Abstract
Background: Paraquat is a herbicide with a good occupational safety record, but a high mortality after intentional ingestion that has proved refractory to treatment. For nearly three decades paraquat concentration–time data have been used to predict the outcome following ingestion. However, none of the published methods has been independently or prospectively validated. We aimed to use prospectively collected data to test the published predictive methods and to determine if any is superior.
Methods: Plasma paraquat concentrations were measured on admission for 451 patients in 10 hospitals in Sri Lanka as part of large prospective cohort study. All deaths in hospital were recorded; patients surviving to hospital discharge were followed up after 3 months to detect delayed deaths. Five prediction methods that are based on paraquat concentration–time data were then evaluated in all eligible patients.
Results: All methods showed comparable performance within their range of application. For example, between 4- and 24-h prediction of prognosis was most variable between Sawada and Proudfoot methods but these differences were relatively small [specificity 0.96 (95% CI: 0.90–0.99) vs. 0.89 (0.82–0.95); sensitivity 0.57 vs. 0.79, positive and negative likelihood ratios 14.8 vs. 7.40 and 0.44 vs. 0.23 and positive predictive values 0.96 vs. 0.92, respectively].
Conclusions: All five published methods were better at predicting death than survival. These predictions may also serve as tools to identify patients who need treatment and for some assessment to be made of new treatments that are trialled without a control group.
Background
Paraquat (1,1'-dimethyl-4,4'-bipyridinium) dichloride is a non-selective contact herbicide that has been widely used in many countries since the 1960s. It is fast-acting, rain-fast and facilitates ‘no-till’ farming, but it has attracted controversy because of a high mortality in cases of self harm (typical case fatality 50–90%).1 It is a common cause of lethal poisoning in some developing countries in Asia, Pacific Islands and the Caribbean.2 Ingestion of >20 ml of a 20% preparation is likely to cause death from multi-organ failure and cardiogenic shock within 1–4 days, while smaller quantities (10–20 ml) may initiate an irreversible lung fibrosis and renal failure resulting in death within several weeks.1 Current treatments for paraquat poisoning focus on reducing absorption from the gastrointestinal tract and increasing its elimination.1 Several other interventions have been proposed but none has been shown to be effective in clinical trials. The most promising is ‘immunosuppressive’ therapy, but this is not widely used due to the lack of evidence supporting its use.3
A reliable predictor of prognosis would be helpful to guide treatment and future clinical research on antidotes and other therapies. For example, early prediction of inevitable death would be important to stop inappropriate treatments in terminal acute paraquat poisoning patients.4 A tool to identify patients who will die from the delayed onset of lung fibrosis might be useful to identify patients most likely to benefit from antidotes directed at preventing lung injury or fibrosis.3
The measurement of plasma paraquat concentration has considerable support as a marker of severity and prognosis. Proudfoot et al.5 produced a nomogram in 1979 that related the outcome to the plasma paraquat concentration on admission and the time from ingestion to blood collection. In 1987, Scherrmann extended the Proudfoot nomogram so that it was applicable to patients who presented >24 h after ingestion.6 Hart et al.7 created six concentration–time curves to represent estimates of the probability of survival ranging from 10 to 90%. Sawada et al.8 developed a Severity Index for Paraquat Poisoning (SIPP) to predict which patients would die and whether the patient would die from acute organ failure or lung fibrosis. In 1999, using multiple logistic regression of published data, Jones et al.4 produced a calculation using concentration and time to give a numeric estimate of the probability of survival (Table 1). The largest and most recently published study by Gil et al.9 demonstrated a very strong relationship between measured concentrations and survival without sequelae but unfortunately did not validate previous measures nor propose a new method of using plasma concentrations to predict outcome.
Table 1.
Nomograms and prognostic formula for prediction of outcome with paraquat concentrations
| References | Location | Patients | Prognostic formula for survival | Validated by authors | Limitations in application |
|---|---|---|---|---|---|
| Proudfoot et al.5 | UK: London, Edinburgh, Scotland | 71 | Patients with paraquat levels less than a line connecting concentrations of 2.0, 0.6, 0.3, 0.16 and 0.1 μg/ml at 4, 6, 10, 16 and 24 h survive. | No | Applicable only between 4 and 24 h. |
| Hart et al.7 | UK | 219 | Graph plasma paraquat levels vs. time since ingestion. Generating contour map lines denoting equal probability of survival. | No | Applicable up to 28 h. Nomogram unable to assign risk to concentrations taken <4 h which are > 5.5 μg/ml. |
| Scherrmann et al.6 | France | 30 | Survivors have plasma paraquat levels less than C μg/ml where C = 1/(0.471 × (h since ingestion) −1.302) | No | Curve is used to extend Proudfoot for use beyond 24 h. Applicable >4 h. |
| Sawada et al.8 | Japan | 30 | SIPP = time to treatment (h) × serum PQ concentration (μg/ml) SIPP < 10 predicts survival SIPP 10–50 death from lung fibrosis, SIPP > 50 death from circulatory failure. | No | Applicable up to 200 h. |
| Jones et al.4 | Review of worldwide literature | 375 | Probability of survival = exp(logit)/[1 + exp(logit)] Logit = 0.58−[2.33 × log10 (plasma paraquat μg/ml)] − [1.15 × log10 (h since ingestion)] | No | Applicable up to 200 h. |
Thus, paraquat concentration–time data have been used to predict outcome for nearly three decades. However, no method has been prospectively validated in a large cohort study nor have their predictive values been compared. We have used data from patients prospectively enrolled in a cohort study in Sri Lanka to test the accuracy of previously published methods of predicting outcome, and to determine if any were superior.
Methods
Patients and setting
We obtained plasma samples for quantification from 451 patients ingesting paraquat while we conducted two other studies in 10 different Sri Lankan hospitals between April 2002 and January 200610,11 (see Appendix 1: Recruitment flow diagram). These studies were both approved by a number of Sri Lankan and overseas Ethical Review Committees. These studies included 809 patients with paraquat poisoning but 13 were lost to follow up and a further 358 either did not have a paraquat concentration measured or the time of ingestion or measurement was not known or not recorded.
Blood was taken from patients on admission, or if this did not occur, during the patient's first day in hospital. The sample was centrifuged, before the plasma was taken off and immediately stored at −20°C until analysis. Plasma samples were sent to the Syngenta CTL (Alderley Park, Macclesfield, Cheshire, UK) for quantitative analysis using HPLC, LC–MS–MS and LC fluorescence.12 The three techniques have different LoQs; the most sensitive is LC–MS–MS, and this was done when other methods did not detect paraquat. A UV detector was used in tandem with a fluorescence detector to measure very high levels of paraquat, and a proportion of the samples measured by fluorescence were also analysed by LC–MS–MS to confirm that the methods gave very similar results.
Clinical data on patients were prospectively recorded in all hospitals, in particular, whether death occurred in hospital and the time to death. All patients who survived to discharge were visited after 3 months to determine if there were any delayed deaths.
Method of validating nomograms
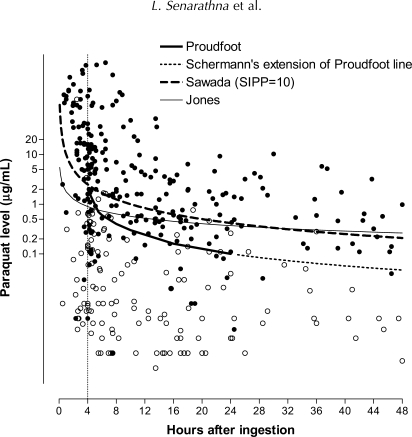
Five methods to predict outcome were evaluated.4–8 We constructed nomograms from the SIPP score calculations of Sawada et al. and the probability score of Jones in order to provide a graphical representation of these prediction tools for visual comparison with the other methods (Figure 1).
Figure 1.
Paraquat concentrations and outcome of patients with paraquat poisoning and Jones, Proudfoot, Scherrmann and Sawada prediction lines. All methods predict those above the lines are more likely to die than survive (filled bullet = death, bullet = alive).
The five methods are applicable over a different range of times. Proudfoot's nomogram can only be applied to levels taken between 4 and 24 h. Hart's nomogram is also difficult to apply outside these times. Thus, we could directly compare the Proudfoot, Hart, Sawada and Jones methods on samples from patients whose plasma paraquat levels were taken between 4 and 24 h (Comparison 1). The methods of Scherrmann, Sawada and Jones could be compared for all patients who had plasma paraquat levels measured at least 4 h after ingestion (Comparison 2).
To calculate sensitivity and specificity 2 × 2 tables were constructed (together with 95% CIs). Sensitivity is defined as the proportion of people who died that were predicted to die, and specificity as the proportion of people who survived that were predicted to survive.13 The positive predictive value is the proportion of those predicted to die who died, and the negative predictive value is the proportion of those predicted to survive who did so. Positive likelihood ratio is the ratio of the likelihood of death in those with a positive test vs. those with a negative test. Negative likelihood ratio is the ratio of the likelihood of death in those with a negative test vs. those with a positive test. All calculations were performed using Graph Pad Prism v 4.0. The Jones and Hart methods for predicting outcome provide varying estimates of risk (very low to moderate to very high). For the Jones equation and Hart nomogram, the line denoting an equal probability of survival or death (50%) was used as the cut off line to create the 2 × 2 table for direct comparison of test performance with the other methods.
The overall performance of the methods for predicting the mortality of the entire cohort (compared to the actual mortality) was also examined. For the Sawada, Proudfoot and Scherrmann methods, this was done by simply counting every person to whom the method could be applied with a concentration above the line as an expected death. For the Jones and Hart methods, we added the expected probability for each individual to provide an overall estimate for the whole cohort.
The formulation of paraquat in Sri Lanka was changed in August 2004 to a new product that was developed to decrease toxicity through a reduction in the amount of paraquat absorbed from the gastrointestinal tract following ingestion.14 Since Tmax values were similar between the two formulations in laboratory studies, we did not expect the nomograms to be affected by variation in formulation ingested. However, to test this hypothesis, we compared data from those ingesting old and new formulations to see if test performance varied with formulation. We had previously noted that gastric lavage may adversely affect outcome,15 and postulated it might alter the performance of the methods. To test this hypothesis, we compared data from a subset of 392 patients that we had recorded the treatment provided to see if test performance varied by whether gastric lavage had been performed.
We also, post hoc, performed a logistic regression of log transformed data to derive a formula that would best have predicted the survival of our population.
Results
Demographic data from the Sri Lankan patients are compared with demographic data from the previous studies in Table 2. The Sri Lankan patients were younger but had a similar gender balance. Reliable information on treatment was available on the 392 patients from the formulation study.10 The most frequent were Fullers’ earth (76.5%; 300/392), and activated charcoal (22.4%; 88); 15.6% (61) received both treatments. Other gastrointestinal decontamination was also common: 12.0% (47) had forced emesis and 61.5% (241) had gastric lavage. Ninety patients (23.0%) were given immunosuppressive treatments and from that group, 11.7% (46) received cyclophosphamide, 11.2% (44) received prednisolone and 2.0% (8) were given both treatments. No patients had haemodialysis. The overall survival rate of 39% in our cohort was comparable to the 30–62% rates observed in the previous studies. There were no statistically significant differences between old and new formulations in specificity and sensitivity of any method (data not shown). Therefore, these groups were combined for all subsequent comparisons.
Table 2.
Demographic data reported in original studies and in the present cohort
| Proudfoot | Hart | Scherrmann | Sawada | Jones | This study | |
|---|---|---|---|---|---|---|
| Total Patients | 73 | 219 | 30 | 30 | 375 | 451 |
| Female | 29 | NA | 8 | 11 | 101 | |
| Male | 42 | NA | 22 | 19 | 350 | |
| Age | NA | |||||
| Mean | 38 | – | 45 | 38.3 | 29 | |
| Median | – | – | – | 25 | ||
| Range (IQR) | 16–81 | 17–75 | – | 1–87 | 12–93 (20–34) | |
| Post ingestion time to blood sampling | NA | NA | ||||
| Median | – | 11 | ||||
| Range (IQR) | 24–360 h | 0.5–335 (4.5–26.0) | ||||
| Number surviving | 45 (62%) | 109 (49.7%) | 9 (30%) | 10 (33%) | 134 (35.7%) | 186 (39.6%) |
The median time from ingestion to blood sampling was 11 h with IQR 4.5–26 h (range 20 min to 335 h). Four samples were taken <1 h and 66 < 4 h post ingestion. The plasma concentrations of patients who lived and died are shown together with the nomogram prediction lines in Figures 1 and 2. It can be seen in Figure 1 that most patients were not near the cut off lines and the use of different methods would change the predicted outcome of only a minority of patients. A higher proportion of the < 4 h samples incorrectly predicted death or survival (Figure 1). In order to directly compare test performance on the same patients, some patients needed to be excluded. There were 451 patients in the cohort but only 385 patients had blood levels taken at least 4 h after ingestion. These 385 patients were used for the comparison of the Jones, Sawada and Scherrmann methods (Comparison 2). One hundred and twenty patients had blood samples more than 24 h after ingestion, therefore 265 patients were used for the direct comparison of Proudfoot, Hart, Sawada and Jones methods (Comparison 1).
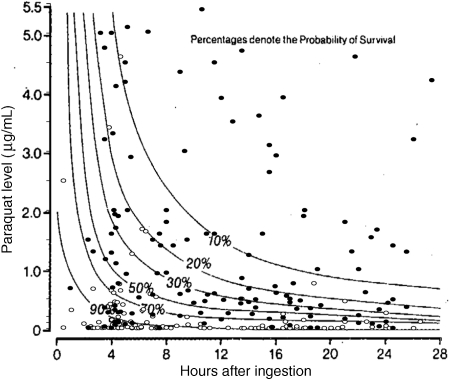
Figure 2.
Paraquat concentrations and outcome from 277 patients (up to 28-h post ingestion) with paraquat poisoning compared with the probability of survival estimated by Hart's nomogram. (filled bullet = death, bullet = alive).
In Comparison 1, the methods of Proudfoot, Hart, Sawada and Jones showed comparable performance. Specificity varied between 0.96 and 0.89 and sensitivity varied between 0.79 and 0.57. Positive predictive values (0.92–0.96) were much better than negative predictive values (0.59–0.73) for all methods (Table 3). Similar overlapping measures of performance were found in Comparison 2 of the Scherrmann, Sawada and Jones methods for all patients with plasma paraquat levels after 4 h (Table 4). There was also no significant change in test performance when all 451 patients were analysed using the methods of Sawada and Jones (Appendix 2).
Table 3.
Comparison of prediction of outcome by four methods in 265 patients with paraquat concentrations between 4- and 24-h post ingestion
| Method | Specificity (95% CI) | Sensitivity (95% CI) | Positive likelihood ratio | Negative likelihood ratio | Positive predictive value (95% CI) | Negative predictive value (95% CI) |
|---|---|---|---|---|---|---|
| Proudfoot et al. | 0.89 (0.82–0.95) | 0.79 (0.72–0.85) | 7.40 | 0.23 | 0.92 (0.86–0.96) | 0.73 (0.64–0.81) |
| Hart et al. | 0.92 (0.85–0.97) | 0.77 (0.69–0.83) | 9.78 | 0.25 | 0.94 (0.88–0.97) | 0.71 (0.63–0.79) |
| Sawada et al. | 0.96 (0.90–0.99) | 0.57 (0.49–0.65) | 14.78 | 0.44 | 0.96 (0.90–0.99) | 0.59 (0.51–0.67) |
| Jones et al. | 0.93 (0.87–0.97) | 0.70 (0.63–0.77) | 10.35 | 0.32 | 0.94 (0.88–0.98) | 0.67 (0.58–0.74) |
Table 4.
Comparison of prediction of outcome by three methods in 385 patients with paraquat concentrations more than 4-h post ingestion
| Method | Specificity (95% CI) | Sensitivity (95% CI) | Positive likelihood ratio | Negative likelihood ratio | Positive predictive value (95% CI) | Negative predictive value (95% CI) |
|---|---|---|---|---|---|---|
| Proudfoot/ Scherrmann | 0.83 (0.76–0.86) | 0.81 (0.76–0.86) | 4.82 | 0.22 | 0.87 (0.82–0.91) | 0.76 (0.69–0.82) |
| Sawada et al. | 0.93 (0.87–0.96) | 0.58 (0.51–0.64) | 7.70 | 0.46 | 0.92 (0.86–0.96) | 0.61 (0.54–0.67) |
| Jones et al. | 0.92 (0.87–0.96) | 0.66 (0.60–0.72) | 8.15 | 0.37 | 0.92 (0.87–0.96) | 0.66 (0.59–0.72) |
All methods under-estimated the actual number of deaths in our cohort (Table 5). Gastric lavage appeared to adversely affect the performance of all methods but the effect was particularly noted in methods using samples taken at <4 h (Table 6). This was reflected in a substantially lower negative predictive value in those who had received gastric lavage. The risk of death was also much further under-estimated in these patients (Table 6).
Table 5.
Comparison of the different methods in terms of estimated total mortality and actual mortality in a Sri Lankan cohort of patients
| Method | Patients to which prognostic method could be applied (n) | Estimated mortality within valid range (%) | Actual mortality (%) |
|---|---|---|---|
| Jones et al. | 451 | 45.8 | 60.3 |
| Sawada et al. | 451 | 40.4 | 60.3 |
| Proudfoot/Scherrmann | 385 | 55.5 | 58.4 |
| Proudfoot et al. | 265 | 52.5 | 61.1 |
| Hart | 277 | 50.2 | 61.7 |
Table 6.
Comparison of nomogram performance depending on whether gastric lavage had been performed, in the subset of patients with data on treatment (392/451)
| Method | Lavaged |
Estimated and actual mortality (%) | No Lavage |
Estimated and actual mortality (%) | ||
|---|---|---|---|---|---|---|
| (n = lavaged, not lavaged) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | ||
| Sawada et al. (n = 266, 126) | 0.94 (0.88–0.98) | 0.50 (0.42–0.58)† | 44, 67 | 0.88 (0.76–0.96) | 0.81 (0.71–0.89)† | 40, 47 |
| Jones et al. (n = 266, 126) | 0.94 (0.88–0.98) | 0.54 (0.46–0.62)† | 38, 67 | 0.89 (0.77–0.96) | 0.85 (0.74–0.92)† | 43, 47 |
| Proudfoot et al. (n = 170, 71) | 0.96 (0.90–0.99)* | 0.62 (0.50–0.73)# | 56, 71 | 0.81 (0.63–0.93)* | 0.90 (0.76–0.97)# | 44, 41 |
| Proudfoot/Scherrmann (n = 251, 104) | 0.90 (0.85–0.95)* | 0.68 (0.58–0.77)* | 59, 66 | 0.76 (0.62–0.87)* | 0.89 (0.77–0.96)* | 48, 42 |
*P < 0.05,#P < 0.005, †P < 0.0001, by Fishers exact test, similar significant differences for these prediction methods were also seen in sensitivity which was lower in the lavaged group.
Our post hoc logistic regression of log-transformed data derived the following probability formula:
Logit = 0.701 + [1.739 × log10 (plasma paraquat μg/ml)] + [0.896 × log10 (h since ingestion)].
This did not change by more than a few percent if the samples taken <4 h from the time of ingestion were excluded. However, when the probabilities were dichotomized to provide a simple line predicting death or survival (Appendix 3), this lower line did not perform much better in terms of clinical prediction for individuals. The better sensitivity (90%) was offset by lower specificity (74%) and the positive likelihood ratio was only 3.37.
Discussion
These results demonstrate that previously published methods to predict a fatal outcome after paraquat poisoning patients using plasma paraquat concentrations are reasonably accurate in predicting death for individuals with paraquat poisoning. However, they all had much better specificity than sensitivity.
The good predictive utility of the measured paraquat concentration is to be expected from the predictable and steep dose-related increase in toxicity. It is therefore valuable to consider why some patients were misclassified. Limited human data suggest that peak plasma concentrations after paraquat ingestion occur within 2–4 h with a distribution half life of 5 h.16 It is therefore likely that plasma paraquat concentrations taken later (at least 4 h) give a better estimate of the total amount of paraquat that has reached the systemic circulation. Four patients survived with a high plasma paraquat concentration within 2–4 h after ingestion; it is likely that these preceded significant distribution. A few patients died with low plasma paraquat concentrations taken within 2–4 h. It is likely that further absorption took place after these blood samples were taken.
Patients misclassified after 4 h may have been atypical. Some may have had a total systemic exposure to paraquat that was greater or less than expected due to the rate of renal paraquat clearance after the blood sample was taken. This might be due to variable pre-morbid renal function and also variable treatments (amount of intravenous fluids) or extent of renal toxicity. Deaths in patients with low plasma paraquat concentrations may relate to individual susceptibilities (older, other co-morbid conditions). In the recent study of a new paraquat formulation10 age over 50 was an independent factor adversely affecting outcome (J. Tomenson, unpublished results), akin to our finding with yellow oleander (Thevetia peruviana) poisoning.17
There may also have been a contribution to death from factors other than paraquat toxicity such as complications of treatment. For example, gastric lavage might have led to aspiration, asphyxia15 or to mediastinal perforation,18 while oxygen therapy may have contributed to free radical damage and lung damage leading later to fibrosis. The adverse effect of gastric lavage on the sensitivity of nomogram negative predictive values and overall mortality (Table 6) might be seen to suggest that lavage is contributing to deaths in patients with low concentrations, however, it is likely to be confounded by other prognostic factors and might also reflect an effect on the pharmacokinetics of paraquat. We have elsewhere attempted to directly address whether gastric lavage leads to benefit or harm after paraquat poisoning,19 but did not find a consistent or large effect. Nevertheless, these data provide further cause for concern about the use of gastric lavage in paraquat poisoning, as it may interfere with prediction of outcome and cause adverse events and the evidence to date does not suggest a benefit is likely.19
It is also possible that the patients used to create the published nomograms represent atypical populations with better than usual survival due to selective reporting. We collected timed (and therefore valuable) samples from 60% of our patients, and the mortality in those who did not have timed samples taken was higher (77.1% vs. 60.3%—Appendix 1). This problem may have been even more of an issue in the selection of patients when the nomograms were derived but the data on the outcome of the total group from which patients were selected were not presented in any of the original studies. Sri Lankan patients themselves may also be different, for example, due to the short time to presentation to hospital. More than 50% of poisoned patients present to a primary care hospital within 2 h of ingestion20 (they were then usually referred on to larger hospitals such as those included in our study). There are no details on the time to presentation in any of the original studies (Table 1). In any case it is clear that all methods substantially underestimated the overall mortality of eligible patients from our unselected cohort of Sri Lankan patients.
Our study is the largest cohort study of paraquat poisoning published; however, it is still not large enough to evaluate the estimates of gradations of risk set out in the Jones equation and the Hart nomogram. Only 123 of the 277 (44.4%) patients (Table 5) that could be evaluated by the Hart nomogram had an estimated risk of death between 10% and 90%. The Jones equation similarly only assigned less than half the patients [223/451 (49.4%)] to an estimated risk of death in this range. However, the underestimate of total mortality by these two methods (Table 5) was comparable to other methods and suggests that they are no more accurate. As most patients do not lie close to the cut off points, a much larger total number of patients would be required to determine if specific Hart risk groups between 10% and 90% were accurate to within 5% or to demonstrate whether the small 5–10% differences in sensitivity and specificity we observed between methods was due to chance or not.21 However, given the overall underestimate of mortality it is unlikely that any of these methods would be the optimal method of prediction of the expected mortality of a cohort.
Prediction may serve a number of purposes. From a clinical perspective it can identify patients who are very likely to survive, where prolonged monitoring or treatment would not be warranted. Conversely, if rapidly available, it might identify patients whose outcome is hopeless so that they may be spared futile life-prolonging measures which have the potential to cause discomfort, such as extracorporeal elimination techniques (e.g. haemodialysis). While these concentrations will give considerable guidance in these decisions, our analysis suggests that they should not be regarded as accurate enough to give such reassurance for life and death decisions.
From a research perspective, patients between those two extremes are those most appropriate to enrol in clinical trials of new treatments. In addition, an accurate method of predicting outcome from paraquat concentrations might allow some estimate to be made of the effectiveness of new treatments that have been studied without a control group. Our study suggests that a plasma paraquat concentration is not sufficiently predictive to infer effectiveness from case reports. However, effectiveness of a new treatment (other than those designed to reduce absorption) might be inferred by much higher survival rates in a large unselected cohort with concentrations predicting death by any method.
Supplementary Data
Supplementary data are available at QJM online.
Funding
Wellcome Trust/National Health and Medical Research Council International Collaborative Research (071669MA to The South Asian Clinical Toxicology Research Collaboration); Wellcome Trust Career Development Fellow (GR063560MA to M.E.); Syngenta Crop Protection AG, Basel, Switzerland (to The second paraquat cohort study). The study sponsors had no involvement in study design, data analysis, data interpretation, or writing of the report.
Conflict of interest: M.F.W. and B.W. are employees of Syngenta, a manufacturer of paraquat. J.A.T. has been a paid consultant to Syngenta. M.E. and N.A.B. have received travel expenses from Syngenta to attend meetings of a scientific advisory group in relation to studies of new paraquat formulations.
Acknowledgements
We thank Prof. Andrew Dawson, Prof. Ravindra Fernando, Prof. P.L. Ariyananda, the research doctors, the medical, nursing and other staff of study hospitals, the SACTRC research coordinators, and research support staff members for their help with this study.
References
- 1.Lock EA, Wilks MF. Handbook of Pesticide Toxicology. 2nd edn. San Diego: Academic Press; 2001. Paraquat. pp. 1559–603. [Google Scholar]
- 2.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–31. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston M, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM. 2003;96:809–24. doi: 10.1093/qjmed/hcg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones AL, Elton R, Flanagan R. Multiple logistic regression analysis of plasma paraquat concentrations as a predictor of outcome in 375 cases of paraquat poisoning. QJM. 1999;92:573–8. doi: 10.1093/qjmed/92.10.573. [DOI] [PubMed] [Google Scholar]
- 5.Proudfoot AT, Stewart MS, Levitt T, Widdop B. Paraquat poisoning: significance of plasma-paraquat concentrations. Lancet. 1979;2:330–2. doi: 10.1016/s0140-6736(79)90345-3. [DOI] [PubMed] [Google Scholar]
- 6.Scherrmann JM, Houze P, Bismuth C, Bourdon R. Prognostic value of plasma and urine paraquat concentration. Hum Toxicol. 1987;6:91–3. doi: 10.1177/096032718700600116. [DOI] [PubMed] [Google Scholar]
- 7.Hart TB, Nevitt A, Whitehead A. A new statistical approach to the prognostic significance of plasma paraquat concentrations. Lancet. 1984;2:1222–3. doi: 10.1016/s0140-6736(84)92784-3. [DOI] [PubMed] [Google Scholar]
- 8.Sawada Y, Yamamoto I, Hirokane T, Nagai Y, Satoh Y, Ueyama M. Severity index of paraquat poisoning. Lancet. 1988;1:1333. doi: 10.1016/s0140-6736(88)92143-5. [DOI] [PubMed] [Google Scholar]
- 9.Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol. 2008;46:515–8. doi: 10.1080/15563650701549403. [DOI] [PubMed] [Google Scholar]
- 10.Wilks MF, Fernando R, Ariyananda PL, Eddleston M, Berry DJ, Tomenson JA, et al. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLoS Med. 2008;5:e49. doi: 10.1371/journal.pmed.0050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, et al. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371:579–87. doi: 10.1016/S0140-6736(08)60270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blake DK, Gallagher RT, Woollen BH. Improved methods for the analysis of paraquat in biological fluids. Chromatographia. 2002;55(Suppl.):S183–5. [Google Scholar]
- 13.Attia J. Moving beyond sensitivity and specificity: using likelihood ratios to help interpret diagnostic tests. Aust Prescriber. 2003;26:111–3. [Google Scholar]
- 14.Heylings JR, Farnworth MJ, Swain CM, Clapp MJ, Elliott BM. Identification of an alginate-based formulation of paraquat to reduce the exposure of the herbicide following oral ingestion. Toxicology. 2007;241:1–10. doi: 10.1016/j.tox.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Eddleston M, Haggalla S, Reginald K, Sudarshan K, Senthilkumaran M, Karalliedde L, et al. The hazards of gastric lavage for intentional self-poisoning in a resource poor location. Clin Toxicol (Phila) 2007;45:136–43. doi: 10.1080/15563650601006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houze P, Baud FJ, Mouy R, Bismuth C, Bourdon R, Scherrmann JM. Toxicokinetics of paraquat in humans. Hum Exp Toxicol JID - 9004560. 1990;9:5–12. doi: 10.1177/096032719000900103. [DOI] [PubMed] [Google Scholar]
- 17.Eddleston M, Dissanayake M, Sheriff MH, Warrell DA, Gunnell D. Physical vulnerability and fatal self-harm in the elderly. Br J Psychiatry. 2006;189:278–9. doi: 10.1192/bjp.bp.105.018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackrill P, Hasleton PS, Ralston AJ. Oesophageal perforation due to paraquat. Br Med J. 1978;1:1252–3. doi: 10.1136/bmj.1.6122.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilks MF, Tomenson JA, Buckley NA, Dawson AH. Influence of gastric decontamination on patient outcome after paraquat ingestion.. Asia Pacific Association of Medical Toxicology (APAMT) meeting; Bangkok. 2007. [Accessed 7 January 2009]. [ http://www.evosof.com/asiatox/papers/Influence of gastric decontamination on patient outcome after paraquat ingestion.pdf. 2007] [Google Scholar]
- 20.Eddleston M, Sudarshan K, Senthilkumaran M, Reginald K, Karalliedde L, Senarathna L, et al. Patterns of hospital transfer for self-poisoned patients in rural Sri Lanka: implications for estimating the incidence of self-poisoning in the developing world. Bull World Health Organ. 2006;84:276–82. doi: 10.2471/blt.05.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carley S, Dosman S, Jones SR, Harrison M. Simple nomograms to calculate sample size in diagnostic studies. Emerg Med J. 2005;22:180–1. doi: 10.1136/emj.2003.011148. [DOI] [PMC free article] [PubMed] [Google Scholar]