Abstract
We describe breast cancer incidence and mortality in the predominantly African-origin population of Barbados, which shares an ancestral origin with African-Americans. Age-standardized incidence rates were calculated from histologically confirmed breast cancer cases identified during a 45-month period (July 2002–March 2006). Mortality rates were estimated from death registrations over 10-years starting January 1995. There were 396 incident cases of breast cancer for an incidence rate of 78.1 (95% confidence interval (CI) 70.5–86.3), standardized to the US population. Breast cancer incidence in African-Americans between 2000 and 2004 was 143.7 (142.0–145.5) per 100,000. Incidence peaked at 226.6 (174.5–289.4) per 100,000 among Barbadian women aged 50–54 years, and declined thereafter, a pattern in marked contrast to trends in African-American women, whose rates continued to increase to a peak of 483.5 per 100,000 in those aged 75–79 years. Incidence rate ratios comparing Barbadian and African-American women showed no statistically significant differences among women aged ≤39 years, marginal statistical differences among women 40–54 years and strongly significant differences among women aged ≥ 55 years (p ≤ 0.001 at all older ages). The age-standardized mortality rate in Barbados was 32.9 (29.9–36.0) per 100,000; similar to reported US rates. The pattern of diverging breast cancer incidence between Barbadian and African-American women may suggest a greater contribution from genetic factors in younger women, and from environmental factors in older women. Studies in intermediate risk populations, such as Barbados, may assist the understanding of racial disparities in breast cancer.
Keywords: breast cancer, African-origin, incidence, mortality
Although breast cancer incidence rates are lower in African-American women than comparable White populations, their morbidity and mortality rates are higher.1–3 African-American women tend to present with late stage disease, which may reflect poor access to or utilization of health services, or indeed more aggressive forms of the disease.4–7 Insight into reasons for these differences may be gained by studying breast cancer patterns in other populations across the African diaspora. Of particular interest are comparisons with African-Caribbean populations, which share a common heredity with African-Americans and West Africans.8 As Caribbean countries undergo developmental changes consistent with the latter stages of the epidemiological transition,9 high rates of lifestyle-related chronic diseases are now prevalent,10–12 underpinning the similar health profiles in African-Caribbean and African-American populations. There remains, however, a dearth of relevant epidemiological data about breast cancer, reported to be the principal malignancy affecting women in the region.13
The aim of this report is to provide the first population-based data of breast cancer incidence and trends in mortality for women in Barbados, West Indies. Barbados is an independent Caribbean island nation in the western Atlantic Ocean, with an estimated population of about 270,000. At the 2000 household census, over 90% of all Barbadians were of African descent, approximately 4% were of European origin, while South Asian and other ethnic groups accounted for less than 2% of the population.14 The geographic features of the island, its comprehensive publicly-funded healthcare system, centralized pathology, radiology and other clinical specialist services, as well as cooperation of clinical colleagues, have facilitated the development of a comprehensive epidemiologic study of breast and prostate cancer. The Barbados National Cancer Study commenced in 2002 with the aim of describing the incidence and risk factors (environmental and genetic) for breast and prostate cancer. Our study provided infrastructure and allowed data collection for this report.
Material and methods
The Barbados National Cancer Study (BNCS) centers are the Clinical Center (Ministry of Health and The University of the West Indies, Bridgetown, Barbados, West Indies), the Local Laboratory Center (The University of the West Indies), the Coordinating Center (University Medical Center, Stony Brook, NY), the National Human Genome Research Institute (NHGRI) Center (Bethesda, MD) and the Gene Discovery Center (Translational Genomics Research Institute, Phoenix, AZ). The BNCS is funded by the NHGRI, with contribution from the Office for Minority Health.
All histologically confirmed cases of breast cancer occurring for the first time (in situ and invasive disease) were ascertained from records held at the Pathology Department of the Queen Elizabeth Hospital, Bridgetown. This is the sole public tertiary care hospital and all pathological samples in the country are evaluated in the department. Other data sources such as patient charts and pathology reports were reviewed and cases of recurrent breast cancer or disease occurring in nonresidents (i.e., domiciled for less than 6 months a year) were excluded. To ascertain mortality from breast cancer, we reviewed all death certificates held at the offices of the Registrar General for the 10-year period commencing January 1, 1995. These data were collected independently of the standard data gathering activities of the BNCS.
Statistical methods
We calculated crude incidence rate per 100,000 years of observation by dividing the number of incident cases by the number of women in the Barbados population and multiplying by 100,000. We calculated age specific incidence rates in 18 age groups (0–4 years, 5–9 years, 10–14 years,…, 85 years and older). The incidence of breast cancer is known to increase with age: to allow comparisons independent of age, we calculated age-standardized rates (with 95% confidence intervals), using the direct method, applied to 3 standard populations: the 2000 US standard population15 and the IARC European and World standard million populations.16 Age-standardized data (using the 2000 US standard population) were compared to the US breast cancer incidence rates from the Surveillance Epidemiology and End Results (SEER) reports for the period of 2000–2004.17 The same techniques were used to calculate age-stratified and age-standardized death rates for the 10-year study period of 1995–2004. For crude and age-stratified rates we calculated exact Poisson confidence intervals, and for age-standardized confidence intervals we used a Gamma approximation, which has improved properties when the number of cases is small.18 We compared Barbados and SEER incidence rates by age and year of diagnosis using incidence rate ratios, calculated from a log-linear model. We performed all analyses using Stata (Version 10, StataCorp LP, College Station, TX).
Results
During the study period, 396 women were diagnosed with histologically confirmed breast cancer. Table I presents age-specific incidence of female breast cancer in Barbados. The age at presentation ranged from 26 to 100 years, with a median age of 54 years (interquartile range of 45–68 years). Crude breast cancer incidence increased from 7.5 (95% CI 1.6–21.9) per 100,000 in women aged 25–29 years, to a peak at 226.6 (95% CI: 174.5–289.4) per 100,000 in women aged 50–54 years, declining thereafter in women of postmenopausal age. The overall crude incidence rate was 75.6 (95% CI: 68.4–83.5) per 100,000, with rates of 78.1 (70.5–86.3), 78.6 (70.7–87.2) and 58.4 (52.5–65.0) per 100,000 standardized to the US, European and World populations, respectively. Comparable US rates varied according to ethnic group, such that overall breast cancer incidence in White and African-American women was 162.7 (95% CI: 162.1–163.4) per 100,000 and 143.7 (95% CI: 142.0–145.5) per 100,000, respectively.
TABLE I.
AGE-SPECIFIC AND AGE-STANDARDIZED INCIDENCE OF FEMALE BREAST CANCER IN BARBADOS BETWEEN JULY 1, 2002 AND MARCH 30, 2006 PER 100,000 PERSON-YEARS OF OBSERVATION
| Age group | Number of cases | Person years1 | Age specific rate | 95% CI |
|---|---|---|---|---|
| 0–4 | 0 | 33,891 | 0 | 0–10.9 |
| 5–9 | 0 | 36,670 | 0 | 0–10.1 |
| 10–14 | 0 | 36,535 | 0 | 0–10.1 |
| 15–19 | 0 | 37,676 | 0 | 0–9.8 |
| 20–24 | 0 | 35,767 | 0 | 0–10.3 |
| 25–29 | 3 | 40,035 | 7.5 | 1.6–21.9 |
| 30–34 | 15 | 40,166 | 37.3 | 20.9–61.6 |
| 35–39 | 25 | 44,333 | 56.4 | 36.5–83.2 |
| 40–44 | 45 | 41,959 | 107.3 | 78.2–143.5 |
| 45–49 | 50 | 35,028 | 142.7 | 106.0–188.2 |
| 50–54 | 64 | 28,239 | 226.6 | 174.5–289.4 |
| 55–59 | 43 | 20,730 | 207.4 | 150.1–279.4 |
| 60–64 | 25 | 19,894 | 125.7 | 81.3–185.5 |
| 65–69 | 34 | 18,780 | 181.1 | 125.4–253.0 |
| 70–74 | 25 | 17,973 | 139.1 | 90.0–205.3 |
| 75–79 | 26 | 13,799 | 188.4 | 123.1–276.1 |
| 80–84 | 19 | 11,068 | 171.7 | 103.4–268.1 |
| 85+ | 17 | 10,974 | 154.9 | 90.2–248.0 |
| Unknown age | 5 | |||
| Crude rate | 396 | 523,517 | 75.6 | 68.4–83.5 |
| Age standardized rates (US) | – | – | 78.1 | 70.5–86.3 |
| Age standardized rates (Europe) | – | – | 78.6 | 70.7–87.2 |
| Age standardized rates (World) | – | – | 58.4 | 52.5–65.0 |
85+ age group rounded down to 10,974 to maintain correct person-year total.
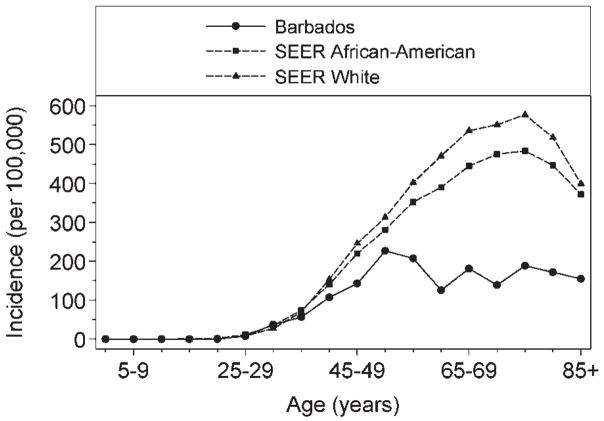
Figure 1 presents breast cancer incidence in Barbadian women and data from the US. Rates were similar in Barbadian and African-American until age 40–44 years, when the incidence among Barbadians was 107.3 (95% CI: 78.2–143.5), compared to 140.6 (135.1–146.2) among African-Americans (log linear model comparison, p = 0.07). Thereafter, breast cancer incidence rates among African-American women rose faster and into later life than in Barbadians. The highest incidence among African-Americans was 483.5 (95% CI: 461.7–505.9) among women aged 75–79; over 250 per 100,000 higher and 25 years later than the peak incidence among Barbadian women. Incidence rates among White Americans diverged even earlier and reached a peak at 576.8 (95% CI: 569.7–583.9) per 100,000.
Figure 1.
Age-specific incidence of female breast cancer in Barbados (2002–2006) and the United States (2000–2004).
Table II presents secular trends in incident breast cancer in Barbadian and African-American women during the period from 2002 to 2006. Rates in African-Americans were consistently around 143 per 100,000 between 2002 and 2004 in contrast to an age-standardized rate of 75.4 (95% CI: 65.9–86.2) per 100,000 in Barbadians during this period.
TABLE II.
SHORT-TERM SECULAR CHANGE IN BREAST CANCER INCIDENCE IN BARBADOS AND IN AFRICAN-AMERICANS
| Year | Follow-up months (days) | Cases | Person-years exposure | Age-standardized incidence rate (2000 US standard population) |
|
|---|---|---|---|---|---|
| Barbados | African-Americans | ||||
| 2002 | 6 (183) | 55 | 69,981 | 79.2 (59.3–103.7) | 143.3 (139.5–147.3) |
| 2003 | 12 (365) | 107 | 139,579 | 80.6 (65.8–97.8) | 143.0 (139.1–146.9) |
| 2004 | 12 (365) | 93 | 139,961 | 67.9 (54.7–83.5) | 143.7 (139.9–147.6) |
| 2005 | 12 (365) | 107 | 139,579 | 79.5 (65.0–96.5) | n/a1 |
| 2006 | 3 (91) | 34 | 34,417 | 101.1 (69.4–143.1) | n/a1 |
| 2002–2006 | 45 (1,369) | 396 | 523,517 | 75.6 (68.4 – 83.5) | – |
All rates based on invasive and in-situ disease.
SEER data for 2005 and 2006 not available (n/a).
Table III compares age-specific and secular breast cancer incidence in African-American and Barbadian women. Incidence rate ratios (IRR) showed no statistically significant differences among women aged 39 or less, marginal statistical differences among women aged 40–54, and strongly significant differences among women aged 55 years and older. At ages 60 years and older, incidence rates among African-American women were consistently between 2 and 4 times greater than in Barbadian women (p < 0.001 in all cases).
TABLE III.
INCIDENCE RATE RATIOS (IRRS) COMPARING AGE-STRATIFIED INCIDENCE RATES, AND SECULAR INCIDENCE RATES BETWEEN BARBADOS AND AFRICAN-AMERICANS (2002–2004)17
| Characteristic | IRR | 95% CI | p Value |
|---|---|---|---|
| Age | |||
| 25–29 | 1.49 | 0.48–4.67 | 0.49 |
| 30–34 | 0.94 | 0.56–1.57 | 0.81 |
| 35–39 | 1.31 | 0.89–1.95 | 0.18 |
| 40–44 | 1.31 | 0.98–1.76 | 0.07 |
| 45–49 | 1.54 | 1.17–2.04 | 0.002 |
| 50–54 | 1.24 | 0.97–1.59 | 0.09 |
| 55–59 | 1.70 | 1.26–2.30 | 0.001 |
| 60–64 | 3.10 | 2.09–4.60 | <0.001 |
| 65–69 | 2.46 | 1.75–3.45 | <0.001 |
| 70–74 | 3.42 | 2.30–5.07 | <0.001 |
| 75–79 | 2.57 | 1.74–3.78 | <0.001 |
| 80–84 | 2.60 | 1.65–4.09 | <0.001 |
| 85+ | 2.40 | 1.49–3.88 | <0.001 |
| All ages | 1.59 | 1.44–1.76 | <0.001 |
| Year | |||
| 2002 | 1.51 | 1.16–1.97 | 0.002 |
| 2003 | 1.59 | 1.31–1.93 | <0.001 |
| 2004 | 1.84 | 1.50–2.26 | <0.001 |
Table IV presents age-stratified and age-standardized death rates in Barbados from breast cancer between 1995 and 2004, per 100,000 person years of observation. In this 10-year period, there were 469 death certifications citing breast cancer as a principal cause of death. The number of deaths from breast cancer progressively increased with older age, with the distribution by 10-year age-groups being: aged 39 or less (33 or 7.0%), 40–49 (79 or 16.8%), 50–59 (78 or 16.6%), 60–69 (83 or 17.7%), 70–79 (86 or 18.3%), 80+ (110 or 23.5%). The crude death rate was 33.6 (95% CI: 30.6–36.8) per 100,000, whereas the age-standardized rate (using the US standard 2,000 population) was 32.9 (95% CI: 29.9–36.0) deaths per 100,000 person years.
TABLE IV.
AGE-STRATIFIED AND AGE-STANDARDIZED DEATH RATES FROM BREAST CANCER IN BARBADOS BETWEEN JANUARY 1, 1995 AND DECEMBER 31, 2004 PER 100,000 PERSON-YEARS OF OBSERVATION
| Age group | Number of cases | Person years | Age specific rate | 95% CI |
|---|---|---|---|---|
| 0–4 | 0 | 90,360 | 0 | 0–4.1 |
| 5–9 | 0 | 97,770 | 0 | 0–3.8 |
| 10–14 | 0 | 97,410 | 0 | 0–3.8 |
| 15–19 | 0 | 100,450 | 0 | 0–3.7 |
| 20–24 | 0 | 95,360 | 0 | 0–3.9 |
| 25–29 | 4 | 106,740 | 3.8 | 1.0–9.6 |
| 30–34 | 13 | 107,090 | 12.1 | 6.5–20.8 |
| 35–39 | 16 | 118,200 | 13.5 | 7.7–22.0 |
| 40–44 | 38 | 111,870 | 34.0 | 24.0–46.6 |
| 45–49 | 41 | 93,390 | 43.9 | 31.5–59.6 |
| 50–54 | 33 | 75,290 | 43.8 | 30.2–61.6 |
| 55–59 | 45 | 55,270 | 81.4 | 59.4–108.9 |
| 60–64 | 47 | 53,040 | 88.6 | 65.1–117.8 |
| 65–69 | 36 | 50,070 | 71.9 | 50.4–99.5 |
| 70–74 | 48 | 47,920 | 100.2 | 73.9–132.8 |
| 75–79 | 38 | 36,790 | 103.3 | 73.1–141.8 |
| 80–84 | 43 | 29,510 | 145.7 | 105.5–196.3 |
| 85+ | 67 | 29,260 | 229.0 | 177.5–290.8 |
| Crude rate | 469 | 1,395,790 | 33.6 | (30.6–36.8) |
| Age standardized rates (US) | – | – | 32.9 | (29.9–36.0) |
| Age standardized rates (Europe) | – | – | 31.6 | (28.6–34.8) |
| Age standardized rates (World) | – | – | 22.6 | (20.4–25.0) |
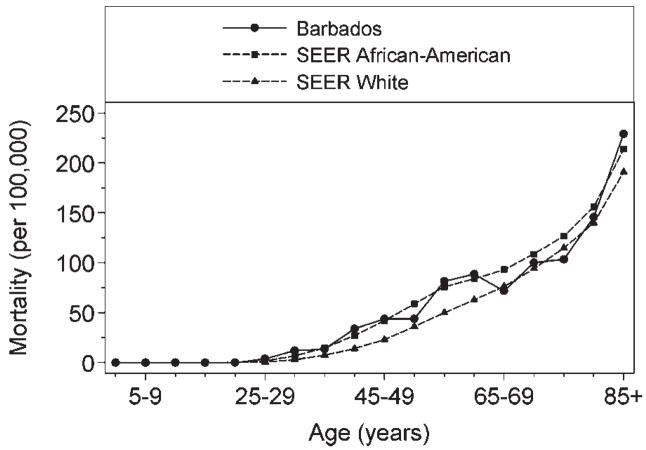
Figure 2 and Table V present data comparing mortality from breast cancer in the Barbadian and US populations between 1995 and 2004. Despite the lower breast cancer incidence among older Barbadians (standardized to the US population), we note the similar age-stratified death rates in the Barbadian, African-American and White American populations. In Barbados, breast cancer mortality ranged from 28.7 to 37.8 per 100,000, and among African-Americans rates ranged from 32.3 to 38.2 per 100,000.
Figure 2.
Age-specific death rates of female breast cancer in Barbados (1994–2004) and the United States (2000–2004).
TABLE V.
ANNUAL AGE-STANDARDIZED DEATH RATES FROM BREAST CANCER IN BARBADOS AND AMONG AFRICAN-AMERICANS BETWEEN JANUARY 1, 1995 AND DECEMBER 31, 2004 PER 100,000 PERSON-YEARS OF OBSERVATION
| Breast cancer death rates |
||||||
|---|---|---|---|---|---|---|
| Year | Barbados |
African-Americans |
||||
| All (95% CI) | <50 years | 50+ years | All | <50 years | 50+ years | |
| 1995 | 28.7 (20.6–39.3) | 11.1 | 74.9 | 38.2 | 11.7 | 107.5 |
| 1996 | 34.6 (25.6–46.0) | 10.0 | 99.0 | 37.1 | 11.7 | 103.6 |
| 1997 | 29.5 (21.3–40.0) | 11.7 | 76.0 | 37.4 | 11.0 | 106.6 |
| 1998 | 33.5 (24.6–44.7) | 10.3 | 94.2 | 35.5 | 10.6 | 100.7 |
| 1999 | 25.6 (17.9–35.6) | 7.0 | 74.3 | 35.2 | 10.3 | 100.5 |
| 2000 | 34.9 (25.8–46.4) | 10.2 | 99.8 | 34.4 | 9.9 | 98.3 |
| 2001 | 37.2 (27.8–49.0) | 14.1 | 97.7 | 34.5 | 9.9 | 99.1 |
| 2002 | 35.9 (26.5–47.6) | 13.3 | 95.0 | 34.1 | 10.0 | 97.2 |
| 2003 | 37.8 (28.2–49.8) | 14.0 | 100.2 | 34.1 | 9.7 | 97.9 |
| 2004 | 31.0 (22.4–42.1) | 10.8 | 83.5 | 32.3 | 9.3 | 92.4 |
Discussion
Breast cancer incidence was lower in the predominantly African ancestry population of Barbados, compared to rates in African and White Americans (78.1 vs. 143.7 and 162.7 per 100,000, respectively). Among Barbadians, age-specific rates reached a peak in the 50–54 year age group and declined thereafter, while rates continued to increase until ages 75–79 in the American populations (Fig. 1). Compared to Barbadian women, overall breast cancer incidence was 1.6 times higher in African-American women (IRR 1.59: 95% CI: 1.44–1.76), with elderly African-Americans (aged 60 years and older) experiencing 2–4 times the breast cancer incidence (Table III). In spite of the lower frequency of disease, Barbadian and African-American women experienced similar breast cancer mortality, with rates of 32.9 and 33.8 per 100,000, respectively (Table V). White American women had slightly lower mortality (25 per 100,000) (Fig. 2).
In our study, we recorded 396 incident cases of breast cancer during the period. Since an objective for this first description of breast cancer incidence in Barbados was complete case ascertainment, we chose to include combined in situ and invasive disease to fulfill this ascertainment goal. Of the total cases, 348 or 88% had basic histology on disease type (in situ or invasive disease): 34 (9.8%) were in situ and 314 (90.2%) were invasive. The remaining 48 could not be assigned a histology type, and would create an incidence undercount had we chosen to focus on known disease alone. We performed a sensitivity analysis to examine the possible influence of unknown histology on incidence rates. Many of the cases with unknown histology had traveled overseas for histological diagnosis, a decision related to socioeconomic status and thus unlikely to be related to histology type. We therefore assumed that the 48 breast cancers of unknown histology had the same ratio of invasive disease as known cases and assigned 43 (90%) as invasive and 5 (10%) as in situ. Restricting to cases with known histology gave incidence rates of 60.0 per 100,000 (95% CI 53.5–67.0) for invasive disease and 6.5 per 100,000 (4.5–9.1) for in situ disease. Adding unknown cases increased invasive disease to 68.2 per 100,000 (61.3–75.7) and increased in situ disease to 7.5 per 100,000 (5.3–10.2). In situ breast cancer therefore accounted for the minority (one-tenth) of cases.
Comparisons of breast cancer incidence across the African diaspora demonstrate a gradient, with rates being lowest in West Africa, higher in the Caribbean and highest among African-Americans.13,16,19 Registry-based data, which provide more accurate disease estimates, are available from only 2 of 18 West African countries (Mali and the Gambia, with world standardized breast cancer incidence of 20 and 7 per 100,000, respectively), and 2 Caribbean countries, Martinique and Cuba. Data available from the cancer registry in Martinique, French West Indies documented an incidence rate of 35.8 per 100,000 (1981–2000; standardized to the world population).20 Comparable data from Villa Clara in Cuba estimated breast cancer incidence (1995–1997) at 28.9 per 100,000.16 From our study, comparable breast cancer incidence in Barbados (standardized to the world population) is 58.4 per 100,000 (Table I). These overall incidences are lower than world standardized rates in African-American women between 2000 and 2004: 104.9 (95% CI: 103.6–106.2) per 100,000. These data have been collected at differing time periods and in populations with varying environmental and other characteristics, such as ancestry and degree of admixture, but they support a gradient in breast cancer incidence across the African diaspora.
Although overall breast cancer incidence is higher in White than African-American women, the latter often present with invasive breast cancer at an earlier age.17 Similar observations have now been reported in Black British women.21 Additionally, breast cancer incidence rates in younger Barbadian and African-American women (aged 50 years and younger) were similar. Biological mechanisms may partly underpin these observations. There are reported racial differences in estrogen and progesterone receptor status, linked to differences in age-specific incident disease, tumor aggressiveness and clinical outcomes.22–24 The role of estrogen in the pathogenesis of breast cancer has been well established, and progesterone and HER2 receptor status are also known to affect breast tumorigenesis.25,26 As such, African-American women younger than 40 years with invasive cancer are more likely than White women to be negative for estrogen, progesterone and HER2 receptors (triple-negative disease), linked to poor survival regardless of disease stage.26 These observations suggest a possible genetic etiology but this area remains poorly understood. African-American women have low rates of confirmed deleterious BRCA1 and BRCA2 mutations, and a spectrum of mutations which differs from those observed in European-origin populations.27 Unique BRCA1 mutations have been described in African-Americans28 as well as specific founder mutations,29 and recently, genetic variants in the insulin-like growth factor (IGF) signaling pathway have been described, which may partly explain racial differences in disease manifestations.30 Much work, however, is needed to elucidate the genetic basis of breast cancer susceptibility in African-origin populations.
Breast cancer incidence was considerably lower in postmenopausal Barbadian women than among African-American women. A possible explanation may be differences in environmental exposures, including reproductive-related factors. Among BNCS female participants with breast cancer, Nemesure31 reported that reproductive profiles were characterized by later menarche, higher parity, earlier age at childbearing and higher frequency of breast feeding relative to African-American women, likely to confer lower disease risk.3,13 Given the expected time sequence between environmental exposures and disease manifestation, environmental factors are likely to contribute to the marked differences in breast cancer incidence among older women.
In spite of the lower disease incidence, breast cancer mortality in Barbados was higher than expected, and similar to the experience of African-American women. Clear disparities in survival after breast cancer diagnosis have been described in the latter group4,5,17,26 and may exist in Barbadian women as well. In addition to biological factors, higher breast cancer mortality in African-American women has been associated with more advanced and aggressive disease at presentation,32 low uptake of mammographic screening,33 diagnostic and treatment delays,34 quality of care35 and comorbid conditions.36 Anecdotally, Barbadian women also present with late stage disease, in spite of easy access to free comprehensive public sector health services. More work is therefore needed to elucidate the reasons for the high breast cancer mortality in Barbados, given the relatively low incidence.
Our study has the strengths of providing comprehensive data on breast cancer patterns in the country. Although a national cancer registry is only now being established on the island, all incident breast cancer cases had a histological diagnosis confirmed at a single nation-wide Pathology Department. This feature reduces the likelihood of under-ascertainment or reporting errors. Unfortunately, this level of rigor was not possible in establishing breast cancer as the cause of death. In spite of this potential underestimation, breast cancer mortality was at rates comparable to those reported in the United States. One limitation is that it was not possible to clarify the ethnic group of all women with breast cancer. However, over 90% of the Barbados population is of African origin, with lower racial admixture than African-Americans.37
Conclusions
The parallel incidence patterns of breast cancer in African-American and Barbadian women of 50 years of age and younger may suggest a common disease etiology. There remains limited understanding of the genetic basis of early-onset clinically aggressive breast cancer in African-origin women, and genetic studies will likely be important in contributing to our understanding of this disease presentation. Although the incidence of breast cancer is lower in older Barbadian women, it would be expected to increase with secular changes in lifestyle risk behaviors as populations such as this continue to adopt more “westernized” lifestyles. Studies in intermediate risk populations such as Barbados can assist our understanding of racial disparities in breast cancer among African-Americans, given the similar heredity, and comparable lifestyle-related risk behaviors and disease outcomes. Variations in environmental risk factors and associated gene-environmental interactions across populations of the diaspora, are also likely to provide insight into breast cancer etiology, treatment and prevention.
Acknowledgments
National Human Genome Research Institute, NIH, Bethesda, MD.
Footnotes
Barbados National Cancer Study Group
Investigators: Coordinating Center: M. Cristina Leske, Barbara Nemesure, Suh-Yuh Wu, Karen Kelleher, Melinda Santoro, Department of Preventive Medicine, University at Stony Brook, Stony Brook, NY. Clinical Center: Anselm Hennis, Celia Greaves, Lynda Williams, Ingrid Cumberbatch, Karen Archer, Karen Bynoe, Nastassia Rambarran, Rachel Harris, Patricia Basdeo, Pissamai Maul, Wendy Browne, Shirley Reeves, Janelle Springer, Mauretta Newton, Sir Winston Scott Polyclinic, Bridgetown, Barbados. Local Laboratory Center: Lyndon Waterman, Andrew Stoute, Ronald Worrell, Carl Walters, Ray Scott, University of the West Indies, Bridgetown, Barbados. Gene Discovery Center: John Carpten, Jeffrey Trent, TGen Translational Genomics Research Institute, Phoenix, AZ. NHGRI: Joan Bailey-Wilson, National Human Genome Research Institute, Bethesda, MD. CRCH: Gita Sharma, Cancer Research Center, Hawaii.
Scientific Advisory Committee: Agnes Baffoe-Bonnie, Merck Corporation, Philadelphia, PA; Louise Brinton, National Cancer Institute, Bethesda, MD; Olufunmilayo Olopade, University of Chicago Medical Center, Chicago, IL; Tim Rebbeck, University of Pennsylvania School of Medicine, Philadelphia, PA; Duncan Thomas, University of Southern California, Los Angeles, CA.
Barbados Advisory Committee: Trevor A. Hassell, Henry Fraser, Beverley Barnett, Jerry Emtage, Selwyn Ferdinand, W. Leroy Inniss, Timothy Roach, Beverley Miller.
References
- 1.Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–83. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Fay MP, Feuer EJ, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2000. Bethesda, MD: National Cancer Institute; 2003. ( http://seer.cancer.gov/csr/1975_2000) [Google Scholar]
- 3.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL, Prentice R. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–48. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 5.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–6. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 6.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–7. [PubMed] [Google Scholar]
- 7.Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance. Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 8.Harris JE. Global dimensions of the African diaspora. 2. Washington, DC: Howard University Press; 2007. [Google Scholar]
- 9.Omran AR. The epidemiologic transition. A theory of the epidemiology of population change, 1971. Bull World Health Organ. 2001;79:161–70. [PMC free article] [PubMed] [Google Scholar]
- 10.Luke A, Cooper RS, Prewitt TE, Adeyemo AA, Forrester TE. Nutritional consequences of the African diaspora. Annu Rev Nutr. 2001;21:47–71. doi: 10.1146/annurev.nutr.21.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Forrester T, Wilks R, Bennett F, McFarlane-Anderson N, McGee D, Cooper R, Fraser H. Obesity in the Caribbean. Ciba Found Symp. 1996;201:17–26. doi: 10.1002/9780470514962.ch2. [DOI] [PubMed] [Google Scholar]
- 12.Cooper RS, Rotimi CN, Kaufman JS, Owoaje EE, Fraser H, Forrester T, Wilks R, Riste LK, Cruickshank JK. Prevalence of NIDDM among populations of the African diaspora. Diabetes Care. 1997;20:343–8. doi: 10.2337/diacare.20.3.343. [DOI] [PubMed] [Google Scholar]
- 13.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 14.BSS. Population and housing census. Barbados census 2001. Vol. 1. Barbados: Barbados Statistical Service; 2002. [Google Scholar]
- 15.Day J. US Bureau of the Census. 1996. Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050. [Google Scholar]
- 16.Parkin DM, Whelan S, Ferlay J, Teppo L, Thomas D. Cancer incidence in five continents, vol. VIII. IARC Scientific Publications No. 155. International Agency for Research on Cancer; Lyon, France: 2002. [Google Scholar]
- 17.Surveillance, epidemiology, and end results (SEER) program (www.seer.cancer.gov). National Cancer Institute, DCCPS, Surveillance Research Program, 2007.
- 18.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the Gamma distribution. Stats Med. 1997;16:791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Porter P. “Westernizing” women’s risks?. Breast cancer in lower-income countries. N Engl J Med. 2008;358:213–16. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 20.Dieye M, Veronique-Baudin J, Draganescu C, Azaloux H. Cancer incidence in Martinique: a model of epidemiological transition. Eur J Cancer Prev. 2007;16:95–101. doi: 10.1097/01.cej.0000236246.78736.51. [DOI] [PubMed] [Google Scholar]
- 21.Bowen RL, Duffy SW, Ryan DA, Hart IR, Jones JL. Early onset of breast cancer in a group of British black women. Br J Cancer. 2008;98:277–81. doi: 10.1038/sj.bjc.6604174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu KC, Anderson WF. Rates for breast cancer characteristics by estrogen and progesterone receptor status in the major racial/ethnic groups. Breast Cancer Res Treat. 2002;74:199–211. doi: 10.1023/a:1016361932220. [DOI] [PubMed] [Google Scholar]
- 23.Tarone RE, Chu KC. The greater impact of menopause on ER- than ER+ breast cancer incidence: a possible explanation (United States) Cancer Causes Control. 2002;13:7–14. doi: 10.1023/a:1013960609008. [DOI] [PubMed] [Google Scholar]
- 24.Yasui Y, Potter JD. The shape of age-incidence curves of female breast cancer by hormone-receptor status. Cancer Causes Control. 1999;10:431–7. doi: 10.1023/a:1008970121595. [DOI] [PubMed] [Google Scholar]
- 25.Wenger CR, Beardslee S, Owens MA, Pounds G, Oldaker T, Vendely P, Pandian MR, Harrington D, Clark GM, McGuire WL. DNA ploidy. S-phase, and steroid receptors in more than 127,000 breast cancer patients. Breast Cancer Res Treat. 1993;28:9–20. doi: 10.1007/BF00666351. [DOI] [PubMed] [Google Scholar]
- 26.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 27.Nanda R, Schumm LP, Cummings S, Fackenthal JD, Sveen L, Ademuyiwa F, Cobleigh M, Esserman L, Lindor NM, Neuhausen SL, Olopade OI. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294:1925–33. doi: 10.1001/jama.294.15.1925. [DOI] [PubMed] [Google Scholar]
- 28.Gao Q, Neuhausen S, Cummings S, Luce M, Olopade OI. Recurrent germ-line BRCA1 mutations in extended African American families with early-onset breast cancer. Am J Hum Genet. 1997;60:1233–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Stoppa-Lyonnet D, Laurent-Puig P, Essioux L, Pages S, Ithier G, Ligot L, Fourquet A, Salmon RJ, Clough KB, Pouillart P, Bonaiti-Pellie C, Thomas G. BRCA1 sequence variations in 160 individuals referred to a breast/ovarian family cancer clinic. Institut Curie Breast Cancer Group. Am J Hum Genet. 1997;60:1021–30. [PMC free article] [PubMed] [Google Scholar]
- 30.Garner CP, Ding YC, John EM, Ingles SA, Olopade OI, Huo D, Adebamowo C, Ogundiran T, Neuhausen SL. Genetic variation in IGFBP2 and IGFBP5 is associated with breast cancer in populations of African descent. Hum Genet. 2008;123:247–55. doi: 10.1007/s00439-008-0468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemesure B. Risk factors for breast cancer in a black population—The Barbados National Cancer Study. Int J Cancer. doi: 10.1002/ijc.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg J, Chia YL, Plevritis S. The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Breast Cancer Res Treat. 2005;89:47–54. doi: 10.1007/s10549-004-1470-1. [DOI] [PubMed] [Google Scholar]
- 33.Smith-Bindman R, Miglioretti DL, Lurie N, Abraham L, Barbash RB, Strzelczyk J, Dignan M, Barlow WE, Beasley CM, Kerlikowske K. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144:541–53. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 34.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–52. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 35.Mandelblatt JS, Kerner JF, Hadley J, Hwang YT, Eggert L, Johnson LE, Gold K. Variations in breast carcinoma treatment in older medicare beneficiaries: is it black or white. Cancer. 2002;95:1401–14. doi: 10.1002/cncr.10825. [DOI] [PubMed] [Google Scholar]
- 36.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–72. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 37.Benn-Torres J, Bonilla C, Robbins CM, Waterman L, Moses TY, Hernandez W, Santos ER, Bennett F, Aiken W, Tullock T, Coard K, Hennis A, et al. Admixture and population stratification in African Caribbean populations. Ann Hum Genet. 2008;72:90–8. doi: 10.1111/j.1469-1809.2007.00398.x. [DOI] [PubMed] [Google Scholar]