Abstract
Connective Tissue Growth Factor (CTGF), a member of the CCN family of secreted matricellular proteins, regulates fibrosis, angiogenesis, cell proliferation, apoptosis, tumor growth and metastasis. However, the role of CTGF and its regulation mechanism in Wilms tumor remains largely unknown. We found that the bioactive lipid sphingosine-1 phosphate (S1P) induced CTGF expression in a concentration- and time-dependent manner in a Wilms tumor cell line (WiT49), while FTY720-P, an S1P analogue that binds all S1P receptors except S1P2, did not. Further, the specific S1P2 antagonist JTE-013 completely inhibited S1P-induced CTGF expression, whereas the S1P1 antagonist VPC44116 did not, indicating this effect was mediated by S1P2. This was confirmed by adenoviral transduction of S1P2 in WiT49 cells, which showed that overexpression of S1P2 increased the expression of CTGF. Induction of CTGF by S1P was sensitive to ROCK inhibitor Y-27632 and JNK inhibitor SP600125, suggesting the requirement of RhoA/ROCK and JNK pathways for S1P-induced CTGF expression. Interestingly, the expression levels of CTGF were decreased in 8 out of 10 Wilms tumor tissues compared with matched normal tissues by quantitative real-time PCR and western blot analysis. In vitro, human recombinant CTGF significantly inhibited the proliferation of WiT49 cells. In addition, overexpression of CTGF resulted in significant inhibition of WiT49 cell growth. Taken together, these data suggest that CTGF protein induced by S1P2 might act as a growth inhibitor in Wilms tumor.
Keywords: connective tissue growth factor, sphingosine 1-phosphate, sphingosine 1-phosphate receptor 2, WiT49, Wilms tumor
Introduction
Connective tissue growth factor (CTGF) is a member of the CCN family, which includes CTGF (also known as CCN2), cysteine-rich 61 (CYR61, also known as CCN1), nephroblastoma overexpressed (NOV, also known as CCN3) and the newly discovered WISP-1/elm1 (CCN4), WISP-2/rCop1 (CCN5) and WISP-3 (CCN6). CCN family members are involved in a variety of biological functions and are thought to be important regulators of tumorigenesis (1, 2). Originally identified as a secreted mitogen from the conditioned media of human umbilical vein endothelial cells (3), CTGF is widely known to play an important role in different fibrotic diseases (4–6). More recently, CTGF has been implicated as a regulatory protein in several human cancers including breast cancer (7), pancreatic cancer (8, 9), melanomas (10) and chondrosarcomas (11). Chang et al. found that reduced expression of CTGF was associated with the risk of advanced-stage disease, lymph node metastasis and shorter survival in lung adenocarcinoma (12, 13). However, the role of CTGF in Wilms tumor is not known.
The bioactive lipid sphingosine-1 phosphate (S1P) is the ligand for five G-protein coupled receptors of the endothelial differentiation genes family (S1P1–5). Interaction of S1P with its different receptors results in regulation of diverse cellular functions, as a consequence of activating different downstream signaling pathways. The successful use of a S1P receptor modulator FTY720 in a murine melanoma model (14) and a biospecific anti-S1P antibody in several murine xenograft and allograft models (15) to inhibit tumor progression suggest that the S1P pathway is a promising new therapeutic target in oncology (16). Recently, it has been shown that S1P can regulate CTGF expression in non-malignant cell types such as human endothelial cells (17), smooth muscle cells (18), and rat mesangial cells (19). The relationship between S1P and CTGF in human cancers remains unexplored.
Wilms tumor is the most common malignant renal tumor in children. While multimodal therapy involving surgery, chemotherapy and radiation has resulted in high overall cure rates, the significant late effects associated with these treatment modalities highlight the need for alternative therapeutic approaches (20). To date, the mechanisms governing growth and metastasis of Wilms tumor are still largely unknown. Interestingly, Zirn et al. found that CTGF was downregulated in advanced Wilms tumors (21) and treatment of Wilms tumor cell lines with the anti-cancer agent all-trasns retinoic acid increased CTGF level by real-time PCR and microarray analysis (22) which suggested that CTGF might act as a negative modulator in Wilms tumor. In this study, we explored the relationship between S1P and CTGF expression in WiT49 cells (23), a metastatic Wilms tumor cell line as well as the role that CTGF might play in Wilms tumorigenesis.
Results
S1P induced CTGF expression and secretion in WiT49 cells
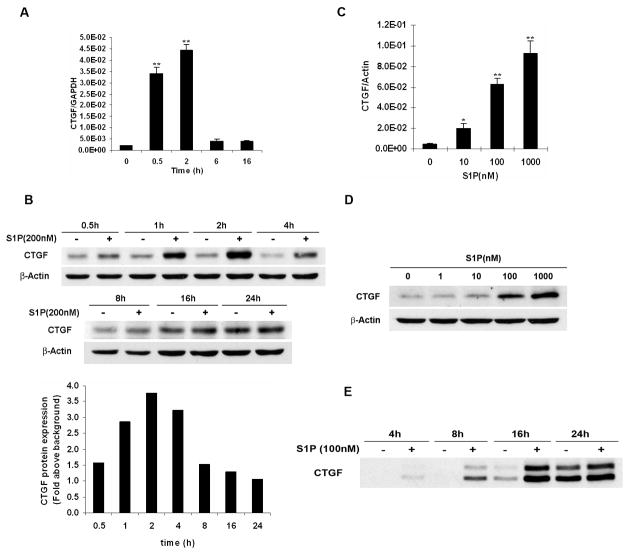
Prior reports have shown that S1P induced CTGF expression in various non-mallignant cell types (17–19). To date it is still unclear whether this effect occurs in human cancers. To address this question, a well-characterized Wilms tumor cell line WiT49 was used (23). We found that S1P induced CTGF mRNA expression in a time-dependent manner (Fig. 1A). Western blot analysis further showed that this effect began at as early as 0.5 h, reached maximum at 1–2 h, and returned to the basal level at 24 h (Fig. 1B). Treatment of WiT49 cells with different concentrations of S1P for 1 h also showed that S1P induced CTGF expression in a concentration-dependent manner by quantitative real-time PCR analysis (Fig. 1C) and western blot analysis (Fig. 1D). Since CTGF is a secreted protein (24, 25), the conditioned media of WiT49 cells was collected and detection of CTGF protein was performed by western blot analysis. It showed that S1P increased CTGF secretion time-dependently (Fig. 1E). Two detectable bands with the molecular weights of about 38 and 42 kDa (Fig. S1), one of which might be its N-glycosylated form (24, 25), can be seen as early as 0.5 h after S1P treatment (data not shown).
FIGURE 1.
S1P induced CTGF expression and secretion in WiT49 cells. A and B. WiT49 cells were serum starved for 24 h and then treated with S1P for different time before quantitative real-time PCR (A, 100 nM S1P) or western blot analysis (B, 200 nM S1P) was done. **, P < 0.01 versus control (0 h). Relative ratio in B represents the fold induction of CTGF by S1P treatment compared to that in non-treatment control at each different time point. CTGF expression was normalized to the housekeeping gene β-Actin. C and D. WiT49 cells were serum starved for 24 h and then treated with different concentrations of S1P for 1 h before quantitative real-time PCR (C) or western blot analysis (D) was done. *, P < 0.05, **, P < 0.01 versus non-treatment control. E. Conditioned media of WiT49 cells were collected and prepared as in Materials and Methods, and followed by western blot analysis.
S1P-induced CTGF expression was mediated by S1P2 receptor
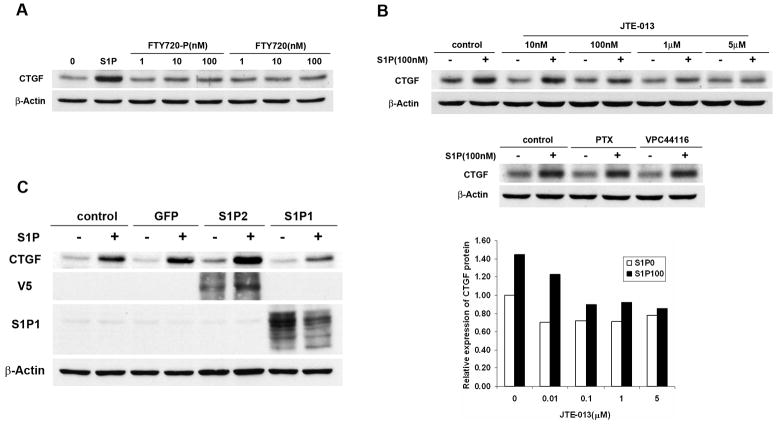
There are five S1P receptors (S1P1–5), which have been identified to bind specifically to S1P (26). We have analyzed S1P receptors expression in Wilms tumor specimens and confirmed that S1P1, S1P2, S1P3, among others, appear to be expressed in vivo (Fig. S2). In order to elucidate which S1P receptor was responsible for this induction, we used several pharmacological agonists and antagonists of S1P receptors. The immunosuppressor FTY720 has been shown to be metabolized in vivo by sphingosine kinase activity. Its phosphorylated derivative, FTY720-phosphate (FTY720-P), is a potent agonist of all S1P receptors except S1P2 (27, 28). In contrast to S1P, stimulation of WiT49 cells with FTY720-P did not increase the CTGF levels (Fig. 2A), suggesting that CTGF expression might be mediated by S1P2. To substantiate this notion, we used the specific S1P2 antagonist JTE-013 (29). Pre-incubation of WiT49 cells with JTE-013 inhibited S1P-induced CTGF expression in a dose-dependent manner (Fig. 2B). In contrast, inhibition of S1P1 signaling by the specific S1P1 antagonist VPC44116 (30) or Gi signaling by pertussis toxin (PTX) did not affect the CTGF expression induced by S1P (Fig. 2B), while these treatments effectively inhibited S1P-induced WiT49 cell migration (data not shown). Finally, we overexpressed S1P2 or S1P1 in WiT49 cells by adenoviral transduction. In agreement with our hypothesis, overexpression of S1P2 into WiT49 cells increased the basal level of CTGF protein (Fig. 2C), most likely due to the autocrine effect of endogenously made S1P (29). In fact, blockade of S1P2 with JTE-013 in the absence of exogenous S1P significantly inhibited this effect (Fig S3). Taken together, these data indicate that S1P-induced CTGF expression is mediated by S1P2.
FIGURE 2.
S1P-induced CTGF expression was mediated by S1P2. A. WiT49 cells were serum starved for 24 h and then treated with 100 nM S1P or different concentrations of FTY720-P or FTY720 for 1 h before western blot analysis was done. B. WiT49 cells were pretreated with S1P2 antagonist JTE-013 (10 nM, 100 nM, 1μM, 5 μM), S1P1 antagonist VPC44116 (1 μM) or Gi protein inhibitor PTX (400 ng/ml) for 0.5 h after serum starvation and then stimulated with 100 nM S1P for another 1 h before western blot analysis was done. The relative expression of CTGF protein in cells treated with or without JTE-013 was normalized to that in control cells without JTE-013 or S1P treatment which was regarded as 1. S1P0 and S1P100 mean no S1P and 100 nM S1P treatment, respectively. C. WiT49 cells were infected with adenovirus overexpressing S1P2, S1P1 or GFP with MOI 100. After 16–24 h, cells were serum starved for 24 h and then stimulated with 100 nM S1P for another 2 h before western blot analysis was done.
RhoA/ROCK and JNK pathways mediated S1P-induced CTGF expression while p38 and ERK MAPK pathways were partially involved
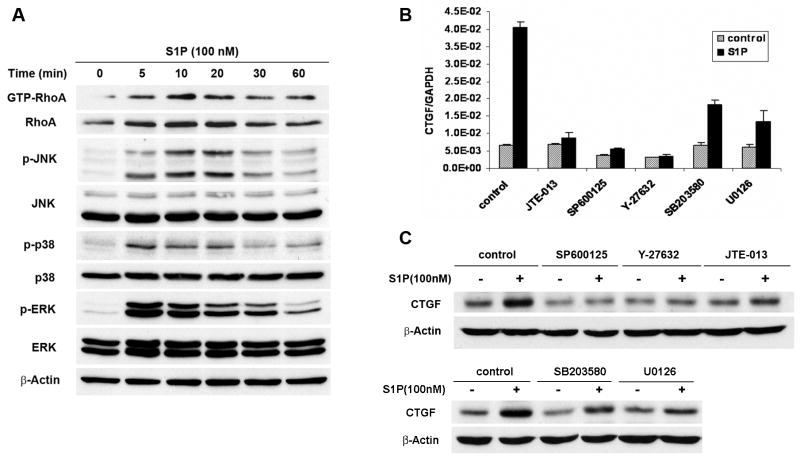
It has been shown that Rho GTPase RhoA and three MAPKs including JNK, p38 and ERK act as the downstream molecules of the S1P2 (31). Pull-down assay showed that RhoA was activated by S1P in a time-dependent manner. Western blot analysis also showed that JNK, p38 and ERK were rapidly activated upon the stimulation by S1P at 5–10 min (Fig. 3A). To delineate the downstream signaling pathways of S1P-induced CTGF expression by S1P2, various pharmacological inhibitors were utilized. Interestingly, inhibition of ROCK and JNK signaling pathways with the ROCK inhibitor Y-27632 and the JNK inhibitor SP600125 completely blocked S1P-induced CTGF mRNA expression as did the S1P2 antagonist JTE-013. Blockade of p38 and ERK pathways with the p38 inhibitor SB203580 and ERK inhibitor U0126 only demonstrated partial inhibition (about 50–65 %) (Fig. 3B), which suggested that ROCK and JNK might be required for CTGF induction by S1P and that p38 and ERK were only partially involved. Consistent with the results of quantitative real-time PCR, western blot analysis also showed that CTGF protein levels were regulated similarly as its mRNA (Fig. 3C).
FIGURE 3.
S1P-induced CTGF expresision was mainly mediated by RhoA/ROCK and JNK pathways. A. WiT49 cells were serum starved for 24 h and then treated with 100 nM S1P for different time before pull-down assay and western blot analysis were done. The blot was stripped and reprobed with β-Actin antibody to confirm equivalent loading of lanes. B and C. WiT49 cells were pretreated with various pharmacological inhibitors (SP600125 20 μM, Y-27632 10 μM, JTE-013 1 μM, SB203580 20 μM, U0126 10 μM) for 0.5 h after serum starvation for 24 h and then stimulated with 100 nM S1P for another 1 h before quantitative real-time PCR (B) and western blot analysis (C) were done.
CTGF expression was suppressed in Wilms tumor tissues
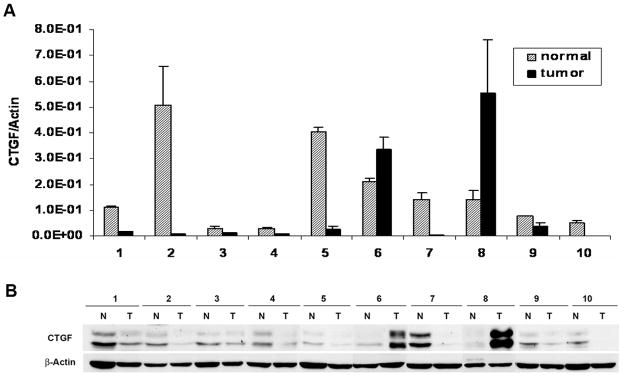
Having demonstrated that CTGF could be induced by S1P/S1P2 pathway in WiT49 cells and on the basis of the findings that S1P, mainly produced from hematopoietic system and vascular endothelium (32–34), is a normal constituent of human plasma and serum (35), we were interested in knowing the expression level of CTGF in vivo. Therefore, ten frozen Wilms tumor tissues were analyzed. Consistent with Zirn’s findings (21), quantitative real-time PCR showed that CTGF mRNA levels were decreased in 8 out of 10 Wilms tumor tissues when compared to their matched normal tissues (Fig. 4A), these results were further confirmed by western blot analysis (Fig. 4B).
FIGURE 4.
CTGF expression was decreased in Wilms tumor tissues compared to matched normal tissues. A. Quantitative real-time PCR for CTGF mRNA expression in 10 Wilms tumor tissues and their matched normal tissues. CTGF expression was normalized to the expression of the housekeeping gene β-Actin. Data are the mean±SD. B. Western blot analysis for CTGF protein in these corresponding tissues. Every small piece of tissue (about 100 mg) was homogenized in 1ml RIPA buffer before western blot analysis was done. N, normal tissue; T, Wilms tumor.
CTGF inhibited cell proliferation in WiT49 cells
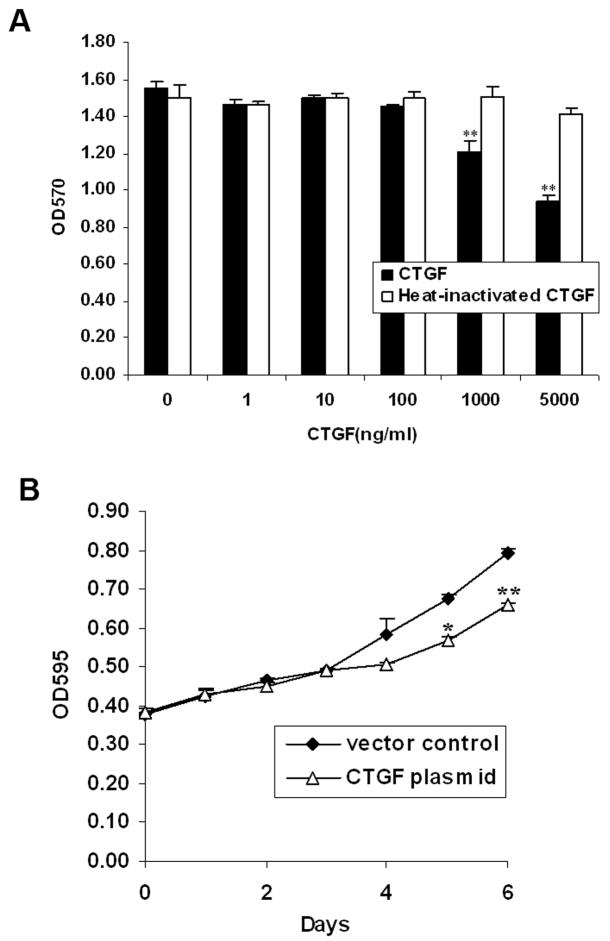
Having shown that significant levels of CTGF were secreted in response to S1P in vitro, and that decreased CTGF levels were detected in tumor tissues in vivo, we tested what the effect of CTGF was on Wilms tumor cell proliferation. MTT assay was done in WiT49 cells using human recombinant CTGF protein. After treatment of WiT49 cells with different concentrations of CTGF for 48 h, we found that CTGF significantly inhibited cell proliferation at 1 μg/ml (22% inhibition) and 5 μg/ml (40% inhibition). However, heat-inactivated CTGF did not have any effect on cell proliferation (Fig. 5A). To confirm that the recombinant CTGF used in our study was biologically active, we checked the active forms of ERK and AKT, which were shown to be the downstream molecules of CTGF signaling (36, 37). Consistent with Yosimichi’s findings (36, 37), 1 μg/ml CTGF activated ERK and AKT phosphorylation in WiT49 cells (Fig S4).
FIGURE 5.
CTGF inhibited cell proliferation in WiT49 cells. A. WiT49 cells were plated to 96-wells. After attachment, they were serum starved for 24 h and then incubated with different concentrations of human recombinant CTGF or heat-inactivated CTGF by being boiled for 10 min for an additional 48 h before the MTT assay was conducted. Data are the mean±SD of triplicates. **, P < 0.01 versus without CTGF. B. WiT49 cells were transfected with either CTGF plasmid or vector control and cultured for indicated days followed by MTT assay. Growth rates were compared between the CTGF plasmid and vector control transfected cells. *, P < 0.05, **, P < 0.01 versus vector control.
In addition, to further confirm the anti-proliferative role of CTGF in WiT49 cells, we transiently transfected WiT49 cells with PCMV-SPORT6-CTGF plasmid. CTGF expression was confirmed by western blot analysis (data not shown). Compared to vector control transfected cells, WiT49 cells overexpressing CTGF showed significant reduction in their growth rate as measured by MTT assay after day 4 (Fig. 5B). Taken together, these data support an anti-proliferative role for CTGF in Wilms tumor.
Discussion
While substantial progress has been made in the treatment of Wilms tumor, the failure of some patients to respond to current treatments along with emerging data regarding long term risks of those treatments, call for the development of new therapeutic approaches. Bioactive lipids such as S1P are increasingly being recognized as therapeutic targets for many diseases including cancer. Several investigators, including our laboratory, have observed the differential effect of ligand-S1P1 and S1P2 interactions. We have previously demonstrated that S1P2 has an inhibitory effect on glioblastoma cell migration while S1P1 stimulates migration (38). In the current study we begin to look beyond S1P-driven cellular migration to explore its regulation of a member of the CCN family of signaling proteins. Our interest in this area was driven by two recent studies which have identified CTGF as a target protein in Wilms tumor (21, 22). Utilizing microarray and real-time PCR analysis of patients from SIOP protocols, Zirn et al. found that diminished expression of CTGF was associated with a higher risk of relapse and disease progression (21). In another related study, treatment of Wilms tumor cell lines with the differentiating agent, retinoic acid, caused an increase in CTGF expression (22), further suggesting that CTGF dysregulation may occur in Wilms tumor. These findings in conjunction with evidence from experiments performed in non-malignant cells (17–19) that suggest regulation of CTGF by S1P, prompted our investigations.
In our study, we used WiT49 cell line as our system model since our previous study showed that in WiT49 cells various S1P receptors were expressed at different levels, in a similar expression pattern of Wilms tumor tissues. Quantitative real-time PCR and western blot ananlysis showed that S1P, which is mainly produced from hematopoietic system and vascular endothelium (32–34), and physiologically detectable at concentrations of about 200 nM in human plasma and 500 nM in human serum (35), could induce CTGF expression in WiT49 cells at the concentration of as low as 10 nM. It only took 2 h to reach the peak level, indicating that CTGF was an immediate early gene during this process (Fig. 1). Western blot analysis of conditioned media from WiT49 cells further showed that S1P induced CTGF secretion in a time-dependent manner, with the modified band (42 kDa) greatly enhanced (Fig 1E, S1). As an exogenous stimulator, the bioactive lipid S1P has various biological functions which are carried out by interaction with its receptors and subsequent activation of downstream pathways. S1P receptors play important roles in angiogenesis, vascular development, lymphocyte trafficking, and cancer (38–41), in which usually S1P1 and S1P2 display opposite effects. However, little is known regarding which S1P receptor is responsible for S1P-mediated CTGF induction. To address this question, we used several techniques including the employment of S1P analogue FTY720-P, specific S1P2 antagonist JTE-013 as well as adenoviral transduction (Fig. 2). Our findings conclusively demonstrate that S1P-induced CTGF expression was mediated by S1P2, not by S1P1 or other receptors.
S1P2 mainly couples G12/13 protein and activates small Rho GTPase RhoA as well as three MAPK signaling pathways (26, 31, 38). In our study, RhoA and three MAPKs (JNK, p38 and ERK) were found to be rapidly activated by S1P in WiT49 cells. To further investigate the downstream pathways of S1P-induced CTGF expression, different pharmacological inhibitors were employed. The results showed the ROCK inhibitor Y-27632 and JNK inhibitor SP600125 completely blocked S1P-induced CTGF expression in WiT49 cells while p38 inhibitor SB203580 and ERK inhibitor U0126 only partially inhibited this induction (Fig. 3), which suggested that S1P-induced CTGF expression might mainly be mediated by RhoA/ROCK and JNK pathways in WiT49 cells.
CTGF is a multifunctional protein that engages a wide variety of biological processes. On one hand, CTGF has been reported to have angiogenic properties. It can stimulate the adhesion, proliferation, migration and tube formation of vascular endothelial cells as well as the neovascularization of chicken chorioallantoic membrane while anti-CTGF antibody blocked all these effects (42). On the other hand, CTGF has been regarded as a tumor suppressor. It can induce apoptosis in human breast cancer cells (43) and suppress cell proliferation in non-small cell lung cancer cells (44) and human oral squamous cell carcinoma-derived cells (45). Moreover, recent studies showed that lower CTGF expression was negatively associated with survival and mortality in lung adenocarcinoma and colorectal cancer (13, 46). Further study in lung adenocarcinoma has shown that inhibition of these effects was likely achieved by promoting HIF-1α protein degradation and thus VEGF inhibition (12). Therefore, the biological function of CTGF seems to be cell-type specific and it is probably due to the complex contextual interactions within a specific tumor milieu. For example, recently integrins were reported to be cell surface receptors of CTGF and a novel integrin α5β1 binding site was thereafter found in module 4 of CTGF (47, 48). However, we can not exclude some other surface receptors, growth factors or cytokines may exist, to interact with CTGF and thus promote or inhibit tumor growth (1, 49).
Interestingly, in our study, we found that CTGF was decreased in 80% (8 out of 10) of Wilms tumor tissues obtained from patients enrolled in COG studies when compared to matched normal tissues (Fig. 4). This prompted us to investigate the role of CTGF in Wilms tumor cell proliferation. MTT assay demonstrated that human recombinant CTGF produced in E.coli, at the concentration of 1 μg/ml and 5 μg/ml, had an inhibitory effect on WiT49 cell proliferation (Fig. 5A). Overexpression of CTGF in WiT49 cells by plasmid transfection further confirmed this result (Fig. 5B). However, the role of CTGF in Wilms tumor may extend beyond an anti-proliferative effect and this role needs to be further investigated. In addition, although S1P could induce CTGF expression in Wilms tumor via S1P2, we do not propose a simple causal relationship between S1P2 and CTGF in Wilms tumorigenesis. In fact, evaluation of S1P2 in a limited number of Wilms tumor specimens and normal tissues suggested that S1P2 mRNA levels were higher in tumor tissues (Fig. S5). We concur with accumulating data suggesting that the role of S1P signaling in tumorigenesis is complex and is likely dependent on many factors such as the level of S1P, differential expression of S1P receptors, resultant impact of downstream effectors, and the specific tumor microenvironments. Our findings do however extend the biologic relevance of S1P2 beyond that of solely an inhibitor of migration and invasion, and suggest that its anti-tumorigenic effects include activation of potential tumor suppressors such as CTGF.
In summary, our results reveal that S1P can induce CTGF expression in human cancer cells. This is the first time that this induction has been shown to be mediated by S1P2, which further extends our knowledge of the biological functions of S1P2 beyond that of inhibition of cellular migration. Moreover, on the basis of the lower levels of CTGF expressed in untreated Wilms tumor patients and the inhibitory effect of CTGF on Wilms tumor cell proliferation, CTGF protein induced by S1P/S1P2 might act as a tumor suppressor in Wilms tumor.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Sigma (Saint Louis, MO). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). Nutrient mixture F-12 and penicillin-streptomycin were purchased from Gibco (Grand Island, NY). S1P was purchased from Biomol (Plymouth Meeting, PA) and JTE-013 was from Tocris Bioscience (Ellisville, MO). Human recombinant CTGF was purchased from BioVendor (Candler, NC). FTY720-P and FTY720 was kindly provided by Dr. Volker Brinkmann (Novartis, Basel) and VPC44116 was obtained from Dr. Kevin R. Lynch. PTX, U0126, SB203580, SP600125 and Y-27632 were purchased from Calbiochem (La Jolla, CA). Primary antibodies for CTGF (sc-14939), RhoA (sc-418), JNK (sc-474), p38 (sc-535) and β-Actin (sc-8432) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for p-JNK (#9251), p-p38 (#4631), p-AKT (#9271), p-ERK (#9106) and ERK (#9102) were from Cell Signaling Technology (Beverly, MA) and V5 antibody was from Invitrogen (Carlsbad CA). S1P1 (E49) monoclonal antibody was developed and characterized as described previously (50). Fresh frozen Wilms tumor specimens and their matched normal tissues, which are those pieces of kidney obtained near the tumors and verified by pathologic evaluation, were obtained through collaboration with the Children’s Oncology Group (COG) Biopathology Center.
Cell culture, adenoviral transduction and plasmid transfection
The WiT49 cell line, derived from a primary lung metastasis of an aggressive Wilms tumor (23), was cultured in 1:1 DMEM/nutrient mixture F-12, 10% FBS, 100 U/ml penicillin, and 100 ug/ml streptomycin. For adenoviral transduction, cells were infected with adenovirus containing GFP, S1P1 or S1P2 for 16–24 h (100 multiplicity of infection, MOI) before western blot analysis was done. For plasmid transfection, WiT49 cells were transfected with either plasmid PCMV-SPORT6-CTGF (Open Biosystems, Huntsville, AL) or vector control using Lipofectamine 2000 ((Invitrogen, Carlsbad CA) according to the manufacturer’s protocol before MTT assay was performed.
Conditioned media collection
WiT49 cells were plated in 60 mm cell culture dishes (Nunc, Rochester, NY) at a density of 1×106 cells/dish. After attachment, they were serum starved for 24 h and then treated with or without 100 nM S1P in 3 ml/dish of serum-free (SF) DMEM for different time. The conditioned media was collected, spin down at 1000 g for 5 min at 4°C and then concentrated by Amicon Ultra-4 10K centrifugal filter device (Millipore, Billerica, MA) at 7000 g for 20 min at room temperature.
Quantitative real-time PCR
Total RNA was isolated from WiT49 cells treated with S1P under different conditions or Wilms tumor tissues using Trizol reagent (Invitrogen, Carlsbad CA) according to the manufacturer’s protocol and cDNA was made as previously described (38). Primers were designed using Primer Express™ 2.0 (Applied Biosystems) according to the software guidelines. Sequences were as follows: 5′-CAGAGTGGAGCGCCTGTTC-3′(forward) and 5′-CTGCAGGAGGCGTTGTCAT-3′ (reverse) for the CTGF gene, 5′-GCAGCAGCAAGATGCGAAG-3′ (forward) and 5′-CGATGAGTGATCCAGGCTTTT-3′ (reverse) for the S1P1gene, 5′-GGCCTAGCCAGTTCTGAAAGC-3′ (forward) and 5′-GCGTTTCCAGCGTCTCCTT-3′ (reverse) for the S1P2 gene, 5′-CTGGTGACCATCGTGATCCTC-3′ (forward) and 5′-ACGCTCACCACAATCACCAC-3′ (reverse) for the S1P3 gene, 5′-TGCACCACCAACTGCTTAGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse) for the GAPDH gene and 5′-GACAGGATGCAGAAGGAGATTACT-3′ (forward) and 5′-TGATCCACATCTGCTGGAAGGT-3′ (reverse) for the β-Actin gene. Real-time PCR was performed using SYBR Green I DNA binding dye technology on an ABI Prism 7900 HT Sequence Detection System (PE Applied Biosystems, Foster City, CA). Results were expressed relative to the internal control gene β-Actin or GAPDH.
Western blot analysis
WiT49 cells were treated with S1P under different conditions after serum starvation for 24 h. Then they were washed with ice-cold PBS and homogenized in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl pH7.5, 500 mM NaCl, 10 mM MgCl2, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM sodium fluoride, and 1 x protease inhibitor mixture). Samples were centrifuged at 14,000 g for 20 min at 4°C, and protein concentrations of supernatants were determined by BCA protein assay Kit (Pierce, Rockford, IL). Equal amounts of protein were separated on 10% SDS-PAGE and blotted to nitrocellulose membranes. The membranes were incubated with the indicated primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Immunoreactivity was visualized by exposure to X-ray film using Pierce ECL Western Blotting Substrate (Pierce Inc, Rockford, IL) according to the manufacturer’s instructions.
RhoA-GTP pull-down assay
WiT49 cells were treated with 100 nM S1P for the indicated time after serum starvation for 24 h. Then they were lysed with RIPA buffer. Measurement of RhoA-GTP activity was performed using the Rhotekin-RBD protein GST beads (Cytoskeleton, Denver, CO) following the manufacturer’s instructions. Total and activated RhoA protein was detected by western blot analysis as described above.
MTT assay
Proliferation of WiT49 cells treated with human recombinant CTGF or transfected with either CTGF plasmid or control vector was determined by the MTT assay. Briefly, after treatment, cells were incubated at 37°C for 2 h in the presence of methylthiazolyldiphenyl-tetrazolium bromide (MTT, Sigma-Aldrich Corporation, Saint Louis, MO), and absorbance was measured according to the manufacturer’s instructions.
Statistical Analysis
All experiments on cells were performed at least twice on separate occasions. The data are presented as means ± SD from a representative experiment. The statistical significance of differences between two groups was determined by Student’s t test using Microsoft Excel software.
Supplementary Material
Acknowledgments
Grant support: NIH grants k08DK070468A and 5R37HL067330-07 and the Seraph Foundation.
We thank Dr. Herman Yeger for the WiT49 cell line, Novartis Pharma for FTY720-P and FTY720 and the Children’s Oncology Group Biopathology Center for the Wilms tumor specimens. T. Sanchez is supported in part by a Scientist Development Grant from the American Heart Association.
References
- 1.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 2.Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- 3.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–94. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denton CP, Abraham DJ. Transforming growth factor-beta and connective tissue growth factor: key cytokines in scleroderma pathogenesis. Curr Opin Rheumatol. 2001;13:505–11. doi: 10.1097/00002281-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Clarkson MR, Duggan J, Brady HR. Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 2000;58:1389–99. doi: 10.1046/j.1523-1755.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 6.Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002;4:136–42. doi: 10.1007/s11926-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 7.Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917–23. [PubMed] [Google Scholar]
- 8.Wenger C, Ellenrieder V, Alber B, et al. Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene. 1999;18:1073–80. doi: 10.1038/sj.onc.1202395. [DOI] [PubMed] [Google Scholar]
- 9.Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5:1108–16. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- 10.Kubo M, Kikuchi K, Nashiro K, et al. Expression of fibrogenic cytokines in desmoplastic malignant melanoma. Br J Dermatol. 1998;139:192–7. doi: 10.1046/j.1365-2133.1998.02354.x. [DOI] [PubMed] [Google Scholar]
- 11.Shakunaga T, Ozaki T, Ohara N, et al. Expression of connective tissue growth factor in cartilaginous tumors. Cancer. 2000;89:1466–73. [PubMed] [Google Scholar]
- 12.Chang CC, Lin MT, Lin BR, et al. Effect of connective tissue growth factor on hypoxia-inducible factor 1alpha degradation and tumor angiogenesis. J Natl Cancer Inst. 2006;98:984–95. doi: 10.1093/jnci/djj242. [DOI] [PubMed] [Google Scholar]
- 13.Chang CC, Shih JY, Jeng YM, et al. Connective tissue growth factor and its role in lung adenocarcinoma invasion and metastasis. J Natl Cancer Inst. 2004;96:364–75. doi: 10.1093/jnci/djh059. [DOI] [PubMed] [Google Scholar]
- 14.LaMontagne K, Littlewood-Evans A, Schnell C, et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–31. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- 15.Visentin B, Vekich JA, Sibbald BJ, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–38. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Sabbadini RA. Targeting sphingosine-1-phosphate for cancer therapy. Br J Cancer. 2006;95:1131–5. doi: 10.1038/sj.bjc.6603400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muehlich S, Schneider N, Hinkmann F, Garlichs CD, Goppelt-Struebe M. Induction of connective tissue growth factor (CTGF) in human endothelial cells by lysophosphatidic acid, sphingosine-1-phosphate, and platelets. Atherosclerosis. 2004;175:261–8. doi: 10.1016/j.atherosclerosis.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury I, Chaqour B. Regulation of connective tissue growth factor (CTGF/CCN2) gene transcription and mRNA stability in smooth muscle cells. Involvement of RhoA GTPase and p38 MAP kinase and sensitivity to actin dynamics. Eur J Biochem. 2004;271:4436–50. doi: 10.1111/j.1432-1033.2004.04382.x. [DOI] [PubMed] [Google Scholar]
- 19.Katsuma S, Ruike Y, Yano T, Kimura M, Hirasawa A, Tsujimoto G. Transcriptional regulation of connective tissue growth factor by sphingosine 1-phosphate in rat cultured mesangial cells. FEBS Lett. 2005;579:2576–82. doi: 10.1016/j.febslet.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 20.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. Jama. 2007;297:2705–15. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 21.Zirn B, Hartmann O, Samans B, et al. Expression profiling of Wilms tumors reveals new candidate genes for different clinical parameters. Int J Cancer. 2006;118:1954–62. doi: 10.1002/ijc.21564. [DOI] [PubMed] [Google Scholar]
- 22.Zirn B, Samans B, Spangenberg C, Graf N, Eilers M, Gessler M. All-trans retinoic acid treatment of Wilms tumor cells reverses expression of genes associated with high risk and relapse in vivo. Oncogene. 2005;24:5246–51. doi: 10.1038/sj.onc.1208725. [DOI] [PubMed] [Google Scholar]
- 23.Alami J, Williams BR, Yeger H. Derivation and characterization of a Wilms’ tumour cell line, WiT 49. Int J Cancer. 2003;107:365–74. doi: 10.1002/ijc.11429. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Segarini P, Raoufi F, Bradham D, Leask A. Connective tissue growth factor is secreted through the Golgi and is degraded in the endosome. Exp Cell Res. 2001;271:109–17. doi: 10.1006/excr.2001.5364. [DOI] [PubMed] [Google Scholar]
- 25.Ball DK, Moussad EE, Rageh MA, Kemper SA, Brigstock DR. Establishment of a recombinant expression system for connective tissue growth factor (CTGF) that models CTGF processing in utero. Reproduction. 2003;125:271–84. doi: 10.1530/rep.0.1250271. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–22. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 28.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–9. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–8. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 30.Foss FW, Jr, Snyder AH, Davis MD, et al. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–77. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 33.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 34.Venkataraman K, Lee YM, Michaud J, et al. Vascular Endothelium As a Contributor of Plasma Sphingosine 1-Phosphate. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yatomi Y, Igarashi Y, Yang L, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem (Tokyo) 1997;121:969–73. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 36.Yosimichi G, Kubota S, Nishida T, et al. Roles of PKC, PI3K and JNK in multiple transduction of CCN2/CTGF signals in chondrocytes. Bone. 2006;38:853–63. doi: 10.1016/j.bone.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Yosimichi G, Nakanishi T, Nishida T, Hattori T, Takano-Yamamoto T, Takigawa M. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK) Eur J Biochem. 2001;268:6058–65. doi: 10.1046/j.0014-2956.2001.02553.x. [DOI] [PubMed] [Google Scholar]
- 38.Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–95. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- 39.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–25. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 42.Shimo T, Nakanishi T, Nishida T, et al. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem (Tokyo) 1999;126:137–45. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- 43.Hishikawa K, Oemar BS, Tanner FC, Nakaki T, Luscher TF, Fujii T. Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J Biol Chem. 1999;274:37461–6. doi: 10.1074/jbc.274.52.37461. [DOI] [PubMed] [Google Scholar]
- 44.Chien W, Yin D, Gui D, et al. Suppression of cell proliferation and signaling transduction by connective tissue growth factor in non-small cell lung cancer cells. Mol Cancer Res. 2006;4:591–8. doi: 10.1158/1541-7786.MCR-06-0029. [DOI] [PubMed] [Google Scholar]
- 45.Moritani NH, Kubota S, Nishida T, et al. Suppressive effect of overexpressed connective tissue growth factor on tumor cell growth in a human oral squamous cell carcinoma-derived cell line. Cancer Lett. 2003;192:205–14. doi: 10.1016/s0304-3835(02)00718-8. [DOI] [PubMed] [Google Scholar]
- 46.Lin BR, Chang CC, Che TF, et al. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology. 2005;128:9–23. doi: 10.1053/j.gastro.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Chen PS, Wang MY, Wu SN, et al. CTGF enhances the motility of breast cancer cells via an integrin-alphavbeta3-ERK1/2-dependent S100A4-upregulated pathway. J Cell Sci. 2007;120:2053–65. doi: 10.1242/jcs.03460. [DOI] [PubMed] [Google Scholar]
- 48.Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut. 2006;55:856–62. doi: 10.1136/gut.2005.079178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 50.Oo ML, Thangada S, Wu MT, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.