Abstract
A new high-load, oligomeric monoamine hydrochloride (OMAm•HCl) derived from ring-opening metathesis polymerization (ROMP) of norbornene methylamine is reported. This oligomeric amine has been shown to be an effective scavenger of acid chlorides, sulfonyl chlorides and isocyanates. The reagent can be synthesized in a straightforward protocol from the Diels-Alder reaction of dicyclopentadiene (DCPD) 1 with allylamine (neat), formation of the corresponding ammonium salt and subsequent ROM polymerization to afford the desired oligomeric ammonium salts.
The growing demand for facile protocols to produce libraries of compounds for high throughput screening related to drug discovery has led to considerable efforts towards the development of new enabling techniques.1,2 In this regard, immobilized reagents/scavengers have emerged as effective tools which streamline purification by sequestering remaining reactant/reagents, leaving the desired pure product in solution.3 While resin-based solid phase organic chemistry (SPOC) has merits for offering a direct purification technique, and is highly amenable to automation, it also presents limitations. Two primary deficiencies are the low load levels of reactive functionality in traditional solid phase resins (typically 1 mmol/g) and non-ideal reaction kinetics due to heterogeneous reaction conditions. These problems are further compounded by restrictions in both the types of solvents that can be used with each resin and extended validation times required when transferring or optimizing solution-phase organic reaction protocols to the heterogeneous environment of resin-based SPOC synthesis.
In order to circumvent these issues, efforts have been directed toward the development of new designer polymeric reagents and scavengers with tunable properties.2 Despite significant advances, resin limitations in non-linear reaction kinetics, low resin-load capacities, means of distributing reagents, and “technological mechanics” behind automating multi-step parallel solution phase sequences continue to warrant the development of novel platforms and improved strategies. Among several technologies that have emerged, ring-opening metathesis (ROM) polymerization,4 of functionalized norbornenes has surfaced as a powerful tool in the generation of high-load, immobilized reagents with tunable properties to overcome the aforementioned limitations.5,6 We herein report the development and application of a new high-load, oligomeric monoamine hydrochloride (OMAm•HCl) 4a which can be produced on large scale from combination of Diels-Alder and ROMP methodologies.
2. Results and Discussion
Our interest in the synthesis of a high-load norbornenyl-tagged amine scavenger was based on the desire to develop a complimentary high-load, nucleophilic scavenger to augment electrophilic scavengers previously reported.7 Resin-based amine scavengers are well known to scavenger/sequester commonly used electrophiles such as acid chlorides, sulfonyl chlorides, isocyanates, anhydrides and chloroformates.8 With this goal in mind, we targeted the oligomeric monoamine 4 shown in Scheme 1. A quick calculation reveals the high-load nature of the targeted oligomeric ROMP scavenger 4 with a theoretical load of 8.3 mmol/g as the free amine. This value represents a significant advantage over commercially available electrophile scavengers: 1.3–1.5 mmol/g for PS-NH2 (polystyrene) 3–5 mmol/g for PS-Trisamine and 2–3 mmol/g for MP-Trisamine (macroporous, Biotage).9
Scheme 1.
The direct synthesis of 4 was envisioned to occur via Diels-Alder reaction of cyclopentadiene with allylamine followed by ROM polymerization. However, it has been well documented that metathesis of free primary amines is problematic.10 To circumvent this problem, we targeted the oligomeric monoamine hydrochloride 4a and envisioned its use as a scavenger in the presence of excess base to generate the free amine in situ. Monomeric ammonium salts have been previously shown to undergo facile ROM polymerization.11 In this regard, the free monomeric amine 2 was readily prepared by the Diels-Alder reaction of dicyclopentadiene (DCPD) 1 with allylamine (neat) in good yield and purity after distillation. The norbornene ammonium salts were prepared by treatment with the corresponding acid. ROM polymerization of the norbornene ammonium salts was achieved by subjecting the hydrochloride salt 3 with the second generation Grubbs catalyst cat-B in monomer:catalyst ratios of 25–70:1.12 The pure oligomeric monoamine hydrochloride cat-B (2GOMAm•HCl, theoretical load 6.3 mmol/g) was found to have low solubility in DCM and was isolated by filtration after treatment with ethyl vinyl ether. Polymerization of the acetate, tosylate and the bis(trimethylsilyl)amide derivatives of 1 was attempted as well. Treatment of the acetate of 3 with catalyst cat-B (monomer:catalyst ratio = 30:1) gave only starting material. The bis(trimethylsilyl)amide of 3 was also prepared and ROM polymerization with cat-B yielded a mixture of the desired polymer and starting material. The tosylate of 3 polymerized cleanly with cat-B (monomer:catalyst ratio = 20–40:1) to yield 2GOMAm•TsOH 4b.
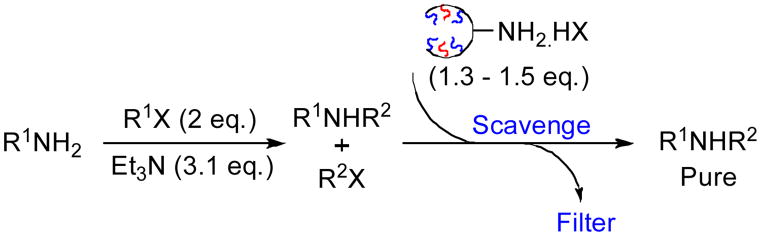
We found that although OMAm•HCl has limited solubility in most organic solvents including CH2Cl2, scavenging reactions were fast and required only a slight excess of the scavenging reagent. The benzoylation of a number of amines was investigated using an excess of benzoyl chlorides in the presence of 3 equivalents of base (entries 1–3, Table 1).13 Upon completion of the reaction, the excess benzyl chloride was scavenged with 1.5 equivalents of 2GOMAm•HCl. After two hours at room temperature, the crude reaction mixture was added to a SiO2 SPE, and flushed with a solvent mixture of 1:1 EtOAc:Hexane. The resulting solution gave the desired amides in good yield and purity. When K2CO3 was used as the base, the reaction times for the scavenging event were longer, but workup involved only a simple filtration through Celite. Additionally, we demonstrated the application of 2GOMAm•HCl for the scavenging of excess sulfonyl chlorides in the sulfonylation of amines. It is worthy to note that only 210 mg (1.5 eq) of the OMAm•HCl was needed to scavenge 1 mmol of the electrophile. In comparison, at least 1.0 gram (3 equivalents) of commercially available resin Argoresin MP-Trisamine (Biotage, 2–3 mmol/g)9 are required for complete consumption of the same amount of electrophile. This data reinforces empirical evidence that ROMP reagents possess characteristics on the fringe between small molecules and that of insoluble polymers.
Table 1.
Formation of substituted amines using 2GOMAm•HCl (4a)a.
| Entry | R1NH2 | R2X | Yield (%)a | Purity (%)b |
|---|---|---|---|---|
| 1 | iPrNH2 | BnC(O)Cl | 94 | >95 |
| 2 | BnNH2 | BnC(O)Cl | 96 | >95 |
| 3 | Pyrrolidine | BnC(O)Cl | 92 | >95 |
| 4 | iPrNH2 | 4-MeOBnCl | 94 | >95 |
| 5 | iPrNH2 | 2,3-MeOBnCl | 93 | >95 |
| 6 | iPrNH2 | 4-NO2BnCl | 92 | >95 |
| 7 | BnNH2 | TsCl | 98 | >95 |
| 8 | BnNH2 | 2-BrBnSO2Cl | 96 | >95 |
| 9 | BnNH2 | 2-Br-4CF3-BnSO2Cl | 92 | >95 |
| 10 | 4-MeBnNH2 | PhNCO | 94 | >95 |
| 11 | 4-MeBnNH2 | 4-MePhNCO | 96 | >95 |
| 12 | 4-MeBnNH2 | BnCHO | 90 | >95 |
| 13 | 4-MeBnNH2 | 4-MeOBnCHO | 91 | >95 |
All reactions were carried out on 0.164 mmol scale, with addition of 1.5 eq of 4a, unless stated otherwise.
Purity by 1H NMR of crude isolated products.
In addition to acid chlorides, OMAm•HCl was also applied as a scavenger to remove excess isocyanate and aldehydes yielding the desired product in good yields and purity (Table 1 Entry 10–13).
In conclusion, we have synthesized a high-load oligomeric amine hydrochloride scavenger (4a) on multi-gram scale from inexpensive starting materials that is highly amenable to kilogram scale-up. The utility of these scavengers for scavenging/sequestering of an assortment of electrophiles has been demonstrated. Ultimately, this reagent has application in facilitated protocols for library production avoiding tedious work-up procedures.
Scheme 2.
Acknowledgments
This investigation was generously supported by funds provided by the NIH STTR grant (R41 GM076765 STTR-Phase-I), NIH COBRE award (P20 RR015563) and the National Institutes of General Medical Sciences (KU Chemical Methodologies and Library Development Center of Excellence, P50 GM069663).
References
- 1.(a) Booth RJ, Hodges JC. Acc Chem Res. 1999;32:18–26. [Google Scholar]; (b) Ley SV, Baxendale IR, Bream RN, Jackson PS, Leach AG, Longbottom DA, Nesi M, Scott JS, Storer RI, Taylor SJ. J Chem Soc, Perkin Trans. 2000;1:3815–4195. [Google Scholar]; (c) Kirschning A, Monenschein H, Wittenberg R. Angew Chem Int Ed. 2001;40:650–679. [PubMed] [Google Scholar]; (d) Eames J, Watkinson M. Eur J Org Chem. 2001:1213–1224. [Google Scholar]
- 2.(a) Gravert DJ, Janda KD. Chem Rev. 1997;97:489–509. doi: 10.1021/cr960064l. [DOI] [PubMed] [Google Scholar]; (b) Toy PH, Janda K. Acc Chem Res. 2000;33:546–554. doi: 10.1021/ar990140h. [DOI] [PubMed] [Google Scholar]; (c) Dickerson TJ, Reed NN, Janda KD. Chem Rev. 2002;102:3325–3344. doi: 10.1021/cr010335e. [DOI] [PubMed] [Google Scholar]; (d) Haag R. Chem Eur J. 2001;7:327–335. doi: 10.1002/1521-3765(20010119)7:2<327::aid-chem327>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; (e) Haag R, Sunder A, Hebel A, Roller S. J Comb Chem. 2002;4:112–119. doi: 10.1021/cc010058p. [DOI] [PubMed] [Google Scholar]; (f) Bergbreiter DE. Chem Rev. 2002;102:3345–3384. doi: 10.1021/cr010343v. [DOI] [PubMed] [Google Scholar]; (g) Yoshida JI, Itami K. Chem Rev. 2002;102:3693–3716. doi: 10.1021/cr0103524. [DOI] [PubMed] [Google Scholar]; (h) van Heerbeek R, Kamer PCJ, van Leeuwen PWNM, Reek JNH. Chem Rev. 2002;102:3717–3756. doi: 10.1021/cr0103874. [DOI] [PubMed] [Google Scholar]
- 3.(a) Baxendale IR, Lee AL, Ley SV. J Chem Soc Perkin Trans 1. 2002:1850–1857. [Google Scholar]; (b) Baxendale IR, Ley S. Ind Eng Chem Res. 2005;44:8588–8592. [Google Scholar]; (c) Creswell MW, Bolton GL, Hodges JC, Meppen M. Tetrahedron. 1998;16:3983–3998. [Google Scholar]; (d) Chaudhry P, Schoenen F, Neuenswander B, Lushington GH, Aubé J. J Comb Chem. 2007;9:473–476. doi: 10.1021/cc060159t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Barrett AGM, Hopkins BT, Köbberling J. Chem Rev. 2002;102:3301–3324. doi: 10.1021/cr0103423. [DOI] [PubMed] [Google Scholar]; (b) Harned AM, Probst DA, Hanson PR. In: Handbook of Metathesis. 2. Grubbs RH, editor. Wiley-VCH: Weinheim; 2003. pp. 361–402. [Google Scholar]; (c) Flynn DL, Hanson PR, Berk SC, Makara GM. Curr Opin Drug Discov Devel. 2002;5:571–579. [PubMed] [Google Scholar]
- 5.(a) Harned AM, Zhang M, Vedantham P, Mukherjee S, Herpel RH, Flynn DL, Hanson PR. Aldrichim Acta. 2005;38:3–16. [Google Scholar]; (b) Zhang M, Flynn DL, Hanson PR. J Org Chem. 2007;72:3194–3198. doi: 10.1021/jo0620260. [DOI] [PubMed] [Google Scholar]; (b) Harned AM, Sherrill WM, Flynn DL, Hanson PR. Tetrahedron. 2005;61:12093–12099. [Google Scholar]
- 6.For use of ROMP reagents in library synthesis, see: Vedantham P, Zhang M, Gor PJ, Huang M, Georg GI, Lushington GH, Mitscher LA, Ye QZ, Hanson PR. J Comb Chem. 2008;10:195–203. doi: 10.1021/cc7000869.
- 7.(a) Herpel RH, Vedantham P, Flynn DL, Hanson PR. Tetrahedron Lett. 2006;47:6429–6432. [Google Scholar]; (b) Moore JD, Byrne RJ, Vedantham P, Flynn DL, Hanson PR. Org Lett. 2003;5:4241–4244. doi: 10.1021/ol0352759. [DOI] [PubMed] [Google Scholar]; (c) Moore JD, Herpel RH, Lichtsinn JR, Flynn DL, Hanson PR. Org Lett. 2003;5:105–107. doi: 10.1021/ol0270273. [DOI] [PubMed] [Google Scholar]
- 8.(a) Zhang J, Zhang L, Zhang S, Wang Y, Liu G. J Comb Chem. 2005;7:657–664. doi: 10.1021/cc050005y. [DOI] [PubMed] [Google Scholar]; (b) Shimomura O, Clapham B, Spanka C, Mahajan S, Janda KD. J Comb Chem. 2002;4:436–441. doi: 10.1021/cc020012b. [DOI] [PubMed] [Google Scholar]; (c) Macquarrie DJ, Rousseau H. Synlett. 2003;2:244–246. [Google Scholar]
- 9.Biotage: http://www.biotage.com/.
- 10.Larroche C, Laval JP, Lattes A, Basset JM. J Org Chem. 1984;49:1886–1890. [Google Scholar]
- 11.(a) Liaw DJ, Tsai C–H. J Mol Catalysis A: Chemical. 1999;147:23–31. [Google Scholar]; (b) Liaw D–J, Huang CC, Wu PL. Macromol Chem Phys. 2002;203:2177–2187. [Google Scholar]; (c) Pontrello JK, Allen MJ, Underbakke ES, Kiessling LL. J Am Chem Soc. 2005;127:14536–14537. doi: 10.1021/ja053931p. [DOI] [PubMed] [Google Scholar]
- 12.General procedure for the polymerization of the ammonium salts: A suspension of the hydrochloride salt (5g, 31.3 mmol) in DCM (200 ml) was degassed with argon for 15 minutes. Metathesis catalyst cat-B (177 mg, 0.21 mmol) was added and the mixture refluxed under argon until the reaction was completed. The mixture was cooled, ethyl vinyl ether (5 ml) was added and the mixture stirred for 30 minutes. The oligomer was filtered, washed with CH2Cl2 and dried under high vacuum to afford 5.0 g as a free-flowing off-white solid.
- 13.General procedure for the benzoylation of amines, followed by scavenging with 4a: Benzoyl chloride (38.1 μl, 0.33 mmol, 2 eq.) was added to a solution of benzylamine (17.9 μl, 0.164 mmol, 1 equiv.), Et3N (68.5 μl, 0.49 mmol, 3 equiv.) and CH2Cl2 (0.36 ml, 0.46 M). After stirring for 2 hours, OMAm50 (41.9 mg, 0.26 mmol, 1.5 equiv.) was added and stirring was continued for two additional hours. The reaction was filtered through a SiO2 SPE using a hexane:EtOAc (1:1) to yield the desired product.