Abstract
Background
C4d is a useful marker of antibody-mediated rejection in cardiac and renal transplants, but clinical studies examining correlations between circulating alloantibodies, C4d deposition, and rejection in lung transplants have yielded conflicting results.
Methods
We studied circulating alloantibody levels and C4d deposition in two rat models of lung transplantation: Brown Norway (BN) to Wistar-Kyoto (WKY) and PVG.R8 to PVG.1U lung allografts. The availability of C6 deficient (C6−) and C6 sufficient (C6+) PVG 1U rats allowed evaluation of the effects of the terminal complement components on graft injury and C4d deposition.
Results
The lung allografts had histologic features resembling human posttransplant capillaritis, characterized by neutrophilic infiltration of alveoli, edema, and hemorrhage. Immunoperoxidase stains on cross sections of allografts showed intense, diffuse, C4d deposition in a continuous linear pattern on the vascular endothelium. C4d deposits were found in both BN to WKY and PVG R8 to 1U allografts, whereas no staining was detectable in WKY to WKY isografts or native lungs. Complement deposition was associated with vascular disruption in C6−, but not in C6+ recipients. The presence of circulating donor-specific alloantibodies was verified by flow cytometry. Cell-specific staining revealed perivascular accumulation of macrophages and T lymphocytes whereas neutrophils were sequestered in the intravascular and alveolar capillary compartments.
Conclusions
The deposition of C4d on vascular endothelium as well as the coincident presence of alloantibodies is consistent with previous findings in antibody-mediated rejection of renal and cardiac transplants. Furthermore, the histological features of our allografts support the concept that posttransplant capillaritis is a form of humoral rejection.
Keywords: Complement, Macrophage, Neutrophil, Lymphocyte, Alloantibody
Antibody and complement-mediated injury is less well investigated in lung transplants than in renal or cardiac transplants. In the last few years, there have been limited reports of clinical evidence of acute antibody and complement-mediated injury to lung transplants (1-3). These reports provide divergent views of the antibodies associated with acute graft injury. Furthermore, other studies have yielded conflicting data regarding the diagnostic value of C4d as a marker for lung allograft rejection, with one group reporting no correlation between C4d staining and lung rejection (4) whereas another has reported C4d staining in lung allografts correlated with the presence of alloantibodies to human leu- kocyte antigen (HLA) (5), and another group reported that C4d deposits correlated with endothelial specific alloantibodies (2). Finally, different patterns of C4d deposition have been reported, varying from linear deposits on endothelial cells to granular deposits including nuclear and membrane staining (1-3, 5).
In light of these limited and diverse findings, we initiated a study of C4d deposition in well-defined models of orthotopic lung transplantation in rats. We purposely included in these studies lung transplants performed by two different transplant centers (Johns Hopkins and Indiana Universities) using different rat strains; namely, Brown Norway (BN) donors to Wistar-Kyoto (WKY) recipients, and PVG.R8 donors to congenic major histocompatibility complex (MHC) class I incompatible PVG.1U recipients. This eliminated the possibility that the findings were limited to a specific transplantation protocol used at a single center or to a single strain of rats. All of these transplants undergo acute rejection in untreated recipients and elicit high titers of alloantibody. The congenic strain combination elicits antibodies to MHC class I antigens (6). Our previous studies in PVG congenic rats used C6 deficient rats to establish that complement activation contributed to the acute rejection of lung transplants in this strain combination. In the current study, we have used a recently described rabbit polyclonal antibody to rat C4d (7) to stain complete cross sections from paraffin-embedded orthotopic unilateral lung transplants. This approach avoids the artifacts in morphology frequently resulting from the mechanics of obtaining small transbronchial biopsies from human transplants. We have further characterized the presence of neutrophil and macrophage infiltrates that are characteristic of antibody-mediated rejection in renal and cardiac allografts. Neutrophils have been described as a feature of posttransplant capillaritis in lungs (8), but this pathological lesion has not been related to donor-specific antibodies and complement deposition.
MATERIALS AND METHODS
Experimental Design
Lung allografts were performed in two different rat strain combinations. In our first set of experiments, BN (RT–1n)lungs were transplanted into WKY (RT–1l)recipients (allografts, n=5). To study graft histology in the absence of rejection, WKY lungs were transplanted into WKY rats (isografts, n=5). Recipient rats were sacrificed 14 days after transplantation.
In our second set of experiments, PVG.R8 (RTl.AaBu) donor lungs were transplanted into C6 sufficient (C6+) PVG.1U (RT1.AuBu) (n=5) or C6 deficient (C6−) (n=5) PVG.1U rat recipients. Recipient rats were sacrificed 5 or 7 days after transplantation.
Animals
BN and WKY rats were purchased from Harlan (Indianapolis, IN) and housed in the Laboratory Animal Resource Center at Indiana University School of Medicine (Indianapolis, IN) in accordance with institutional guidelines.
The derivation of PVG congenic rat strains with C6 deficiency has been described previously (9). The PVG.R8 (RTl.AaBu) and PVG.1U (RT1.AuBu) rats used in these study are mismatched at MHC class I antigens. Donor and recipient rats were always the same gender. C6 levels in the sera were confirmed by a sandwich enzyme-linked immunosorbent assay, and MHC phenotypes of these congenic rats were confirmed by flow cytometry as described previously (9,10). Rats were 200 to 300 g at the time of transplantation.
All animals received humane care in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals prepared and formulated by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication 86-23, revised 1985).
Unilateral Orthotopic Lung Transplantation
In the experiments using BN and WKY rats, orthotopic transplantation of left-lung isografts (WKY→WKY) or allografts (BN→WKY) was performed in the same manner as previously reported (11,12). Survival was more than 90% in all transplantation groups. No immunosuppressive, antibiotic, or anti-inflammatory therapy was given at any time during the experimental period. Details of anesthesia, ventilator management, and other surgical details have been reported elsewhere (13).
In the studies using PVG.R8 and PVG.1U rats, donor left lungs were transplanted orthotopically into the left hemithorax of recipients using a modified technique of Marck et al. (14), and the pulmonary artery and vein were anastomosed using the cuff technique of Reis et al. (15). The mean graft ischemic time was 45±10 min. A single dose of 10,000 U penicillin G was administrated intramuscularly immediately after surgery, and 10 mg/day gentamicin was administered intramuscularly from days 0 to 4 after transplantation.
Serum Samples
Blood samples were collected by tail bleeding before surgery and at the time of killing. Blood was allowed to clot for 30 min at 37°C and then for 1 hr at 4°C. Serum was separated by centrifugation and was stored at −80°C until use.
Alloantibody Assay
Alloantibodies were measured by flow cytometry on single-cell suspensions of cervical lymph nodes from BN or PVG.R8 rats as described previously (16,17). Briefly, the cells were incubated with 50 μL diluted heat-inactivated sera (1/4, 1/16, 1/64, and 1/256). The washed cells were reacted with 50 μL phosphate buffer saline containing 0.2% bovine serum albumin and 0.02% NaN3 containing a mixture of fluorescein isothiocyanate-conjugated F(ab′)2 of goat anti-rat IgG and PE-conjugated F(ab′)2 of goat anti-rat μ-chain of rat IgM (Jackson ImmunoResearch Laboratories, West Grove, PA). The cells were analyzed using an FACScan flow cytometer (BD Biosciences, MountainView, CA). Transplant recipients were determined to be positive for alloantibodies at a given titer if the mode channel fluorescence for IgM or IgG was greater than two standard deviations above the mean for the isograft group in the BN to WKY transplants. Serum drawn from the transplant recipients pretransplant (day 0) served as controls for the PVG.R8 to 1U transplants.
Histological Evaluation of Tissue Sections
At the time of killing, the recipient rat was anesthetized and placed on a ventilator. The transplanted lung was exposed for gross evaluation. The PVG transplants were fixed by perfusion with 60% methanol with 10% acetic acid; whereas the BN transplants were fixed in 10% formalin as is routine in the two transplant centers. Tissues were embedded in paraffin and sectioned at 7 μm. Paraffin sections were stained with hematoxylin-eosin. The slides were evaluated by a pathologist blinded to the individual groups and were evaluated for signs of allograft rejection.
The paraffin sections were also stained by standard immunoperoxidase techniques using avidin-biotinylated enzyme complex for four markers: (1) rat C4d using a polyclonal rabbit antibody described previously (7) (2) myeloperoxidase (MPO), in rodents, marker specific for neutrophils (Biomeda, Burlingame, CA), (3) rat CD68 (ED1), which is a lysosomal membrane antigen expressed in rat macrophages (Antibodies Direct, Raleigh, NC), and (4) rat CD8 (clone OX-8), for detection of CD8+ cytotoxic T lymphocytes (BD Biosciences, San Jose, CA). These samples were evaluated by a pathologist blinded to individual groups, and were scored on a scale of 0 to 4, using criteria based on the clinical classification of lung transplant rejection (18). A score of 0 designated the absence of inflammatory infiltrate, 1 designated rare, scattered cell infiltrates that are not obvious at low magnification, 2 designated significant cellular infiltrates involving bronchioles, arteries, or alveolae that were evident at low magnification, 3 designated dense cellular infiltrates encompassing bronchioles, arteries, or alveolae, and 4 designated diffuse cellular infiltration. This scoring was conducted for each of the individual lung compartments, namely, the perivascular, intravascular, alveolar, and alveolar capillary regions of the lung.
In addition to the above stains, trichrome staining (Sigma-Aldrich, St. Louis, MO) was conducted on PVG allograft lungs.
RESULTS
Lung Allografts Elicit Alloantibody Responses to Donor Antigens
IgG and IgM alloantibodies were detected in serum of transplant recipients by flow cytometry using lymph node leukocytes from the donors. BN to WKY allograft recipients had circulating donor specific IgM and IgG alloantibodies detectable at titers greater than 1:256, whereas WKY to WKY isograft recipients had no detectable levels of alloantibody (data not shown).
All PVG R8 to 1U allograft recipients had donor-specific IgM and IgG titers greater than 1:256. IgM antibodies were detectable in the circulation by day 5. No significant differences in antibody titers were noted between C6+ and C6− allograft recipients.
C4d is Deposited on Vascular Endothelium in Acutely Rejected Lung Transplants
BN to WKY allografts were visibly rejected as evidenced by hyperemia and edema at the time of killing (2 weeks). Histologically, the grafts had significant leukocytic infiltration and extensive destruction of the alveolar walls. This rejection time is consistent with previous results in this strain combination (Wilkes et al., unpublished data). Staining of the lung allografts for C4d showed linear, diffuse C4d deposition on the vascular endothelium (Fig. 1a, b). Isografts from WKY rats showed no visible signs of rejection and no C4d deposition was detected on any structures in isografts (Fig. 1c) or native lungs (Fig. 1d). Each of the transplanted lungs were evaluated for extent of C4d staining and scored on a scale of 0 to 4. Tabulation of the results demonstrate that all BN to WKY allograft lungs had significant C4d staining (Fig. 3a) in the arteries and capillaries whereas no C4d staining was visible in any of the isograft or native lung controls.
FIGURE 1.
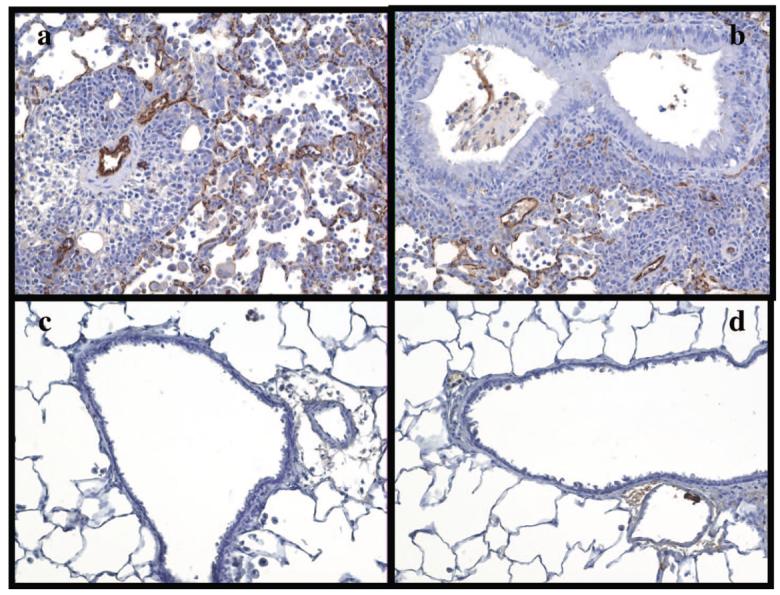
C4d staining of transplanted lung tissue from WKY recipients at 14 days after transplantation. BN to WKY allograft lungs (a and b) show strong linear deposits of C4d in both capillaries and arteries, combined with significant epithelial, perivascular, septal, and intra-alveolar infiltration of inflammatory cells. C4d staining was limited to vascular endothelium, with no detectable C4d deposition on the bronchioles (b). WKY to WKY lung isografts (c), and WKY native lungs (d) served as negative controls, and showed no detectable C4d deposition. No cellular infiltration or tissue damage was noted in the isograft groups.
FIGURE 3.
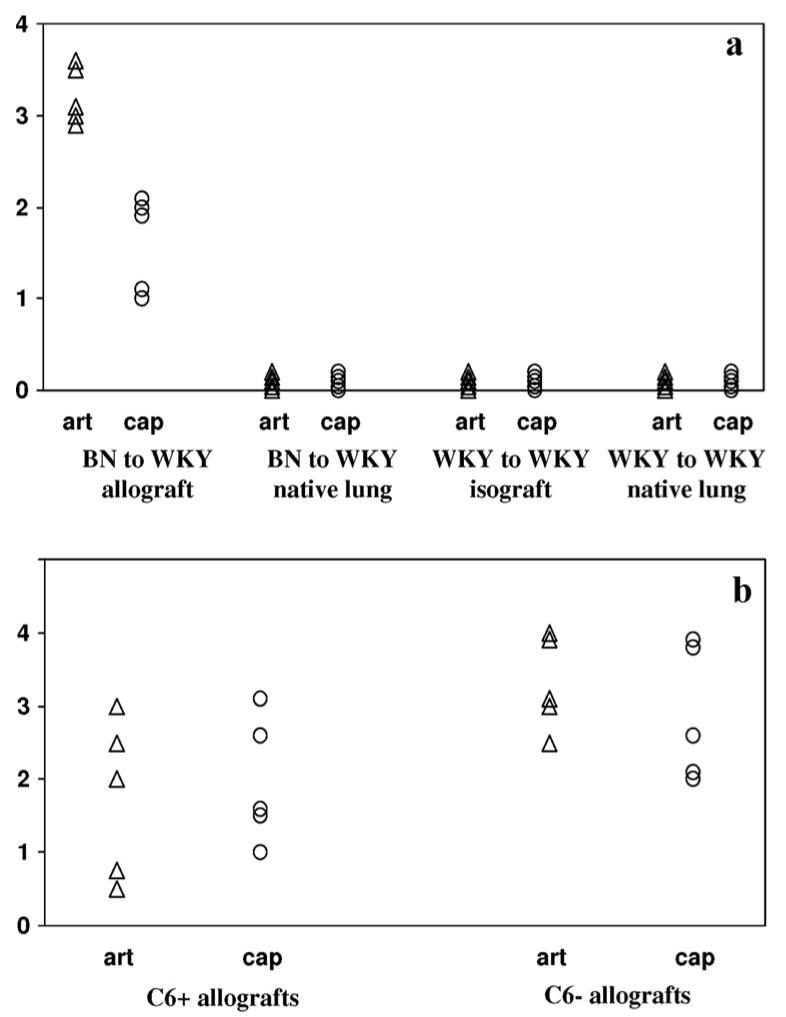
Scoring of transplanted lung tissue for levels of C4d staining. Transplanted lung tissue harvested from both WKY (a) and 1U (b) recipients were scored individually for levels of C4d staining from a scale of 0 to 4, with a score of 0 indicating no staining and a score of 4 indicating strong, linear deposition of C4d on the vascular endothelium throughout the lung. Significant C4d staining was noted in the arteries as well as in capillaries of BN to WKY lung allografts whereas no C4d staining was seen in either the native lung of lung transplant recipients or in the WKY to WKY lung isografts. Strong C4d staining was also noted in the PVG R8 to 1U transplants in both C6− and C6+ recipients. The lower scores in the C6+ group reflect the fragmented staining because of the disruption of the arterial and capillary endothelium. Each symbol represents one recipient.
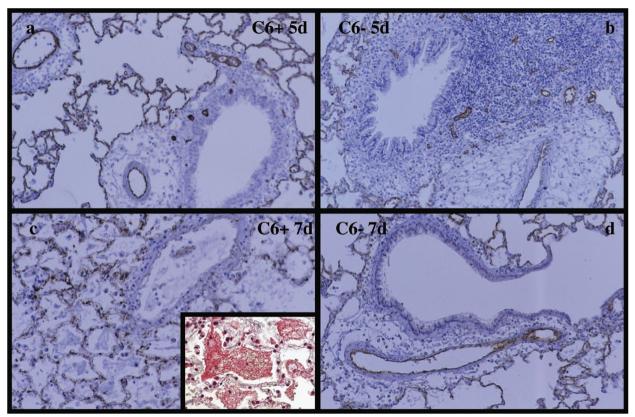
Orthotopic lung transplants from PVG.R8 donors were rejected within 6 to 7 days by C6+ PVG.1U rats in the absence of immunosuppression. In contrast, PVG.R8 lungs transplanted into C6− recipients had good vascular perfusion and air inflation at day 7 (6). Some of the C6+ recipients, as well as the C6− recipients, were killed at 5 days after transplantation to study the initial pattern of C4d deposition, whereas others were killed at 7 days to examine the pattern after rejection was complete. Strong, diffuse, and linear staining of vascular endothelium by C4d was noted in the arteries as well as capillaries of both C6+ and C6− recipients at 5 days after transplantation (Fig. 2a, b, respectively). By 7 days after transplantation, C4d staining was patchy in C6+ recipients, which was reflective of the extensive destruction of vascular endothelium contributed by the terminal membrane attack complex (Fig. 2c). Trichrome staining of the C6+ tissue revealed significant leakage of fibrin into the alveolar compartment, also indicating vascular damage (Fig. 2c, inset). C4d staining remained strong, linear, and diffuse at 7 days after transplantation in the lung allografts of C6− recipients (Fig. 2d). The PVG R8 to 1U allografts were scored for C4d staining in a manner similar to the BN to WKY groups, and significant C4d staining was noted in both C6+ and C6− groups. The lower scores in the C6+ group reflect the fragmented staining because of the disruption of the arterial and capillary endothelium (Fig. 3b).
FIGURE 2.
C4d staining of lung allografts in C6+ and C6− PVG 1U recipients. Lung allografts were taken from C6+ and C6− recipients 5 days (a and b, respectively) and 7 days (c and d, respectively) after transplantation. The C6+ group showed extensive endothelial damage and diffuse alveolar edema and hemorrhage on the 7th day. Trichrome staining revealed extensive leakage of fibrin into the alveolar compartment, indicating severe vascular damage (c, inset). In contrast, the alveolar setpa are largely intact in the C6− recipients. Strong, linear staining of vascular endothelium is seen at both 5 and 7 days for the C6− group, and at 5 days for the C6+ group. In the C6+ group at 7 days, there is patchy staining of alveolar capillaries and no staining of arteries because of extensive endothelial damage.
Cellular Infiltration in Acutely Rejected PVG R8 to 1U Lung Transplants
The presence of neutrophils and macrophages in allograft biopsy tissue is a key feature in the diagnosis of antibody-mediated rejection in cardiac (19) and renal (20) transplantations. Furthermore, our laboratory has shown previously that activated macrophages can be found in rejecting lung allografts (6).
In previous studies, we established with the use of C6 deficient donors and recipients that the antibodies to MHC class I generated in the recipients of R8 lung allografts contributed to rejection. Therefore, we used the PVG.R8 to 1U model to define the cellular responses associated with antibody-mediated rejection.
Using ED1, a mouse monoclonal to rat CD68, we found macrophage infiltration to be strongest in the perivascular region of the PVG R8 to 1U lung allografts, with a lower concentration of macrophages located in the alveolar and vascular compartments (Fig. 4a).
FIGURE 4.
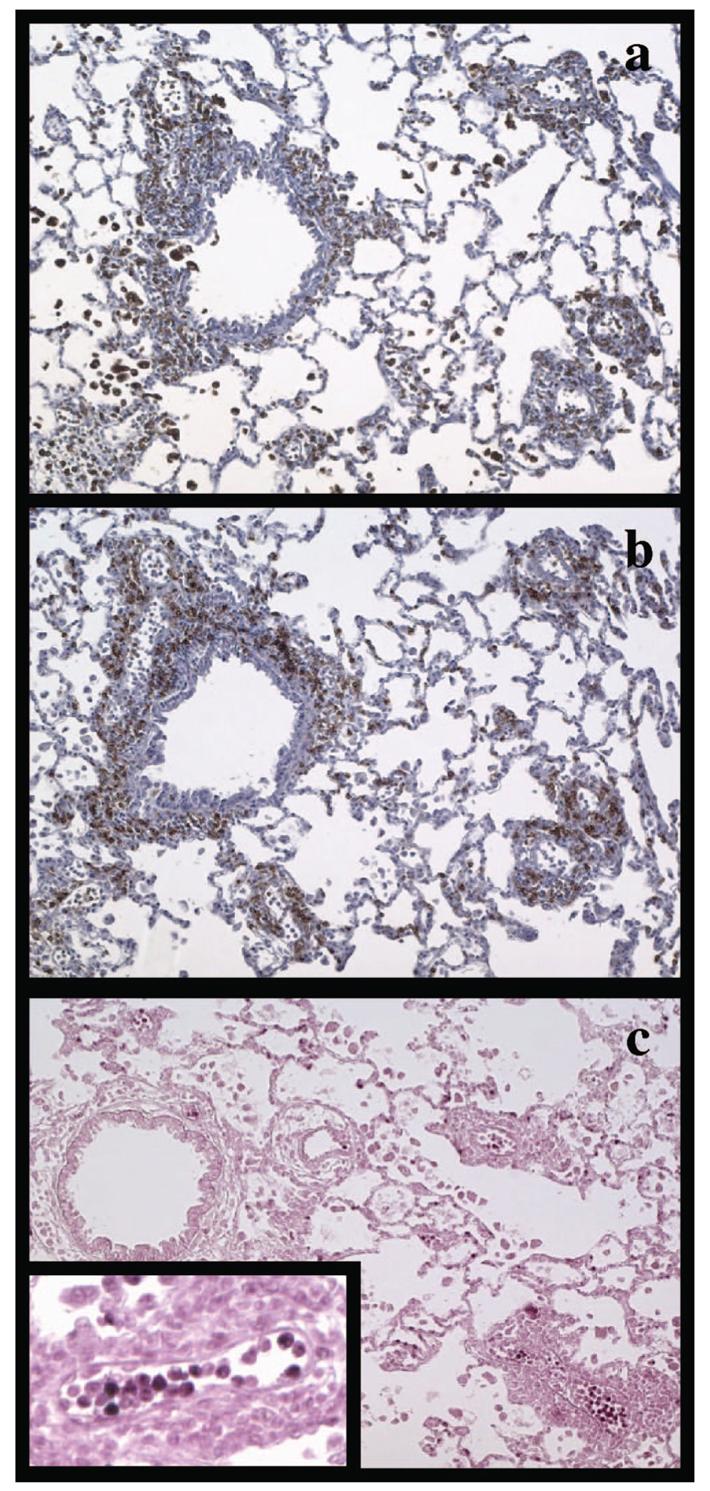
Immunohistochemical staining of lung allograft tissue from C6− recipients. Lung allografts were stained for CD68+ macrophages (a), CD8+ T lymphocytes (b), and MPO+ neutrophils (c). Significant perivascular and peribronchiolar infiltrates by macrophages and cytotoxic T cells were noted, whereas neutrophils were found predominantly in the intravascular compartment of the allografted lungs (c, inset).
Neutrophils are also seen frequently in clinical biopsies from kidneys undergoing antibody-mediated rejection (20). We have used antibodies directed against MPO to stain for neutrophils in our rat lung tissue specimens. Significant numbers of intravascular neutrophils were noted in lungs transplanted into C6 deficient recipients (Fig. 4c inset). Some neutrophils were noted in the alveolar capillaries. Few neutrophils were noted in the perivascular regions of the graft.
The PVG.R8 to 1U strain combination is mismatched for MHC class I antigens. Thus, an antibody to rat CD8 was used to stain for cytotoxic T lymphocytes, which recognize foreign antigens in the context of class I MHC, in our lung transplants. These stains showed extensive perivascular infiltration of lymphocytes (Fig. 4b).
All stained tissue sections were scored semi-quantitatively for degree of infiltration by macrophages, CD8+ T cells, and neutrophils in each of the three different lung compartments (intravascular, perivascular, and alveolar capillaries). This demonstrated that macrophages and T cells were found predominantly in the perivascular region, whereas neutrophils were found primarily in the intravascular compartment (data not shown).
DISCUSSION
We have used rat models of orthotopic lung transplantation to examine whether C4d correlates with antibody-mediated rejection of lung allografts. This was prompted by conflicting reports regarding the utility of C4d as a clinical diagnostic marker for acute lung rejection. To date, all of these studies have used clinical biopsy material. However, studies using biopsies only sample very small amounts of tissue from restricted sites in the organ that do not necessarily reflect pathological changes in the entire lung. Transbronchial biopsies, in particular, are limited to sampling a small area adjacent to the bronchi. These biopsies capture primarily a network of capillaries with a few small arterioles that frequently are distorted by “crush artifacts.” The use of rat models of lung transplantation allowed us to examine entire cross sections of a lung in different segments of the lung, allowing us to gain a more complete picture of histopathological changes occurring within the lung.
The fact that we found similar patterns of C4d deposition in two models of orthotopic lung transplantation that were performed in two different transplant centers and used different strains of rats indicates that these findings are not dependent on surgical technique or methods of fixation. The staining for C4d was strong, in both formalin fixed and acetic methanol fixed tissues. Likewise, strong staining was found in the PVG congenic strain combination that has only an MHC class I histoincompatibility (PVG.R8; RT1.AaBu donor to PVG.1U; RT1.AuBu recipients), and the completely antigenically disparate BN (RT-1n) to WKY (RT-1l) combination.
C4d, the final split product of C4, has been the most widely reported marker for humoral immune responses to renal and cardiac transplants (19-21). Data for the use of C4d in lung transplants, however, have not been as conclusive. The first clinical studies of C4d in lung transplants reported widespread staining on structures beyond the vasculature (1, 2). In addition, the pattern was reported as granular and sometimes nuclear. This pattern is different from the diffuse, linear pattern of C4d deposition localized to vascular endothelial cells that is the usual pattern described in renal or cardiac transplants (19-21). The C4d deposits in lung transplants were associated with septal capillary necrosis and deposition of IgG, C1q, C3, andC5b-9. The lung transplant recipients were distinguished by the fact that none of them had detectable antibodies to HLA in their circulation by ELISA and bead-based flow cytometry. Instead, it was suggested by the authors that endothelial cells were the antigenic target of the antibodies based on positive granular nuclear staining of fixed human umbilical vein cells by serum obtained from a patient during a rejection episode. The authors have also reported in a separate publication based on the same patient population that of 12 biopsy specimens obtained from these patients who were positive for C4d deposition in the septal capillaries, all showed signs of acute humoral rejection as demonstrated by septal capillary necrosis (2).
A subsequent study by Ionescu et al. (5) described subendothelial deposition of C4d that correlated with the presence of donor-specific HLA antibodies in the serum of the recipient. All patients with biopsies staining positive for C4d had detectable circulating donor-specific HLA antibodies, but only 31% of patients testing positive for donor-specific alloantibodies had subendothelial deposition of C4d. Thus, the investigators suggested that C4d is a specific, but not very sensitive marker for antibody-mediated rejection. Miller et al. (3) measured C4d levels in serial bronchoalveolar lavage samples from a small group of patients and found that elevated C4d levels were correlated with antibodies to HLA.
In contrast, Wallace et al. (4) studied 68 lung transplant biopsies and concluded that C4d staining of paraffin-embedded allograft biopsies does not identify acute, chronic, or humoral rejection in lung allograft tissue. Although the authors reported that serum from many of these patients was screened for the presence of antibodies to HLA, no data were presented on whether they correlated with C4d staining.
Most recently, the International Society for Heart and Lung Transplantation issued a concensus statement that included a discussion of acute antibody-mediated rejection of the lung (22). Based on the available literature, including the studies discussed above, as well as extrapolation from data available from kidney and heart transplantation, the group suggested that immunologic staining for C4d may be of limited clinical use in protocol biopsies because of the patchy nature and low sensitivity of C4d staining in the lung, although the possibility of its use as a marker of co-existent AMR in patients with refractory acute cellular was discussed. However, no, definitive recommendations were made by the group regarding the diagnosis or treatment of antibody-mediated rejection in the lung.
The discrepancies between these clinical studies and findings from our study may stem from a couple of factors. We have shown previously in rat models of cardiac transplantation that C4d is cleared within 3 to 5 days from the vascular endothelium after elimination of alloantibodies from circulation (7). Such rapid clearance mechanisms could decrease C4d to levels that are below detection by staining in human lung transplants when the antibody titers are low. The degree of rejection and tissue destruction may also significantly affect the pattern of C4d staining. As we have shown in our PVG.R8 to 1U lung allografts, the terminal complement components can contribute significantly to tissue destruction. As destruction of vascular endothelium progressed, the fragmented tissue imparted a patchy, coarse granular staining pattern for C4d in the C6+ recipient group. However, lungs that were obtained early in the rejection process had diffuse, linear staining for C4d that was restricted to the endothelium of arteries, capillaries, and veins. These findings may in part account for the granular pattern of staining for C4d as well as the staining in extravascular compartments described by Magro et al. Although we were able to stain allografts early in the rejection process, the clinical specimens stained for C4d by Magro et al. were from patients with clinical and histological features indicative of severe rejection. At this point, enough destruction of lung tissue may have occurred that alloantibodies previously contained in the vascular compartment may leak out into extravascular spaces. Thus, it is possible that strong, linear staining of vascular endothelium for C4d is an early pattern of graft rejection.
The presence of macrophages in biopsies is now included as one of the diagnostic criteria for humoral rejection in cardiac (23) and kidney (24) transplantation. Alveolar macrophages have been shown to play an important role in lung allograft rejection through the release of proinflammatory molecules (25, 26). More recently, Maruyama et al. (27) have shown in a tracheal implant model of bronchiolitis obliterans that the administration of antibodies to HLA class I can induce macrophage and granulocyte infiltration. In our study, we have used CD68 as a marker to study macrophage infiltrates in lung transplants, and found significant perivascular infiltration of macrophages in rejecting lung allografts correlating with the presence of serum alloantibodies and C4d deposition on lung vascular endothelium. These findings further support a role for macrophages in rejection of solid organ transplants, probably initiated through the interaction of alloantibodies and complement split products with Fc and complement receptors on the surface of macrophages.
The influx of neutrophils into lung allograft tissue is associated with the development of acute (28) as well as chronic (29, 30) rejection. Persistently high concentrations of neutrophils in bronchoalveolar lavages have also been shown to be a predictor of higher mortality after transplantation (31). The histological findings in our rat lung allografts resembles posttransplant pulmonary capillaritis, which has been previously described in the clinical literature as a form of acute allograft rejection that is distinct from acute cellular rejection (8). Patients with pulmonary capillaritis in transplants typically present with clinical symptoms that are indistinguishable from acute cellular rejection, such as dyspnea, hypoxemia, malaise, with a significant decrease in FEV1 and signs of significant infiltrates on chest X-rays (8). Histologically, however, pulmonary capillaritis is characterized by intense neutrophilic infiltration of the alveolar walls, edema, alveolar septal fibrinoid necrosis, and free red blood cells (8), whereas in acute cellular rejection, the histologic pattern typically consists of perivascular lymphocytic infiltration. These similarities in histologic patterns between pulmonary capillaritis and that of our rat allograft lungs are not surprising, because of the contribution of complement and antibody to the severity of rejection in our lung allograft model. It has been hypothesized that pulmonary capillaritis is also a form of humoral rejection because of several circumstantial pieces of evidence. Magro et al. demonstrated that patients with this form of capillary injury have C4d deposition in their septal capillaries (2). In addition, patients with capillaritis tend to have lower rates of response to intravenous corticosteroid therapy (32). Finally, both Magro et al. and Astor et al. have shown that those patients with capillaritis who were unresponsive to corticosteroid therapy often responded well to plasmapheresis (2, 8). Our study links neutrophils in pulmonary capillaries to both donor-specific alloantibodies and C4d deposits.
In summary, we have presented data from two models of orthotopic lung allografts in rats that demonstrate C4d is deposited in a diffuse, linear pattern on capillary and arterial endothelium before the vasculature is disrupted during rejection. We have also characterized the localization of macrophages and neutrophils relative to the complement deposits. These findings suggest the need for further study of C4d as a diagnostic marker of acute allograft rejection as well as pulmonary capillaritis in human lung transplantation.
REFERENCES
- 1.Magro CM, Deng A, Pope-Harman A, et al. Humorally mediated post-transplantation septal capillary injury syndrome as a common form of pulmonary allograft rejection: A hypothesis. Transplantation. 2002;74:1273. doi: 10.1097/00007890-200211150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Magro CM, Klinger DM, Adams PW, et al. Evidence that humoral allograft rejection in lung transplant patients is not histocompatibility antigen-related. Am J Transplant. 2003;3:1264. doi: 10.1046/j.1600-6143.2003.00229.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller GG, Destarac L, Zeevi A, et al. Acute humoral rejection of human lung allografts and elevation of C4d in bronchoalveolar lavage fluid. Am J Transplant. 2004;4:1323. doi: 10.1111/j.1600-6143.2004.00508.x. [DOI] [PubMed] [Google Scholar]
- 4.Wallace WD, Reed EF, Ross D, et al. C4d staining of pulmonary allograft biopsies: An immunoperoxidase study. J Heart Lung Transplant. 2005;24:1565. doi: 10.1016/j.healun.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 5.Ionescu DN, Girnita AL, Zeevi A, et al. C4d deposition in lung allografts is associated with circulating anti-HLA alloantibody. Transpl Immunol. 2005;15:63. doi: 10.1016/j.trim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima S, Qian Z, Rahimi S, et al. Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol. 2002;169:4620. doi: 10.4049/jimmunol.169.8.4620. [DOI] [PubMed] [Google Scholar]
- 7.Minami K, Murata K, Lee CY, et al. C4d deposition and clearance in cardiac transplants correlates with alloantibody levels and rejection in rats. Am J Transplant. 2006;6:923. doi: 10.1111/j.1600-6143.2006.01281.x. [DOI] [PubMed] [Google Scholar]
- 8.Astor TL, Weill D, Cool C, et al. Pulmonary capillaritis in lung transplant recipients: Treatment and effect on allograft function. J Heart Lung Transplant. 2005;24:2091. doi: 10.1016/j.healun.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Qian Z, Jakobs FM, Pfaff-Amesse T, et al. Complement contributes to the rejection of complete and class I major histocompatibility complex-incompatible cardiac allografts. J Heart Lung Transplant. 1998;17:470. [PubMed] [Google Scholar]
- 10.Qian Z, Pfaff-Ameese T, Behrens E, et al. C6 produced by macrophages contributes to cardiac allograft rejection. Transplantation. 1998;65:S77. doi: 10.1016/S0002-9440(10)65231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasufuku K, Heidler KM, O'Donnell PW, et al. Oral tolerance induction by type V collagen downregulates lung allograft rejection. Am J Respir Cell Mol Biol. 2001;25:26. doi: 10.1165/ajrcmb.25.1.4431. [DOI] [PubMed] [Google Scholar]
- 12.Yasufuku K, Heidler KM, Woods KA, et al. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation. 2002;73:500. doi: 10.1097/00007890-200202270-00002. [DOI] [PubMed] [Google Scholar]
- 13.Sekine Y, Yasufuku K, Heidler KM, et al. Monocyte chemoattractant protein-1 and RANTES are chemotactic for graft infiltrating lymphocytes during acute lung allograft rejection. Am J Respir Cell Mol Biol. 2000;23:719. doi: 10.1165/ajrcmb.23.6.3825. [DOI] [PubMed] [Google Scholar]
- 14.Marck KW, Prop J, Wildevuur CR, et al. Lung transplantation in the rat: Histopathology of left lung iso- and allografts. J Heart Transplant. 1985;4:263. [PubMed] [Google Scholar]
- 15.Reis A, Giaid A, Serrick C, et al. Improved outcome of rat lung transplantation with modification of the nonsuture external cuff technique. J Heart Lung Transplant. 1995;14:274. [PubMed] [Google Scholar]
- 16.Wray DW, Baldwin WM, III, Sanfilippo F. IgM and IgG alloantibody responses to MHC class I and II following rat renal allograft rejection. Effects of transplantectomy and posttransplantation blood transfusion. Transplantation. 1992;53:167. doi: 10.1097/00007890-199201000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Wray DW, Baldwin WM, III, Sanfilippo F. Different patterns of sensitization following renal allograft rejection in an inbred rat strain combination. Transplantation. 1993;55:1132. doi: 10.1097/00007890-199305000-00038. [DOI] [PubMed] [Google Scholar]
- 18.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1. [PubMed] [Google Scholar]
- 19.Fishbein MC, Kobashigawa J. Biopsy-negative cardiac transplant rejection: Etiology, diagnosis, and therapy. Curr Opin Cardiol. 2004;19:166. doi: 10.1097/00001573-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 21.Feucht HE. Complement C4d in graft capillaries—The missing link in the recognition of humoral alloreactivity. Am J Transplant. 2003;3:646. doi: 10.1034/j.1600-6143.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 22.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Fahim T, Bohmig GA, Exner M, et al. The cellular lesion of humoral rejection: Predominant recruitment of monocytes to peritubular and glomerular capillaries. Am J Transplant. 2007;7:385. doi: 10.1111/j.1600-6143.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 25.Nicod LP, Joudrier S, Isler P, et al. Upregulation of CD40, CD80, CD83 or CD86 on alveolar macrophages after lung transplantation. J Heart Lung Transplant. 2005;24:1067. doi: 10.1016/j.healun.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Frachon I, Fattal-German M, Magnan A, et al. Emergence of inflammatory alveolar macrophages during rejection or infection after lung transplantation. Transplantation. 1994;57:1621. [PubMed] [Google Scholar]
- 27.Maruyama T, Jaramillo A, Narayanan K, et al. Induction of obliterative airway disease by anti-HLA class I antibodies. Am J Transplant. 2005;5:2126. doi: 10.1111/j.1600-6143.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 28.Hirayama S, Shiraishi T, Shirakusa T, et al. Prevention of neutrophil migration ameliorates rat lung allograft rejection. Mol Med. 2006;12:208. doi: 10.2119/2006-00036.Hirayama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiGiovine B, Lynch JP, III, Martinez FJ, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: Role of IL-8. J Immunol. 1996;157:4194. [PubMed] [Google Scholar]
- 30.Hirsch J, Elssner A, Mazur G, et al. Bronchiolitis obliterans syndrome after (heart-)lung transplantation. Impaired antiprotease defense and increased oxidant activity. Am J Respir Crit Care Med. 1999;160:1640. doi: 10.1164/ajrccm.160.5.9902012. [DOI] [PubMed] [Google Scholar]
- 31.Henke JA, Golden JA, Yelin EH, et al. Persistent increases of BAL neutrophils as a predictor of mortality following lung transplant. Chest. 1999;115:403. doi: 10.1378/chest.115.2.403. [DOI] [PubMed] [Google Scholar]
- 32.Sibley RK, Berry GJ, Tazelaar HD, et al. The role of transbronchial biopsies in the management of lung transplant recipients. J Heart Lung Transplant. 1993;12:308. [PubMed] [Google Scholar]