Abstract
The serine/threonine kinase PKB/Akt is a key mediator of survival and resistance to cancer therapy. Pharmacological inhibition of Akt and its biologic sequelae may significantly impact the treatment of cancer. The use of molecular imaging technologies has contributed significantly to drug discovery research with emphasis on drug efficacy, on the mechanism of action and on target validation studies. We have constructed a genetically engineered hybrid bioluminescent reporter molecule (BAR) which reports on Akt serine/throenine kinase activity. Based on the fact that Akt is recruited to the plasma membrane upon activation, we here describe a modified version of this reporter molecule (MyrPalm-BAR) that is membrane bound and whose bioluminescence activity can be used to monitor Akt activity at the cell membrane. Utilizing changes in Akt activation status with small molecule inhibitors of Akt we demonstrated that the membrane targeted Akt reporter was more sensitive and quantitative. In addition, inhibition of upstream signaling kinases such as EGFR and PI-3-Kinase activity resulted in changes in Akt activity which was quantitatively monitored by bioluminescence imaging. Based on these results we propose that the membrane associated Akt reporter may be better suited for identification of novel compounds that modulate the Akt pathway by high throughput screening.
Keywords: Akt, non-invasive molecular imaging, bioluminescence, kinase activity
INTRODUCTION
Genetic alterations that result in changes within signaling pathways have been implicated in tumor initiation, propagation and resistance to cancer therapy. In particular, amplification and/or over-expression of receptor tyrosine kinases (RTK) of which EGFR and Her-2 are prototypic examples, has been implicated as being initiating events in a variety of human malignancies1. These kinases as well as their downstream signaling kinases therefore make a very attractive target for therapeutic interventions. Indeed, recent clinical success of Trastuzumab in Her-2 over-expressing breast cancer2,3 and Erlotinib in EGFR over-expressing cancers4 as well as Gleevec for chronic myeloid leukemia5 provides impetus for targeted inhibition of kinases as a viable therapeutic paradigm. The serine/threonine kinase PKB/Akt is a key mediator of survival and resistance to therapy. Akt functions as a signaling hub wherein many upstream signaling pathways such as those activated in response to RTK activation, eventually converge. The integration of these intracellular signals at the level of Akt and its kinase activity, regulates the phosphorylation of several downstream effectors, such as NF-kappa B, mTOR, Forkhead, Bad, GSK-3 and MDM-26-12. These phosphorylation events in turn mediate the effects of Akt on cell growth, proliferation, protection from pro-apoptotic stimuli, and stimulation of neo-angiogenesis13. The activation of Akt is regulated by both translocation to the plasma membrane and phosphorylation at Thr308 and Ser473. Constitutive phosphorylation of Akt at these sites is frequently observed in a wide range of solid tumors and hematologic malignancies. Ongoing efforts have focused on therapeutically targeting Akt and its biologic sequelae, either at the level of Akt itself or at the levels of its upstream regulators and downstream effectors14. Because Akt is also important for proliferative and anti-apoptotic signaling pathways critical for normal cells, particular emphasis is placed on the fine-tuning the targeting of individual components of this pathway to maximize the therapeutic index of anti-cancer strategies based on the Akt pathway. Although, there have been major developments in our understanding of the biology of Akt and its role in human malignancies, the development of molecular imaging technologies to monitor and quantify Akt activity is still in its infancy.
Non-invasive technologies like MRI, MRS, PET and Optical Imaging will contribute significantly to drug discovery research with emphasis on drug efficacy, on the mechanism of action and on target validation studies in animal disease models in vivo15-24. Molecular imaging techniques bridge the gap between preclinical and clinical research for the development of successful drug candidates that have target specificity, optimal safety, pharmacokinetics/pharmacodynamics and efficacy25-28. The use of molecular imaging endpoints instead of time-consuming dissection and histology significantly decreases the workload involved in tissue analysis and thereby speeds up the evaluation of drug candidates. As imaging methods are non-invasive, they allow for longitudinal studies in a single animal. This increases the statistical relevance of a study, allows for more clinically relevant study designs and decreases the number of animals required. These tools also provide much earlier surrogate markers of therapeutic success and thus speed up the process of drug discovery and clinical evaluation.
In previous studies we described a reporter molecule whose expression provides the ability to non-invasively image Akt activity using bioluminescence imaging (BLI)29. This bioluminescent Akt reporter (BAR) was designed to be expressed within the cytosolic compartment. Based on the reasoning that Akt, when activated is recruited to the plasma membrane, here we describe the development of a membrane targeted bioluminescent Akt reporter (MyrPalm-BAR). Fusion of 10 amino-terminal residues of Lyn, which enables recruitment of Lyn to the plasma membrane by virtue of myristoylation and palmitoylation30, to the amino-terminus BAR resulted in membrane association of the reporter. Using various inhibitors of the Akt pathway we demonstrate that membrane targeted BAR is a more sensitive reporter of Akt activity. MyrPalm-BAR would therefore be better suited for studies wherein Akt activity was being evaluated in a biological context as well as studies for the identification of novel compounds targeting the Akt pathway.
RESULTS
Construction of membrane targeting Akt reporter
Akt/PKB is recruited to the plasma membrane following production of phosphatidylinositol (3,4,5) trisphosphate (PIP3) and phosphatidylinositol (3,4) bisphosphate. At the cell membrane, Akt is activated upon phosphorylation13. To investigate if membrane recruitment of the bioluminescent Akt reporter (BAR) would enhance the sensitivity of the reporter, we constructed a membrane-targeted bioluminescent Akt reporter (MyrPalm-BAR) by fusing 10 amino-terminal residues of the Lyn kinase to BAR (Fig. 1a). This sequence contains signals for myristoylation and palmitoylation, which has been previously demonstrated to effectively targeting a non-membrane protein to the plasma membrane30. The functional basis of the reporter, which is similar to that previously described, is shown schematically in Figure 1b. In the presence of Akt kinase activity, phosphorylation of Akt consensus substrate sequence within the reporter would result in its interaction with the FHA2 domain, thus stearically preventing reconstitution of the luciferase reporter molecule. In the absence of Akt kinase activity, loss of this stearic constraint would allow reconstitution of the luciferase reporter molecule, whose activity can be detected noninvasively by bioluminescence imaging.
Figure 1.
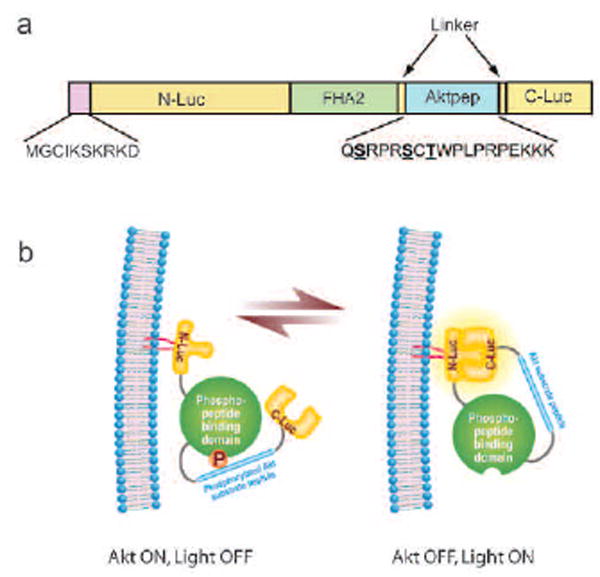
MyrPalm-BAR. (a) The domain structure representation of MyrPalm-BAR. MyrPalm-BAR is generated by adding 10 amino acids from the N terminus of Lyn kinase BAR plasmid. N-Luc and C-Luc are the animo- and carboxyl- terminal domains of firely luciferase that are fused to the appropriate ends of the reporter. The Aktpep domain constitutes a consensus Akt substrate sequence33. On either side of the substrate sequence, flexible linker sequence was included (GGSGG). At the amino- terminal of the Aktpep domain the yeast FHA2 phospho-ser/thr binding domain (reisdues 420 to 582)30 was included. (b) The proposed mechanism of action for the MyrPalm-BAR reporter involves Akt-dependent phosphorylation of the Aktpep domain which results in its interaction with the FHA2 domain. In this form (Akt-ON) the reporter has minimal bioluminescence activity. In the absence of Akt activity, association of the N-Luc and C-Luc domains restores bioluminescence activity.
MyrPalm-BAR is membrane located
The expression plasmid for this hybrid reporter, pEF-MyrPalm-BAR was stably transfected to D54 human glioma cells and resulting clones (D54- MyrPalm-BAR) were analyzed by western blot. D54-MyrPalm-BAR as well as D54-BAR yielded an 84 kD anti-luciferase reactive polypeptide. Mock transfected D54 cells failed to show a luciferase specific polypeptide (Fig. 2a).
Figure 2.
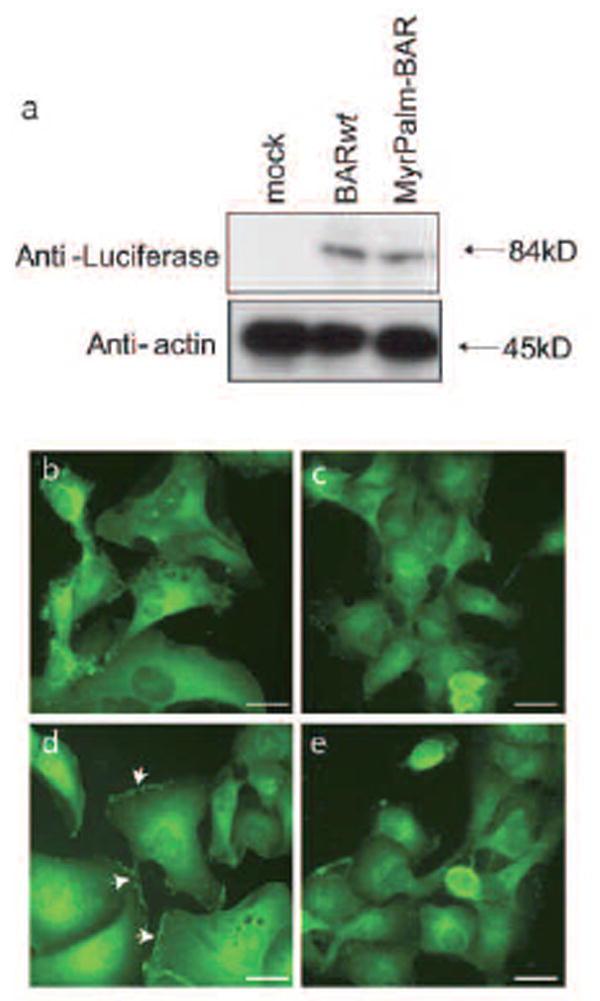
Sub-cellular localization of MyrPalm-BAR. (a) D54 glioma cells stably expressing MyrPalm-BAR and BAR analyzed by western blot using a luciferase specific antibody revealed the expected polypeptides (84 kDa). D54-BAR cells treated with control vehicle (0.1% DMSO in the medium) (b) or 10μM 2- bromopalmitate overnight (c) and D54-MyrPalm-BAR cells treated with control vehicle (0.1% DMSO in the medium) (d) or 10μM 2- bromopalmitate overnight (e) were fixed, permeabilized and immunostained with luciferase antibody and monitored under a fluorescence microscope.
To confirm the membrane targeting of the repoter, we examined the subcellular distribution of MyrPalm-BAR in D54 cells and compared it with BAR localization by immunofluorescence microscopy. The distinct membrane staining with luciferase antibody was only seen in cells expressing MyrPalm-BAR while BAR was predominately localized in the cytoplasm (Fig. 2b and 2d). To address the requirement of palmitoylation for targeting to plasma membrane of MyrPalm-BAR, D54-MyrPalm-BAR cells were treated with the palmitoylation inhibitor, 2-bromopalmitate (2-BP)31. Such treatment of D54-MyrPalm-BAR cells resulted in loss of membrane fluorescence signal (Fig. 2e). This effect was specific for palmitoylated BAR, because the distribution of BAR that lacks a palmitoylatable N-terminal sequence remained the same in the absence and presence of 2-BP treatment (Fig. 2c).
MyrPalm-BAR is more sensitive to Akt activity
To compare the sensitivity of MyrPalm-BAR and BAR to Akt inhibition, we imaged D54-MyrPalm-BAR as well as D54-BAR cells after treatment with an Akt inhibitor, perifosine. Treatment of D54-BAR resulted in a two fold increase of bioluminescence activity, whereas treatment of D54-MyrPalm-BAR resulted in a five fold increase of bioluminescence activity (Fig. 3a and b). Western blot analysis confirmed that perifosine treatment resulted in a similar decrease of phospho-Akt levels in both cell lines (Fig. 3c). We further treated D54-MyrPalm-BAR with LY294002, a PI-3K inhibitor, which resulted in a 9 fold increase in bioluminescence activity. However, treatment of D54-BAR cells resulted in 6 fold increase of bioluminescence activity (Fig. 3d and e). These changes in bioluminescence activity correlated with phospho-Akt as determined by western blotting analysis (Fig. 3f).
Figure 3.
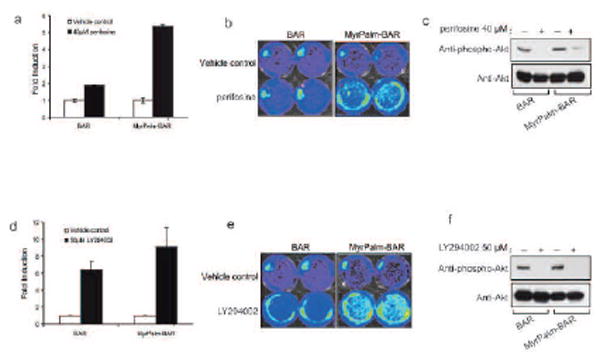
Imaging of Akt activity. (a) D54 cells expressing MyrPalm-BAR as well as D54 cells expressing BAR was treated with 40 μM perifosine or control vehicle (0.1% PBS in the medium) for 1 h. The change in bioluminescence activity compared with the pretreatment value was plotted as fold induction. Data were derived from minimum of five experiments. (b) D54-MyrPalm-BAR cells as well as D54-BAR cells were treated with 40 μM perifosine or control vehicle (0.1% PBS in the medium). Images of representative cells are shown before and after treatment. (c) Cells from one of experiments of 3a were used for western blot analysis with antibodies specific for phospho-Akt and total Akt. (d) D54-MyrPalm-BAR cells as well as D54-BAR cells was treated with 50 μM LY294002 or control vehicle (0.1% DMSO in the medium) for 1 h, and the bioluminescence activity before and after treatment was compared and plotted as fold induction. Data were derived from minimum of five experiments. (e) Images of representative cells from 3d are shown before and after treatment. (c) Western blotting analysis of samples from 3d using antibodies specific for phospho-Akt and total Akt.
Monitor EGFR activity by MyrPalm-BAR
We further investigated the ability of the MyrPalm-BAR to detect changes in Akt status upon inhibition of upstream signal from growth factor receptors. D54-MyrPalm-BAR as well as D54-BAR cells were treated with the EGFR inhibitor, erlotinib and increase in BLI of reporters was monitored. D54-BAR cells showed a 5 fold induction of BLI activity while D54-MyrPalm-BAR cells displayed 11 fold increase in BLI activity within 1hr of erlotinib treatment (Fig. 4a). Fig. 4c demonstrates that this discrepancy in BLI was not due to differences in the sensitivity of the two cell lines to erlotinib, since changes in phospho-Akt levels and phospho-EGFR levels were comparable. To verify that increased sensitivity of MyrPalm-BAR is due to its membrane targeting ability, we pretreated both D54-MyrPalm-BAR and D54-BAR cells with the palmitoylation inhibitor, 2-BP, and then monitored the induction of BLI of reporter activated by erlotinib. The pretreatment D54-MyrPalm-BAR with 2-BP resulted in a lower fold induction, while D54 cells had a similar induction in bioluminescence activity in the presence or absence of 2-BP.
Figure 4.
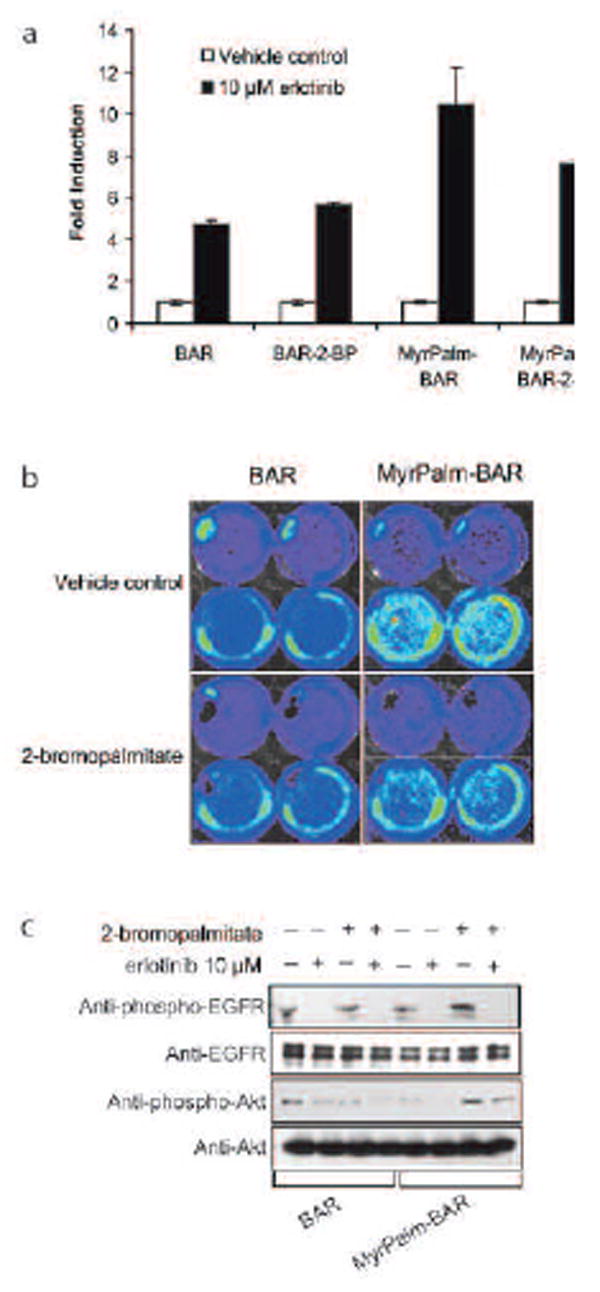
Molecular imaging of RTK (a) D54 cells expressing MyrPalm-BAR as well as D54 cells expressing BAR was preincubated overnight with or without 10 μM 2-BP, treated with 10 μM erlotinib or control vehicle (0.1% DMSO in the medium), and imaged after 1 h. The change in bioluminescence activity compared with the pretreatment value was plotted as fold induction. (b) Images of representative cells from a are shown before and after treatment. (c) Western blot analysis of samples from a using antibodies specific for phospho-EGFR, total EGFR, phospho-Akt and total Akt.
DISCUSSION
In our previous studies, we have developed a split firefly luciferase based reporter (BAR) wherein Akt activity can be detected by bioluminescence imaging. We showed that the inhibition of Akt activity using various kinase inhibitors resulted in an increase of bioluminescence activity in a time- and dose-dependent manner, which indicated that BAR provides a surrogate for Akt activity in terms of quantity and dynamics. In the present study, we modified BAR by fusing the 10 amino-terminal residues of Lyn kinase. This sequence contains signals for myristorylation and palmitoylation, effectively targeting BAR to the cell membrane, where the activated form of Akt is recruited. Using an Akt inhibitor and a PI-3K inhibitor, the sensitivity of BAR and MyrPalm-BAR reporter is studied by bioluminsent imaging, as well as by traditional western blot (Fig. 3). The MyrPalm-BAR showed a higher fold induction in bioluminescence than BAR reporter. These results suggested that MyrPalm-BAR is more sensitive than BAR. Furthermore, we evaluated the erlotinib-mediated inhibition of EGFR using both reporters. When compared to BAR, MyrPalm-BAR generated a higher increase in bioluminescence, and pretreatment of a palmitoylation inhibitor, 2-BP, resulted in lower activation of MyrPalm-BAR. Taken together, these results suggested that membrane targeting ability of MyrPalm-BAR confers the increased sensitivity in reporting Akt activity. Targeting of kinase reporters to appropriate sub-cellular compartments has been successfully used previously to enhance their sensitivity30.
Molecular imaging of Akt in cells and live animals will greatly facilitate the process of target validation, dose and schedule optimization as well as rapid identification of novel compounds from a library of inhibitors using cell based high throughput screening. We suggest that since signal to noise ratio of myr-palm-BAR is higher than BAR alone, the membrane targeted Akt reporter may be better suited for optimization for high throughput screening to identify Akt inhibitors.
METHODS
Gene construction and DNA plasmid
MyrPalm-BAR reporter is cloned in mammalian expression vector PEF. MyrPalm-BAR is generated by adding 5’ 30 bp of Lyn kinase to the 5’ end of BAR plasmid. Generation of BAR plasmid was described in our previous paper29.
Cell culture and transfection
D54 (human glioma) cells is maintained in RPMI (Gibco) supplemented with 10% fetal bovine serum (Gibco). To generate stable cell line, MyrPalm-BAR plasmid is transfected into D54 cell using Fugene (Roche Diagnostics) and selected the resulting stable clones using 200 μg/ml G418 (Invitrogen).
Antibodies and chemicals
Rabbit polyclonal antibodies to Akt, phospho-Akt (phosphorylated on Ser473) and phospho-EGFR (Y845) were purchased from Cell Signaling Technology, and rabbit polyclonal antibody to EGFR (SC-03) was purchased from Santa Cruz biotechnology. Perifosine were from Cayman Chemical. LY294002 was obtained from Cell Signaling Technology. Luciferin was obtained from Biosynth. Erlotinib was kindly provided by Genetech. 2-Bromopalmitic acid is bought from Sigma.
Immunostaining
Cells grown on a coverslip were fixed with 3.5% paraformaldehyde in PBS for 15 min at room temperature. Chilled methanol was then added, and cells were incubated at -20°C for 10 min. Cells were then blocked by using 10% donkey serum and incubated with 1:50 anti-Luciferase antibodies for 1 h at 37°C in a humidified environment. After washing, cells were incubated with donkey anti-Goat antibody coupled to Cy3 (Jackson ImmunoResearch) was monitored with a Nikon fluorescence microscope using filters with an excitation wavelength of 533 nm.
Bioluminescence imaging
Live-cell luminescent imaging was achieved by adding D-luciferin (100 μl/ml) to the growth medium. Photon counts for each condition were acquired 5 min after incubation with D-luciferin using an IVIS imaging system (Xenogen).
Western blot analysis
Cell lysates were prepared in NP40 lysis buffer (1% NP40, 150mM NaCl, 25mM Tris pH 8.0) supplemented with protease inhibitors (Calbiochem, CA) and phosphatase inhibitors (Sigma, MO). Proteins were resolved by SDS/PAGE and analyzed by western blotting using appropriate antibodies. Detection of bound antibody was using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Amersham Pharmacia, Uppsala, Sweden).
Data analysis
Percent changes in signal intensity were calculated using pretreatment values as baseline and plotted as means ± SEM for each of the groups. Statistical comparisons were made by using the unpaired Student’s t test with a value of p< 0.05 being the cut off for significance.
Abbreviations
- BAR
bioluminescent Akt reporter
- MyrPalm-BAR
Myristoylated and palmitoylated bioluminescent Akt reporter
- BLI
bioluminescence imaging
- N-Luc
amino-Luciferase
- C-Luc
carboxyl-Luciferase
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- PI-3K
Phosphoinositide-3 kinase
- 2-BP
2-bromopalmitate
References
- 1.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Buzdar AU, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Kim TE, Murren JR. Erlotinib OSI/Roche/Genentech. Curr Opin Investig Drugs. 2002;3:1385–95. [PubMed] [Google Scholar]
- 5.Radford IR. Imatinib. Novartis. Curr Opin Investig Drugs. 2002;3:492–9. [PubMed] [Google Scholar]
- 6.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 7.Brunn GJ, et al. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. Embo J. 1996;15:5256–67. [PMC free article] [PubMed] [Google Scholar]
- 8.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 9.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 10.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–28. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 11.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 12.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 14.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 15.Ross BD, Chenevert TL, Rehemtulla A. Magnetic resonance imaging in cancer research. Eur J Cancer. 2002;38:2147–56. doi: 10.1016/s0959-8049(02)00387-8. [DOI] [PubMed] [Google Scholar]
- 16.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–20. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 17.Shields AF, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 18.Luker GD, Piwnica-Worms D. Molecular imaging in vivo with PET and SPECT. Acad Radiol. 2001;8:4–14. doi: 10.1016/s1076-6332(03)80738-9. [DOI] [PubMed] [Google Scholar]
- 19.Becker A, et al. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat Biotechnol. 2001;19:327–31. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 20.Weissleder R. Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer. 2002;2:11–8. doi: 10.1038/nrc701. [DOI] [PubMed] [Google Scholar]
- 21.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–8. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 22.Contag CH, Ross BD. It’s not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16:378–87. doi: 10.1002/jmri.10178. [DOI] [PubMed] [Google Scholar]
- 23.Laxman B, et al. Noninvasive real-time imaging of apoptosis. Proc Natl Acad Sci U S A. 2002;99:16551–5. doi: 10.1073/pnas.252644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Bhojani MS, Ross BD, Rehemtulla A. Molecular imaging of protein kinases. Cell Cycle. 2008;7:314–7. doi: 10.4161/cc.7.3.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckmann N, Mueggler T, Allegrini PR, Laurent D, Rudin M. From anatomy to the target: contributions of magnetic resonance imaging to preclinical pharmaceutical research. Anat Rec. 2001;265:85–100. doi: 10.1002/ar.1059. [DOI] [PubMed] [Google Scholar]
- 26.Chenevert TL, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–36. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 27.Moffat BA, et al. Diffusion imaging for evaluation of tumor therapies in preclinical animal models. Magma. 2004;17:249–59. doi: 10.1007/s10334-004-0079-z. [DOI] [PubMed] [Google Scholar]
- 28.Morgan B, Horsfield MA, Steward WP. The role of imaging in the clinical development of antiangiogenic agents. Hematol Oncol Clin North Am. 2004;18:1183–206. x. doi: 10.1016/j.hoc.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, et al. Molecular imaging of Akt kinase activity. Nat Med. 2007;13:1114–9. doi: 10.1038/nm1608. [DOI] [PubMed] [Google Scholar]
- 30.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275:261–70. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 32.Luker KE, et al. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci U S A. 2004;101:12288–93. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe MB, et al. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat Biotechnol. 2001;19:348–53. doi: 10.1038/86737. [DOI] [PubMed] [Google Scholar]


