Abstract
Objective
To determine the effects of bone morphogenetic protein-2 (BMP-2), insulin-like growth factor (IGF-I), and transforming growth factor-β1 (TGF-β1) on the biochemical and biomechanical properties of engineered articular cartilage constructs under serum free conditions.
Methods
A scaffoldless approach for tissue engineering, the self-assembly process, was employed. The study consisted of two phases. In the first phase, the effects of BMP-2, IGF-I, and TGF-β1, at two concentrations and two dosage frequencies each were assessed on construct biochemical and biomechanical properties. In phase II, the effects of growth factor combination treatments were determined. Compressive and tensile mechanical properties, glycosaminoglycan (GAG) and collagen content, histology for GAG and collagen, and immunohistochemistry (IHC) for collagen types I and II were assessed.
Results
In phase I, BMP-2 and IGF-I treatment resulted in significant, >1-fold increases in aggregate modulus, accompanied by increases in GAG production. Additionally, TGF-β1 treatment resulted in significant, ~1-fold increases in both aggregate modulus and tensile modulus, with corresponding increases in GAG and collagen content. In phase II, combined treatment with BMP-2 and IGF-I increased aggregate modulus and GAG content further than either growth factor alone, while TGF-β1 treatment alone remained the only treatment to also enhance tensile properties and collagen content.
Discussion
This study determined systematically the effects of multiple growth factor treatments under serum-free conditions, and is the first to demonstrate significant increases in both compressive and tensile biomechanical properties as a result of growth factor treatment. These findings are exciting as coupling growth factor application with the self-assembly process resulted in tissue engineered constructs with functional properties approaching native cartilage values.
Keywords: Articular cartilage, tissue engineering, growth factors, extracellular matrix, mechanical properties
INTRODUCTION
Articular cartilage has a limited ability for self-repair, and injuries to articular cartilage result in the formation of mechanically inferior fibrocartilage.1 Since current clinical treatments are limited, tissue engineering is a promising strategy for articular cartilage regeneration.
To alleviate some of the potential issues associated with scaffold use, our lab has developed and employed a scaffoldless process for tissue engineering, called the self-assembly process.2-4 Using this process, the goal is to create engineered constructs with biochemical and biomechanical properties approaching those of native tissue. Growth factor application appears to be a promising approach for enhancing these properties.
Previous studies5, 6 systematically assessed the effects of several growth factors at different concentrations on chondrocyte-seeded PGA scaffolds, and indicated that treatment with BMP-2 and IGF-I enhanced GAG production, while TGF-β1 enhanced collagen production. However, these studies employed fetal bovine serum (FBS) in the medium, potentially confounding the effects of exogenous growth factor application. Also, a prior study by Ng et al.7 indicated beneficial effects from temporal application of IGF-I and TGF-β1.
Although many studies have demonstrated beneficial effects of growth factor application, no studies have systematically assessed the effects of growth factors alone and in combination under serum-free conditions. Furthermore, no studies have examined growth factor effects on both compressive and tensile properties. The objective of this study was to determine the effects of growth factor application on the biomechanical and biochemical properties of self-assembled articular cartilage constructs. This study utilized a 2-phase approach to determine the effects of single growth factor treatments followed by the determination of the effects of combined growth factor treatments. Based on the results of prior studies,5, 6, 8-10 in phase I, it was hypothesized that BMP-2 and IGF-I treatment would enhance compressive properties by increasing GAG production, and TGF-β1 treatment would enhance both compressive and tensile properties by increasing GAG production and collagen production respectively. It was further hypothesized that growth factor concentration and dosage frequency would have significant effects on construct biochemical and biomechanical properties, based on prior work.11, 12 In phase II, it was hypothesized that combined growth factor treatment would have beneficial effects on construct properties, by increasing biochemical and biomechanical properties further than any growth factor alone. To test these hypotheses, three experiments were performed in phase I and one experiment was performed in phase II. In phase I, BMP-2, IGF-I, and TGF-β1 were all assessed at two concentrations and two dosage frequencies each, with the best treatment for each growth factor selected for use in phase II. In phase II, the growth factor treatments selected from phase I were assessed in combinations of two and three.
METHODS
Chondrocyte Isolation and Seeding
Chondrocytes were obtained from the distal femur of wk-old male calves13-15 (Research 87, Boston, MA), and digested with collagenase type 2 (Worthington, Lakewood, NJ). Each leg yielded roughly 150 million chondrocytes, and animal variability was reduced by pooling cells from five legs of different animals to yield a mixture of chondrocytes for each study (see descriptions below). The pooled cells were counted on a hemocytometer, and viability >90% was found using a trypan blue exclusion test. Chondrocytes were frozen in culture medium supplemented with 20% FBS (Biowhittaker) and 10% DMSO at −80°C for 3 days before use in phase I, and for 3 wks before use in phase II. After thawing, viability remained greater than 85%. A polysulfone die consisting of 5 mm dia. × 10 mm long cylindrical prongs was used to construct each agarose mold. Sterile, molten 2% agarose was introduced into a well fitted with the polysulfone die. The agarose was allowed to gel at room temperature for 60 min, and two exchanges of culture medium were used to completely saturate the agarose well with culture medium by the time of cell seeding. To each well, 5.5 × 106 cells in 100 μl of culture medium were added. The cells self-assembled within 24 hrs in the agarose wells and were maintained in the same well for t=10 days; t=0 was defined as 24 hrs after seeding. The culture medium was DMEM with 4.5 g/L-glucose and L-glutamine (Biowhittaker/Cambrex, Walkersville, MD), 100 nM dexamethasone (Sigma, St. Louis, MO), 1% Fungizone/Penicillin/Streptomycin (Biowhittaker), 1% ITS+ (BD Scientific, Franklin Lakes, NJ), 50 μg/mL ascorbate-2-phosphate, 40 μg/mL L-proline, and 100 μg/mL sodium pyruvate (Fisher Scientific, Pittsburgh, PA).
Phase I: Individual Growth Factor Effects
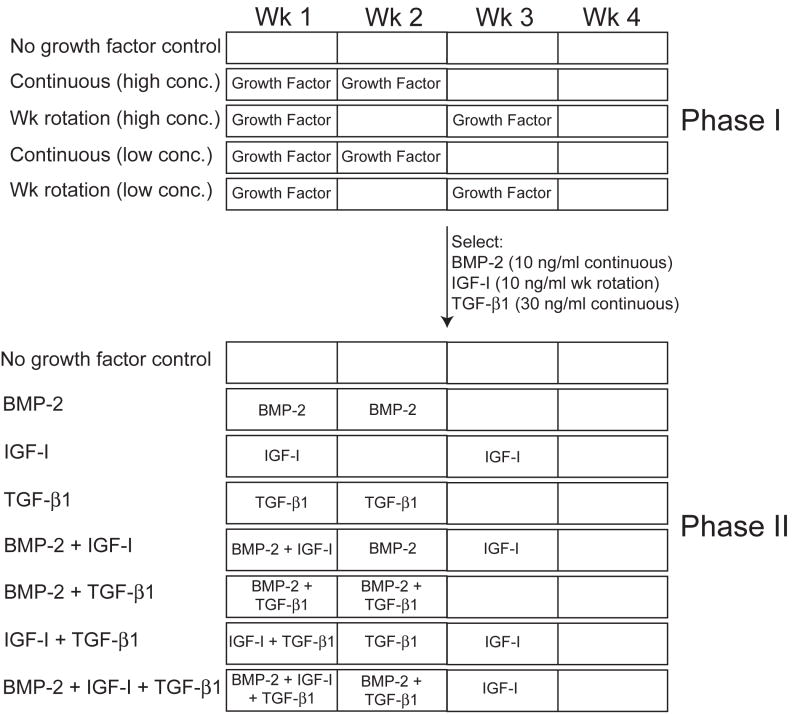
This phase included three separate studies to assess the individual effects of BMP-2, IGF-I and TGF-β1 at different concentrations and dosage frequencies. All growth factors were obtained from Peprotech Inc. (Rocky Hill, NJ), and were applied in the culture medium. For each growth factor, the effects of two concentrations (low and high) and two dosage frequencies were assessed, with separate no growth factor controls for each study, yielding a total of five treatment groups for each growth factor study (Fig. 1). The concentrations used were 10 and 100 ng/ml for BMP-2 and IGF-I, and 10 and 30 ng/ml for TGF-β1, selected from prior studies.5, 6 The dosage regimens were 2 wks continuous application followed by 2 wks of no growth factor (continuous), or growth factor application only during the 1st and 3rd wk of culture (wk rotation), which were chosen based on pilot studies and current ongoing work in our group as well as adapted from prior studies using intermittent growth factor application by Lieb et al.11, 12
Fig. 1.
Schematic diagram indicating experimental designs of phases I and II. The experimental design depicted in phase I was carried out for each individual growth factor separately (blocked by growth factor). The best treatment for each growth factor was selected for phase II. Phase II assessed the effects of each growth factor individually and in all combinations of two and three.
For all studies, at t=10 days, self-assembled constructs (n=6/group) were removed from confinement in 5 mm dia. agarose wells and transferred to individual 2% agarose coated wells of a 48-well culture plate for the remainder of the study. Per construct, 500 μl of medium was changed daily, and all constructs were assessed at t=4 wks. The “best” treatment for each growth factor was selected, using a functionality index as described below, for use in phase II.
Phase II: Growth Factor Combination Effects
One treatment for each growth factor was selected from phase I to be compared individually, as well as in combinations of two and three in phase II (Fig. 1). The specific application treatments selected were 10 ng/ml continuous BMP-2, 10 ng/ml wk rotation IGF-I, and 30 ng/ml continuous TGF-β1. As in phase I, constructs were unconfined from agarose wells at t=10 days, and transferred to individual 2% agarose coated wells of a 48-well culture plate for the remainder of the study. Again, 500 μl of medium per construct was changed daily, and all constructs were assessed at t=4 wks.
Histology and Immunohistochemistry
Samples were frozen and sectioned at 14 μm. Safranin-O and fast green staining were used to examine GAG distribution.16, 17 Picrosirius red was used for qualitative examination of collagen content. A von Kossa stain was used to assess for mineralization. Slides were also processed with IHC to test for the presence of collagen types I, II, and X. After fixing in chilled acetone, the slides were rinsed with IHC buffer (Biogenex), quenched of peroxidase activity with hydrogen peroxide/methanol, and blocked with horse serum (Vectastain ABC kit, Vector Laboratories, Burlingame, CA). The slides were then incubated with either mouse anti-collagen type I (Accurate Chemicals, Westbury, NY), rabbit anti-collagen type II (Cedarlane Labs, Burlington, NC), or rabbit anti-collagen X (Abcam Inc., Cambridge, MA) antibodies. The secondary antibody (anti-mouse or anti-rabbit IgG, Vectastain ABC kit) was applied, and color was developed using the Vectastain ABC reagent and DAB (Vectastain kit).
Quantitative Biochemistry
Samples were frozen overnight and lyophilized for 72 hrs, followed by re-suspension in 0.8 mL of 0.05 M acetic acid with 0.5 M NaCl and 0.1 mL of a 10 mg/mL pepsin solution (Sigma) at 4°C for 72 hrs. Next, 0.1 mL of 10× TBS was added along with 0.1 mL pancreatic elastase and mixed at 4°C overnight. From this digest, total DNA content was measured by Picogreen® Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR). Total sulfated GAG was then quantified using the Blyscan Glycosaminoglycan Assay kit (Biocolor), based on 1,9-dimethylmethylene blue binding.18, 19 After being hydrolyzed by 2 N NaOH for 20 min at 110°C, samples were assayed for total collagen content by a chloramine-T hydroxyproline assay.20
Indentation Testing
Samples were evaluated with an indentation apparatus.21 A step mass of 0.7 g (0.007 N) was applied with a 1 mm flat-ended, porous indenter tip, and specimens were allowed to creep until equilibrium, as described elsewhere.2 Preliminary estimations of the aggregate modulus of the samples were obtained using the analytical solution for the axisymmetric Boussinesq problem with Papkovich potential functions.22, 23 The aggregate modulus (HA), permeability, and Poisson’s ratio of the samples were then determined using the linear biphasic theory.24
Tensile Testing
Tensile tests were performed using a uniaxial materials testing system (Instron Model 5565, Canton, MA) with a 50 N load cell as described previously.25 Briefly, samples were cut into a dog-bone shape with a 1-mm-long gauge length. Samples were attached to paper tabs for gripping with cyanoacrylate glue outside of the gauge length. The 1-mm-long sections were pulled at a constant strain rate of 0.01 s-1. Stress-strain curves were created from the load-displacement curve and the cross-sectional area of each sample, and Young’s modulus (EY) was calculated from the linear region of each stress-strain curve.
Functionality Index (FI)
A functionality index (Eq. 1) was used to determine the “best” treatment condition for each growth factor in phase I, for use in phase II. The index was only used as a selection tool within each experiment, without making comparisons among experiments. It was weighted using normalized collagen and GAG content, tensile stiffness, and creep indentation compressive stiffness. The index served as a quantified comparison between the properties of the engineered constructs and native tissue. In the functionality index, G represents GAG/WW, C represents collagen/WW, ET represents tensile modulus, and EC represents compressive stiffness (aggregate modulus). The subscripts nat and sac are used to denote native and self-assembled construct values, respectively. Using immature bovine cartilage explants, native tissue values were 5% and 15% for GAG/WW and collagen/WW respectively, and 213 kPa and 12.1 MPa for EC and ET respectively. Although different weights may be afforded to each component of the FI, they are equally weighted in this study. Since the eventual goal of our tissue engineering approach is in vivo construct implantation, as cartilage experiences both compressive and tensile loading in the joint, these properties are equally weighted. Furthermore, the biochemical characteristics are equally important as constructs with biochemical characteristics divergent from native tissue may present problems in construct integration with native tissue. However, due to the flexibility of the FI, the exact weights can easily be modified based on the results of future studies.
| (1) |
Statistical Analysis
All samples were assessed biochemically and biomechanically (n=6 or 7). In each phase, a single factor ANOVA was used to analyze the samples, and a Fisher LSD post hoc test was used when warranted. Significance was defined as p < 0.05.
RESULTS
Gross Appearance and Histology
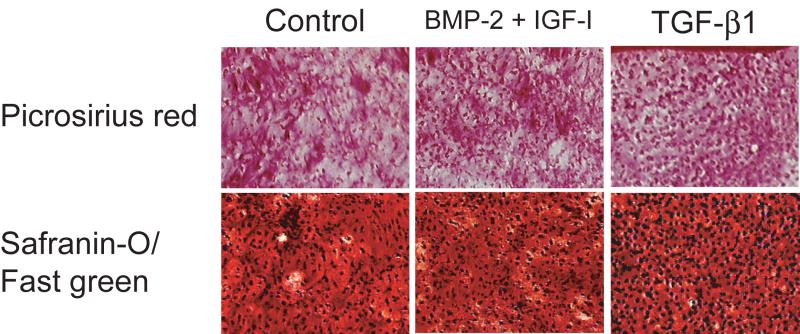
Construct diameter was approximately 6 mm in all studies. In phase I, BMP-2 at all concentrations and dosages increased construct wet weight (WW) and thickness slightly, as demonstrated in Table 1. IGF-I treatment led to a slightly decreased construct WW, with no differences in construct thickness, as shown in Table 2. Finally, treatment with TGF-β1 resulted in a concentration dependent decrease in construct WW and thickness, as indicated in Table 3. In phase II, there were no differences in construct WW or thickness among any of the treatment groups (Table 4). In all studies, constructs stained positive for collagen and GAG throughout their thickness (Fig. 2), and based on IHC, collagen II was expressed throughout each construct, with no collagen I production. Similar images can be observed in our previous work.4 Additionally, no constructs demonstrated mineralization and no chondrocyte hypertrophy was noted with BMP-2 treatment.
Table 1.
Properties of constructs treated with BMP-2 in phase I.
| Group | WW (mg) | Thickness (mm) | Total Cells (×106) | FI |
|---|---|---|---|---|
| Control | 21.8±2.4 | 0.67±0.04 | 4.6±0.5 | 0.75±0.18 |
| 100 ng/ml Continuous | 23.7±1.8 | 0.71±0.07 | 5.3±0.3 | 0.94±0.06a |
| 100 ng/ml Wk Rotat. | 22.6±1.9 | 0.71±0.04 | 4.8±0.9 | 0.96±0.08a |
| 10 ng/ml Continuous | 23.3±2.0 | 0.73±0.07 | 4.4±1.0 | 0.97±0.10a |
| 10 ng/ml Wk Rotat. | 23.8±1.3 | 0.72±0.04 | 4.8±0.3 | 0.92±0.06a |
Significantly different from control
Wk Rotat., 2-wk rotation dosage; Col., total collagen
Table 2.
Properties of constructs treated with IGF-I in phase I.
| Group | WW (mg) | Thickness (mm) | Total Cells (×106) | FI |
|---|---|---|---|---|
| Control | 25.7±1.0 | 0.74±0.06 | 5.2±0.6 | 0.59±0.19 |
| 100 ng/ml Continuous | 24.5±1.4 | 0.65±0.08 | 5.3±0.7 | 0.93±0.12a |
| 100 ng/ml Wk Rotat. | 22.7±1.6a | 0.71±0.09 | 5.0±0.4 | 0.92±0.14a |
| 10 ng/ml Continuous | 23.2±1.7a | 0.75±0.10 | 4.6±0.6 | 0.78±0.19a |
| 10 ng/ml Wk Rotat. | 24.1±1.9 | 0.75±0.12 | 5.0±0.3 | 0.96±0.08a |
Significantly different from control
Wk Rotat., 2-wk rotation dosage; Col., total collagen
Table 3.
Properties of constructs treated with TGF-β1 in phase I.
| Group | WW (mg) | Thickness (mm) | Total Cells (×106) | FI |
|---|---|---|---|---|
| Control | 24.9±2.8 | 0.88±0.14 | 5.7±0.3 | 0.60±0.08 |
| 30 ng/ml Continuous | 12.6±0.7a | 0.57±0.06a | 6.6±0.4a | 0.82±0.07a |
| 30 ng/ml Wk Rotat. | 13.9±0.6a | 0.57±0.02a | 6.5±0.8a | 0.81±0.14a |
| 10 ng/ml Continuous | 17.8±1.3a | 0.64±0.09a | 5.7±0.4 | 0.62±0.07 |
| 10 ng/ml Wk Rotat. | 17.9±4.6a | 0.76±0.17 | 5.8±0.8 | 0.55±0.06 |
Significantly different from control
Wk Rotat., 2-wk rotation dosage; Col., total collagen
Table 4.
Phase II construct properties.
| Group | WW (mg) | Thickness (mm) | Total Cells (×106) | FI |
|---|---|---|---|---|
| Control | 13.3±1.3 | 0.45±0.09 | 5.6±0.5 | 0.53±0.06 |
| BMP-2 | 15.0±1.6 | 0.55±0.06 | 5.2±0.4 | 0.76±0.07a |
| IGF-I | 13.3±3.1 | 0.55±0.05 | 5.7±0.9 | 0.73±0.12a |
| TGF-β1 | 14.5±1.6 | 0.58±0.05 | 5.5±0.3 | 0.72±0.04a |
| BMP-2 + IGF-I | 16.7±1.3a | 0.59±0.05 | 5.9±0.4 | 0.80±0.08a |
| BMP-2 + TGF-β1 | 14.9±1.4 | 0.57±0.04 | 5.8±0.6 | 0.66±0.02a |
| IGF-I + TGF-β1 | 14.2±1.4 | 0.57±0.06 | 5.5±0.3 | 0.59±0.04 |
| BMP-2 + IGF-I + TGF-β1 | 13.0±1.3 | 0.53±0.08 | 6.1±0.4 | 0.70±0.05 |
Significantly different from control
Col., total collagen
Fig. 2.
Photomicrographs of collagen and GAG staining for no growth factor control constructs, BMP-2 + IGF-I treated constructs, and TGF-β1 treated constructs. 10× original magnification.
Quantitative Biochemistry
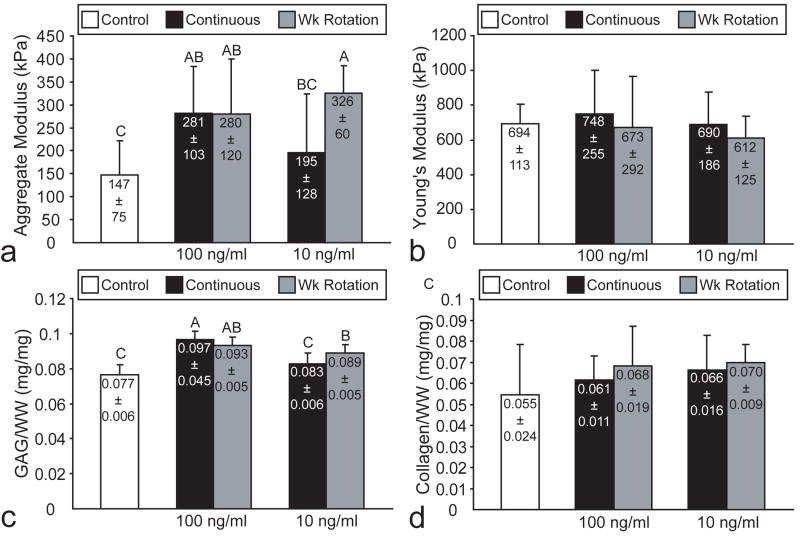
In phase I, there were no differences in cells/construct among the different treatment groups in the BMP-2 study (Table 1). Treatment with 10 ng/ml continuous BMP-2 led to the greatest increase in GAG/WW, although all BMP-2 treatments significantly increased GAG/WW (Fig. 3c). There were no differences in collagen/WW among any of the treatment groups (Fig. 3d). In the IGF-I study, there were no differences in cells/construct among any of the treatment groups (Table 2). All IGF-I treatments significantly increased GAG/WW, with the exception of 10 ng/ml continuous treatment (Fig. 4c). There were no differences in collagen/WW among any of the treatment groups (Fig. 4d). In the TGF-β1 study, 30 ng/ml treatment led to an approximately 14% increase in cells/construct (Table 3). Additionally, 30 ng/ml TGF-β1, at either continuous or 2 wk rotation dosages, significantly increased both collagen/WW and GAG/WW (Figs. 5c and 5d).
Fig. 3.
Biomechanical and biochemical properties of BMP-2 treated constructs in phase I. All BMP-2 treatments significantly increased (a) aggregate modulus with no effect on (b) Young’s modulus. Likewise, all BMP-2 treatments significantly increased (c) GAG/WW with no effect on (d) collagen/WW. Columns and error bars represent means and standard deviations. Groups denoted by different letters are significantly different (p<0.05).
Fig. 4.
Biomechanical and biochemical properties of IGF-I treated constructs in phase I. All IGF-I treatments, except 10 ng/ml continuous, significantly increased (a) aggregate modulus with no effect on (b) Young’s modulus. Likewise, all IGF-I treatments, except 10 ng/ml continuous, significantly increased (c) GAG/WW with no effect on (d) collagen/WW. Columns and error bars represent means and standard deviations. Groups denoted by different letters are significantly different (p<0.05).
Fig. 5.
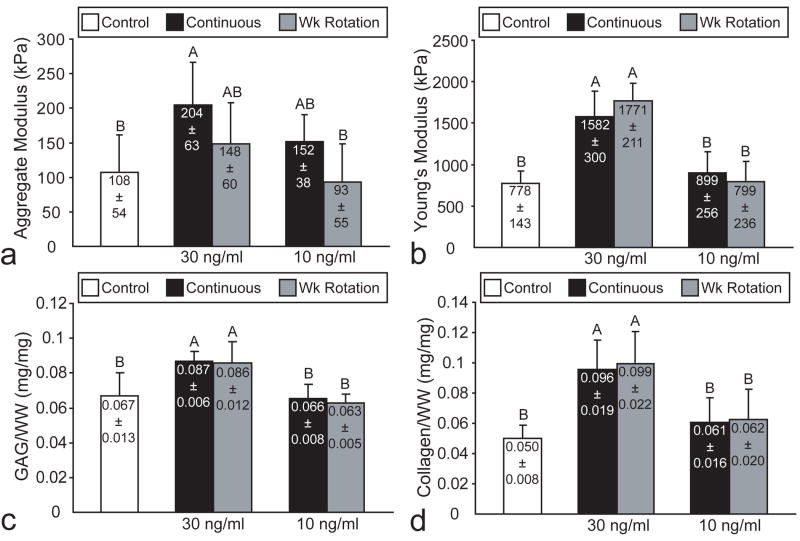
Biomechanical and biochemical properties of TGF-β1 treated constructs in phase I. TGF-β1 treatment at 30 ng/ml and 2-wk continuous dosage significantly increased (a) aggregate modulus and (b) Young’s modulus, with corresponding increases in (c) GAG/WW and (d) collagen/WW. Columns and error bars represent means and standard deviations. Groups denoted by different letters are significantly different (p<0.05).
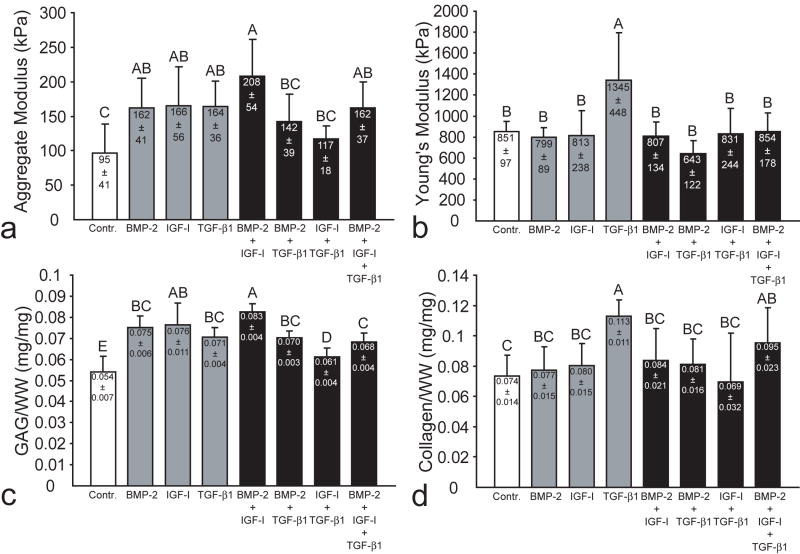
In phase II, there were no differences in cells/construct among any of the treatment groups (Table 4). All growth factor treatments significantly increased GAG/WW, although combined BMP-2 and IGF-I treatment led to the greatest increase in GAG/WW (Fig. 6c). However, both treatment with TGF-β1 alone and combined application of all three growth factors significantly increased collagen/WW (Fig. 6d).
Fig. 6.
Biomechanical and biochemical properties of constructs in phase II. Combined treatment with BMP-2 and IGF-I led to the greatest enhancement of aggregate modulus and GAG/WW, while TGF-β1 alone was the only treatment to enhance both compressive and tensile stiffness. (a) aggregate modulus, (b) Young’s modulus, (c) GAG/WW and (d) collagen/WW. Columns and error bars represent means and standard deviations. Groups denoted by different letters are significantly different (p<0.05).
Mechanical Evaluation
In phase I, all BMP-2 treatments significantly increased aggregate modulus, although BMP-2 at 10 ng/ml continuous application led to the greatest increase (Fig. 3a). There were no differences in Poisson’s ratio or permeability noted among the different groups, with ranges of 0.15-0.28 and 4.1×10-14-1.2×10-13, respectively. Furthermore, there were no differences in Young’s modulus among any of the treatment groups in the BMP-2 study (Fig. 3b). In the IGF-I study, all IGF-I treatments except for 10 ng/ml continuous significantly increased aggregate modulus, while application at 10 ng/ml wk rotation led to the greatest increase in aggregate modulus (Fig. 4a). There were no differences in Poisson’s ratio or permeability noted among the different groups, with ranges of 0.19-0.26 and 8.0×10-14-1.2×10-13, respectively. Additionally, there were no differences in Young’s modulus among any of the treatment groups in the IGF-I study (Fig. 4b). In the TGF-β1 study, only 30 ng/ml continuous treatment significantly increased aggregate modulus (Fig. 5a). However, both TGF-β1 treatments at 30 ng/ml exhibited a significant increase in Young’s modulus (Fig. 5b). There were no differences among the treatment groups for Poisson’s ratio and permeability, with ranges of 0.09-0.22 and 2.3×10-14-7.2×10-14, respectively.
In phase II, all three individual growth factor treatments significantly increased aggregate modulus (Fig. 6a), replicating the results of phase I. However, combined BMP-2 and IGF-I treatment led to the greatest enhancement of aggregate modulus. Only individual application of TGF-β1 significantly increased Young’s modulus (Fig. 6b). There were no differences in Poisson’s ratio or permeability among the treatment groups, with ranges of 0.09-0.26 and 5.1×10-14-1.3×10-13, respectively.
DISCUSSION
The objective of this study was to assess systematically the effects of growth factors on the biochemical and biomechanical properties of self-assembled articular cartilage constructs. The study utilized a 2-phase approach to determine the effects of different growth factors, concentrations, and dosage frequencies, as well as to examine the effects of growth factor combination treatment. This approach allowed for a methodical growth factor examination under serum-free conditions. To the best of our knowledge, this study is the first to demonstrate significant increases in both compressive and tensile biomechanical properties as a result of growth factor treatment.
In phase I, all BMP-2 treatments led to significant increases in construct compressive stiffness and GAG/WW. The greatest enhancement was observed with 2 wk continuous treatment at 10 ng/ml, resulting in a 104% increase in compressive stiffness. Despite the increased compressive properties, no increases in tensile properties or collagen/WW were noted for any of the treatment groups. These results supported our hypothesis that BMP-2 would only increase the compressive properties of the constructs by increasing the GAG/WW, as increased GAG production without changes in collagen synthesis has previously been observed with BMP-2 treatment.6, 26 BMP-2 treatment of 2 wk continuous dosage at 10 ng/ml was selected for use in phase II as it demonstrated the greatest increase in the functionality index.
Similarly, in phase I, all IGF-I treatments except for 10 ng/ml continuous application significantly increased construct compressive stiffness and GAG/WW. However, the greatest increase was observed with the wk rotation dosage at 10 ng/ml, with a 122% increase in compressive stiffness. As with BMP-2 treatment, no increases in tensile properties or collagen/WW were observed for any of the treatment groups. These results supported our hypothesis that IGF-I would increase only the compressive properties of the constructs by increasing the GAG/WW, as previous studies demonstrated enhanced GAG production without changes in collagen synthesis from IGF-I treatment in both tissue engineered constructs and explants.5, 27, 28 IGF-I treatment of wk rotation dosage at 10 ng/ml was selected for use in phase II as it demonstrated the greatest increase in the functionality index.
Finally, in phase I, 30 ng/ml TGF-β1 treatment, at either dosage frequency, significantly increased tensile stiffness and collagen/WW, as well as GAG/WW. However, only 30 ng/ml TGF-β1 treatment at the 2 wk continuous dosage significantly increased compressive stiffness. These results demonstrate that the enhancement of compressive properties likely requires a lag period, as suggested previously,7 following TGF-β1 treatment; both 30 ng/ml treatments increased GAG/WW, but only the 2 wk continuous application, with 2 wks between cessation of growth factor treatment and construct evaluation, demonstrated an increase in compressive stiffness. It is likely that the increased lag time is required to incorporate and organize the GAG and collagen into the ECM.7 Based on these results, 2 wk continuous TGF-β1 treatment at 30 ng/ml was selected for use in phase II as it demonstrated the greatest increase in the functionality index, and was the only treatment in phase I that increased both compressive and tensile properties. This result supported our hypothesis that TGF-β1 treatment would increase both compressive and tensile properties by increasing both GAG and collagen content, respectively. Additionally this finding corresponds with previous work that has demonstrated that TGF-β1 treatment increases collagen synthesis or gene expression,5, 29-31 while TGF-β1 treatment only under serum free conditions increases proteoglycan synthesis.29
In phase I, the different dosage frequencies had profound effects on the biochemical and biomechanical properties of the constructs. For example, 10 ng/ml IGF-I applied at the 2 wk continuous dosage significantly increased compressive stiffness and GAG/WW, while 10 ng/ml IGF-I applied at the wk rotation dosage had no effect on compressive stiffness and GAG/WW. Additionally, as described above, only 30 ng/ml TGF-β1 treatment at the 2 wk continuous dosage increased the compressive stiffness. A possible explanation is that different dosages may mimic temporal patterns of growth factor expression during wound healing32 as well as during chondrogenesis, as reviewed by Goldring et al.33
TGF-β1 and the combination of BMP-2 and IGF-I were identified as the winners in terms of construct functionality in this study. These results were primarily obtained in phase II, where BMP-2, IGF-I, and TGF-β1 were applied at the selected conditions from phase I in combinations of one, two, or three. Combined BMP-2 and IGF-I treatment had beneficial effects, demonstrating the greatest increase in aggregate modulus (119%), accompanied by the greatest increase in GAG/WW (54%). However, as with the use of these growth factors individually, there was no difference in tensile properties or collagen/WW. As in phase I, only treatment with TGF-β1 alone led to a significant increase in tensile properties and collagen/WW. There was a disparity in values obtained for the individual growth factor treatments between phases I and II, likely as a result of different donor tissue from which the cells were isolated. However, although the values for the properties of the control constructs vary between the phases, similar percent increases in properties are observed for the individual growth factors in each phase.
It is also interesting to note that combining TGF-β1 with either of the other growth factors did not have additive or synergistic effects, negating the increased compressive and tensile stiffness observed with TGF-β1 treatment alone. This result agrees with prior work by Blunk et al.5 which noted that combined TGF-β1 and IGF-I treatment decreased GAG and collagen fractions. Additionally, TGF-β1 has been shown to regulate the autocrine/paracrine axis of IGF-I,34 and it is likely that combined growth factor treatment may alter these intracellular pathways, potentially leading to the reduced effects observed in this study. Prior work by Suzuki et al.35 also supports our results, as it was demonstrated that BMP-2 signal transduction was inhibited by application of TGF-β1. However, it is possible that there is a concentration-dependence of our results; for example, if TGF-β1 was applied at much higher or lower concentrations than used in the manuscript, IGF-I and BMP-2 may have different responses than what was reported in this study.
It is important to note that our results differ from several prior growth factors studies5, 6 which have utilized culture medium containing FBS. This medium already contains growth factors, potentially confounding the effects of additional growth factor application. In this study, we utilized serum-free medium to control for any confounding from the presence of FBS in the medium and to enable us to look solely at the effects of the growth factor supplementation. The use of serum-free medium may explain some of the differences between our results and those of prior studies. Additionally, the self-assembly process may modulate some of the effects of growth factors differently. For example, Gooch et al.6 found that treatment with BMP-2 at 100 ng/ml led to the presence of hypertrophic chondrocytes; however, we found no differences in chondrocyte morphology nor any other histological properties. Furthermore, it has previously been shown that growth factor application at higher concentrations significantly increases construct WW.5, 6 We did not observe this WW increase, and in fact found that TGF-β1 treatment actually decreased the construct WW. It is possible that these responses are due to the combined effects of FBS and supplemental growth factors, and that the use of growth factors in serum-free conditions mitigates the hypertrophic response at the concentrations used in the present study.
Although multiple studies have examined the effects of various growth factors on monolayer, explant, and engineered construct gene expression and biochemical properties, this study systematically assessed the effects of different growth factors, concentrations, dosages, and combinations, leading to construct biochemical and biomechanical properties in the range of native tissue values. Since most other investigations of engineered cartilage have not achieved the biochemical and biomechanical properties found in this study in only 4 wks, the results presented here likely are due to the combination of the self-assembling process, serum-free media, and the selected growth factor regimens. Only treatment with TGF-β1 was found to enhance both the compressive and tensile properties of engineered constructs, while combined treatment with BMP-2 and IGF-I led to adjunctive enhancement of construct compressive stiffness and GAG content. As previous studies have demonstrated beneficial effects of combined growth factor treatment and direct compression,14, 36 future studies should assess the effects of these growth factor treatments when combined with mechanical stimulation, such as hydrostatic pressure and direct compression.
Acknowledgments
The authors would like to acknowledge funding from NIH NIAMS R01 AR053286 and funding from the NIH Biotechnology Training Grant. Additionally, the authors would like to acknowledge Dustin Baldridge and the laboratory of Dr. Brendan Lee for assistance with immunohistochemistry.
Grant Support: National Institute of Arthritis and Musculoskeletal and Skin Disease, R01 AR053286
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 2.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12(4):969–79. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 3.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12(5):1337–44. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 4.Elder BD, Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26(2):238–46. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blunk T, Sieminski AL, Gooch KJ, et al. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002;8(1):73–84. doi: 10.1089/107632702753503072. [DOI] [PubMed] [Google Scholar]
- 6.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Vunjak-Novakovic G, Freed LE. Bone morphogenetic proteins-2, -12, and -13 modulate in vitro development of engineered cartilage. Tissue Eng. 2002;8(4):591–601. doi: 10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 7.Ng KW, O’Conor CJ, Kugler LE, Ateshian GA, Hung CT. The response of engineered cartilage to a timed application of transforming and insulin-like growth factors. Transactions of the ORS. 2008;33:588. [Google Scholar]
- 8.Wagner DR, Lindsey DP, Li KW, et al. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36(5):813–20. doi: 10.1007/s10439-008-9448-5. [DOI] [PubMed] [Google Scholar]
- 9.Darling EM, Athanasiou KA. Growth factor impact on articular cartilage subpopulations. Cell Tissue Res. 2005;322(3):463–73. doi: 10.1007/s00441-005-0020-4. [DOI] [PubMed] [Google Scholar]
- 10.Chubinskaya S, Hakimiyan A, Pacione C, et al. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2007;15(4):421–30. doi: 10.1016/j.joca.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieb E, Milz S, Vogel T, Hacker M, Dauner M, Schulz MB. Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. I. Culture conditions and tissue formation. Tissue Eng. 2004;10(910):1399–413. doi: 10.1089/ten.2004.10.1399. [DOI] [PubMed] [Google Scholar]
- 12.Lieb E, Vogel T, Milz S, Dauner M, Schulz MB. Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: Osteoblastic differentiation. Tissue Eng. 2004;10(910):1414–25. doi: 10.1089/ten.2004.10.1414. [DOI] [PubMed] [Google Scholar]
- 13.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25(3):293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 14.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9(4):597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 15.Saini S, Wick TM. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 2004;10(56):825–32. doi: 10.1089/1076327041348545. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Minakuchi K, Kaji S, Koga J. Chondrocyte migration to fibronectin, type I collagen, and type II collagen. Cell Struct Funct. 1997;22(3):309–15. doi: 10.1247/csf.22.309. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;(53):69–82. [PubMed] [Google Scholar]
- 18.Brown AN, Kim BS, Alsberg E, Mooney DJ. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000;6(4):297–305. doi: 10.1089/107632700418029. [DOI] [PubMed] [Google Scholar]
- 19.Pietila K, Kantomaa T, Pirttiniemi P, Poikela A. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164(1):30–6. doi: 10.1159/000016640. [DOI] [PubMed] [Google Scholar]
- 20.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 21.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12(3):340–9. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 22.Sneddon I. The relaxation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;(3):47–57. [Google Scholar]
- 23.Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5(5):541–51. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 24.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J Biomech Eng. 1980;102(1):73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 25.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13(9):2195–205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 26.Luyten FP, Yu YM, Yanagishita M, Vukicevic S, Hammonds RG, Reddi AH. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992;267(6):3691–5. [PubMed] [Google Scholar]
- 27.Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308(1):137–47. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 28.Fortier LA, Lust G, Mohammed HO, Nixon AJ. Coordinate upregulation of cartilage matrix synthesis in fibrin cultures supplemented with exogenous insulin-like growth factor-I. J Orthop Res. 1999;17(4):467–74. doi: 10.1002/jor.1100170403. [DOI] [PubMed] [Google Scholar]
- 29.Fortier LA, Nixon AJ, Mohammed HO, Lust G. Altered biological activity of equine chondrocytes cultured in a three-dimensional fibrin matrix and supplemented with transforming growth factor beta-1. Am J Vet Res. 1997;58(1):66–70. [PubMed] [Google Scholar]
- 30.Galera P, Redini F, Vivien D, et al. Effect of transforming growth factor-beta 1 (TGF-beta 1) on matrix synthesis by monolayer cultures of rabbit articular chondrocytes during the dedifferentiation process. Exp Cell Res. 1992;200(2):379–92. doi: 10.1016/0014-4827(92)90186-c. [DOI] [PubMed] [Google Scholar]
- 31.Galera P, Vivien D, Pronost S, et al. Transforming growth factor-beta 1 (TGF-beta 1) up-regulation of collagen type II in primary cultures of rabbit articular chondrocytes (RAC) involves increased mRNA levels without affecting mRNA stability and procollagen processing. J Cell Physiol. 1992;153(3):596–606. doi: 10.1002/jcp.1041530322. [DOI] [PubMed] [Google Scholar]
- 32.Bos PK, van Osch GJ, Frenz DA, Verhaar JA, Verwoerd-Verhoef HL. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage. 2001;9(4):382–9. doi: 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- 33.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 34.Tsukazaki T, Usa T, Matsumoto T, et al. Effect of transforming growth factor-beta on the insulin-like growth factor-I autocrine/paracrine axis in cultured rat articular chondrocytes. Exp Cell Res. 1994;215(1):9–16. doi: 10.1006/excr.1994.1307. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Nagano T, Yamakoshi Y, et al. Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-{beta} J Dent Res. 2005;84(6):510–4. doi: 10.1177/154405910508400605. [DOI] [PubMed] [Google Scholar]
- 36.Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19(1):11–7. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]