, is unlikely to easily cross the endothelial barrier found in noninflamed tissues without specialized transport mechanisms [87, 88]. However, during inflammation when the endothelial layer allows leakage of plasma into the extracellular tissues, SAP may readily enter an inflammatory site. The appearance of fibrocytes within experimental inflammatory lesions within 3–7 days of initiation indicates that the levels of biologically active SAP, or any other molecules that inhibit fibrocyte differentiation, soon fall below a threshold necessary to inhibit fibrocyte differentiation [4, 5, 13, 15, 55, 56, 59]. This then suggests that increasing levels of fibrocyte-inhibitory molecules, such as SAP, might then inhibit fibrosis, and conversely, decreasing levels of fibrocyte-inhibitory molecules or increasing the levels of fibrocyte-promoting molecules may promote wound healing.
Acknowledgments
This work was supported by grant number C-1555 from the Robert A. Welch Foundation, grant 005/04 from the Scleroderma Foundation, and National Institutes of Health grant HL083029. R. H. G. was an Investigator of the Howard Hughes Medical Institute. We thank Anu Maharjan for enumeration of fibrocytes and Deen Bakthavatsalam for size-exclusion column chromatography.
References
- 1.Kumar V, Abbas AK, Fausto N, Kumar V, Abbas AK, Fausto N. Robbins and Cotran: Pathological Basis of Disease. Vol. 7. Philadelphai, PA, USA: Elsevier Saunders; 2005. Tissue renewal and repair: regeneration, healing, and fibrosis; pp. 87–118. [Google Scholar]
- 2.Chettibi S, Ferguson MW, Gallin JI, Snyderman R. Inflammation: Basic Principles and Clinical Correlates. Vol. 3. Philadelphia, PA, USA: Lippincott, Williams and Wilkins; 1999. Wound repair: an overview; pp. 865–881. [Google Scholar]
- 3.Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol. 1990;259:L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- 4.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 6.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 8.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 9.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 11.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79:1242–1251. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilling D, Gomer RH. Regulatory pathways for fibrocyte differentiation. In: Bucala R, editor. FIBROCYTES—New Insights into Tissue Repair and Systemic Fibroses. World Scientific Publishing; Singapore: 2007. pp. 37–60. [Google Scholar]
- 15.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 18.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 19.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 20.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, Lord JM, Gordon C, Buckley CD, Salmon M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–R795. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simms RW, Korn JH. Cytokine directed therapy in scleroderma: rationale, current status, and the future. Curr Opin Rheumatol. 2002;14:717–722. doi: 10.1097/00002281-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z, Kavanagh E, Zang Y, Dolan SM, Kriynovich SJ, Mannick JA, Lederer JA. Burn injury promotes antigen-driven Th2-type responses in vivo. J Immunol. 2003;171:3983–3990. doi: 10.4049/jimmunol.171.8.3983. [DOI] [PubMed] [Google Scholar]
- 24.Tredget EE, Yang L, Delehanty M, Shankowsky H, Scott PG. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interferon Cytokine Res. 2006;26:179–189. doi: 10.1089/jir.2006.26.179. [DOI] [PubMed] [Google Scholar]
- 25.Zurawski SM, Vega F, Jr, Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993;12:2663–2670. doi: 10.1002/j.1460-2075.1993.tb05927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 27.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 28.Horvath CM, Darnell JE. The state of the stats: recent developments in the study of signal transduction to the nucleus. Curr Opin Cell Biol. 1997;9:233–239. doi: 10.1016/s0955-0674(97)80067-1. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann TR, Coffman RL. Th1-cell and Th2-cell— different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 30.Salmon M, Pilling D, Mappin C, Akbar AN, Gordon J, Whetton AD. Human T cell differentiation and cytokine regulation. In: Harris JR, editor. Hemopoietic Cell Growth Factors. New York, NY, USA: Plenum; 1996. pp. 203–215. [Google Scholar]
- 31.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-{γ}: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 32.Pilling D, Kitas GD, Salmon M, Bacon PA. The kinetics of interaction between lymphocytes and magnetic polymer particles. J Immunol Methods. 1989;122:235–241. doi: 10.1016/0022-1759(89)90269-x. [DOI] [PubMed] [Google Scholar]
- 33.Akbar AN, Salmon M, Ivory K, Taki S, Pilling D, Janossy G. Human CD4+ CD45RO+ and CD4+ CD45RA+ T cells synergize in response to alloantigens. Eur J Immunol. 1991;21:2517–2522. doi: 10.1002/eji.1830211031. [DOI] [PubMed] [Google Scholar]
- 34.Salmon M, Pilling D, Borthwick NJ, Viner N, Janossy G, Bacon PA, Akbar AN. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994;24:892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- 35.Pilling D, Akbar AN, Bacon PA, Salmon M. CD4+ CD45RA+ T cells from adults respond to recall antigens after CD28 ligation. Int Immunol. 1996;8:1737–1742. doi: 10.1093/intimm/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 36.Salmon M, Scheel-Toellner D, Huissoon AP, Pilling D, Shamsadeen N, Hyde H, D’Angeac AD, Bacon PA, Emery P, Akbar AN. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997;99:439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 38.Irish JM, Hovland R, Krutzik PO, Perez OD, Bruserud O, Gjertsen BT, Nolan GP. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–228. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Krutzik PO, Hale MB, Nolan GP. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J Immunol. 2005;175:2366–2373. doi: 10.4049/jimmunol.175.4.2366. [DOI] [PubMed] [Google Scholar]
- 40.Faint JM, Pilling D, Akbar AN, Kitas GD, Bacon PA, Salmon M. Quantitative flow cytometry for the analysis of T cell receptor Vβ chain expression. J Immunol Methods. 1999;225:53–60. doi: 10.1016/s0022-1759(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 41.Pilling D, Akbar AN, Girdlestone J, Orteu CH, Borthwick NJ, Amft N, Scheel-Toellner D, Buckley CD, Salmon M. Interferon-β mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–1050. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 43.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18:60–65. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 44.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 45.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 47.Lehtonen A, Ahlfors H, Veckman V, Miettinen M, Lahesmaa R, Julkunen I. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J Leukoc Biol. 2007;82:710–720. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- 48.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 49.Schleicher U, Hesse A, Bogdan C. Minute numbers of contaminant CD8+ T cells or CD11b+CD11c+ NK cells are the source of IFN-{γ} in IL-12/IL-18-stimulated mouse macrophage populations. Blood. 2005;105:1319–1328. doi: 10.1182/blood-2004-05-1749. [DOI] [PubMed] [Google Scholar]
- 50.Bogdan C, Schleicher U. Production of interferon-[γ] by myeloid cells—fact or fancy? Trends Immunol. 2006;27:282–290. doi: 10.1016/j.it.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- 52.Thomas GL, Yang B, Wagner BE, Savill J, El Nahas AM. Cellular apoptosis and proliferation in experimental renal fibrosis. Nephrol Dial Transplant. 1998;13:2216–2226. doi: 10.1093/ndt/13.9.2216. [DOI] [PubMed] [Google Scholar]
- 53.Salmon M, Koto H, Lynch OT, Haddad EB, Lamb NJ, Quinlan GJ, Barnes PJ, Chung KF. Proliferation of airway epithelium after ozone exposure. Effect of apocynin and dexamethasone. Am J Respir Crit Care Med. 1998;157:970–977. doi: 10.1164/ajrccm.157.3.9704067. [DOI] [PubMed] [Google Scholar]
- 54.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 56.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–520. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- 62.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 63.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Varcoe RL, Mikhail M, Guiffre AK, Pennings G, Vicaretti M, Hawthorne WJ, Fletcher JP, Medbury HJ. The role of the fibrocyte in intimal hyperplasia. J Thromb Haemost. 2006;4:1125–1133. doi: 10.1111/j.1538-7836.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 65.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curnow SJ, Falciani F, Durrani OM, Cheung CM, Ross EJ, Wloka K, Rauz S, Wallace GR, Salmon M, Murray PI. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46:4251–4259. doi: 10.1167/iovs.05-0444. [DOI] [PubMed] [Google Scholar]
- 67.Banerjee S, Savant V, Scott RA, Curnow SJ, Wallace GR, Murray PI. Multiplex bead analysis of vitreous humor of patients with vitreoretinal disorders. Invest Ophthalmol Vis Sci. 2007;48:2203–2207. doi: 10.1167/iovs.06-1358. [DOI] [PubMed] [Google Scholar]
- 68.Curnow SJ, Scheel-Toellner D, Jenkinson W, Raza K, Durrani OM, Faint JM, Rauz S, Wloka K, Pilling D, Rose-John S, Buckley CD, Murray PI, Salmon M. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J Immunol. 2004;173:5290–5297. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 69.Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P, Holden DA, Forrester JM, Lazarus A, Wysocka M, Trinchieri G, Karp C. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol. 1996;156:4952–4960. [PubMed] [Google Scholar]
- 70.Shigehara K, Shijubo N, Ohmichi M, Takahashi R, Kon S-i, Okamura H, Kurimoto M, Hiraga Y, Tatsuno T, Abe S, Sato N. IL-12 and IL-18 are increased and stimulate IFN-γ production in sarcoid lungs. J Immunol. 2001;166:642–649. doi: 10.4049/jimmunol.166.1.642. [DOI] [PubMed] [Google Scholar]
- 71.Sakkas LI, Tourtellotte C, Berney S, Myers AR, Platsoucas CD. Increased levels of alternatively spliced interleukin 4 (IL-4δ 2) transcripts in peripheral blood mononuclear cells from patients with systemic sclerosis. Clin Diagn Lab Immunol. 1999;6:660–664. doi: 10.1128/cdli.6.5.660-664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu MC. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–2694. [PubMed] [Google Scholar]
- 73.De Jager W, Hoppenreijs EPAH, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66:589–598. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szodoray P, Alex P, Chappell-Woodward CM, Madland TM, Knowlton N, Dozmorov I, Zeher M, Jarvis JN, Nakken B, Brun JG, Centola M. Circulating cytokines in Norwegian patients with psoriatic arthritis determined by a multiplex cytokine array system. Rheumatology (Oxford) 2007;46:417–425. doi: 10.1093/rheumatology/kel306. [DOI] [PubMed] [Google Scholar]
- 75.Pepys MB, Dash AC, Markham RE, Thomas HC, Williams BD, Petrie A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol. 1978;32:119–124. [PMC free article] [PubMed] [Google Scholar]
- 76.Nelson SR, Tennent GA, Sethi D, Gower PE, Ballardie FW, Amatayakul-Chantler S, Pepys MB. Serum amyloid P component in chronic renal failure and dialysis. Clin Chim Acta. 1991;200:191–199. doi: 10.1016/0009-8981(91)90090-y. [DOI] [PubMed] [Google Scholar]
- 77.Hawkins PN, Rossor MN, Gallimore JR, Miller B, Moore EG, Pepys MB. Concentration of serum amyloid P component in the CSF as a possible marker of cerebral amyloid deposits in Alzheimer’s disease. Biochem Biophys Res Commun. 1994;201:722–726. doi: 10.1006/bbrc.1994.1760. [DOI] [PubMed] [Google Scholar]
- 78.Kiernan UA, Nedelkov D, Tubbs KA, Niederkofler EE, Nelson RW. Proteomic characterization of novel serum amyloid P component variants from human plasma and urine. Proteomics. 2004;4:1825–1829. doi: 10.1002/pmic.200300690. [DOI] [PubMed] [Google Scholar]
- 79.Strachan AF, Johnson PM. Protein SAP (serum amyloid P-component) in Waldenstrom’s macroglobulinaemia, multiple myeloma and rheumatic diseases. J Clin Lab Immunol. 1982;8:153–156. [PubMed] [Google Scholar]
- 80.Cathcart ES, Wollheim FA, Cohen AS. Immunoassay of P-component in amyloidotic sera. Proc Soc Exp Biol Med. 1967;125:1123–1125. doi: 10.3181/00379727-125-32292. [DOI] [PubMed] [Google Scholar]
- 81.Khan AM, Walker F. Age related detection of tissue amyloid P in the skin. J Pathol. 1984;143:183–186. doi: 10.1002/path.1711430305. [DOI] [PubMed] [Google Scholar]
- 82.Kendall CH, Walker F. Amyloid P component in human thyroid. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:75–78. doi: 10.1007/BF02889853. [DOI] [PubMed] [Google Scholar]
- 83.Melvin T, Kim Y, Michael AF. Amyloid P component is not present in the glomerular basement membrane in Alport-type hereditary nephritis. Am J Pathol. 1986;125:460–464. [PMC free article] [PubMed] [Google Scholar]
- 84.Al-Mutlaq H, Wheeler J, Robertson H, Watchorn C, Morley AR. Tissue distribution of amyloid P component as defined by a monoclonal antibody produced by immunization with human glomerular basement membranes. Histochem J. 1993;25:219–227. doi: 10.1007/BF00163818. [DOI] [PubMed] [Google Scholar]
- 85.Butler MG, D’Ardenne AJ, Scott DL. P component in the synovium in rheumatoid and osteoarthritis. Ann Rheum Dis. 1988;47:463–467. doi: 10.1136/ard.47.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pepys MB, Booth DR, Hutchinson WL, Gallimore JR, Collins PM, Hohenester E. Amyloid P component. A critical review. Amyloid. 1997;4:274–295. [Google Scholar]
- 87.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 88.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]