Renal dysfunction has long been a recognized feature of essential hypertension as first suggested by Dr Richard Bright’s mid-19th century observations of small, shrunken, scarred kidneys associated with cardiac hypertrophy and the seminal studies in the 1930s by Dr Harry Goldblatt directly linking the kidney to chronic hypertension. Attention was originally and appropriately focused on the renal cortex, which represents more than 90% of the renal mass, is the site of glomerular filtration, and receives more than 95% or renal blood flow. Reductions of glomerular filtration and the morphological changes within cortical structures such as vascular medial wall hypertrophy, hyperplasia, and fibrinoid necrosis of the glomeruli have been well described,1 especially in patients with chronic hypertension and glomerulosclerosis.
By comparison, interest in the involvement of the renal medulla in the initiation and development of hypertension evolved much later and was stimulated by two different observations. First, studies in the 1960s by Dr Eric Muirhead showed that the medullary interstitial cells produced an antihypertensive principle that he called medullipin.2 Second, studies by our own group in the mid-1980s reported that renal medullary blood flow (MBF) was importantly linked to the phenomena of pressure-natriuresis and diuresis and that MBF was reduced in several forms of experimental hypertension.3,4
Before these studies, the physiological importance of the renal medullary circulation was focused on the unique countercurrent structure of the vasa recta vessels as first appreciated in the 1950s by Hargitay and Kuhn5 and Wirz et al,6 who introduced the modern concept of the urinary concentrating mechanism. Subsequent studies have shown that the structure-function relationships of the outer medulla make this region especially vulnerable to chronic ischemia and tubulointerstitial injuries.7 MBF was found to be a critical requirement for an adequate O2 delivery to match the high metabolic needs of the outer medulla, where nearly 33% of the filtered NaCl is reabsorbed by the medullary thick ascending limbs (mTAL) of Henle.8 This unique vulnerability to ischemia within the renal medulla is magnified by a hematocrit of only 26% in the vasa recta vessels9 and by O2 shunting that occurs between descending and ascending vasa recta contributing to a steep pO2 gradient from the cortex to the outer medulla and the papilla (45 to 32 to <10 mm Hg).8,10,11 This vulnerability to ischemia is believed to be one of the important reasons that tubulonterstitial injuries occur in the outer medulla during the early development of hypertension in rats12 and man.13 The purpose of this review is to summarize and emphasize what is new and relevant regarding the role that MBF plays in pressure-natriuresis. I will review recent evidence of the importance of local medullary production of nitric oxide (NO), superoxide (O2·−), and hydrogen peroxide (H2O2) on these mechanisms and their relationship to hypertension and renal injury.
Role of the Renal Medullary Circulation in Pressure-Natriuresis and Sodium Homeostasis
Red cell velocity through large thoroughfare single vasa recta vessels is maintained relatively constant9,14 as renal perfusion pressure is increased from 100 to 150 mm Hg, but there is marked recruitment of previously unperfused descending vasa recta capillaries and an increase in total perfusion to the renal medulla.9 Overall blood flow to the renal medulla of the rat is poorly autoregulated, such that increases of renal perfusion pressure are transmitted to the vasa recta circulation, a phenomenon that is exaggerated in the volume expanded state.3,4 As renal perfusion pressure and vasa recta flow increase, there are consequent elevations of vasa recta capillary hydrostatic pressure which lead to parallel increases in renal interstitial hydrostatic fluid pressure (RIHP) attribute-able to a net filtration of fluid into the renal interstitial space.3,15,16 This rise of RIHP, albeit small (≈5 to 6 mm Hg),15 is sufficient to signal within seconds a rapid decrease in sodium transport in the proximal tubule and in the deep loops of Henle.17,18 Recent studies found that this proximal tubular reduction of sodium transport results from an increase of 20-HETE production within the tubules of the renal cortex triggered by the increases of RIHP.19 20-HETE inhibits Na+ K+-ATPase activity by protein kinase C–induced phosphorylation of the serine 23 residue in the enzyme20 resulting in the internalization of the sodium/hydrogen exchanger from the brush border.19,21 Removal of the renal capsule, which blunts the overall increase in RIHP, reduces pressure-natriuresis by 50% because the RIHP signal for 20-HETE stimulation is uncoupled. The mechanisms responsible for the remaining reduction of NaCl reabsorption are less clearly understood but micropuncture studies have demonstrated that this occurs in the loops of Henle of deep medullary nephrons and may be coupled to washout of the medullary solute gradient that affects the passive reabsorption of sodium in this portion of the nephron.3 Some investigators have found similar relationships between renal perfusion pressure and MBF in dogs,22 whereas others have not.23 However, the results of numerous studies in rats have been consistent, and although influenced by the state of hydration these results indicate that changes of medullary blood flow provide the signal for the pressure-natriuresis response and are the vital link to the long-term control of sodium excretion and arterial blood pressure.
Medullary Blood Flow in Hypertension
Pallone et al24 first demonstrated that descending vasa recta when isolated from outer medullary vascular bundles were capable of constricting at various foci when exposed to contractile agonists. It has since been shown that this is mediated by contraction of vasa recta pericytes.25,26 The vasa recta capillaries are thereby capable of exerting regional control of blood flow to the renal medulla.
The role of the renal medullary circulation in hypertension remained poorly understood until the development of laser-Doppler flowmetry techniques that allowed for continuous measurement of blood flow in discrete areas of tissue (approx 1 mm3) in a relatively noninvasive manner. These techniques rely on the Doppler shift imparted to monochromatic light by backscatter from moving red blood cells (RBCs) in a localized area of tissue. As described elsewhere in detail,27 with the implantation of either acute or chronic optical fibers into both the cortical and medullary tissue it is possible to measure small changes in regional capillary RBC density, velocity and flux in different regions of the kidney, albeit not as an absolute measure of flow. Importantly, these techniques have enabled the continuous measurement of changes of blood flow to both the renal cortex and medulla in the same animal and are now the most prevalent method for measuring regional perfusion of the kidney.
Using these techniques, reduced medullary blood flow (MBF) was observed in several genetic forms of hypertension. Anesthetized spontaneously hypertensive rats (SHR) exhibited reduced MBF even at 3 to 5 weeks of age compared to normotensive WKY rats,28 which was further reduced at 6 to 9 and 12 to 16 weeks of age. Because pressure-natriuresis was reduced even in the youngest age group, these findings suggested that reduction of MBF contributed to a reduction of excretory function and to the development of hypertension in SHR. As techniques for chronic implantation of the optical fibers into both the cortex and the medulla were developed,29 it became possible to measure the sequential changes of regional blood flow in unanesthetized rats. When the ACE inhibitor captopril was infused chronically into the medullary interstitial space of a single remaining kidney of SHR,29 MBF increased by 40% without altering cortical blood flow. This resulted in a 50% reduction of mean arterial pressure and a leftward shift of the steady-state pressure-natriuresis-diuresis relationship, whereby sodium and water balance were achieved at a mean arterial pressure of 130 mm Hg. Because intravenous infusion of captopril in SHR at the same dose had no effect on MBF, cortical blood flow, or arterial pressure, it was concluded that these antihypertensive actions of captopril were attributable to the improved perfusion of the renal medulla.
The role of the medullary circulation in the development of hypertension in the Dahl salt-sensitive (SS) rat has recently received much attention. SS rats mimic human salt-sensitive forms of hypertension that are particularly prevalent in black individuals.30 Neither Dahl salt-resistant (DR) nor Sprague Dawley rats exhibit significant changes of MBF in response to increases of salt diet31,32 indicating that increases of MBF are not normally required to achieve long-term sodium homeostasis. However, in SS rats, sequential measurements of MBF and cortical blood flow in response to an increase of daily NaCl intake (from 0.4% to 4.0% of diet) resulted in nearly a 30% reduction of MBF over the first 24 to 48 hours after switching to the high-salt diet.31 When the reduction of MBF in SS rats was prevented by the chronic intramedullary infusion of L-arginine, salt-induced hypertension was prevented as discussed below.31
These studies have shown that reductions of MBF can have a profound effect on the long-term control of arterial pressure in these commonly used genetic rat models of hypertension. Although such data have not yet been obtained in human subjects, it would be of great interest to evaluate MBF in human hypertension as this may be possible using noninvasive imaging techniques such as electron beam computer tomography.33
NO Is a Major Determinant of MBF and Sodium Excretion
Among the many factors that can influence MBF, NO is currently the most studied. There is considerable evidence that NO production within the renal medulla plays a major role in the regulation of MBF and protects this region from ischemic injury.34 The highest levels of NOS activity are found in the medullary collecting ducts.35 NOS activity is 26 times higher in the inner medulla and 4 times higher in the outer medulla than in the renal cortex of Sprague-Dawley rats.34 In vivo microdialysis of renal interstitial fluids using a hemoglobin-trapping technique found NO to be twice as high in the renal outer medulla as in the cortex.36 This method of NO quantitation based on a stoichiometric interaction of NO with oxyhemoglobin thereby forming methemoglobin is the method of choice for measuring renomedullary NO,36 although NO microelectrode sensors have also detected nearly 3-fold differences between these 2 regions.37
The high levels and wide range of NO concentrations found within the medullary tissue play an important role in the regulation of MBF and sodium excretion. Chronic intravenous administration of the nitric oxide synthase inhibitor N-G-nitro-l-arginine methyl ester (l-NAME), at a dose that produced no change of cortical blood flow, resulted in a sustained 30% reduction of MBF with a subsequent reduction in sodium excretion and chronic hypertension.38 Chronic intramedullary infusions of l-NAME into a single remaining kidney of Sprague Dawley rats resulted in a 30% reduction of MBF with no change of cortical blood flow resulting in sodium retention and hypertension.39 The implication of this study is that although NO production may be altered in other regions of the body, dysfunction of this system in the renal medulla alone can have a global impact on the entire cardiovascular system. This concept becomes important when considering the pathophysiological basis of hypertension in genetic salt-sensitive forms of hypertension, such as in the Dahl salt-sensitive rat model discussed below.
Inhibition of renal NOS activity produces an immediate reduction of sodium excretion by reducing tubular Na+ transport in the medullary thick ascending limb.40,41 Responses to changes of NO production in the proximal tubule remain controversial.42 These antinatriuretic responses are associated with parallel reductions of medullary blood flow.34,39 Together, these effects account for the salt sensitivity that occurs with chronic inhibition of NOS activity as seen by the reduction in the slope of the pressure-natriuresis relationship in Sprague Dawley rats.43 The opposite is also observed in Dahl S rats when medullary NO production is enhanced by medullary infusion of L-arginine which abolishes salt-induced hypertension.31
The powerful effects of NO on the acute and chronic pressure-diuresis relationship suggested that it may be a mediator of pressure-natriuresis.44 This idea is consistent with observations that an increase in vascular wall shear stress results in enhanced NO production.45 It is also consistent with observations that the natriuretic response to acute elevations in arterial pressure is greatly attenuated with acute inhibition of NOS.46,47 To further support mediation of pressure-natriuresis by NO, it was reported that increases of renal perfusion pressure resulted in increased urinary excretion of nitrate and nitrite and cortical NO levels as determined by tissue microelectrodes.48 Despite these associations, support for this theory was considerably reduced when the work of Guarasci and Kline demonstrated that the slope of the pressure-natriuresis relationship remained intact in rats treated either acutely or chronically with l-NAME.43 Other investigators have also repeatedly confirmed that l-NAME substantially decreased sodium excretion and renal interstitial pressure if renal perfusion pressure was maintained constant; however, the more usual response to l-NAME is natriuresis as systemic pressure increases. The pressure natriuretic response typically associated with l-NAME administration, despite blockade of intrarenal NOS activity, strongly argues against the view that NO is the mediator of this response. Overall, the available data support the importance of NO as a modulator of pressure-natriuresis, but not as an essential mediator of pressure-natriuresis.
Role of NO in Buffering Actions of Vasoconstrictor Hormones
In addition to its important role in modulating pressure-natriuresis, medullary NO production is also important in protecting the kidney from inappropriate reductions of MBF and ischemia in the face of physiological increases of circulating vasoconstrictor agents. This is perhaps best illustrated by observations that angiotensin II (Ang II) and norepinephrine can both reduce diameters of isolated perfused vasa recta25 but have little effect on MBF when infused intravenously at doses that cause a substantial reduction of cortical blood flow.10,49 The reason for these minimal in vivo effects on MBF can be explained by stimulation of medullary NO production, as shown by a number of studies as described in a previous review.34
The importance of NO in buffering the vasoconstrictor actions of circulating hormones has also been observed in studies with arginine vasopressin (AVP). In contrast to Ang II and norepinephrine, AVP when administered in low nonpressor amounts produces significant reductions of MBF, a response mediated via vasopressin V1 receptor stimulation50 despite an immediate stimulation of NO production in the renal medulla.50 When administered over several days, the acute medullary vasoconstrictor effects of AVP were not sustained and only a transient elevation of arterial pressure was observed,51 consistent with many reports of failure to produce hypertension with chronic administration of this peptide.50 This “escape” from both volume retention and hypertension may be mediated in part by a progressive upregulation of a V2 receptor-, Ca2+-dependent pathway mediating NO production.52 In the absence of V2 receptor stimulation, chronic infusion of a specific V1 receptor agonist in Sprague Dawley rats produced a sustained reduction of MBF and chronic hypertension.51 Consistent with these observations, the only situation in which chronic infusion of AVP has been found to produce sustained hypertension in the presence of normal kidneys is when medullary NOS activity was reduced. That is, intravenous infusion of AVP at low doses in uninephrectomized Sprague Dawley rats receiving a continuous infusion of l-NAME into the renal medulla resulted in sustained hypertension.53
There are undoubtedly other situations in which medullary NO production protects this region of the kidney from ischemia. It is known, for example, that NO produced in the inner medullary collecting duct cells protects the medulla from the vasoconstrictor actions of endothelin.54 It appears, therefore, that the ischemic actions of a variety of hormonal and paracrine factors are buffered by NO production although the pattern of these responses may differ depending on the specific stimuli.
Other Endogenous Systems Available to Protect Perfusion of the Renal Medulla in Face of Vasoconstrictor Influences
In addition to NO production, a number of other paracrine or autocrine systems may participate in the regulation of MBF, pressure-natriuresis, and in protection from medullary ischemia. Cyclooxygenase (COX-2) is highly expressed in the renal medulla,55 and prostaglandin E2 (PGE2) is present in high concentrations in the medullary interstitial cells of the kidney.56 Intramedullary infusion of PGE2 increases MBF and counteracts the vasopressor actions of Ang II.57 Rats pretreated with the cyclooxygenase inhibitor meclofenamate exhibit enhanced vasoconstrictor sensitivity to circulating Ang II.57,58 Similar responses have been observed with kinin receptor antagonists.58,59 Levels of hemoxygenase (HO) are also present at nearly twice the concentrations in the renal medulla than in the cortex.60,61 Inhibition of medullary HO by ZnDPBG reduces MBF in Sprague Dawley rats.60 The production of medullary carbon monoxide (CO) via the hemoxygenase pathway also appears to protect the renal medulla from oxidative stress.62 Mice with targeted overexpression of HO-1 in mTAL cells were able to significantly attenuate oxidative damage induced by Ang II.63 HO-1 in Ang II–stimulated O2− production within the renal medulla was also reduced by induction of HO-1 with cobalt protoporphyrin (CoPP).62
We have found that endogenous medullary adenosine increases MBF via stimulation of adenosine A2 receptors resulting in a natriuretic response that overrides tubular adenosine A1 receptor-mediated antinatriuretic effects.64 Although poorly understood at this time, ATP has also been reported to influence MBF whereby exogenous ATP increased MBF in rats fed a low salt diet (mediated by purinergic P2Y receptors) and had the opposite effects in rats fed a high salt diet.63
The NOS, COX-2, and HO enzyme pathways have all been found to be upregulated in response to high salt intake.36,55,61,65,66 Inhibition of any of these pathways within the renal medulla reduces sodium excretion and when inhibited chronically produce salt-sensitive hypertension.55,61,65,67–69 There is also good evidence that the hypoxia inducible factor (HIF)-1 α, the master regulator of many oxygen-sensitive genes, is highly expressed in the renal medulla.70,71 HIF-1 α has been found to be importantly involved in the regulation of oxygen-sensitive genes such as NOS, COX-2, and HO.72,73,74 In addition, recent studies show that transfection of a decoy of HIF-1α into the renal medulla of a single remaining kidney of Sprague Dawley rats in amounts that reduced the expression of this transcription factor by 45%, resulted in a blunting of pressure-induced increases of MBF and sodium excretion by 50% and 37%, respectively.69 High-salt diet–stimulated protein medullary transcription levels of NOS-2 and HO were shown to be reduced by 70% and 61% in these rats. In chronic studies, a high salt intake (8% NaCl) resulted in sodium retention, and mean arterial pressure rose to levels of 154 mm Hg over 10 days of hypertension. Decoy rats fed a normal salt diet did not develop hypertension, nor did control rats (scrambled decoy).70
It appears, therefore, that in addition to NO, a number of redundant systems have evolved to protect the renal medulla from ischemia, all orchestrated by HIF-1α in this region of the kidney. However, the relative importance and contribution of each of these systems in response to different stimuli remains to be determined.
Vulnerability to Renal Medullary Ischemia Determined by Genetic Predisposition
Inbred salt-sensitive Dahl S (SS) rats exhibit one-third the levels of NOS enzyme activity in the outer medulla compared to inbred salt-resistant Brown Norway rats.75,76 The mRNA and protein for each of the 3 NOS isoforms is also significantly less in the outer medulla.75,76 While basal medullary tissue NO levels did not differ between rat strains, the stimulatory actions of Ang II on medullary NO production were substantially blunted in SS rats.76 The consequences of this reduced buffering capacity of NO within the renal medulla renders the SS rat more sensitive to small elevations of circulating vasoconstrictor agents. Ang II, when chronically infused intravenously to SS rats at a dose that fails to increase mean arterial pressure in the salt-insensitive Brown Norway (BN) rats or Sprague-Dawley rats, produced sustained hypertension in SS rats.76 Similar studies with chronic IV infusion of AVP in SS rats maintained on a 0.4% salt diet found that a 20-mm Hg sustained hypertension occurred over the 16-day study.75 These were remarkable findings because this was the first time that AVP had been found to produce sustained hypertension without pharmacological manipulations or surgical reduction of renal mass.
The substantial importance of the reduced NO production in the development of hypertension in the SS rat was demonstrated when NO production in the renal medulla was restored by chronic infusion of L-arginine into the renal medulla of a single remaining kidney of the SS rats. Restoration of NO production within the renal medulla prevented salt-induced hypertension in the SS rat.31 The specificity of this effect to the renal medulla was demonstrated by control studies in which the same amount of L-Arg administered intravenously failed to prevent salt-induced hypertension in the SS rats.31 These results are consistent with observations that large doses of either orally or intravenously administered L-Arg blunt pressure-natriuresis and reduce salt-induced hypertension in SS rats.77 It has been puzzling that this can occur because intracellular L-Arg (100 to 3800 µmol/L)78 is much greater than the Km of NOS for L-Arg (<5 µmol/L),79 and it has been thought that L-Arg levels are not rate-limiting. There is now evidence, however, demonstrating that NO production in the renal medulla of rats is limited by L-Arg substrate levels.80 It was found in these studies that NO concentration in the renal medulla of the anesthetized rat was decreased by renal medullary interstitial infusion of competitive inhibitors of the cationic amino acids transporters such as L-ornithine, L-lysine, or L-homoarginine. More importantly, NO production in the renal medulla increased when exogenous L-Arg was infused into the renal medulla.
It is likely that the COX-2 and HO-1 pathways behave in a similar manner, but there is a dearth of such data regarding their role in the regulation of MBF and salt-sensitive forms of hypertension in genetically inbred rats or in human subjects. However, there is presently a general understanding that all of these medullary protective enzymes play critical roles in regulating MBF and tubular activity and are essential in sodium and water homeostasis and in the regulation of arterial pressure.
Medullary Production and Actions of Superoxide
The production of superoxide (O2·−) plays an important role in determining MBF and sodium excretion. Abundant basal amounts of O2·− are found within the medullary tissue under normal conditions.81,82,83 Intramedullary infusion of the O2·− scavenger Tiron in anesthetized rats increases MBF and sodium excretion, suggesting that even basal levels of tissue O2·− production may participate in the maintenance of these parameters.83 Conversely, increased levels of O2·− within the renal medulla produced by intramedullary infusion of the SOD inhibitor DETC results in reduction of MBF and antinatriuresis83 and when administered chronically produces hypertension.84 Consistent with these observations, increased tubular production of O2·− was shown to stimulate Na+ reabsorption in medullary thick ascending limb (mTAL), a response mediated through activation of the Na/K/2Cl cotransporter85 and a PKC-α pathway.86
The source of O2·− in the renal medulla was found to be primarily from NAD(P)H-oxidase and mitochondrial respiratory chain enzymes.83,87,88,89 NAD(P)H-oxidase–derived O2·− appears to increase Na-K-ATPase activity in the medulla by reducing availability of NO.90 O2·− derived from mitochondria, but not NADP(H)-oxidase, mediate hypertonicity-induced phosphorylation of MAP kinase and the stimulation of COX-2 expression.91 The observed NO stimulation of COX-2 in collecting duct cells appears to be mediated through mechanisms involving MAP kinase and O2·− rather than a GMP.92 As reviewed below, we are just beginning to define the role of increased metabolic activity related to sodium transport in the mTAL, the effects of mechanical factors (shear stress, stretch, deformation), and the effects of local oxygen availability on mTAL-O2·− production.
The functional consequences of elevations of O2·− within the renal medulla are clear. An immediate reduction of sodium excretion occurs with acute intramedullary infusion of DETC83 to raise tissue O2·− levels along with a 50% reduction of MBF. Sustained hypertension results with intramedullary infusions of this SOD inhibitor.84 Medullary O2·− levels were measured in interstitial fluid collected by microdialysis in the latter study by determining the rate of conversion of the fluorescent O2·−-sensitive dye dihydroethidium (DHE), to oxyethidium and interstitial O2·− was found to be elevated nearly 8-fold above basal levels.84
Prehypertensive SS rats exhibit O2·− levels in the outer medulla that are significantly higher than levels in the inbred control, consomic salt-insensitive SS.13BN rat strain, as determined by tissue oxyethidium production and by tissue lucigenin chemiluminescence analysis.81,82 Time resolved fluorescence videomicroscopy techniques used to determine the rates of change of DHE to ethidium fluorescence found that mTAL of SS exhibit greater rates of O2·− production compared to SS.13BN rats,93 consistent with observed elevations of urinary 8-isoprostane levels in SS rats.94 Furthermore, oral administration of the SOD mimetic, tempol, has been found to reduce salt-induced hypertension in SS rats,95,96 although results using this compound are confounded by nonspecific effects97 discussed below.
Both O2·− and H2O2 production within the renal medulla appear to contribute importantly to the salt-sensitivity of the SS rat. NAD(P)H-oxidase appears to account for most of the enhanced O2·− production in the medulla of SS rats.81,82 Inhibition of this enzyme by chronic intramedullary infusion of apocynin resulted in a 50% reduction of salt-induced hypertension in SS rats.81 Apocynin has been traditionally used to inhibit NAD(P)H-oxidase, although it has been found recently to act as an antioxidant in endothelial and vascular smooth muscle cells as a consequence of a lack of myeloperoxidase to convert the prodrug to an active dimer in these cells.98 However, this is not likely to be an important issue in intact renal interstitial tissue of SS rats which contains abundant leukocytes.12,99 Leukocytes contain high levels of myeloperoxidase activity enabling the conversion of apocynin to the active dimer required to inhibit NADP(H)-oxidase.100 Outer medullary tissue mRNA levels of both the membrane (gp91 and p22) and cytosolic (p47) subunits of NAD(P)H-oxidase were greater in SS rats compared to salt-insensitive control SS.13BN rats.81 Protein levels of p22 and p47 and NAD(P)H-oxidase enzyme activity were found to be significantly higher within the renal medulla of SS rats.81 O2·− levels in the medulla of SS rats are further enhanced by reduced protein levels of SOD and catalase as determined by Western blot analysis.81,94,96 In hypertensive SS rats fed a high 4% salt diet for 3 to 6 weeks, outer medullary tissue O2·− and H2O2 levels were further increased to levels 2- to 3-fold higher than measured in the prehypertensive (0.4% salt diet) state.81,94 Increased levels of oxidative stress are further driven in the outer medulla of SS rats by NOS uncoupling as reflected by increased concentrations of BH2, reduced ratios of BH4/BH2, and reduced tissue O2·− production following NOS inhibition with l-NAME.82
The mechanisms responsible for initiating this excess production of O2·− and H2O2, and the related reduction of NO bioavailability, are only now becoming understood. It is recognized that 20% to 25% of filtered NaCl is reabsorbed within the mTAL with as much as 75% of this reabsorption attributed to the Na/K/2Cl cotransporter and about 25% attributed to the Na/H apical exchangers (NHE).101 Exposure of isolated mTAL of SD rats to increased bath concentrations of NaCl within a physiological range stimulates O2·− production.102 Physiological increases in the luminal sodium concentration from 60 to 149 mmol/L/L at a fixed luminal flow rate resulted in an increased production of O2·− in isolated microperfused mTAL,103 whereas concurrent reductions in mTAL NO production were observed. Increases of luminal flow independent of changes in NaCl concentrations also stimulate production of O2·− production within the mTALs.103,104,105 Recent studies have found that activation of Na-K-2Cl transport along with stretch-induced mechanical factors contribute equally to increased O2·− production.104,105
SS rats exhibit enhanced Cl− reabsorption in the loop of Henle,106,107 increased renal Na/K-2Cl transporter activity,108 and overexpression of the ROMK channel.109 Na-K-2CL cotransporter activity is enhanced in mTAL by O2·−.110 Together, these observations suggest that a high-salt diet, which increases delivery of NaCl to mTAL, also increases O2·− production and that this is exaggerated in the mTAL of SS rats. The consequences of these enhanced levels of oxidative stress as described below would be expected to reduce NO bioavailability and contribute importantly to observed reduction of MBF,31 reduced sodium excretion, and hypertension. Furthermore, the interstitial fibrosis found in the outer medulla early in the development of hypertension in SS rats12 is related to an excess production of O2·− in the region of the outer medulla.94
Medullary Actions and Consequences of H2O2
H2O2 is produced in the renal medulla from the SOD dismutation of O2·− and possibly via certain oxidases through a 2-electron reduction of O2. eNOS could also be a source of H2O2 production, as found within arterioles of the mesenteric circulation, although this has not yet been shown to be the case in the kidney.111 The chemical activity of H2O2 favors its role as an oxidant and because it is relatively stable in aqueous solutions and lipophilic, it can diffuse out into the interstitial space and subsequently to the blood vessels. H2O2 produces either vasodilation or vasoconstriction depending on the concentrations used and the vascular bed studied. Vasodilator effects are observed within the coronary circulation112 and in the mesenteric113 and skeletal muscle arterioles,114 and H2O2 is viewed as an important endothelium-derived hyperpolarizing factor within the heart.112,115 This response occurs through the activation of a calcium-dependent K+ channel113 and is thought to occur via a redox mechanism involving thiol oxidation by H2O2.115 Conversely, vasoconstrictor effects have also been observed within the mesenteric111,113 and skeletal muscle arterioles.111 The vasoconstrictor responses are thought to be mediated by tyrosine phosphorylation and cyclooxygenase products, including thromboxane116 because such a response has been blocked using T×A2 receptor blockers, thromboxane synthase inhibitors, and cyclooxygenase inhibitors.111,113 Some of the reported regional differences in responses to H2O2 may be explained by the doses used in various studies because biphasic responses have been found in both the mesenteric circulation113 in which constrictor responses occur at lower doses of H2O2 (10 to 100 µmol/L) whereas vasodilator responses predominate at concentrations above 0.3 mmol/L. Removal of the endothelium did not eliminate the biphasic response. Similar biphasic responses are observed in skeletal muscle arterioles.114
Until recently, the role of H2O2 within the renal medulla has received little attention. H2O2 levels in microdialysate of the renal medullary interstitial fluid appear to range between 50 to 300 nmol/L.94,117 Medullary interstitial infusion of H2O2 in Sprague Dawley rats, at a dose that increased H2O2 concentrations in renal medullary dialysates from 116 nmol/L to 211 nmol/L, decreased MBF, and reduced urinary flow and sodium excretion.117 H2O2 produced from within the mTAL could be expected to diffuse more widely than the highly reactive and unstable O2·− radical, although tissue catalase levels within the outer medulla of the kidney are known to be rather high.82 A role for H2O2 in the regulation of MBF was first suggested when administration of the membrane-permeable SOD mimetic, tempol, into the renal medulla was unable to prevent hypertension induced by local medullary inhibition of SOD (by DETC) even though medullary tissue O2·− levels were reduced to normal levels.84 Involvement of H2O2 was resvealed when it was found that DETC-induced hypertension could only be prevented when catalase was chronically infused together with tempol into the renal medulla.97 This study demonstrated the limitations of using tempol as a chemical SOD mimetic because in the presence of high levels of O2·− production it is capable of dismuting 2 O2·− molecules by a direct reaction with O2·− or its .OOH form thereby producing H2O2.39 After these observations it was determined that acute intramedullary infusions of H2O2 in Sprague Dawley rats reduced medullary blood flow and sodium excretion in a dose-dependent manner.117 Importantly, it has now been shown that chronic intramedullary infusion of H2O2 directly into the renal medulla produces blood pressure salt-sensitivity in rats.81,97 H2O2 in these studies was administered in amounts to produce the elevations that were found in the renal medulla of the DETC+tempol model of hypertension97 and in the renal medulla of SS rats.81,82
Tissue O2·− concentrations are elevated within the outer medulla of SS rats81,82 so it is not surprising that H2O2 concentrations have also been found to be nearly twice as great in the dialysate of SS rats compared to SS.13BN rats even in the prehypertensive state when rats were maintained on a 0.4% NaCl diet.94 H2O2 was measured (using Amplex red fluorescence) in interstitial fluid collected from a microdialysis fiber implanted into the renal outer medulla.94 Concentrations more than doubled in both the SS and SS.13BN control strain after 1 week of a 4% salt diet, but levels remained significantly higher in SS rats.94 The contribution of these elevated H2O2 levels to the salt-induced hypertension in SS rats was illustrated when it was found that salt-induced hypertension was blunted nearly 50% in SS rats receiving a chronic medullary infusion of catalase into a single remaining kidney.94 The extent to which O2·− versus H2O2 is responsible for the hypertensive effects remains unclear because salt-induced hypertension was attenuated by nearly the same extent (≈50%) in SS rats receiving an intramedullary infusion of the NADP(H)-oxidase inhibitor apocynin81 as those that received catalase.94 Because it is likely that the predominant source of H2O2 is via the dismutation of O2·−, apocynin would be expected to reduce both O2·− and H2O2. However, in a steady-state of increased production of O2·−, both O2·− and H2O2 would be elevated and both could contribute additively or synergistically to the reduction of MBF and sodium excretion found in SS rats.
Evidence That O2·− and NO Produced Within the mTAL Can Diffuse to Surrounding Vasa Recta Vessels and Influence MBF
A series of ex vivo studies using thin tissue strips obtained from the inner stripe of the outer medulla of rats have found that O2·− and NO produced in the mTAL can diffuse to the pericytes of surrounding vasa recta capillaries. Evidence of this so-called “tubulovascular cross-talk”118 was obtained using real-time fluorescence imaging techniques with dyes sensitive to changes of intracellular NO, O2·−, and Ca2+ in the epithelial cells of the mTAL and pericytes of the vasa recta.34,52,102,103,118 It was found that Ang II (1 µmol/L per L) failed to stimulate NO production in vasa recta pericytes as determined by DAF fluorescence. This same stimulus, however, significantly enhanced NO levels within the epithelial cells of isolated mTAL of Sprague Dawley rats.118 Ang II was found to stimulate increases of vasa recta pericyte NO only when adjacent to a mTAL tubule indicating that the source of the increased pericyte NO was from the adjacent mTAL. The vasa recta endothelial cells were ruled out as a source of the pericyte NO because pericyte NO increased in deendothelialized vasa recta and Ang II was not able to increase NO in vasa recta endothelial cells of isolated intact vessels, a phenomenon attributable to a paradoxical reduction in intracellular Ca2+.118,119 Together these studies indicate that NO produced by the mTAL epithelial cells can diffuse to the pericytes of the surrounding vasa recta, a distance ranging from 50 to 200 µmol/L.119
Similarly, it was also found that O2·− tubular-vascular cross-talk could also occur provided that tissue NO levels were kept at low levels to avoid scavenging of O2·−.93,120 As with NO, Ang II failed to stimulate O2·− production in pericytes of isolated vasa recta yet significantly increased O2·− production in isolated mTAL.120 This response (and responses stimulated by NaCl102) appeared to be NAD(P)H-oxidase–dependent because it was inhibited by apocynin120 and Tiron.102 Ang II stimulation of tissue strips containing mTAL with surrounding vasa recta vessels provided no evidence of O2·− cross-talk because even with proximity of vasa recta to mTAL, O2·− levels failed to rise within the vasa recta pericytes. It was only when the tissue strips were bathed with the NO scavenger, carboxy-PTIO, that Ang II stimulation of mTAL produced an increase in O2·− in the surrounding vasa recta pericytes. Conversely, when tissue O2·− levels were reduced with the O2·− scavenger tempol, there was a significant increase in the diffusion of NO from mTAL to the pericytes indicating that cross-talk of NO from mTAL to the vasa recta is also reduced by O2·−.120 These studies indicate that significant diffusion of O2·− from mTAL to the surrounding vasa recta can occur, but only when tissue NO levels are at low levels as are present in the renal outer medulla of SS rats.75,76 Interactions of O2·− and NO therefore appear to importantly modify the bioavailability of each other in a reciprocal manner within this outer medullary region.
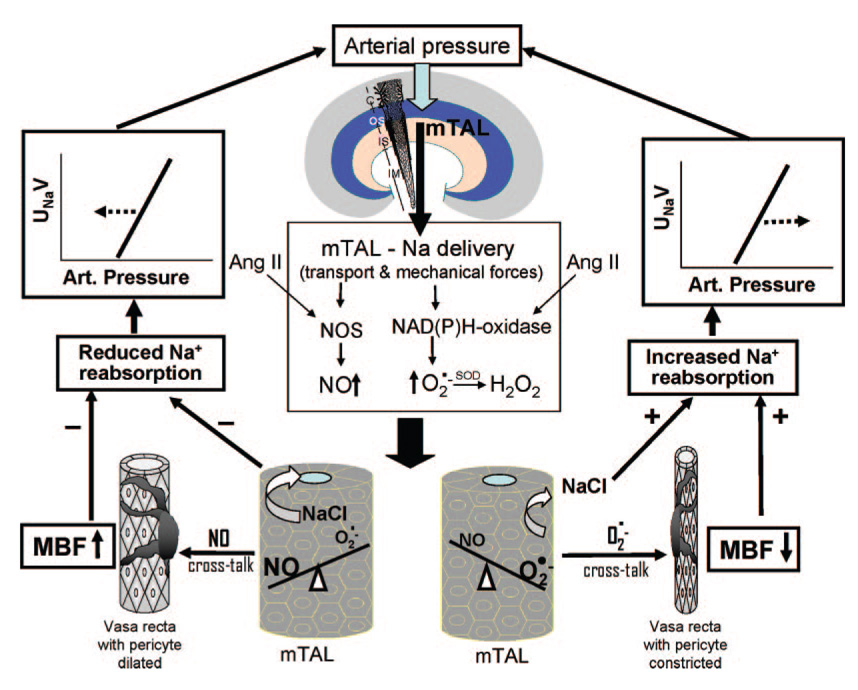
These observations predicted that O2·− cross-talk from mTAL to vasa recta might be exaggerated in the tissue of SS rats which were found to produce less NO and greater amounts of O2·− within the outer medulla compared to control SS.13BN rats.75,76,81,94 This was found to be the case with Ang II stimulation of outer medullary tissue strips that resulted in O2·− tubular-vascular cross-talk in SS rats but not in SS.13BN control rats.93 Conversely, given the greater basal rates of O2·− production in the mTAL of SS rats, NO cross-talk from mTAL to pericytes was observed in SS rats only when O2·− in the tissue was scavenged with tiron.93 These studies emphasize how important it is to understand the relative capacity of each tissue to intrinsically produce O2·− and NO to meaningfully interpret the responses to various stimuli. These interrelationships are illustrated in the accompanying Figure.
Figure 1.
This figure portrays the proposed relationship between arterial pressure and sodium delivery to the medullary thick ascending limb (mTAL) of the kidney. The impact of this relationship on the NO and superoxide (O2·−) systems and the subsequent cross-talk between the tubules and the vasa recta is depicted. When NO is produced by the mTAL and diffuses to the pericytes of the vasa recta, medullary blood flow (MBF) increases thereby reducing sodium reabsorption and shifting the renal function curve to the left. However, when NO is present at low levels, O2·− then diffuses to the vasa recta and MBF is reduced, resulting in an increase of sodium reabsorption shifting the renal function curve to the right. These relationships can in turn be modulated by factors that stimulate or reduce nitric oxide synthase (NOS) and NAD(P)H-oxidase activity such as angiotensin II (Ang II).
Summary and Clinical Implications
The evidence has been reviewed supporting the view that the renal medulla plays an important role in the long-term control of arterial pressure and hypertension. As summarized in the accompanying Figure, this is a consequence of several important factors: (1) the contribution of medullary blood flow to pressure-natriuresis; (2) the ability of genetic factors, neuroendocrine factors, or pharmacological agents to alter the redox state of the outer medulla and thereby alter the balance of NO and O2·− production; (3) tubular-vascular cross-talk of NO and O2·− from the mTAL to the surrounding vasa recta vessels providing an important link between sodium delivery and transport in the mTAL to the capillary delivery of oxygen to the nephron segments of the outer medulla. Reactive oxygen species appear to affect tubular sodium reabsorption by directly altering transport in the mTAL and indirectly by altering MBF, and the relative contribution of these components at this time remains to be determined. A number of antihypertensive factors are enzymatically produced in the renal medulla that normally offset the ravages of oxidative stress. The ability of an individual to prevent the development of many forms of hypertension appears to depend importantly on the underlying buffering capability of NO production system. The HO and COX-2 systems also appear to contribute to counterbalancing the consequences of O2·− production within medulla, but the contribution of these systems is less well understood. If the balance swings from a prevailing production of medullary NO (HO, kinins, and prostaglandins) to that of O2·−, vasa recta flow will be reduced together with the ability of the kidney to excrete normal amounts of sodium. Normalization of this redox imbalance specifically within the renal medulla would be expected to slow the progression of hypertension and renal injury. These issues have not been considered in selecting antihypertensive therapies and may explain why some drugs such as renin-angiotensin system blockers have had a greater effect in reducing proteinuria in patients with diabetic nephropathy in whom pressure was relatively high, but not in those with relatively normal pressures.121
Acknowledgments
The present review was the result of efforts of many outstanding graduate students and research fellows in my laboratory who have contributed to the various parts of this work as cited in the references. I also acknowledge the many enriching conversations on all of these subjects with my close colleagues and collaborators here in the Department of Physiology.
Sources of Funding
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL-29587, HL-081091, HL-66579, and HL-082798.
Footnotes
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://hyper.ahajournals.org/cgi/content/full/52/5/777
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Rojo-Ortega JM, Hatt P-Y. Histopatholgy of cardiovascular lesions in hypertension. In: Genest G, Koiw E, Kuchel O, editors. Hypertension. New York: McGraw-Hill; 1977. [Google Scholar]
- 2.Cowley AW., Jr Franz Volhard lecture: Evolution of the medullipin concept of blood pressure control: a tribute to Eric Muirhead. J Hypertens. 1994;12:S25–S34. [PubMed] [Google Scholar]
- 3.Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- 4.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 5.Hargitay B, Kuhn W. The multiplication principle as the basis for concentrating urine in the kidney. J Am Soc Nephrol. 2001;12:1566–1586. doi: 10.1681/ASN.V1271566. English translation of original publication "Das Multipikationsprinzip als Grundlage der Harnkonzenteierung in der Niere" in Z. Elektrochem. 1951;55:539–558. [DOI] [PubMed] [Google Scholar]
- 6.Wirz H, Hargitay B, Huhn W. Localization of the concentration process in the kidney by direct kryoscopy. Helv Physiol Pharmacol Acta. 1951;9:196–207. [PubMed] [Google Scholar]
- 7.Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol. 2005;67:531–555. doi: 10.1146/annurev.physiol.67.031103.154456. [DOI] [PubMed] [Google Scholar]
- 8.Jamison RL, Kriz W. Urinary Concentrating Mechanism: Structure and Function. New York: Oxford Univ Press; 1982. [Google Scholar]
- 9.Roman RJ, Cowley AW, Jr, Garcia-Estan J, Lombard JH. Pressure-diuresis in volume expanded rats: cortical and medullary hemodynamics. Hypertension. 1988;12:168–176. doi: 10.1161/01.hyp.12.2.168. [DOI] [PubMed] [Google Scholar]
- 10.Zou AP, Wu F, Cowley AW., Jr Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension. 1997;31:271–276. doi: 10.1161/01.hyp.31.1.271. [DOI] [PubMed] [Google Scholar]
- 11.Welch WJ, Mendonca M, Aslam S, Wilcox CS. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K, 1C kidney. Hypertension. 2003;41:692–696. doi: 10.1161/01.HYP.0000052945.84627.8F. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Gordon KL, Giachelli C, Kurth T, Skelton MM, Cowley AW., Jr Tubulo-interstitial injury and loss of nitric oxide synthases parallel the development of hypertension in the Dahl SS rat. J Hypertens. 2000;18:1497–1505. doi: 10.1097/00004872-200018100-00019. [DOI] [PubMed] [Google Scholar]
- 13.Bohle A, Ratschek M. The compensated and the decompensated form of benign nephrosclerosis. Pathol Res Pract. 1982;1174:357–367. doi: 10.1016/S0344-0338(82)80017-4. [DOI] [PubMed] [Google Scholar]
- 14.Cupples WA, Marsh DJ. Autoregulation of blood flow in renal medulla of the rat: no role for angiotensin II. Can J Physiol Pharmacol. 1988;66:833–836. doi: 10.1139/y88-133. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Estan J, Roman RJ. Role of renal interstitial hydrostatic pressure in the pressure diuresis response. Am J Physiol. 1989;256:F60–F70. doi: 10.1152/ajprenal.1989.256.1.F63. [DOI] [PubMed] [Google Scholar]
- 16.Granger JP. Pressure natriuresis. Role of renal interstitial hydrostatic pressure. Hypertension. 1992;19:I9–I17. doi: 10.1161/01.hyp.19.1_suppl.i9. [DOI] [PubMed] [Google Scholar]
- 17.Chou CL, Marsh DJ. Role of proximal convoluted tubule in pressure diuresis in the rat. Am J Physiol. 1986;251:F283–F289. doi: 10.1152/ajprenal.1986.251.2.F283. [DOI] [PubMed] [Google Scholar]
- 18.Haas JA, Granger JP, Knox FG. Effect of renal perfusion pressure on sodium reabsorption from proximal tubules of superficial and deep nephrons. Am J Physiol. 1986;250:F425–F429. doi: 10.1152/ajprenal.1986.250.3.F425. [DOI] [PubMed] [Google Scholar]
- 19.Williams JM, Sarkis A, Lopez B, Ryan RP, Flasch AK, Roman RJ. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49:687–694. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 20.Nowicki S, Chen SL, Aizman O, Chen XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+, K+, ATPase. J Clin Invest. 1997;99:1224–1230. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YB, Magyar CE, Holstein-Rathlou NH, McDonough AA. The cytochrome P-450 inhibitor cobalt chloride prevents inhibition of renal Na, K-ATPase and redistribution of apical NHE-3 during acute hypertension. J Am Soc Nephrol. 1998;9:531–537. doi: 10.1681/ASN.V94531. [DOI] [PubMed] [Google Scholar]
- 22.Lerman LO, Bently MD, Fiksen-Olsen MJ, Strick DM, Ritman EL, Romero JC. Pressure dependency of canine intrarenal blood flow within the rang of autoregulation. Am J Physiol. 1995;268:F404–F409. doi: 10.1152/ajprenal.1995.268.3.F404. [DOI] [PubMed] [Google Scholar]
- 23.Majid DS, Said KE, Omoro SA, Navar LG. Nitric oxide dependency of arterial pressure-induced changes in renal interstitial hydrostatic pressure in dogs. Circ Res. 2001;88:347–351. doi: 10.1161/01.res.88.3.347. [DOI] [PubMed] [Google Scholar]
- 24.Pallone TL. Vasoconstriction of outer medullary vasa recta by angiotensin II is modulated by prostaglandin E2. Am J Physiol. 1994;266:F850–F857. doi: 10.1152/ajprenal.1994.266.6.F850. [DOI] [PubMed] [Google Scholar]
- 25.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol. 2003;284:F253–F266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- 26.Park F, Mattson DL, Roberts LA, Cowley AW., Jr Evidence for the presence of smooth muscle alpha-actin within pericytes of the renal medulla. Am J Physiol. 1997;273:R1742–R1748. doi: 10.1152/ajpregu.1997.273.5.R1742. [DOI] [PubMed] [Google Scholar]
- 27.Roman RJ, Mattson DL, Cowley AW., Jr . Measurement of regional blood flow in the kidney using laser-Doppler flowmetry. In: Wang DH, editor. Methods in Molecular Medicine. Totowa, NJ: Humana Press, Inc.; 2000. pp. 407–426. [DOI] [PubMed] [Google Scholar]
- 28.Roman RJ, Kaldunski ML. Renal cortical and papillary blood flow in spontaneously hypertensive rats. Hypertension. 1988;11:657–663. doi: 10.1161/01.hyp.11.6.657. [DOI] [PubMed] [Google Scholar]
- 29.Lu S, Mattson DL, Cowley AW., Jr Renal medullary captopril delivery lowers blood pressure in spontaneously hypertensive rats. Hypertension. 1994;23:337–345. doi: 10.1161/01.hyp.23.3.337. [DOI] [PubMed] [Google Scholar]
- 30.Campese VM. Salt-sensitivity in hypertension. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 31.Miyata N, Cowley AW., Jr Renal medullary interstitial infusion of L-arginine prevents reduction of medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension. 1999;33:446–450. doi: 10.1161/01.hyp.33.1.446. [DOI] [PubMed] [Google Scholar]
- 32.Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW., Jr Effects of daily sodium intake and Ang II on cortical and medullary renal blood flow in conscious rats. Am J Physiol. 1998;274:R1317–R1323. doi: 10.1152/ajpregu.1998.274.5.R1317. [DOI] [PubMed] [Google Scholar]
- 33.Lerman LO, Taler SJ, Textor SC, Sheedy PF, II, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996;49:846–854. doi: 10.1038/ki.1996.117. [DOI] [PubMed] [Google Scholar]
- 34.Cowley AW, Jr, Mori T, Mattson D, Zou A-P. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol. 2003;284:R1355–R1369. doi: 10.1152/ajpregu.00701.2002. [DOI] [PubMed] [Google Scholar]
- 35.Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol. 1999;276:F874–F881. doi: 10.1152/ajprenal.1999.276.6.F874. [DOI] [PubMed] [Google Scholar]
- 36.Zou A-P, Cowley AW., Jr Nitric oxide in renal cortex and medulla: an in vivo microdialysis study. Hypertension. 1997;29:194–198. doi: 10.1161/01.hyp.29.1.194. [DOI] [PubMed] [Google Scholar]
- 37.Grzelec-Mojzesowica M, Sadowski J. Renal tissue NO and intrarenal haemodynamics during experimental variations of NO content in anesthetized rats. J Physiol Pharmacol. 2007;58:149–163. [PubMed] [Google Scholar]
- 38.Nakanishi K, Mattson DL, Cowley AW., Jr Role of renal medullary blood flow in the development of L-NAME hypertension in rats. Am J Physiol. 1995;268:R317–R323. doi: 10.1152/ajpregu.1995.268.2.R317. [DOI] [PubMed] [Google Scholar]
- 39.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW., Jr Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol. 1994;266:H1918–H1919. doi: 10.1152/ajpheart.1994.266.5.H1918. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz PA, Garvin JL. NO inhibits NaCl absorption by rat thick ascending limb through activation of cGMP stimulated phosphodiesterase. Hypertension. 2003;37:467–471. doi: 10.1161/01.hyp.37.2.467. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz PA, Garvin JL. Interaction of O(2)(−) and NO in the thick ascending limb. Hypertension. 2002;39:591–596. doi: 10.1161/hy0202.103287. [DOI] [PubMed] [Google Scholar]
- 42.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol. 2002;282:F777–F784. doi: 10.1152/ajprenal.00334.2001. [DOI] [PubMed] [Google Scholar]
- 43.Guarasci G, Kline RL. Pressure-natriuresis following acute and chronic inhibition of nitric oxide synthase in rats. Am J Physiol. 1996;270:R469–R478. doi: 10.1152/ajpregu.1996.270.2.R469. [DOI] [PubMed] [Google Scholar]
- 44.Miyata N, Zou AP, Mattson DL, Cowley AW., Jr Renal medullaryinterstitial infusion of L-arginine prevents hypertension in Dahl salt-sensitive rats. Am J Physiol. 1998;275:R1667–R1673. doi: 10.1152/ajpregu.1998.275.5.R1667. [DOI] [PubMed] [Google Scholar]
- 45.Hecker M, Mulsch A, Bassenge E, Busse R. Vasoconstriction and increased flow: two principal mechanisms of shear stress-dependent endothelial autacoid release. Am J Physiol. 1993;265:H828–H833. doi: 10.1152/ajpheart.1993.265.3.H828. [DOI] [PubMed] [Google Scholar]
- 46.Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol. 1993;264:F79–F87. doi: 10.1152/ajprenal.1993.264.1.F79. [DOI] [PubMed] [Google Scholar]
- 47.Salom MG, Lahera V, Miranda-Guardiola F, Romero JC. Blockade of pressure natriuresis induced by inhibition of renal synthesis of nitric oxide in dogs. Am J Physiol. 1992;262:F718–F722. doi: 10.1152/ajprenal.1992.262.5.F718. [DOI] [PubMed] [Google Scholar]
- 48.Navar LG, Inscho EW, Majid DSA, Imig JD, Harrison-Bernard L, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 49.Zou A-P, Cowley AW., Jr α2-Adrenergic receptor-mediated increase in NO production buffers renal medullary vasoconstriction. Am J Physiol. 2000;279:R769–R777. doi: 10.1152/ajpregu.2000.279.3.R769. [DOI] [PubMed] [Google Scholar]
- 50.Cowley AW., Jr Control of the renal medullary circulation by vasopressin V1 and V2 receptors in the rat. Exp Physiol. 2000;85:223S–231S. doi: 10.1111/j.1469-445x.2000.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 51.Cowley AW, Jr, Skelton MM, Kurth TM. Effects of long-term vasopressin receptor stimulation on medullary bllod flow and arterial pressure. Am J Physiol. 1998;275:R1420–R1424. doi: 10.1152/ajpregu.1998.275.5.R1420. [DOI] [PubMed] [Google Scholar]
- 52.O’Connor PM, Cowley AW., Jr Vasopressin-induced nitric oxide production in rat inner medullary collecting duct is dependent on V2 receptor activation of the phosphoinositide pathway. Am J Physiol. 2007;293:F526–F532. doi: 10.1152/ajprenal.00052.2007. [DOI] [PubMed] [Google Scholar]
- 53.Szentivanyi M, Jr, Park F, Maeda CY, Cowley AW., Jr Nitric oxide in the renal medulla protects from vasopressin-induced hypertension. Hypertension. 2000;35:740–745. doi: 10.1161/01.hyp.35.3.740. [DOI] [PubMed] [Google Scholar]
- 54.Vassileva I, Mountain C, Pollock DM. Functional role of ETB receptors in the renal medulla. Hypertension. 2003;41:1359–1363. doi: 10.1161/01.HYP.0000070958.39174.7E. [DOI] [PubMed] [Google Scholar]
- 55.Zwede T, Mattson DL. Inhibition of cyclooxygenase-2 in the rat renal medulla leads to sodium-sensitive hypertension. Hypertension. 2004;44:424–428. doi: 10.1161/01.HYP.0000140924.91479.03. [DOI] [PubMed] [Google Scholar]
- 56.Daniels EG, Hinman JW, Leach BE, Muirhead EE. Identification of prostaglandin E2 as the principal vasodepressor lipid of rabbit renal medulla. Nature. 1967;215:1298–1299. doi: 10.1038/2151298a0. [DOI] [PubMed] [Google Scholar]
- 57.Badzynska B, Sadowski J. Renal hemodynamic responses to intrarenal infusion of acetylcholine: comparison with effects of PGE2 and NO donor. Kidney Int. 2006;69:1774–1779. doi: 10.1038/sj.ki.5000338. [DOI] [PubMed] [Google Scholar]
- 58.Mattson DF, Roman RJ. Role of kinins and AII in the renal hemodynamic response to captopril. Am J Physiol. 1991;260:F670–F679. doi: 10.1152/ajprenal.1991.260.5.F670. [DOI] [PubMed] [Google Scholar]
- 59.Fenoy FJ, Scicli G, Carretero O, Roman RJ. Effect of angiotensin II and a kinin receptor antagonist on the renal hemodynamic response to Captropril. Hypertension. 1991;17:1038–1044. doi: 10.1161/01.hyp.17.6.1038. [DOI] [PubMed] [Google Scholar]
- 60.Zou A-P, Billington H, Su N, Cowley AW., Jr Expression and actions of heme oxygenase in the renal medulla of rats. Hypertension. 2000;35:342–347. doi: 10.1161/01.hyp.35.1.342. [DOI] [PubMed] [Google Scholar]
- 61.Li N, Yi F, dos Santos EA, Donley DK, Li PL. Role of renal medullary heme oxygenase in the regulation of pressure natriuresis and arterial blood pressure. Hypertension. 2007;49:148–154. doi: 10.1161/01.HYP.0000250086.06137.fb. [DOI] [PubMed] [Google Scholar]
- 62.Vera T, Kelsen Silvia, Yanes LL, Reckelhoff JF, Stec DE. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am J Physiol. 2007;292:R1472–R1478. doi: 10.1152/ajpregu.00601.2006. [DOI] [PubMed] [Google Scholar]
- 63.Quan S, Yang L, Shouda S, Schwartzman ML, Nasjletti A, Goodman AI, Abraham NG. Expression of human heme oxygenase-1 in the thick ascending limb attenuates angiotensin II-mediated increase in oxidative injury. Kidney Int. 2004;65:1628–1639. doi: 10.1111/j.1523-1755.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 64.Zou AP, Nithipatikom K, Li PL, Cowley AW., Jr Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol. 1999;276:R790–R798. doi: 10.1152/ajpregu.1999.276.3.R790. [DOI] [PubMed] [Google Scholar]
- 65.Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension. 1996;27:688–692. doi: 10.1161/01.hyp.27.3.688. [DOI] [PubMed] [Google Scholar]
- 66.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol. 2001;281:F1–F11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 67.Tan DY, Meng S, Cason GW, Manning RD., Jr Mechanisms of salt-sensitive hypertension: role of inducible nitric oxide synthase. Am J Physio. 2000;279:R2297–R2303. doi: 10.1152/ajpregu.2000.279.6.R2297. [DOI] [PubMed] [Google Scholar]
- 68.Yao B, Harris RC, Zhang MZ. Interactions between 11 beta-hydroxysteroid dehydrogenase and COX-2 in kidney. Am J Physiol. 2005;288:R1767–R1773. doi: 10.1152/ajpregu.00786.2004. [DOI] [PubMed] [Google Scholar]
- 69.Li N, Chen L, Yi F, Xia M, Li PL. Salt-sensitive hypertension induced by decoy of transcription factor hypoxia-inducible factor-1(alpha) in the renal medulla. Circ Res. 2008;102:1101–1108. doi: 10.1161/CIRCRESAHA.107.169201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manotham K, Tanaka T, Ohse T, Kojma I, Miyata T, Inagi R, Tanaka H, Sassa R, Fujita T, Nangaku M. A biologic role of HIF-1 in the renal medulla. Kidney Int. 2005;67:1428–1439. doi: 10.1111/j.1523-1755.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 71.Zou AP, Yang ZZ, Li PL, Cowley AW., Jr Oxygen-dependent expression of hypoxia-inducible factor-1-alpha in renal medullary cells of rats. Physiol Genomics. 2001;6:159–168. doi: 10.1152/physiolgenomics.2001.6.3.159. [DOI] [PubMed] [Google Scholar]
- 72.Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ Res. 2000;86:319–325. doi: 10.1161/01.res.86.3.319. [DOI] [PubMed] [Google Scholar]
- 73.Komatsu DE, Hadjiargyrou M. Activation of the transcription fator HIG-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34:680–688. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 74.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, Bazan NG. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Opthalmol Vis Sci. 2003;44:4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 75.Yuan B, Cowley AW., Jr Evidence that reduced renal medullary nitric oxide synthase activity of Dahl S rats enables small elevations of arginine vasopressin to produce sustained hypertension. Hypertension. 2001;37:524–528. doi: 10.1161/01.hyp.37.2.524. [DOI] [PubMed] [Google Scholar]
- 76.Szentivanyi M, Jr, Zou A-P, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW., Jr Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol. 2002;283:R266–R272. doi: 10.1152/ajpregu.00461.2001. [DOI] [PubMed] [Google Scholar]
- 77.Chen PY, Sanders PN. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension. 1993;22:812–818. doi: 10.1161/01.hyp.22.6.812. [DOI] [PubMed] [Google Scholar]
- 78.Baydoun AR, Emery PW, Pearson JD, Mann GE. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- 79.Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990;87:8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kakoki M, Kim HS, Arendshorst W, Mattson DL. L-arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol. 2004;287:R1478–R1485. doi: 10.1152/ajpregu.00386.2004. [DOI] [PubMed] [Google Scholar]
- 81.Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 82.Taylor N, Maier KG, Roman RJ, Cowley AW., Jr NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension. 2006;48:1066–1071. doi: 10.1161/01.HYP.0000248751.11383.7c. [DOI] [PubMed] [Google Scholar]
- 83.Zou A-P, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension. 2001;37:547–553. doi: 10.1161/01.hyp.37.2.547. [DOI] [PubMed] [Google Scholar]
- 84.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 85.Juncos NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol. 2006;290:R79–R83. doi: 10.1152/ajpregu.00447.2005. [DOI] [PubMed] [Google Scholar]
- 86.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaClabsorption in the thick ascending limb via activation of protein kinase C. Hypertension. 2006;48:467–472. doi: 10.1161/01.HYP.0000236646.83354.51. [DOI] [PubMed] [Google Scholar]
- 87.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle’s loop in rat kidney. Am J Physiol. 2002;282:F1111–F1119. doi: 10.1152/ajprenal.00218.2001. [DOI] [PubMed] [Google Scholar]
- 88.Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol. 2005;289:F1048–F1056. doi: 10.1152/ajprenal.00416.2004. [DOI] [PubMed] [Google Scholar]
- 89.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol. 2005;289:913–935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 90.Beltowski J, Jamroz-Wisniewska A, Nazor J, Wojcicka G. Spectrophotometric assay of renal ouabain-resistant Na(+)-ATPase and its regulation by leptin and dietary-induced obesity. Acta Biochim Pol. 2004;51:1003–1014. [PubMed] [Google Scholar]
- 91.Yang T, Zhang A, Honeggar M, Kohan DE, Mizel D, Sanders K, Hoidal JR, Briggs JP, Schnermann JB. Hypertonic induction of COX-2 in collecting duct cells by reactive oxygen species of mitochondrial origin. J Biol Chem. 2005;280:34966–34973. doi: 10.1074/jbc.M502430200. [DOI] [PubMed] [Google Scholar]
- 92.Yang T, Zhange A, Pasumarthy A, Zhang L, Warnock Z, Schnermann JB. Nitric oxide stimulates COX-2 expression in cultured collecting duct cells through MAP kinases and superoxide but not cGMP. Am J Physiol. 2006;291:F891–F895. doi: 10.1152/ajprenal.00512.2005. [DOI] [PubMed] [Google Scholar]
- 93.Mori T, O’Connor PM, Abe M, Cowley AW., Jr Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension. 2007;49:1336–1341. doi: 10.1161/HYPERTENSIONAHA.106.085811. [DOI] [PubMed] [Google Scholar]
- 94.Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol. 2005;289:R1573–R1579. doi: 10.1152/ajpregu.00525.2005. [DOI] [PubMed] [Google Scholar]
- 95.Hoagland KM, Maier KG, Roman RJ. Contribution of 20-HETE to the antihypertensive effects of tempol in Dahl salt-sensitive rats. Hypertension. 2003;41:697–702. doi: 10.1161/01.HYP.0000047881.15426.DC. [DOI] [PubMed] [Google Scholar]
- 96.Manning RD, Jr, Meng S, Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand. 2003;179:243–250. doi: 10.1046/j.0001-6772.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 97.Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension. 2003;42:25–30. doi: 10.1161/01.HYP.0000074903.96928.91. [DOI] [PubMed] [Google Scholar]
- 98.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular reduced nicotinamide-adenine dinucleotide phosphare oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 99.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in Dahl salt-sensitive rats. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 100.Son RG, Zou Y, Yu BP, Lee J, Chung HY. Aging effect on myeloperoxidase in rat kidney and its modulation by calorie restriction. Free Radical Research. 2005;39:282–289. doi: 10.1080/10715760500053461. [DOI] [PubMed] [Google Scholar]
- 101.Molony DA, Reeves WB, Andreoli TE. Na+K+:2Cl- cotransport and the thick ascending limb. Kidney Int. 1989;36:418–426. doi: 10.1038/ki.1989.211. [DOI] [PubMed] [Google Scholar]
- 102.Mori T, Cowley AW., Jr Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension. 2004;43:341–346. doi: 10.1161/01.HYP.0000113295.31481.36. [DOI] [PubMed] [Google Scholar]
- 103.Abe M, O’Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW., Jr Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol. 2006;291:F350–F357. doi: 10.1152/ajprenal.00407.2005. [DOI] [PubMed] [Google Scholar]
- 104.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na/K/2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol. 2007;293:F993–F998. doi: 10.1152/ajprenal.00383.2006. [DOI] [PubMed] [Google Scholar]
- 105.Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension. 2008;51:488–493. doi: 10.1161/HYPERTENSIONAHA.107.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kirchner KA. Increased loop chloride uptake precedes hypertension in Dahl salt-sensitive rats. Am J Physiol. 1992;262:R263–R268. doi: 10.1152/ajpregu.1992.262.2.R263. [DOI] [PubMed] [Google Scholar]
- 107.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension. 1991;17:1018–1024. doi: 10.1161/01.hyp.17.6.1018. [DOI] [PubMed] [Google Scholar]
- 108.Alvarez-Guerra M, Garay RP. Renal Na-K-Cl cotransporter NKCC2 in Dahl salt-sensitive rats. J Hyperten. 2002;20:721–727. doi: 10.1097/00004872-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 109.Hoagland KM, Flasch AK, Dahly-Venon AJ, dos Santos EA, Knepper MA, Roman RJ. Elevated BSC-1 and ROMK expression in Dahl salt-sensitive rat kidneys. Hypertension. 2004;43:860–865. doi: 10.1161/01.HYP.0000120123.44945.47. [DOI] [PubMed] [Google Scholar]
- 110.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl co-transporter activity in the thick ascending limb. Am J Physiol. 2005;288:F983–F987. doi: 10.1152/ajprenal.00348.2004. [DOI] [PubMed] [Google Scholar]
- 111.Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase-dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension. 2007;49:893–901. doi: 10.1161/01.HYP.0000259669.40991.1e. [DOI] [PubMed] [Google Scholar]
- 112.Kokusho Y, Komaru T, Takeda S, Takahashi K, Koshida R, Shirato K, Shimokawa H. Hydrogen peroxide derived from beating heart mediates coronary microvascular dilation during tachycardia. Arterioscler Thromb Vasc Biol. 2007;27:1057–1063. doi: 10.1161/ATVBAHA.0000261570.85983.4f. [DOI] [PubMed] [Google Scholar]
- 113.Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol. 2003;138:1085–1092. doi: 10.1038/sj.bjp.0705147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cseko C, Bagi Z, Koller A. Biphasic effect of hydrogen peroxide on skeletal muscle areteriolar tone via activation of endothelial and smooth muscle signaling pathways. J Appl Physiol. 2004;97:1130–1137. doi: 10.1152/japplphysiol.00106.2004. [DOI] [PubMed] [Google Scholar]
- 115.Saitoh S, Kiyooka T, Rocic P, Rogers PA, Zhang C, Swafford A, Dick GM, Viswanathan C, Park Y, Chilian WM. Redox-dependent coronary metabolic dilation. Am J Physiol. 2007;293:H3720–H372. doi: 10.1152/ajpheart.00436.2007. [DOI] [PubMed] [Google Scholar]
- 116.Zhang L, Fujii S, Igarashi J, Kosaka H. Effects of thiol antioxidant on reduced nicotinamide adenine dinucleotide phosphate oxidase in hypertensive Dahl Salt-sensitive rats. Free Radic Biol Med. 2004;37:1813–1820. doi: 10.1016/j.freeradbiomed.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 117.Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol. 2003;285:R827–R833. doi: 10.1152/ajpregu.00636.2002. [DOI] [PubMed] [Google Scholar]
- 118.Dickhout JB, Mori T, Cowley AW., Jr Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced vasoconstriction. Circ Res. 2002;91:487–493. doi: 10.1161/01.res.0000035243.66189.92. [DOI] [PubMed] [Google Scholar]
- 119.Pallone TL, Silldorff EP, Zhang Z. Inhibition of calcium signaling in descending vasa recta endothelia by Ang II. Am J Physiol. 2000;278:H1248–H1255. doi: 10.1152/ajpheart.2000.278.4.H1248. [DOI] [PubMed] [Google Scholar]
- 120.Mori T, Cowley AW., Jr Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension. 2003;42:588–593. doi: 10.1161/01.HYP.0000091821.39824.09. [DOI] [PubMed] [Google Scholar]
- 121.Ogawa S, Takeuchi K, Mori T, Nako K, Tsubono Y, Ito S. Effects of monotherapy of temocapril or candesartan with dose increments or combination therapy with both drugs on the suppression of diabetic nephropathy. Hypertens Res. 2007;30:325–334. doi: 10.1291/hypres.30.325. [DOI] [PubMed] [Google Scholar]