Abstract
Catastrophic epilepsy due to cortical dysplasia is often intractable to anticonvulsant treatment. Many of the medications used unsuccessfully in treating this disorder are thought to exert at least a portion of their action through enhancement of inhibitory GABAA neurotransmission. In the present study, GABAA receptor properties in resected brain tissue from four infants with infantile spasms and intractable epilepsy due to cortical dysplasia were measured to determine if this clinical resistance to pharmacologic treatment correlates with alterations in receptor function. Results from epileptic cortex were compared with those from autopsy control samples. To perform these studies, we utilized the technique of injection of brain cellular membrane preparations into the Xenopus oocyte, which results in the incorporation of human GABAA receptors in their native configuration into the oocyte plasma membrane. Two-electrode voltage clamp electrophysiology analysis was then performed to assess GABAA receptor pharmacologic properties. The intrinsic properties of affinity, reversal potential, current decay, and current rundown were unchanged in the epileptic infants. Current enhancement by benzodiazepines was also unaltered, as was the response to barbiturates. However, a significant decrease was found in the degree of GABAA current enhancement by neurosteroids in the epileptic infants, along with an increase in current inhibition by zinc. These findings may contribute to the mechanisms of intractability in catastrophic infantile epilepsy due to cortical dysplasia, and suggest alternative therapeutic approaches.
1. Introduction
The catastrophic epilepsies of infancy, including infantile spasms, are frequently refractory to pharmacologic treatment and have devastating developmental outcomes (Glauser, 2004, Mackay et al., 2004, Riikonen, 2005). One of the potential underlying etiologies of infantile spasms is cortical dysplasia, particularly if it is widespread (D'Agostino et al., 2004). While surgical resection of dysplastic brain may result in freedom from seizures, this is often at the expense of neurologic deficits such as hemiplegia and visual field loss (Wheless, 2004, Gonzalez-Martinez et al., 2005, Lettori et al., 2007).
Abnormalities in inhibitory GABAA receptor mediated signaling have been implicated as contributing to the epileptogenicity of cortical dysplasia (Crino et al., 2002, Najm et al., 2004, Calcagnotto et al., 2005). Several of the medications used in the attempted treatment of catastrophic infantile epilepsy, such as barbiturates, benzodiazepines, topiramate, vigabatrin, and ACTH, may exert their actions at least partially through enhancement of GABAergic transmission (Treiman, 2001, Rogawski and Reddy, 2002, Conry, 2004). Investigation of potential alterations in GABAA receptor function and causes of anticonvulsant failure in epilepsy due to cortical dysplasia has been limited by a lack of animal models that faithfully replicate the disorder and the inherent difficulties of working with human tissue (Cepeda et al., 2006, Najm et al., 2007). As a result, detailed pharmacologic analysis of GABAA receptors from human cortical dysplasia tissue has not previously been reported.
In the present study, we examine the intrinsic physiologic properties and responses to pharmacologic agents of GABAA receptors from four infants with infantile spasms and refractory epilepsy who underwent surgical resection of areas of cortical dysplasia. Results from the epileptic infants were compared with those from age matched autopsy control brain samples. To do this, we utilized the technique of injection of brain cellular membrane preparations into the Xenopus oocyte, which results in the “microtransplantation” of human GABAA receptors in their native configuration into the oocyte plasma membrane (Miledi et al., 2002). The appearance of receptor currents utilizing this method does not depend on mRNA translation, but instead arises from incorporation into the oocyte membrane of the injected membrane vesicles harboring native receptors (Marsal et al., 1995). This allows analysis by standard two-electrode voltage clamp electrophysiology. Receptor properties measured after membrane injection into Xenopus oocytes are similar to those measured in situ in neurons from brain slices (Palma et al., 2004, Ragozzino et al., 2005) or in stably-expressing cell lines (Palma et al., 2003). Through the use of this innovative technique, this study reveals previously undescribed abnormalities in GABAA receptor function in infants with catastrophic epilepsy due to cortical dysplasia, which may contribute to the pathogenesis of this disorder and its resistance to anticonvulsant treatment.
2. Methods
2.1 Pediatric cortical brain specimens
Infants with intractable epilepsy were evaluated and underwent brain surgery at Children's Hospital and Regional Medical Center in Seattle, WA. Informed consent for the use of a portion of the resected tissue for research purposes was obtained under the guidance of the hospital's Institutional Review Board. After excision, the fresh tissue was frozen in liquid nitrogen and stored at -80° C. Control frozen autoptic brain tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, Maryland.
2.2 Membrane isolation
Membrane isolation was performed using the method of (Miledi et al., 2002), with modifications, as follows. Frozen cortical brain tissue from each specimen (50 to 300 mg) was weighed and homogenized in 1 mL of glycine buffer containing (in mM) glycine 200, NaCl 150, EGTA 50, EDTA 50, and sucrose 300 (pH 9), plus 10 μL of protease inhibitor cocktail (Sigma P2714). The samples were centrifuged at 9,500 × g, 4°C, for 15 minutes. The supernatant was then centrifuged at 100,000 × g, 4°C, for 2 hours. The resulting membrane pellet was resuspended in 5 mM glycine, using 100 μL per 100 mg original tissue weight. The membrane samples were frozen and stored at − 80°C until use.
2.3 Oocyte preparation and injection
Female Xenopus laevis were euthanized in a bath of 0.2% tricaine methane sulfonate (MS-222). The ovaries were surgically isolated and dissociated for approximately 1 hour at 18°C by 2 mg/mL collagenase A in a solution containing (in mM) NaCl 96, KCl 2, MgCl2 1, and Hepes 5 (pH 7.4). Stage V-VI oocytes were selected and stored at 18°C in the above solution with the addition of 1.8 mM CaCl2 and 50 μg/mL gentamycin. Oocytes were injected with 50-100 nL of membrane in 5 mM glycine and incubated at 18°C for 1-3 days before use.
2.4 Electrophysiology
Injected oocytes were placed in a recording chamber and bathed in oocyte Ringer's solution containing (in mM) NaCl 82.5, KCl 2.5, CaCl2 2.5, MgCl2 1, and Hepes 5 (pH 7.4). Two-electrode voltage-clamp recordings were made at room temperature using a GeneClamp 500B amplifier, Digidata 1322A data acquisition system, and pCLAMP9 data analysis software (Axon Instruments, Molecular Devices Corporation, Sunnyvale, CA). Electrodes with a resistance of 0.5 - 2.5 MΩ were fashioned from borosilicate capillary glass and were filled with 3 mM KCl. Oocyte membrane potential was held at −80 mV unless otherwise stated. Solutions were applied using a gravity-driven, valve-controlled perfusion system.
2.5 Statistical analysis
The significance of any detected difference between oocytes incorporating control or epileptic receptors was determined through the use of a two-tailed, unpaired t-test, or one way ANOVA with posttests, at a significance level of 0.05 (InStat, GraphPad Software, Inc., San Diego, CA). ANOVA was used whenever multiple comparisons were made. Curve fitting and comparison of best-fit parameters was done using GraphPad Prism software.
3. Results
3.1 Patient characteristics
Cortical tissue from four epileptic infants was analyzed, with clinical characteristics as summarized in Table 1. All epileptic infants had onset of catastrophic epilepsy within the first weeks of life which progressed to infantile spasms refractory to ACTH and multiple anticonvulsants. All were found to have unilateral abnormalities on EEG and brain MRI, and underwent surgical resection of the epileptogenic regions at less than a year of age (average age at surgery 5.2 months, range 3-7 months). On pathologic analysis, all were found to have extensive areas of focal cortical dysplasia type 2A (dyslamination, columnar disorganization, immature and dysmorphic neurons, but without balloon cells) (Palmini et al., 2004). Two of the four infants returned to the operating room for completion of functional hemispherectomy due to persistent seizures. All were seizure free at the time of last follow up (14-27 months post surgery).
Table 1.
Clinical data
| Case | Sex | Seizure Onset | Seizure Types | Seizure Frequency | Age at surgery | AED at Surgery | Previous AED | Tissue Studied |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 2 mo | CP, IS | 25-50/day | 7 mo | PB, ZON | ACTH, CBZ, TPM | L. Lateral Temporal |
| 2 | M | 3 days | CP, IS, M, T | 75-100/day | 3 mo | PB, ZON, LEV, MDZ | ACTH, PYR, CLZ | R. Posterior Temporal |
| 3 | F | 4 mo | IS | 3/day | 8 mo | LTG, ZON, LEV | ACTH | L. Parietal |
| 4 | M | 3 wk | CP, IS | 14/day | 3 mo | ACTH, ZON, CLZ, PB | TPM, PYR | L. Lateral Temporal |
| Control | Sex | Age | Cause of Death | Postmortem Interval | Tissue Studied |
|---|---|---|---|---|---|
| Abbreviations: AED, Antiepileptic drugs; PB, Phenobarbital; ZON, Zonisamide; LEV, Levetiracetam; MDZ, Midazolam; LTG, Lamotrigine; ACTH, Adrenocorticotropic hormone; CBZ, Carbamazepine; TPM, Topiramate; CLZ, Clonazepam; PYR, Pyridoxine; CP, Complex partial; IS, Infantile spasms; M, Myoclonic; T, Tonic; SIDS, Sudden Infant Death Syndrome; SMA, Spinal Muscular Atrophy | |||||
| 1 | F | 6 mo | SIDS | 1 hr | Occipital cortex |
| 2 | M | 5 mo | SMA I | 3hr | Parietal cortex |
| 3 | F | 6 mo | SIDS | 6hr | Parietal cortex |
| 4 | M | 10 mo | SMA I | 3hr | Occipital cortex |
| 5 | F | 2 mo | SIDS | 5hr | Temporal cortex |
Frozen postmortem control cortical tissue was analyzed from five infants 2 to 10 months of age (average 6.2 months), with causes of death of sudden infant death syndrome (SIDS) or spinal muscular atrophy type 1, which are not expected to involve significant cortical pathology. Postmortem interval averaged 3.6 hours (range 1-6 hours).
3.2 Intrinsic GABAA Receptor Properties
After control and epileptic membrane injection into Xenopus oocytes, robust GABA-mediated currents were elicited that were not present in uninjected oocytes. These currents were completely blocked by bicuculline and picrotoxin, confirming their mediation by GABAA receptors (data not shown). The GABA reversal potential was calculated for each injected oocyte, and was not significantly different between control and epileptic samples at −22.2 ± 5.3 mV and −25.9 ± 2.5 mV, respectively. These values are similar to those obtained after expression of GABAA receptors in oocytes following injection of brain mRNA (Miledi et al., 1982, Parker et al., 1986) and are equivalent to the chloride reversal potential (Barish, 1983).
Dose-response curves were generated for GABA concentrations between 0.1 and 1000 μM in oocytes expressing control and epileptic receptors (Figure 1A, B). The half-maximal effective GABA concentration (EC50) was the same in the two groups (Control = 45.6 μM, 95% CI = 36.6 - 56.8 μM); Epileptic = 38.3 μM, 95% CI = 29.8 - 49.2 μM; p > 0.05), as was the Hill Slope (1.5 ± 0.2 vs. 1.6 ± 0.3; p > 0.05). These values are similar to that obtained from dissociated human temporal cortical neurons (25 μM) (Gibbs et al., 1996, Gibbs et al., 1998), but substantially lower than that reported from adult temporal cortex using the membrane injection technique (100-120 μM) (Palma et al., 2005, Ragozzino et al., 2005). The reasons for these discrepancies are unknown.
Figure 1.
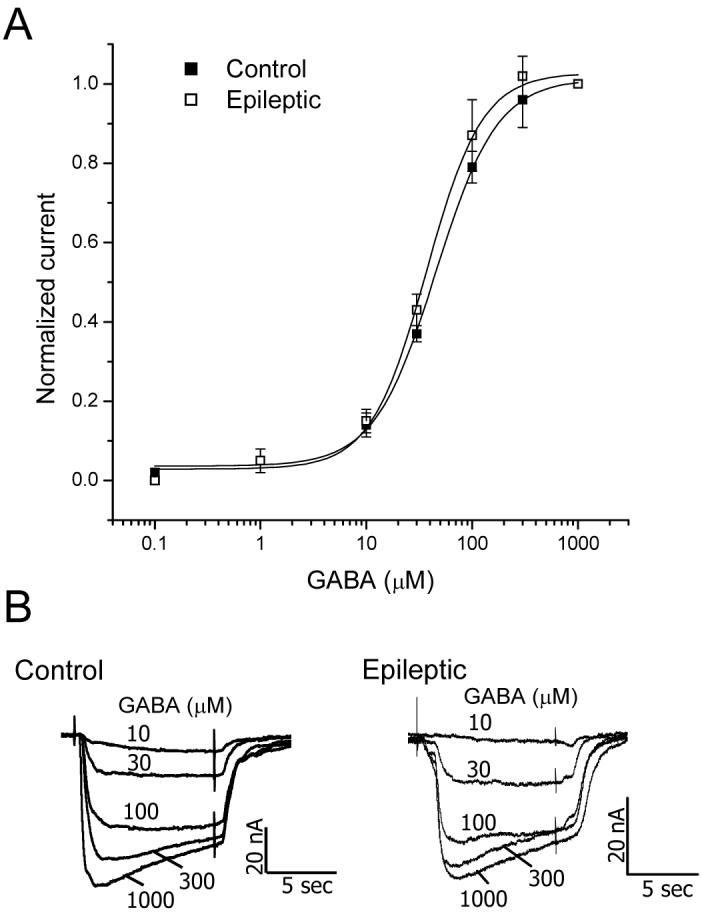
GABA affinity is unchanged in oocytes incorporating GABAA receptors from infants with catastrophic epilepsy due to cortical dysplasia. A, GABAA receptor current produced by GABA concentrations from 0.1 to 1000 μM. Each point represents the average current ± SEM normalized to that produced by 1000 μM GABA, obtained from five control and four epileptic patients (at least 4 injected oocytes analyzed per patient). B, Representative traces of current elicited by increasing concentrations of GABA from oocytes incorporating receptors from a Control (Control 5) and an Epileptic (Case 1) specimen.
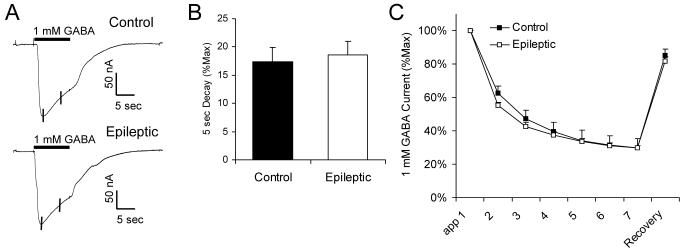
At higher GABA concentrations, the current exhibited a significant amount of decay during the 10 second application, the rate of which was again not significantly different between the two groups (Figure 2A, B). Repeated application of a high concentration of GABA results in a decline in current amplitude, described as current rundown, which recovers after prolonged washout (Huang and Dillon, 1998, Ragozzino et al., 2005). The degree of rundown elicited by 1 mM GABA applied every 40 seconds was also not different between the control and epileptic specimens (Figure 2C).
Figure 2.
GABAA current decay and rundown are unchanged in oocytes incorporating receptors from epileptic infants. A, Sample traces from oocytes incorporating receptors from a Control (Control 4) and an Epileptic (Case 2) specimen showing current decay during a 10 second application of 1 mM GABA. The amount of decay was measured over the 5 seconds following attainment of the peak current, as shown by the vertical lines. B, Average current decay over 5 seconds, shown as the percent of the peak current (%Max) ± SEM. Data for each point was obtained from five control and four epileptic patients (at least 3 injected oocytes analyzed per patient). C, Rundown of GABAA current during repeated 10 second applications of 1 mM GABA every 40 seconds, followed by recovery of current after 5 minute wash-out. Each point represents the average current ± SEM normalized to that produced by the first GABA application, obtained from five control and four epileptic patients (at least 5 injected oocytes analyzed per patient).
In summary, analysis of several intrinsic physiologic properties of GABAA receptors revealed no significant differences between control infants and those with catastrophic epilepsy due to cortical dysplasia.
3.3 Pharmacologic GABAA receptor properties
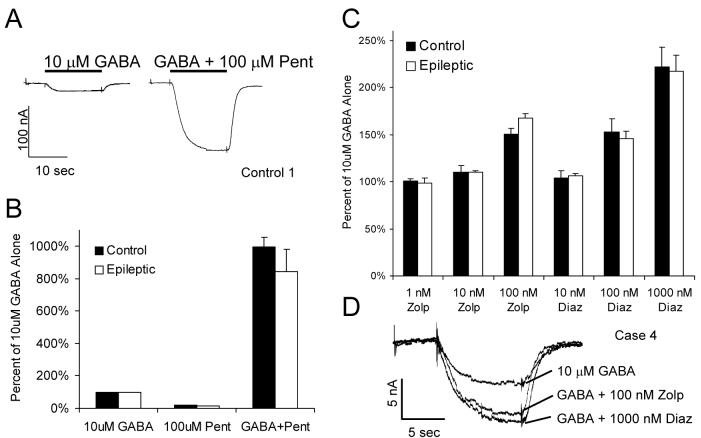
Next, the modulation of GABAA receptor currents by endogenous and exogenous agents, including antiepileptic medications, was investigated. Three of the four infants had been treated unsuccessfully with the barbiturate phenobarbital. Barbiturates can either potentiate GABA responses, directly activate GABAA receptors, or block the GABA chloride channel, depending on concentration and receptor subunit composition (Thompson et al., 1996). In this study, pentobarbital produced a sizeable enhancement of the current elicited by 10 μM GABA, which was not different between oocytes incorporating GABAA receptors from control or epileptic specimens (Figure 3A, B). There was no significant current elicited by 100 μM pentobarbital in the absence of GABA in any specimen tested (Figure 3B), consistent with previous studies demonstrating that its direct agonist effect is primarily seen with receptors expressing the α6 subunit (Drafts and Fisher, 2006), which are found exclusively in the cerebellum (Fritschy and Brunig, 2003).
Figure 3.
GABAA current enhancement by barbiturates and benzodiazepines. A, Representative traces from an oocyte incorporating receptors from Control 1 showing current generated by 10 μM GABA in the absence and presence of 100 μM pentobarbital (Pent). B, Enhancement of current by pentobarbital. Average current ± SEM produced by 10 μM GABA, 100 μM pentobarbital alone, and 10 μM GABA + 100 μM pentobarbital, shown as the percent of the current produced by 10 μM GABA alone. Data was obtained from five control and four epileptic patients (at least 5 injected oocytes analyzed per patient). C, Enhancement of current by the benzodiazepines zolpidem (Zolp) and diazepam (Diaz). Average current ± SEM produced by 10 μM GABA plus the indicated benzodiazepine concentrations, shown as the percent of the current produced by 10 μM GABA alone. Data was obtained from five control and four epileptic patients (at least 5 injected oocytes analyzed per patient). D, Sample traces from an oocyte incorporating receptors from Case 4, demonstrating the increase in GABA current by zolpidem and diazepam.
Benzodiazepines, which exert anticonvulsant effects through enhancement of GABA current by binding to a site formed by the α and γ subunits (Barnard et al., 1998, Jones-Davis and Macdonald, 2003, Rudolph and Mohler, 2004), were used chronically in the treatment of two of the four epileptic infants. The effects of zolpidem, which acts preferentially on α1 containing receptors, and diazepam, which acts equally at receptors containing α1, α2, α3, and α5 subunits, were tested. The degree of enhancement of GABA current by either zolpidem or diazepam was not significantly different between the control and epileptic samples (Figure 3C, D).
The antiepileptic drug topiramate, which has variably been reported to increase GABAA currents (Gordey et al., 2000, White et al., 2000, Simeone et al., 2006), was used clinically in two of the four patients. At concentrations of up to 30 μM, which includes the expected therapeutic serum drug level (Simeone et al., 2006), topiramate had no significant effect on currents elicited by 10 μM GABA (data not shown).
ACTH injections are often effective in the treatment of infantile spasms, which was unfortunately not the case in any of these infants. One mechanism of action proposed for the suppression of spasms by ACTH is through decreasing corticotrophin releasing hormone (CRH) levels (Brunson et al., 2001); however, it also increases levels of endogenous neurosteriods, which are particularly potent enhancers of GABAA receptor function (Torres et al., 2001, Belelli et al., 2002, Rogawski and Reddy, 2002). In addition, a synthetic neurosteroid, ganaxolone, has been found to be effective in treatment-resistant infantile spasms (Kerrigan et al., 2000). Enhancement of the current elicited by 10 μM GABA by the neurosteroid 5α-pregnan-3α-ol-20-one (5α3α) was found to be present, but reduced, in this group of patients with infantile spasms as compared with controls (E(max) Control = 546.2 ± 36.0%; Epileptic = 427.8 ± 17.6%, p < 0.05; Figure 4A). This occurred without a change in the EC50 value (313.8 nM, 95% CI 196.6 - 500.8 nM, vs. 254.7 nM, 95% CI 187.9 - 345.3 nM; p > 0.05.) or Hill Slope (1.2 ± 0.2 vs. 1.4 ± 0.2, p > 0.05). Of note, higher concentrations of the neurosteroid induced a significant amount of GABA current decay (Figure 4B), the degree of which was not different between the control and epileptic samples (5 second decay with 3 μM 5α3α: 28.3 ± 3.9% vs. 29.4 ± 6.1%, p > 0.05.). No current was elicited by 3 μM 5α-pregnan-3α-ol-20-one in the absence of GABA (data not shown).
Figure 4.
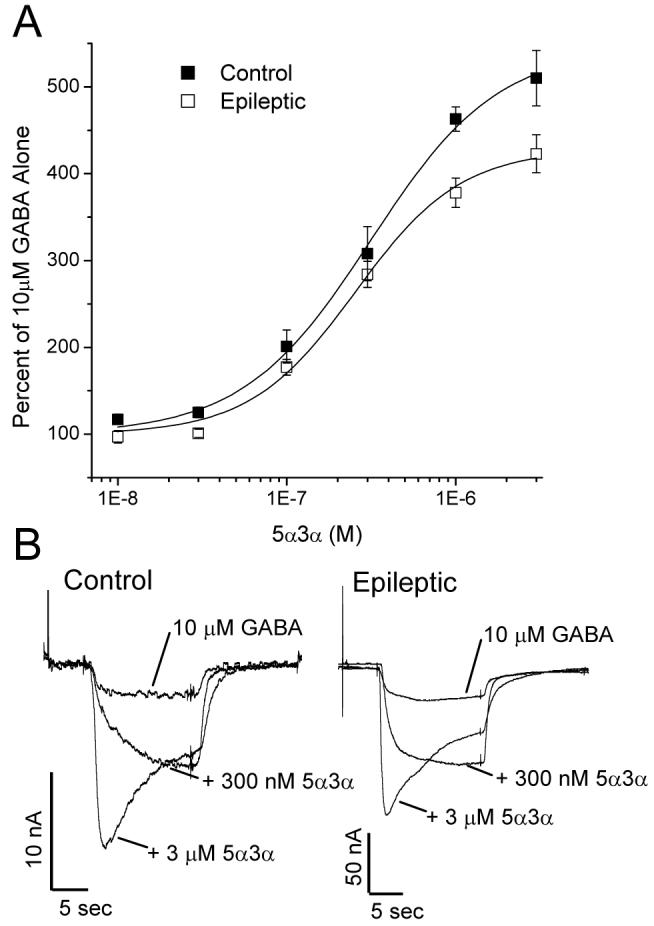
GABAA current enhancement by neurosteroids is reduced in oocytes incorporating receptors from infants with catastrophic epilepsy. A, Current produced by 10 μM GABA in the presence of 5α-pregnan-3α-ol-20-one (5α3α) at concentrations from 10 nM to 3 μM. Each point represents the average current ± SEM normalized to that produced by 10 μM GABA alone, obtained from five control and four epileptic patients (at least 4 injected oocytes analyzed per patient). B, Sample traces from oocytes incorporating receptors from a Control (Control 3) and an Epileptic (Case 2) specimen, demonstrating the increase in GABA current by 5α3α.
Zinc is another endogenous modulator of GABAA receptors, which interacts directly with the ion channel to inhibit chloride entry (Smart et al., 2004). The antiepileptic medication levetiracetam, used in two of the four infants, has been reported to reverse this zinc-mediated inhibition (Rigo et al., 2002). Increased sensitivity of hippocampal GABA currents to zinc has been reported in several models of temporal lobe epilepsy (Buhl et al., 1996, Gibbs et al., 1997, Brooks-Kayal et al., 1998, Shumate et al., 1998, Cohen et al., 2003). Similarly, in cortical specimens from epileptic infants, the degree of reduction in GABA current by zinc was significantly increased as compared with controls (Figure 5A, B).
Figure 5.
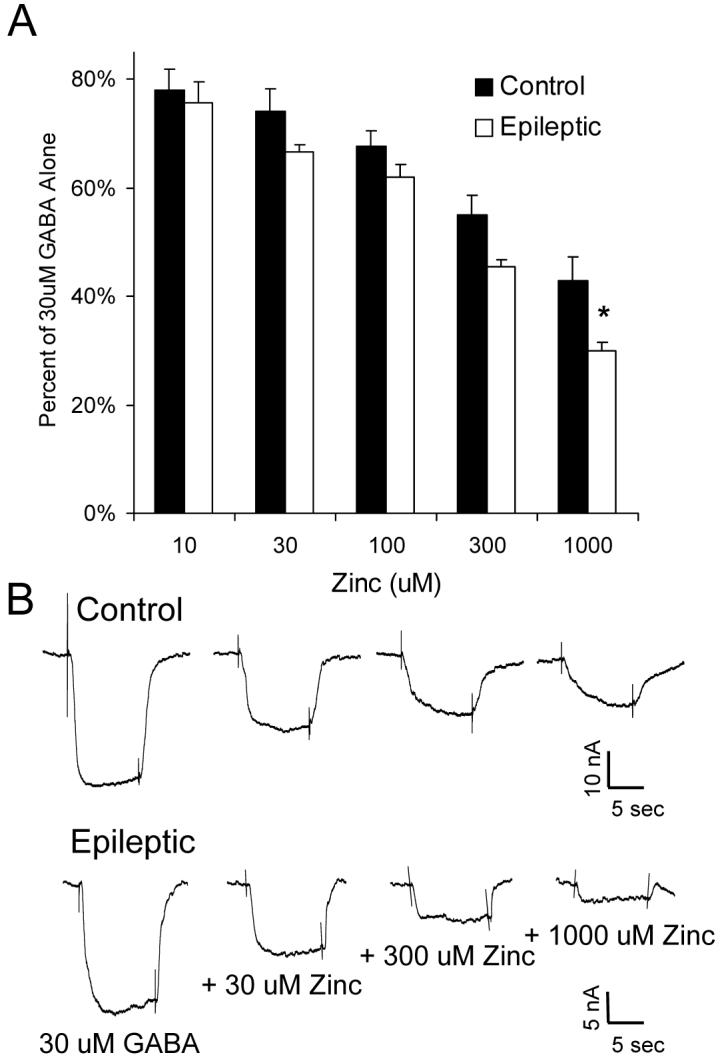
GABAA current inhibition by zinc is increased in oocytes expressing receptors from infants with catastrophic epilepsy. A, Current produced by 30 μM GABA in the presence of ZnCl2 (Zinc) at concentrations from 10 to 1000 μM. Each point represents the average current ± SEM normalized to that produced by 30 μM GABA alone, obtained from five control and four epileptic patients (at least 5 injected oocytes analyzed per patient). * p < 0.05 as compared with corresponding control. B, Sample traces from oocytes incorporating receptors from a Control (Control 2) and an Epileptic (Case 3), demonstrating the inhibition of GABA current by ZnCl2.
In summary, analysis of the functional GABAA receptor properties of four infants with infantile spasms and intractable epilepsy due to cortical dysplasia revealed unchanged intrinsic physiologic properties, but altered pharmacology, including a decreased degree of current enhancement by neurosteroids and an increased degree of current inhibition by zinc.
4. Discussion
4.1 GABAA receptor functional studies
In this study we characterized cortical GABAA receptor properties in four infants with catastrophic epilepsy including infantile spasms due to widespread areas of cortical dysplasia. These findings demonstrate that the intrinsic physiologic receptor properties of GABA affinity, reversal potential, current decay, and rundown are unchanged between control and epileptic samples. In addition, responses to anticonvulsant barbiturates and benzodiazepines were similar. Conversely, the responses of GABAA receptors from the epileptic infants to modulation by neurosteroids and zinc were significantly altered.
ACTH is effective in the short-term treatment of infantile spasms in 42-93% of patients, although with a fairly high relapse rate after completion of treatment and serious side effects (Mackay et al., 2004). One of the possible mechanisms of action of ACTH is via increases in endogenous neurosteroids, which are know to be very potent enhancers of GABAA receptor currents (Torres et al., 2001, Belelli et al., 2002, Rogawski and Reddy, 2002). GABAA current augmentation by the neurosteroid 5α-pregnan-3α-ol-20-one was decreased in these epileptic infants, suggesting that a portion of their resistance to ACTH treatment may be due to this deficit. This also suggests that the synthetic neurosteroid ganaxolone may be less effective in the treatment of infants with infantile spasms due to cortical dysplasia as opposed to other pathologies.
Another significant difference between the infant control and epileptic specimens in this study was an increased sensitivity to GABAA current inhibition by zinc. A reversal of this inhibition has been reported as a mechanism of action of the anticonvulsant levetiracetam (Rigo et al., 2002). Based on these results, other agents targeting the interaction between zinc and GABAA receptors may be beneficial in the treatment of epilepsy due to cortical dysplasia.
The observed differences in GABAA receptor modulation by neurosteroids and zinc in the epileptic infants did occur at fairly high concentrations that are reasonably unlikely to be relevant under normal physiological conditions. However, they may come into play in pathologic states such as during repetitive seizures, exogenous administration of synthetic neurosteroids, or stimulation of endogenous neurosteroid synthesis with ACTH. These situations have been found to increase zinc or neurosteroid levels into the ranges found to differentially affect the epileptic infants in this study (Assaf and Chung, 1984, Kerrigan et al., 2000, Torres et al., 2001).
Unfortunately, the findings of this study do not completely explain the refractoriness to treatment of these infants. Three of the four infants were being treated unsuccessfully with the barbiturate phenobarbital at the time of surgery, but did not exhibit reduced GABAA current enhancement by barbiturates on direct testing. Somewhat unexpected was the intact receptor responsiveness to benzodiazepines in the epileptic infants, particularly in the two infants with innumerable daily seizures despite scheduled benzodiazepine dosing (one on a continuous midazolam infusion the days prior to surgery). This is in contrast to studies of human and experimental temporal lobe epilepsy, where decreased sensitivity to benzodiazepines, in particular the α1-subunit preferring agent zolpidem, has frequently been seen (Buhl et al., 1996, Gibbs et al., 1997, Brooks-Kayal et al., 1998, Shumate et al., 1998, Cohen et al., 2003). The findings of the present study indicate that the failure of barbiturates and benzodiazepines to control seizures in these infants with catastrophic epilepsy due to cortical dysplasia was not due to an inherent inability of their GABAA receptors to respond to these agents.
Previous studies using recombinant GABAA receptors have demonstrated variable effects of topiramate. Depending on subunit composition, an enhancement or inhibition of current was reported, although generally only at supratherapeutic concentrations (Gordey et al., 2000, White et al., 2000, Simeone et al., 2006). In the control and epileptic infants tested in this study, topiramate at a concentration within the range of the usual therapeutic level had no effect GABA currents, suggesting that direct enhancement of GABAA current is unlikely to be a significant mechanism of action for this anticonvulsant.
4.2 Correlation with previous studies on GABAA receptors in human cortical dysplasia
As discussed above, a detailed pharmacologic analysis of GABAA receptors from human cortical dysplasia tissue has not previously been reported. Using a single-cell mRNA amplification technique, dysplastic neurons dissected from adult cortical dysplasia specimens were shown to have decreased GABAA α1, α2, β1, and β2 subunit mRNA expression when compared to postmortem control pyramidal neurons, with no change in α3, α4, α5 or β3 levels (Crino et al., 2001). However, it is difficult to extrapolate these results to predict associated pharmacologic alterations. A recent paper investigated GABA receptor-mediated synaptic function in resected brain slices from pediatric cortical dysplasia patients, identifying a predominance of GABAergic over glutamatergic synaptic activity, spontaneous GABA-mediated depolarizations, and a depolarized GABA reversal potential, all thought to represent features of developmentally immature cortex (Cepeda et al., 2007). Interestingly, increased sensitivity to GABAA current inhibition by zinc and decreased sensitivity to current enhancement by neurosteroids, as observed in the present study, are features of immature rat hippocampal dentate granule cells (Kapur and Macdonald, 1999, Brooks-Kayal et al., 2001, Mtchedlishvili et al., 2003). This suggests that these observed pharmacologic properties may be consistent with a developmental immaturity of the dysplastic tissue. However, immature dentate granule cells are also reported to be less sensitive to diazepam and zolpidem (Kapur and Macdonald, 1999, Brooks-Kayal et al., 2001), which was not the case in the cortical dysplasia samples.
Cellular chloride homestasis, modulated by the NKCC1 and KCC2 cotransporters, also determines neuronal responses to GABAA receptor activation. In immature neurons, high levels of NKCC1 expression and low levels of KCC2 expression result in relatively high intracellular chloride concentrations, and a corresponding depolarizing, excitatory response to GABA during development (Dzhala et al., 2005, Ben-Ari, 2006). Increased NKCC1 and decreased KCC2 levels have been reported in an immunohistochemical study of focal cortical dysplasia (Aronica et al., 2007), as has a depolarizing shift in GABA reversal potential in a subset of neurons in resected dysplastic tissue (Cepeda et al., 2007). In contrast, in the present study, no significant change in GABA reversal potential was found in the epileptic infants. One possible explanation for this disparity is that the Xenopus oocyte expresses its own endogenous set of chloride transporters (Mercado et al., 2001), so that a comparably small difference in levels of “transplanted” transporters is insufficient to substantially change intracellular chloride levels and thus affect GABA reversal potential.
4.3 Significance of findings
It is important to emphasize that the observed alterations in GABAA receptor function in this study may be primary, contributing to epileptogenesis and resistance to pharmacotherapy, or secondary, as a consequence of repeated seizures or antiepileptic medication exposure. Indeed, GABAA properties have been shown to change after episodes of status epilepticus or exposure to benzodiazepines, barbiturates, or steroids (Kapur and Macdonald, 1997, Jones-Davis and Macdonald, 2003, Leroy et al., 2004, Zhang et al., 2004, Raol et al., 2005, Shen et al., 2005, Maguire and Mody, 2007).
Although the sample size in the present study is small, these four patients were chosen due to their relative homogeneity in terms of age (less than 1 year), seizure type (infantile spasms), pathology (FCD 2A), and drug exposure (ACTH). The ability of this study to detect significant alterations in receptor properties in the epileptic patients is likely attributable to this homogeneity. Further studies are underway on a larger population of patients in an attempt to correlate clinical factors with observed GABAA receptor functional properties.
4.4 Discussion of the methods used in this study
The technique of examination of human receptors incorporated in the Xenopus oocyte after injection of cellular membranes has several advantages over other methods previously used to study GABAA receptor function in epileptic human tissue. Techniques which study acutely dissociated neurons or brain slices require fresh tissue, which degrades rapidly, limiting the number of experiments which can be performed. In contrast, with the oocyte membrane injection technique, fresh or recently frozen tissue can be used, as well as autopsy tissue or tissue maintained frozen for many years. Experiments which have looked at GABAA receptor subunit mRNA expression or immunohistochemistry are not able to provide information on the functional status of the detected subunits, which is easily analyzed using this technique. Finally, experiments which isolate mRNA from human tissue and then express it in Xenopus oocytes for analysis depend on the translational, post-translational, protein trafficking, and assembly machinery of the oocyte, which likely does not reproduce that of the host tissue. With the technique of membrane injection, the receptors are incorporated into the oocyte plasma membrane in their native configuration, and their functional properties therefore likely better reflect those of the receptors in situ. Furthermore, RNA is much more susceptible to degradation than are membrane-imbedded proteins.
As with all human studies, in the present study ideal control brain tissue could not be obtained, as healthy children do not generally undergo brain surgery. In previous electrophysiology studies of human cortical dysplasia, histologically “normal” brain tissue from the “least epileptogenic area” was used as a control (Mathern et al., 2000, Cepeda et al., 2003). However, there are problems with this approach, as the tissue is still from a brain that experienced repetitive seizures and which was exposed to antiepileptic medications, and therefore does not serve well as a “control”. Because of the techniques used in the present study, autopsy control cortical tissue was able to be used, allowing examination of age-matched tissues from infants without known central nervous system disease. Of course, this does introduce possible complicating factors of perimortem and postmortem events which could themselves alter receptor function. We have tried to limit this by using tissue with the shortest available postmortem periods before freezing (< 6 hrs).
One drawback to this technique is its inability to adequately determine GABA efficacy; i.e. the maximal GABAA current evoked by GABA application. This is due to the large variability which has been observed to occur in the amplitude of the currents measured (> 10 fold), even when the same membrane preparation is used to inject oocytes from the same frog on the same day. The amplitude of the GABA current does not directly depend on the volume or protein concentration of the membrane preparation injected (Miledi et al., 2002). As a result, these currents do not accurately reflect amplitudes within the source tissue. In contrast, relative responses to experimental perturbations can be reliably determined by normalizing current amplitudes to the current obtained under specified conditions for each oocyte.
A final inherent limitation of this technique is that it does not allow discrimination of GABAA receptor properties based on cell type, e.g. neuronal versus glial, pyramidal cell versus interneuron, or immature versus dysmorphic neuron. However, the actions of the antiepileptic drugs given to patients with epilepsy also do not discriminate in these ways. Therefore, these experiments function to provide global information about cortical GABAA receptor function in pediatric epilepsy, rather than cell-specific or synapse-specific information. As such, alterations observed in GABAA receptor pharmacology in children with intractable epilepsy using this technique can provide guidance for further studies or targeted development of new therapies.
Acknowledgements
This work was supported by National Institutes of Health Grant K08 NS052454 to L.A.J. We thank Elizabeth Limbacher, Nadine Nielsen, Katherine Pearce, Eileen Reichert, Mary Gross, and Mike Bobola for assistance in obtaining consent and collecting surgical tissue, and members of the Children's Hospital Epilepsy Center for clinical evaluations. A special thank you goes to the children and families contributing to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronica E, Boer K, Redeker S, Spliet WG, van Rijen PC, Troost D, Gorter JA. Differential expression patterns of chloride transporters, Na+-K+-2Cl--cotransporter and K+-Cl--cotransporter, in epilepsy-associated malformations of cortical development. Neuroscience. 2007;145:185–196. doi: 10.1016/j.neuroscience.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Barish ME. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Basic developmental rules and their implications for epilepsy in the immature brain. Epileptic Disord. 2006;8:91–102. [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA. gamma-Aminobutyric acid(A) receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J Neurochem. 2001;77:1266–1278. doi: 10.1046/j.1471-4159.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Baram TZ. How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis. Brain Dev. 2001;23:533–538. doi: 10.1016/s0387-7604(01)00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- Calcagnotto ME, Paredes MF, Tihan T, Barbaro NM, Baraban SC. Dysfunction of synaptic inhibition in epilepsy associated with focal cortical dysplasia. J Neurosci. 2005;25:9649–9657. doi: 10.1523/JNEUROSCI.2687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Levine MS, Salamon N, Miyata H, Vinters HV, Mathern GW. Epileptogenesis in pediatric cortical dysplasia: the dysmature cerebral developmental hypothesis. Epilepsy Behav. 2006;9:219–235. doi: 10.1016/j.yebeh.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Wu N, Yamazaki I, Uzgil B, Vinters HV, Levine MS, Mathern GW. Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia. Epilepsia. 2007;48(Suppl 5):79–85. doi: 10.1111/j.1528-1167.2007.01293.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Boylan MK, Calvert CR, Jocoy EL, Nguyen OK, Andre VM, Vinters HV, Ariano MA, Levine MS, Mathern GW. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res. 2003;72:472–486. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABA(A) receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–1616. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conry JA. Pharmacologic treatment of the catastrophic epilepsies. Epilepsia. 2004;45(Suppl 5):12–16. doi: 10.1111/j.0013-9580.2004.05004.x. [DOI] [PubMed] [Google Scholar]
- Crino PB, Duhaime AC, Baltuch G, White R. Differential expression of glutamate and GABA-A receptor subunit mRNA in cortical dysplasia. Neurology. 2001;56:906–913. doi: 10.1212/wnl.56.7.906. [DOI] [PubMed] [Google Scholar]
- Crino PB, Miyata H, Vinters HV. Neurodevelopmental disorders as a cause of seizures: neuropathologic, genetic, and mechanistic considerations. Brain Pathol. 2002;12:212–233. doi: 10.1111/j.1750-3639.2002.tb00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino MD, Bastos A, Piras C, Bernasconi A, Grisar T, Tsur VG, Snipes J, Juhasz C, Chugani H, Guerrini R, Cross H, Andermann E, Dubeau F, Montes J, Olivier A, Andermann F. Posterior quadrantic dysplasia or hemi-hemimegalencephaly: a characteristic brain malformation. Neurology. 2004;62:2214–2220. doi: 10.1212/01.wnl.0000130459.91445.91. [DOI] [PubMed] [Google Scholar]
- Drafts BC, Fisher JL. Identification of structures within GABAA receptor alpha subunits that regulate the agonist action of pentobarbital. J Pharmacol Exp Ther. 2006;318:1094–1101. doi: 10.1124/jpet.106.104844. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Morton LD, Amaker B, Ward JD, Holloway KL, Coulter DA. Physiological analysis of Rasmussen's encephalitis: patch clamp recordings of altered inhibitory neurotransmitter function in resected frontal cortical tissue. Epilepsy Res. 1998;31:13–27. doi: 10.1016/s0920-1211(98)00009-6. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABA(A) receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Zhang YF, Kao CQ, Holloway KL, Oh KS, Coulter DA. Characterization of GABAA receptor function in human temporal cortical neurons. J Neurophysiol. 1996;75:1458–1471. doi: 10.1152/jn.1996.75.4.1458. [DOI] [PubMed] [Google Scholar]
- Glauser TA. Following catastrophic epilepsy patients from childhood to adulthood. Epilepsia. 2004;45(Suppl 5):23–26. doi: 10.1111/j.0013-9580.2004.05005.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez JA, Gupta A, Kotagal P, Lachhwani D, Wyllie E, Luders HO, Bingaman WE. Hemispherectomy for catastrophic epilepsy in infants. Epilepsia. 2005;46:1518–1525. doi: 10.1111/j.1528-1167.2005.53704.x. [DOI] [PubMed] [Google Scholar]
- Gordey M, DeLorey TM, Olsen RW. Differential sensitivity of recombinant GABA(A) receptors expressed in Xenopus oocytes to modulation by topiramate. Epilepsia. 2000;41(Suppl 1):S25–29. [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Maintenance of recombinant type A gamma-aminobutyric acid receptor function: role of protein tyrosine phosphorylation and calcineurin. J Pharmacol Exp Ther. 1998;286:243–255. [PubMed] [Google Scholar]
- Jones-Davis DM, Macdonald RL. GABA(A) receptor function and pharmacology in epilepsy and status epilepticus. Curr Opin Pharmacol. 2003;3:12–18. doi: 10.1016/s1471-4892(02)00015-2. [DOI] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Postnatal development of hippocampal dentate granule cell gamma-aminobutyric acidA receptor pharmacological properties. Mol Pharmacol. 1999;55:444–452. [PubMed] [Google Scholar]
- Kerrigan JF, Shields WD, Nelson TY, Bluestone DL, Dodson WE, Bourgeois BF, Pellock JM, Morton LD, Monaghan EP. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res. 2000;42:133–139. doi: 10.1016/s0920-1211(00)00170-4. [DOI] [PubMed] [Google Scholar]
- Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol. 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettori D, Battaglia D, Sacco A, Veredice C, Chieffo D, Massimi L, Tartaglione T, Chiricozzi F, Staccioli S, Mittica A, Di Rocco C, Guzzetta F. Seizure. 2007. Early hemispherectomy in catastrophic epilepsy A neuro-cognitive and epileptic long-term follow-up. [DOI] [PubMed] [Google Scholar]
- Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, Baram TZ, Duchowny M, Hirtz D, Pellock JM, Shields WD, Shinnar S, Wyllie E, Snead OC., 3rd Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsal J, Tigyi G, Miledi R. Incorporation of acetylcholine receptors and Cl- channels in Xenopus oocytes injected with Torpedo electroplaque membranes. Proc Natl Acad Sci U S A. 1995;92:5224–5228. doi: 10.1073/pnas.92.11.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Cepeda C, Hurst RS, Flores-Hernandez J, Mendoza D, Levine MS. Neurons recorded from pediatric epilepsy surgery patients with cortical dysplasia. Epilepsia. 2000;41(Suppl 6):S162–167. doi: 10.1111/j.1528-1157.2000.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Mercado A, de los Heros P, Vazquez N, Meade P, Mount DB, Gamba G. Functional and molecular characterization of the K-Cl cotransporter of Xenopus laevis oocytes. Am J Physiol Cell Physiol. 2001;281:C670–680. doi: 10.1152/ajpcell.2001.281.2.C670. [DOI] [PubMed] [Google Scholar]
- Miledi R, Eusebi F, Martinez-Torres A, Palma E, Trettel F. Expression of functional neurotransmitter receptors in Xenopus oocytes after injection of human brain membranes. Proc Natl Acad Sci U S A. 2002;99:13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Parker I, Sumikawa K. Synthesis of chick brain GABA receptors by frog oocytes. Proc R Soc Lond B Biol Sci. 1982;216:509–515. doi: 10.1098/rspb.1982.0089. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Sun CS, Harrison MB, Kapur J. Increased neurosteroid sensitivity of hippocampal GABAA receptors during postnatal development. Neuroscience. 2003;118:655–666. doi: 10.1016/s0306-4522(03)00043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm I, Ying Z, Babb T, Crino PB, Macdonald R, Mathern GW, Spreafico R. Mechanisms of epileptogenicity in cortical dysplasias. Neurology. 2004;62:S9–13. doi: 10.1212/01.wnl.0000114506.49267.bb. [DOI] [PubMed] [Google Scholar]
- Najm IM, Tilelli CQ, Oghlakian R. Pathophysiological mechanisms of focal cortical dysplasia: a critical review of human tissue studies and animal models. Epilepsia. 2007;48(Suppl 2):21–32. doi: 10.1111/j.1528-1167.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- Palma E, Ragozzino DA, Di Angelantonio S, Spinelli G, Trettel F, Martinez-Torres A, Torchia G, Arcella A, Di Gennaro G, Quarato PP, Esposito V, Cantore G, Miledi R, Eusebi F. Phosphatase inhibitors remove the run-down of gamma-aminobutyric acid type A receptors in the human epileptic brain. Proc Natl Acad Sci U S A. 2004;101:10183–10188. doi: 10.1073/pnas.0403683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Torchia G, Limatola C, Trettel F, Arcella A, Cantore G, Di Gennaro G, Manfredi M, Esposito V, Quarato PP, Miledi R, Eusebi F. BDNF modulates GABAA receptors microtransplanted from the human epileptic brain to Xenopus oocytes. Proc Natl Acad Sci U S A. 2005;102:1667–1672. doi: 10.1073/pnas.0409442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Trettel F, Fucile S, Renzi M, Miledi R, Eusebi F. Microtransplantation of membranes from cultured cells to Xenopus oocytes: a method to study neurotransmitter receptors embedded in native lipids. Proc Natl Acad Sci U S A. 2003;100:2896–2900. doi: 10.1073/pnas.0438006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Luders HO, Prayson R, Spreafico R, Vinters HV. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- Parker I, Gundersen CB, Miledi R. Actions of pentobarbital on rat brain receptors expressed in Xenopus oocytes. J Neurosci. 1986;6:2290–2297. doi: 10.1523/JNEUROSCI.06-08-02290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Palma E, Di Angelantonio S, Amici M, Mascia A, Arcella A, Giangaspero F, Cantore G, Di Gennaro G, Manfredi M, Esposito V, Quarato PP, Miledi R, Eusebi F. Rundown of GABA type A receptors is a dysfunction associated with human drug-resistant mesial temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2005;102:15219–15223. doi: 10.1073/pnas.0507339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Budreck EC, Brooks-Kayal AR. Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase. Neuroscience. 2005;132:399–407. doi: 10.1016/j.neuroscience.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Rigo JM, Hans G, Nguyen L, Rocher V, Belachew S, Malgrange B, Leprince P, Moonen G, Selak I, Matagne A, Klitgaard H. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br J Pharmacol. 2002;136:659–672. doi: 10.1038/sj.bjp.0704766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen R. The latest on infantile spasms. Curr Opin Neurol. 2005;18:91–95. doi: 10.1097/01.wco.0000162847.91531.9c. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Reddy DS. Neurosteroids and infantile spasms: the deoxycorticosterone hypothesis. Int Rev Neurobiol. 2002;49:199–219. doi: 10.1016/s0074-7742(02)49014-9. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumate MD, Lin DD, Gibbs JW, 3rd, Holloway KL, Coulter DA. GABA(A) receptor function in epileptic human dentate granule cells: comparison to epileptic and control rat. Epilepsy Res. 1998;32:114–128. doi: 10.1016/s0920-1211(98)00045-x. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Wilcox KS, White HS. Subunit selectivity of topiramate modulation of heteromeric GABA(A) receptors. Neuropharmacology. 2006;50:845–857. doi: 10.1016/j.neuropharm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Smart TG, Hosie AM, Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Whiting PJ, Wafford KA. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br J Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ruiz E, Ortega E. Effects of CRH and ACTH administration on plasma and brain neurosteroid levels. Neurochem Res. 2001;26:555–558. doi: 10.1023/a:1010925331768. [DOI] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Wheless JW. Nonpharmacologic treatment of the catastrophic epilepsies of childhood. Epilepsia. 2004;45(Suppl 5):17–22. doi: 10.1111/j.0013-9580.2004.05003.x. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41(Suppl 1):S17–20. [PubMed] [Google Scholar]
- Zhang G, Raol YH, Hsu FC, Coulter DA, Brooks-Kayal AR. Effects of status epilepticus on hippocampal GABAA receptors are age-dependent. Neuroscience. 2004;125:299–303. doi: 10.1016/j.neuroscience.2004.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]