Abstract
Aims
To evaluate clinical and arrhythmic outcomes in post-infarction cardiomyopathy patients implanted with a defibrillator (ICD) for primary prevention of sudden death.
Methods and results
The SEARCH-MI registry is a European multi-centre, prospective, observational study enrolling patients after myocardial infarction, chronic left ventricular dysfunction and an ICD implanted for primary prevention of sudden death. Data on 556 patients with at least one recorded follow-up are presented. Survey to Evaluate Arrhythmia Rate in High-risk MI (SEARCH-MI) patients were sicker than those enrolled in MADIT-II with higher New York Heart Association class and left bundle branch block. Total mortality was 10.4%. Close to one-third (30%) of patients experienced episodes of sustained ventricular arrhythmia. One-quarter (23%) received at least one appropriate therapy and 10% inappropriate therapy. Gender (25% males vs. 5% females, P = 0.0009) and history of non-sustained ventricular tachycardia (24% with vs. 18% without P = 0.037) were predictive of appropriate ventricular therapy.
Conclusion
SEARCH-MI represents the current clinical management of post-MI patients with left ventricular dysfunction indicated to defibrillator implant for primary prevention. European routine clinical practice was influenced by landmark trials and guidelines which impacted on the implantation of cardiac resynchronization therapy in over 25% of such patients. Non-sustained ventricular tachycardia identifies subjects with a higher incidence of appropriate ICD therapy.
Keywords: Post-infarction cardiomyopathy, Ventricular arrhythmias, Sudden death, Implantable cardioverter defibrillator
Introduction
Patients with previous myocardial infarction (MI) and depressed left ventricular ejection fraction (LVEF) are at increased risk of death both from heart failure and from malignant ventricular arrhythmias.1 Several studies have been conducted to identify patients at increased risk of sudden death and to improve their clinical management.2–7
In early 2002, the results of the MADIT-II trial were published: this was the first study that randomly assigned patients to receive a defibrillator or optimal medical therapy on the basis of reduced LVEF alone. After a mean follow-up of 20 months, the study was terminated when the pre-defined reduction of 30% in all cause mortality was reached by patients in the defibrillator arm.8
After MADIT-II investigators published their conclusions, a debate emerged on the translation of the expected benefits from strictly controlled trial to ‘routine’ clinical practice. Because of the potentially large number of patients eligible for this form of therapy, there was also some concern with the financial burden arising from broadening indications for ICD implants.9–12 Additional risk stratification was sought to better target the candidates for implantation, aiming to increase the cost-effectiveness of defibrillator therapy.9,13–16 The prognostic value of several techniques (microvolt T-wave alternans, QRS width, baroreflex sensitivity tests) has been investigated without strong evidence of benefits in terms of patient selection.17–19 Programmed ventricular stimulation also played an uncertain role in these selection as previous studies6,7 had enrolled patients when inducible, with no direct comparison performed between inducible and non-inducible subjects. The MUSTT registry20 included also non-inducible patients to demonstrate that inducibility was a strong risk factor for cardiac arrest. Conclusions, however, were that non-inducible patients were also found at increased risk, albeit a risk lower than that observed when inducible.
The high number of defibrillators implanted in the last 5 years should provide an important source of data to compare with MADIT-II patients.
The Survey to Evaluate Arrhythmia Rate in High-risk MI patients (SEARCH-MI) was created in 2002 to document such a comparison by prospectively evaluating the arrhythmic rate, ICD interventions, and adherence to trial-based recommendations on post-MI patients in regular clinical practice. The aim of this paper is to present the interim data on a selection of patients approximately matching the MADIT-II enrolment criteria and to compare the baseline characteristics and outcome of patients found during routine clinical practice with those from the defibrillator arm of the MADIT-II population.
Methods
The SEARCH-MI registry was designed as a multi-centre, prospective, observational study, sponsored by Medtronic. Patient enrolment started in July 2002 (as a consequence of the MADIT-II publication) and this interim analysis was set as of 1 October 2006 for patients with at least one recorded follow-up beyond 70 days.
The only inclusion criteria applicable to this observational study were those related to the MADIT-II publication:8 MI one month or more before entry, LVEF lower than 30%, without coronary revascularization within the preceding 3 months. Exclusion criteria were: implantation of ICD as secondary prevention of sudden death, age <18 years, unwillingness or inability to participate in data collection, and any condition listed as class III in guidelines for defibrillator implantation.21 There was no upper age-limit.
The primary objective of this registry was to prospectively evaluate adherence to trial-based recommendations on post-MI patients in routine clinical practice. Arrhythmic rates, ICD intervention, and other clinical data were collected to establish device usage and report data on device therapy for ventricular tachyarrhythmia. A second goal was to qualify time to the first appropriate treatment for ventricular arrhythmia, detailing anti-tachycardia pacing (ATP) and the more symptomatic cardioversion shocks; cumulative percentage of ventricular pacing (as stored in device memory); clinical events (death due to any cause, rate, and length of hospitalizations for heart failure).
The patients enrolled underwent defibrillator implantation according to standard techniques: single-chamber, dual-chamber, or biventricular devices were implanted per treating physician prescription. All the devices were Medtronic Inc. market-released defibrillators.
Device programming was empirical. Follow-ups were scheduled and performed according to the standard follow-up visit scheme of the participating centres. No additional procedures beyond regular practice were required. Data on demographic and clinical characteristics (medical history, LVEF, NYHA class, QRS width, medications, arrhythmic history) were collected at baseline. At each follow-up examination, patient related data (clinical status, NYHA class, heart failure and other-related hospitalizations, drug therapy changes, atrial fibrillation occurrence) and device-derived data (ventricular arrhythmia documented by the ICD, ICD interventions, percentage of ventricular pacing) were collected. Data obtained from ICD interrogation were stored centrally on a diskette.
The cause of death was provided by the attending physician or collected from clinical records. Deaths were classified according to the following scheme:
Death occurring in the first hour from the onset of symptoms was defined as sudden death;22
Death resulting from a cardiac event was defined as cardiac death.
Unwitnessed deaths were classified as sudden and to qualify unreported mortality and patients lost to follow-up, patient's information was retrieved from the regional demographic service.
Ventricular arrhythmias appropriately fulfilling the detection criteria of ICD devices were defined as ‘sustained ventricular arrhythmias’. This definition is different from the conventional definition of sustained ventricular arrhythmias (i.e. tachycardia lasting more than 30 s or causing hemodynamic collapse).
Classification of the spontaneous episodes and ICD therapies (both appropriate and inappropriate) stored in the device memory were adjudicated by a committee of five physicians in a blinded review process based on an internet platform (Web-EGM database); each episode was independently reviewed by at least two physicians: in the event of disagreement, a third expert contributed to the final adjudication. Arrhythmic events were reported separately as appropriate detections and appropriate therapies to avoid bias from devices programmed in the monitoring state.
The interim survival analysis of patients was based on data available on the stated analysis cut-off date (1 October 2006) including follow-up status, normal study termination, and reported mortality. The protocol was approved by the local ethics committees where required by national law and all patients gave their informed consent.
Statistical analysis
Descriptive statistics were used to characterize the patient population. Univariate comparisons between the baseline characteristics of the patients, with and without ventricular tachyarrhythmia were performed by means of the log-rank test for categorical variables and non-parametric or parametric t-test for continuous non-Gaussian or Gaussian variables, respectively. Relative risk and 95% confidence intervals were calculated for each categorical variable as a predictor of life-threatening arrhythmia in the Cox regression model. Kaplan–Meier estimates of the time from baseline to endpoints were computed by means of a log-rank analysis. A value of P < 0.05 was considered significant and no correction for multiple testing were applied. Univariate comparisons of the baseline characteristics between the patients enrolled in the MADIT-II trial and the SEARCH-MI patients were made by means of the χ2 test.
Study limitations
The study has limitations inherent to its design which include its observational nature, the non-consecutive enrollment, the potential influence of changing guidelines and landmark trials on the enrollment and inclusion of the patients.
The study was a sponsored registry with data electronically collected from Medtronic devices: it was not designed to provide data from devices produced by other manufacturers. For this reason, there is a doubt that 100% of the post-MI patients admitted in the hospital with potential guideline criteria were enrolled in the study. In addition, the study was initiated just after the publication of the MADIT-II trial: it is highly probable that a certain number of patients with indication to ICD implant may not have received an ICD.
The non-consecutive screening and the non-randomized design prevent the interpretation that ICDs improved outcomes.
Results
This interim analysis covers clinical and follow-up data of 556 patients enrolled between July 2002 and October 2005. These were derived from 48 participating centres in four countries with the major contribution from Italy (75%), Germany (22%), Israel (2%), and Austria (1%) completing the patient cohort. The mean follow-up period was 17 ± 10 months and the patients were followed on average 4.8 ± 2.3 times including enrollment (implant) visit (range 1–14). Demographic and baseline data and the comparison with those of the ICD arm of the MADIT-II trial are shown in Table 1.
Table 1.
Baseline comparison between SEARCH-MI and MADIT-II (ICD arm)
| MADIT-II (ICD arm) | Search-MI | P-value | |
|---|---|---|---|
| 742 patients | 556 patients | ||
| Age (years) | 64 ± 10 | 66 ± 10 | 0.00038 |
| Male gender (%) | 84 | 89 | 0.00896 |
| Time from last MI (years)§ | 6.7 ± 6.5 | 7.4 ± 7.5 | 0.05947 |
| LVEF (%) | 23 ± 5 | 26 ± 6 | P < 0.0001 |
| ICD mode %VR | 56 | 50 | 0.03407 |
| DR (%) | 44 | 25 | P < 0.0001 |
| CRT (%) | 0 | 25 | P < 0.0001 |
| Follow-Up (months) | 20 ± 12 | 17 ± 10 | P < 0.0001 |
| NYHA class distribution | |||
| NYHA class I (%) | 35 | 9 | P < 0.0001 |
| NYHA class II (%) | 35 | 46 | P < 0.0001 |
| NYHA class III (%) | 25 | 43 | P < 0.0001 |
| NYHA class IV (%) | 5 | 2 | 0.00449 |
| Hypertension (%) | 53 | 49 | 0.14919 |
| Diabetes (%) | 33 | 29 | 0.11829 |
| CABG (%) | 58 | 35 | P < 0.0001 |
| PCI (%) | 45 | 37 | 0.00397 |
| AF (permanent) (%) | 9 | 7 | 0.18954 |
| LBBB (%) | 19 | 31 | P < 0.0001 |
| Amiodarone (%) | 13* | 25 | P < 0.0001 |
| Statins (%) | 67* | 48 | P < 0.0001 |
| Diuretics (%) | 72* | 86 | P < 0.0001 |
| Beta blockers (%) | 70* | 76 | 0.01426 |
*Comparison assumes unequal variance. Parameters for the ICD arm of MADIT-II from Moss et al.8 except for §: the ‘Time from MI’ and standard deviation is reported from published38 data available for patients from both arms of the MADIT-II cohort (n = 1159). *Medication Reported at last contact for MADIT-II.
The most significant difference in patient characteristics is the increased high-degree NYHA classification (NYHA II = 35% in MADIT-II vs. 46% in SEARCH-MI, P < 0.0001; NYHA III = 25% in MADIT-II vs. 43% in SEARCH-MI, P < 0.0001) and more left bundle branch block (LBBB) (19% in MADIT-II vs. 31% in SEARCH-MI, P < 0.0001). SEARCH-MI patients were less likely to have coronary artery by-pass grafting, were more frequently treated with diuretics (86% vs. 72%, P < 0.0001) and less frequently with statins (48% vs. 67%, P < 0.0001); the treatment rate with β-blockers (P = 0.014) and amiodarone (P < 0.0001) was slightly higher in SEARCH-MI. Twenty-five per cent of SEARCH-MI patients received a dual-chamber ICD vs. 44% in MADIT-II (P < 0.0001); 25% of the SEARCH-MI population received a biventricular device (such devices were not used in the MADIT-II trial). The mean time between MI and ICD implantation was 7.4 years, comparable to the 6.7 years reported in MADIT-II (P = 0.059).
Main clinical events
No deaths or major complications (infections, cardiac tamponade, hemo-pneumothorax) due to the ICD implantation procedure were reported. During follow-up, 33 patients (5.9%) had their device replaced: 27 patients for industry recall; 4 patients for up-grading to biventricular pacing; 2 patients for device end of life.
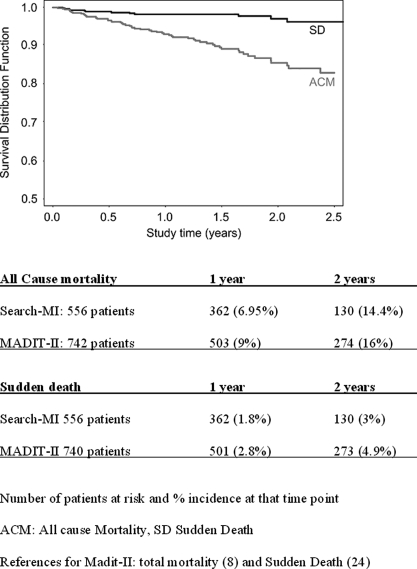
Total mortality was 10.4% (58 out of 556 patients); Kaplan–Meier analysis showed a 2-years ‘all cause mortality’ of 14.4%, close to the 16% reported by MADIT-II (P = ns) (Figure 1).23
Figure 1.
SEARCH-MI registry Kaplan–Meier estimates of the probability of survival from sudden cardiac death and all cause mortality.
The cardiac mortality rate was 3.42% (19 out of 556 patients); 12 patients experiencing sudden death. The Kaplan–Meier estimate showed a 2-year sudden cardiac death of 2.95%, compared with the 4.9% of MADIT-II (Figure 1).23
The mean percentage of ventricular pacing observed in SEARCH-MI patients was 89% in the CRT group and 16% in the non-CRT group.
Arrhythmic events
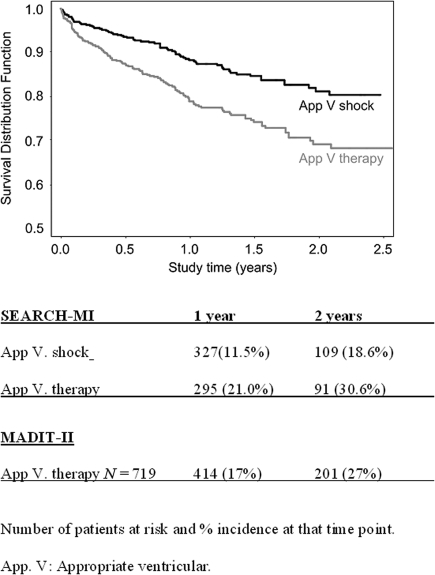
A total of 992 episodes were adjudicated as true ventricular episodes: 167 out of 556 patients (30%) presented with at least one true ventricular arrhythmic detection. In 127 patients (23%) at least one appropriate therapy was delivered: ATP in 85 patients (15%) and shock in 74 patients (13%). These include 32 patients (5.8%) which received both ATP and shock. Several patients had multiple events. The Kaplan–Meier curves illustrate a 2-years incidence of 31% for appropriate therapy (Figure 2). These results are close to those observed in MADIT-II.24
Figure 2.
SEARCH-MI registry Kaplan–Meier estimates of first appropriate ventricular therapy and shock.
In 102 out of 556 patients (18%), a total of 516 tachyarrhythmia episodes were inappropriately detected and stored by the ICD. Overall, an inappropriate device therapy was delivered in 58 of 556 patients (11%), of which 32 patients (6%) received inappropriate shocks.
Ventricular oversensing resulted in 26 events (8 patients) being treated with inappropriate shock. There were 178 episodes of inappropriately treated Atrial Tachy-arrhythmia distributed in 45 patients receiving 103 ATP and in 25 patients who received 75 inappropriate shocks.
These episodes were further classified by the expert reviewers as Sinus Tachycardia (49 episodes, 27%), Atrial Fibrillation/flutter (74 episodes, 41%), and Atrial Tachycardia (55 episodes, 31%).
The mean cycle length of arrhythmic episodes treated by ATP was 328 ± 33 ms for appropriate events and 369 ± 34 ms for inappropriate therapy (P < 0.0001). The mean cycle length of arrhythmic episodes treated by shock was 322 ± 47 ms for appropriate interventions and was slightly longer at 350 ± 31 ms for inappropriate shocks (P < 0.0001).
The incidence of inappropriate device detections and therapies may have related to device programming.
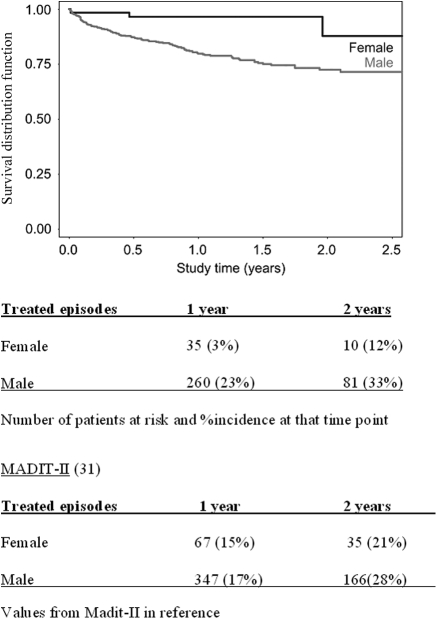
The only two baseline characteristics predictive of appropriate defibrillator therapy were gender (Figure 3) (25% incidence in males vs. 5% in females, P = 0.0009) and history of non-sustained ventricular tachycardia (NSVT) (24% incidence in patients with NSVT vs. 18% in patients without NSVT, P = 0.037). NYHA class, QRS width, history of syncope, diabetes, cumulative ventricular pacing, and LBBB were not predictive of arrhythmic events.
Figure 3.
SEARCH-MI registry Kaplan–Meier probability of appropriate ICD therapy for VT/VF stratified by gender.
No differences were observed when stratified by device type (single/dual-chamber /biventricular) for inappropriate defibrillator intervention.
Discussion
The SEARCH-MI registry represents the routine management, between 2002 and 2005, of (mostly) Italian and German patients with post-MI cardiomyopathy and depressed LVEF treated with ICD for the primary prevention of sudden death. No mandatory device selection, programming, or medical therapy was defined in the protocol. This approach is expected to complete data published by selective, prospective, randomized clinical trials and is able to evaluate the adherence to guidelines in medical practice. For this reason, the data obtained by our registry have been mainly compared with those coming from the MADIT-II study, the first large scale randomized clinical trial for primary prevention of sudden death in ischaemic patients.
The incidence of ventricular tachyarrhythmia therapy is similar in SEARCH-MI and in MADIT-II and can be interpreted as a confirmation of the high arrhythmic risk of these patients. Notwithstanding the poorer clinical status of SEARCH-MI patients, the analysis showed a similarity in all cause mortality with the populations of MADIT-II and of another recently published study.25 This observation might be explained by different hypotheses: about a quarter of the patients were treated with CRT devices while no MADIT-II patients were treated with CRT; moreover the SEARCH-MI registry started in 2002, 5 years after MADIT-II started. In this time interval the clinical management of ischaemic cardiomyopathy has improved. In the light of these important clinical differences, the comparison with the MADIT-II population should be viewed with caution.
In addition, enrolment started just after the publication of MADIT-II trial results: at that time, according to ACC guidelines,21 the implantation of an ICD in this setting had a class IIa (with a level of evidence B) indication and clinicians did not extensively treat their post-MI patients with defibrillators to prevent sudden death. The vast majority of enrolled patients received the indication to implantation mainly during hospital admission for other causes (almost always chest pain or decompensated heart failure): perhaps this explains the more advanced NYHA class of registry patients compared with the MADIT-II population.
It also highlights the strength of an observational study which is expected to follow the routine cardiology patient and reflect changes in clinical practice. Hence the SEARCH-MI enrolment was affected by the results of the SCD-HeFT,26 CARE-HF,27 and COMPANION28 trials which were published in this period, along with the amended implant guidelines.29
A total of 992 ventricular tachyarrhythmia episodes were recorded over the 17 months follow-up period. Considering that the time lapsed between the last MI and ICD implantation was much longer than the study follow-up period, this finding confirms that not all ventricular tachyarrhythmia episodes should be regarded as potentially lethal events. In support to this is the observation that only 23% of the patients received device treatment while 30% of patients showed sustained ventricular arrhythmias fulfilling the device detection criteria. Some patients also had VT therapy programmed off with detection programmed as monitoring-only, up to the first arrhythmic event. We could speculate that arrhythmogenic device effects should be low in view of the low (16%) ventricular pacing observed in the non-CRT patients during follow-up.
The evolving nature of the left ventricular remodelling process overtime and the fact that the substrate may take time to form may explain why devices stored such an amount of arrhythmias after implantation; moreover, some ventricular tachyarrhythmia would have been self-limited but lasted long enough to prompt ICD intervention.
The incidence of inappropriate therapy in our series was comparable to that observed in the MADIT-II trial and was not influenced by the device type (dual- vs. single-chamber). Almost all inappropriate interventions were triggered by supraventricular tachyarrhythmia with cycle lengths significantly longer than appropriately treated ones.
These observations suggest a need to tailor device programming, perhaps by narrowing the VT/VF detection window and prolonging detection time, which could help reduce inappropriate or unnecessary ICD interventions.
The only significant risk stratifiers and predictors of appropriate ICD firing were gender30 and history of non-sustained VT before enrolment, confirming other published results.
Although ICD firing and device therapies (ATP) are not considered appropriate surrogate endpoints for sudden cardiac death, some parallel can be drawn considering the risk stratification markers reported in the literature.
There is conflicting literature on the relationship to arrhythmic events: the fact that QRS width was not predictive of appropriate ICD firing in the SEARCH-MI comes as a confirmation of many other studies on ischaemic patients. Some comparisons may be done with baseline characteristics of other study populations: the PAIN-Free II sub-analysis reported no association between prolonged QRS duration and rapid VT/VF events31 and a poor association between QRS duration and SCD in the ICD-implanted arm of the MADIT-II study,32 whereas QRS duration was only associated to the non-device arm of the EVEREST Heart failure study,33 and has not been found useful for predicting all cause mortality in SCD-HeFT, also following heart failure patients.
Numerous explanations come into play: as QRS duration has been used, in the past, to select patients for implantation, there is the statistical possibility of a ‘regression to the mean’ and considerable bias in the accuracy and round-off errors for these manual measurements. In addition, QRS is only measured once at baseline and this may not reflect the time-dependence and evolution of the ischaemic disease.
SEARCH-MI also confirms, in routine practice, that fewer women seem to be enrolled with ICDs and that they were less likely to experience ventricular arrhythmia, thus confirming previous observations.30–34 They seem consistent with some epidemiological observations which point to a reduced susceptibility of women to ventricular arrhythmia in the setting of post-MI cardiomyopathy.35 The lower incidence of women in these studies is also reflected in the almost 3-fold higher probability of receiving an ICD in the US centers for Medicare & Medicaid report (1991–2005) observed in the 136 421 patients sample from their primary prevention cohort sample.36
The study shows that ICD implantation is very safe as previously reported.37 In fact, no deaths or major complications due to the procedure were reported in the registry.
In conclusion, the observational SEARCH-MI registry demonstrates that routine clinical practice in European patients can replicate the results of therapeutic interventions observed in the more selected MADIT-II trial populations.
Conflict of interest: M.L. has received consulting fees and honoraria from Medtronic, Sorin and St. Jude Medical. E.S. is a Medtronic employee. M.M. holds stock in and is an employee of Medtronic.
Funding
Funding to pay the Open Access publication charges for this article was provided by Medtronic.
References
- 1.Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Saunders and Co; 2004. [Google Scholar]
- 2.Bigger JT, Jr, Fleiss JL, Kleiger R, Miller JP, Rolnitzky LM. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69:250–8. doi: 10.1161/01.cir.69.2.250. [DOI] [PubMed] [Google Scholar]
- 3.Buxton AE, Marchlinski FE, Waxman HL, Flores BT, Cassidy DM, Josephson ME. Prognostic factors in non-sustained ventricular tachycardia. Am J Cardiol. 1984;53:1275–9. doi: 10.1016/0002-9149(84)90078-x. [DOI] [PubMed] [Google Scholar]
- 4.Ruskin JN, McGovern B, Garan H, DiMarco JP, Kelly E. Antiarrhythmic drugs: a possible cause of out-of-hospital cardiac arrest. N Engl J Med. 1983;309:1302–6. doi: 10.1056/NEJM198311243092107. [DOI] [PubMed] [Google Scholar]
- 5.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 6.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. The Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 7.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. The Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 8.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 9.Essebag V, Eisenberg MJ. Expanding indications for defibrillators after myocardial infarction: risk stratification and cost effectiveness. Cardiac Electrophysiol Rev. 2003;7:43–8. doi: 10.1023/a:1023639006565. [DOI] [PubMed] [Google Scholar]
- 10.Prystowsky EN. Primary prevention of sudden cardiac death: the time of your life. Circulation. 2004;109:1073–5. doi: 10.1161/01.CIR.0000121314.13795.F1. [DOI] [PubMed] [Google Scholar]
- 11.Bigger JT, Kleiger RE. Individualizing decisions for patients with prophylactic implantable cardiac defibrillators subject to device advisories: a commentary. Am J Cardiol. 2006;98:1291–3. doi: 10.1016/j.amjcard.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Proclemer A, Ghidina M, Cicuttini G, Gregori D, Fioretti PM. Impact of the main implantable cardioverter-defibrillator trials for primary and secondary prevention in Italy: a survey of the national activity during the years 2001-2004. Pacing Clin Electrophysiol. 2006;29:S20–S28. doi: 10.1111/j.1540-8159.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 13.Cohen RJ. Enhancing specificity without sacrificing sensitivity: potential benefits of using microvolt T-wave alternans testing to risk stratify the MADIT-II population. Cardiac Electrophysiol Rev. 2003;7:438–42. doi: 10.1023/B:CEPR.0000023161.35685.68. [DOI] [PubMed] [Google Scholar]
- 14.Chan PS, Stein K, Chow T, Fendrik M, Bigger JT, Vijan S. Cost-effectiveness of a microvolt T-wave alternans screening strategy for implantable cardioverter-defibrillator placement in the MADIT-II eligible population. J Am Coll Cardiol. 2006;48:112–21. doi: 10.1016/j.jacc.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 15.Al-Khatib SM, Anstrom KJ, Eisenstein EL, Peterson ED, Jollis JG, Mark DB, et al. Clinical and economic implications of the Multicenter Automatic Defibrillator Implantation Trial-II. Ann Intern Med. 2005;142:593–600. doi: 10.7326/0003-4819-142-8-200504190-00007. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds MR, Josephson ME. MADIT II (Second Multicenter Automated Defibrillator Implantation Trial) debate risk stratification, costs and public policy. Circulation. 2003;108:1779–83. doi: 10.1161/01.CIR.0000086777.82110.F5. [DOI] [PubMed] [Google Scholar]
- 17.Al-Khatib SM, Sanders GD, Bigger JT, Buxton AE, Califf RM, Carlson M, et al. Preventing tomorrow's sudden cardiac death today: Part I: current data on risk stratification for sudden cardiac death. Am Heart J. 2007;153:941–50. doi: 10.1016/j.ahj.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Sanders GD, Al-Khatib SM, Berliner E, Bigger JT, Buxton AE, Califf RM, et al. Preventing tomorrow's sudden cardiac death today: Part II: translating sudden death risk assessment strategies into practice and policy. Am Heart J. 2007;153:951–9. doi: 10.1016/j.ahj.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, Kaufman ES, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II Conundrum. Circulation. 2004;110:1885–9. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 20.Buxton AE, Lee KL, DiCarlo L, Gold MR, Greer GS, Prystowsky EN, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. N Engl J Med. 2000;342:1937–45. doi: 10.1056/NEJM200006293422602. [DOI] [PubMed] [Google Scholar]
- 21.Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, et al. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers Antirrhythmia Devices: Summary Article. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) J Cardiovasc Electrophysiol. 2002;13:1183–99. doi: 10.1046/j.1540-8167.2002.01183.x. [DOI] [PubMed] [Google Scholar]
- 22.Myerburg RJ, Castellanos A. Cardiac Arrest and Sudden Cardiac Death in Braunwald's Heart Disease – A Textbook of Cardiovascular Medicine. 6th ed. Philadelphia: WS Saunders Company; 2001. pp. 890–931. [Google Scholar]
- 23.Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML, et al. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II) J Am Coll Card. 2004;43:1459–65. doi: 10.1016/j.jacc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 24.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long term clinical course of patients after termination of ventricular tachy arrhythmia by an implantable defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 25.Groeneveld PW, Farmer SA, Suh JJ, Matta MA, Yang F. Outcomes and costs of implantable cardioverter-defibrillators for primary prevention of sudden cardiac death among the elderly. Heart Rhythm. 2008;5:646–53. doi: 10.1016/j.hrthm.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 27.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 28.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 29.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): Developed in Collaboration With the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 30.Lampert R, McPherson CA, Clancy JF, Caulin-Glaser TL, Rosenfeld LE, Batsford WP. Gender differences in ventricular arrhythmia recurrence in patients with coronary artery disease and implantable cardioverter-defibrillators. J Am Coll Card. 2004;43:2293–9. doi: 10.1016/j.jacc.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 31.Buxton AE, Sweeney MO, Wathen MS, Josephson ME, Otterness MF, Hogan-Miller E, et al. QRS duration does not predict occurrence of ventricular tachyarrhythmias in patients with implanted cardioverter-defibrillators. J Am Coll Cardiol. 2005;46:310–6. doi: 10.1016/j.jacc.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 32.Dhar R, Alsheikh-Ali AA, Estes NA, III, Moss AJ, Zareba W, Daubert JP, et al. Association of prolonged QRS duration with ventricular tachyarrhythmias and sudden cardiac death in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) Heart Rhythm. 2008;5:807–13. doi: 10.1016/j.hrthm.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC, Jr, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656–66. doi: 10.1001/jama.299.22.2656. [DOI] [PubMed] [Google Scholar]
- 34.Zareba W, Moss AJ, Hall WJ, Wilber DJ, Ruskin JN, McNitt S, et al. Clinical course and implantable cardioverter defibrillator therapy in postinfarctionwomen with severe left ventricular dysfunction for the MADIT II Investigators. J Cardiovasc Electrophysiol. 2005;16:1265–70. doi: 10.1111/j.1540-8167.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB, Wilson PW, D'Agostino RB, Kobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–12. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 36.Curtis LH, Al-Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter-defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007;298:1517–24. doi: 10.1001/jama.298.13.1517. [DOI] [PubMed] [Google Scholar]
- 37.Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–55. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 38.Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, et al. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082–4. doi: 10.1161/01.CIR.0000121328.12536.07. [DOI] [PubMed] [Google Scholar]