Abstract
Following L. major infection, the early LACK (Leishmania homolog of receptors for activated C kinase)- induced IL-4 response appears to determine disease susceptibility in BALB/c mice. Therefore, we sought to manipulate the pathogenic T cell responses to the immunodominant epitope with the use of altered peptide ligands (APLs). Conservative and non-conservative substitutions for each amino acid of the LACK 161–175 peptide determinant were tested for their stimulatory capacity in four different LACK-reactive T cell systems. From these results, we propose a likely LACK 163–171/I-Ad core peptide register and show that APLs with changes at putative T cell receptor (TCR) contacts provide the greatest potential for immune deviation. In particular, the TCR-contact H164V APL expanded Th1 cells upon in vitro recall of naïve splenocytes from LACK-specific BV4 T cell receptor transgenic mice and stimulated IFN-γ secretion from a Th2-committed LACK-reactive T cell line. We also observed that non-conservative substitutions flanking the core determinant had strong agonistic effects for proliferation and Th1/Th2 modulation. However, upon immunization, the H164V APL considerably downregulated proliferation and cytokine responses to the wild type LACK 161–175 peptide, while immunization with the weak agonist, MHC contact APL S171K, increased the IFN-γ/IL-4 ratio to the wild type peptide. In these instances, a hyporesponsive T cell response to the wild-type peptide was achieved by immunizing with an APL possessing non-conservative substitutions at TCR contact sites, while immune deviation was accomplished using a weak-agonist APL that retained the core determinant. Thus, certain LACK-APLs are able to induce T cell responses with a protective phenotype in an infectious disease such as leishmaniasis.
Keywords: LACK, Leishmania, flanking residues, core determinant, altered peptide ligands, agonists, I-Ad, T cell receptor, Th1/Th2
1. Introduction
Leishmania are obligate intracellular protozoan parasites that can produce cutaneous, mucocutaneous and visceral disease in humans. In the murine model of leishmaniasis, disease outcome is determined in large part by the ensuing Th1/Th2 T cell responses to infection. BALB/c (H-2d) mice develop a polarized and fatal Th2 T cell response to subcutaneous L. major infection. The outcome is in contrast to other strains such as C57BL/6 (H-2b) or B10.D2 (H-2d) mice that control early dissemination and resolve the infection with a Th1 T cell response; this subject has been nicely reviewed by Sacks (Sacks and Noben-Trauth, 2002). The Th1/Th2 immune decision is central for promoting the ‘healer’ phenotype, partially because Leishmania amastigotes survive in the endo-lysosomal compartment of macrophages. IFNγ, the hallmark cytokine in a Th1 response, is known to activate macrophages by promoting an oxidative burst and the release of free radicals such as nitric oxide, which helps to eliminate intracellular microbes. Conversely, Th2 cytokines IL-4 and IL-13 drive the alternative pathway for macrophage activation and IL-4 treated macrophages have distinctly lower leishmanicidal activity (Holscher et al., 2006).
Previous observations suggested the possibility that a single Leishmania protein epitope controlled disease outcome when an expansion of Vβ4 Vα8-JαTA72 TCRs was detected in both susceptible BALB/c and resistant C57BL/6 mice at early stages of infection (Reiner et al., 1993). Through the use of a Leishmania expression library, the primary antigen recognized by a Leishmania-specific Vβ4 Vα8 CD4+ T cell clone was an intracellular protein called LACK (Leishmania homolog of the receptor for Activated C Kinase) and was able to confer protection when administered with IL-12 as a vaccine (Mougneau et al., 1995). Further evidence suggesting that LACK was critical for immune recognition came from thymic tolerance studies. Transgenic BALB/c mice designed to express the LACK protein under the major histocompatibility complex class II (MHC II) I-Eα promoter were protected from Leishmania infection, implying that tolerance induction of LACK-reactive T cells protects the host from disease (Julia et al., 1996). The dominant determinant of the T cell proliferative response was shown to lie within the 156–173 region of the LACK protein, presented by the I-Ad MHC-II molecule (Mougneau et al., 1995). Mutation of the histidine at position 164 of the LACK 156–173 determinant to asparagine or lysine creates antagonistic peptides, which can halt both cytokine secretion and proliferation of a T cell clone in the presence of otherwise stimulatory amounts of the wild type peptide (Pingel et al., 1999). Subsequent in vivo studies using LACK TCR Vβ4/Vα8 transgenic (ABLE) mice, demonstrated that only the antagonistic rLACK N-164 could attenuate leishmaniasis (Pingel et al., 1999). However, L. major LACK-N164 mutant parasites, despite demonstrating diminished amastigote viability, had no effect in preventing subsequent infection with wild-type L. major when used for priming (Kelly and Locksley, 2004).
Therefore, although LACK-reactive T cells could be antagonized by N164, priming with this variant in BALB/c mice failed to generate T cell clones that promoted the ‘healer’ IFNγ phenotype when encountered with the wild type epitope present during infection. For these reasons we sought to determine guidelines for the use of epitope derivatives, known as altered peptide ligands (APLs), that generate a polyspecific (Wucherpfennig et al., 2007) and potentially protective IFNγ-response to the LACK immunodominant determinant.
It has been known for some time that altering residues within the peptide ligand in contact with MHC-II or TCR molecules has an effect on the type of activation displayed by a T cell. Kinetic thresholding is a dominant concept in the field, postulating that small peptide differences are recognized by the TCR signaling components, with high affinity peptides increasing the lifetime of the MHC-peptide complex on the surface of the APC, thus allowing for a longer interaction with the TCR, (recently referred to as the “lifetime dogma”) (Feinerman et al., 2008). Certain ligands can also affect the Th1 versus the Th2 nature of the ensuing immune response. It has been shown through the use of these substituted derivatives, that peptides with high affinity for the MHC will skew the immune response towards the Th1 phenotype while peptides with low affinity have the opposite effect (Kumar et al., 1995; Murray et al., 1994; Pfeiffer et al., 1995).
TCR affinity for the MHC-peptide complex has also been suggested to determine the Th1/Th2 phenotype (Schountz et al., 1996). T cells from Leishmania-infected LACK Vβ4 single chain TCR transgenic (LACK-TCR BV4) BALB/c and B10.D2 mice, (both I-Ad restricted), displayed low and high affinity TCRs for the LACK peptide/MHC complex, respectively. Furthermore, in vitro recall with the LACK peptide of T cells taken from BALB/c and B10.D2 transgenic mice predominately secreted IL-4 and IFNγ respectively (Malherbe et al., 2000). These results strongly suggest a linkage between TCR affinity for the LACK peptide and Th1/Th2 T cell differentiation.
In this study, we screened a large panel of LACK APLs for their stimulatory capacity in a variety of LACK-reactive T cell populations derived from the Leishmania-susceptible BALB/c background. We observed that certain amino acid substitutions, most notably at TCR contact sites, were capable of preferentially inducing IFNγ secretion by naïve Th0 and committed Th2 T cells. Furthermore, when used for immunization, APLs with substitutions that flanked the core determinant or at MHC contacts were able to generate recall T cell responses to the wild type peptide, and affect cytokine responses.
2. Materials and Methods
2.1. Animals and immunizations
BALB/c mice (4 to 6 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in our animal facility. Transgenic BALB/c mice carrying the rearranged TCR BV chain gene of the LACK-reactive LMR16.2 T cell hybridoma (LACK-BV4) were kindly provided by Dr. Nicolas Glaichenhaus (Malherbe et al., 2000) (University of Nice, France) and bred in our animal facility. Offspring were monitored for predominance of the LACK-BV4 TCR by flow cytometric analysis of peripheral blood cells using anti-CD4, anti-CD3 and anti-BV-4 monoclonal antibodies (mAb) (eBioscience, San Diego, CA). Where indicated, LACK-BV4 animals were immunized subcutaneously (s.c.) with 500 ng of LACK 161–175 wild type peptide or with LACK APLs emulsified in incomplete Freund’s adjuvant (IFA).
2.2. Antigens
The wild type LACK 161–175 peptide: SLEHPIVVSGSWDNT and 161–175 APLs with conservative and non-conservative mutations at each residue encompassing the 161–172 region were synthesized (Chiron, San Diego, CA): S161V; S161K; L162A; L162K; E163A; E163K; H164A; H164V; P165A; P165K; I166A; I166E; I166L; V167A; V167E; V167I; V167F; V168A; V168G; V168L; V168K; S169V; S169K; G170S; G170L; S171V; S171K; W172K and LACK 161–175. All peptides were diluted by weight in double distilled sterile water to 2.0 mg/ml and kept frozen in aliquots at −80 °C.
2.3. Cell lines
LACK-reactive T cell hybridomas LMR16.D2 and LMR17.1D12 (both BV4 AV8), were kindly provided by Dr. Glaichenhaus (Mougneau et al., 1995). LACK-BV4-reactive T cell lines were derived from LACK-BV4 transgenic mice by limiting dilution. Briefly, irradiated naïve BALB/c splenocytes as antigen presenting cells (APCs) and limiting dilutions of naïve LACK-BV4 transgenic splenocytes were stimulated with 10 μg/ml of wild type LACK 161–175 peptide and cultured in RPMI medium (Hyclone) supplemented with 7.5% fetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5×10−5 M 2-beta-mercaptoethanol (2-ME) (Sigma). After 24 h of initial peptide stimulation in vitro, rIL-2 (Cetus) was added to the culture to a final concentration of 10 ng/ml. LACK-reactive T cell lines were maintained every 10 to 14 days by restimulation with 10 μg/ml wild type LACK peptide, irradiated naïve BALB/c splenocytes as APCs and rIL-2 as for the initiation of the lines.
2.4. Assessment of T cell proliferative capacity
LACK-reactive T cell lines (105 per well) and APCs (irradiated BALB/c splenocytes - 4×105 per well) were plated in triplicate in 96-well microtiter plates with increasing concentrations of wild type (w/t) LACK peptide, APLs (from 0.1 ng to 10 μg/ml) or without antigen (background) in supplemented RPMI media. Proliferation was assessed on day 3 by addition of 3H-thymidine (International Chemical and Nuclear, Irvine, CA), 1 μCi per well, for the last 16 h of culture. Incorporation of label was measured by liquid scintillation counting, and the results expressed as counts per minute (CPM). One × 106 naïve splenocytes from LACK-BV4 transgenic mice were stimulated in vitro with increasing concentrations of LACK 161–175 or APLs in HL-1 serum free medium (Bio-Whittaker, Walkersville, MD) supplemented with 2×10−5 M 2-ME and the proliferative responses were analyzed on day 4 by 3H-thymidine incorporation during the last 16 h of culture. Nine to 11 days following peptide-IFA s.c. immunization, 2×105 draining lymph node cells from immunized mice were stimulated in vitro with increasing concentrations of w/t LACK 161–175 peptide or APLs in HL-1 serum free medium and the proliferative responses were analyzed on day 4 by 3H-thymidine incorporation.
2.5. Cytokine detection by ELISA spot or bioassay
IFNγ and IL-4-producing cells were enumerated in cell lines, naïve transgenic splenocytes and draining lymph node cells from peptide-IFA-immunized mice by a cellular ELISA spot assay as previously described (Gabaglia et al., 1999). Briefly, cell lines plus irradiated APCs, or LACK-BV4 naïve splenocytes or lymph node cells from immunized mice were cultured for 48 h in 96 well plates at cell densities described for the proliferation assays with medium alone (background) or 10 μg/ml of peptides before transfer to ELISAspot plates. “Millititer” HA Nitrocellulose plates (Millipore, Bedford, MA) were coated O/N at 4 °C with anti-IFNγ or anti-IL-4 antibody (eBioscience) and blocked with 10% FCS in PBS before transferring the cells. Cells were washed 3 times with medium in the antigen-stimulation plate before being transferred to IFNγ or IL-4 ELISA spot test plates. After an incubation period of 24 h at 37 °C, the ELISA spot plates were washed to remove all cells and the wells were then coated with biotin-conjugated anti-IFNγ or anti-IL-4 antibody followed by incubation with avidin-peroxidase (Vector, Burlingame, CA). Spots were developed by addition of 400 μg/ml of 3-amino-9-ethylcarbazole substrate (Sigma) and enumerated by a computerized image analysis system (Lightools Research, Encinitas, CA) using the image analyzer program NIHImage 1.61. The cell numbers, background wells with medium only deducted, were adjusted for frequency of cytokine-producing cells per 106 cells.
IL-2 secretion by peptide-stimulated LACK-reactive hybridomas was screened in a bioassay. Briefly, 2×104 LACK hybridomas and 5×104 antigen presenting cells (B cell lymphoma LB27.4) plus titrated antigen (from 0.1 ng to 10 μg/ml) were incubated at 37 °C for 48 h in supplemented RPMI media. One hundred μl of supernatant were collected and assayed with an IL-2 dependent cell line, HT-2. HT-2 (2×104) cells were cultured with supernatants from the hybridoma or to an IL-2 standard curve and incubated for 30 h. 1 μCi per well of 3H-thymidine was added for the last 16 h of culture. Incorporation of label was measured by liquid scintillation counting. The results were expressed as CPM.
2.6. Data Analysis
The following method of classification was used to categorize the ‘potency’ of each APL for all T cell populations assayed (displayed in Figure 1). The dose response curves obtained from the hybridoma, T cell lines, naïve LACK-BV4 transgenic splenocytes and lymph node cells from immunized animals to each APL were plotted in CPM and used to calculate the half maximal response (HMR). The HMR was defined as the concentration of APL needed to induce a proliferative response of equal magnitude to that of the wild type peptide at the midpoint between the y maxima and minima of the wild type peptide dose-response curve. Although these categories are arbitrary, we defined them as follows: strong agonist = at least 1 log fold lower HMR compared to the wild type LACK peptide; agonist = within a log range of the wild type peptide HMR; weak agonist = at least 1 log fold higher HMR compared to the wild type peptide; and, non-agonist = no proliferative response.
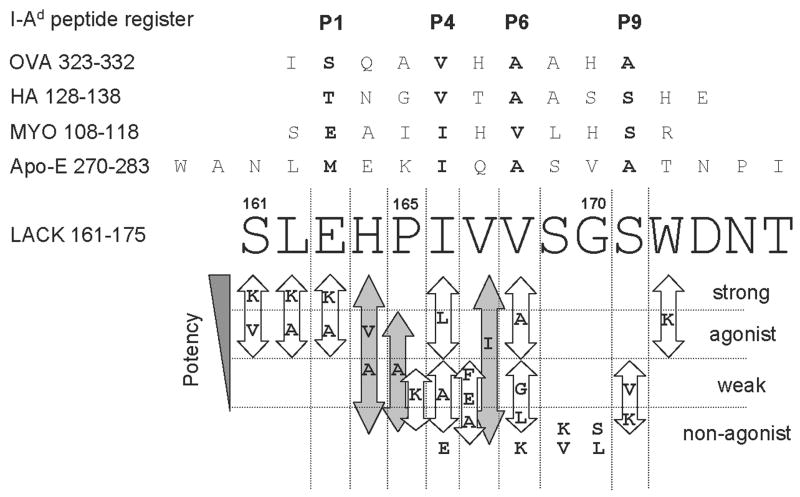
Figure 1. LACK APLs reveal the core determinant spanning residues 163–171 and summary of the proliferative responses of all tested cell systems: LMR17, “Moses”, “Bruce” and naïve LACK-BV4 transgenic T cells.
The I-Ad peptide register of OVA 323–332 (Sette et al., 1987), HA 128–138 (Sette et al., 1988), MYO 108–118 (England et al., 1995; Sette et al., 1988) and Apo-E 270–283 (Hunt et al., 1992) are referenced in comparison to LACK 161–175. The amino acids contacting P1, P4, P6 and P9 I-Ad pockets are in bold type. Log fold differences in the half maximal peptide dose response values with respect to wild type peptide distinguish strong-agonist, agonist and weak-agonist (see Material and Methods). Substitutions at the base of the figure elicited no proliferative response in any of the cell types tested. Substitutions that induced varying degrees of proliferation are grouped within arrows; grey arrows indicate substitutions that ranged from non-agonist to strong agonist (i.e. ≤100 fold differences in potency) in the four T cell systems tested.
3. Results
3.1. A deduced LACK 161–173/I-Ad register
Our preliminary data testing truncated versions of the 156–173 LACK peptide (data not shown) indicated that amino acids 161 at the amino terminus and 171 at the carboxy terminus were crucial for maintaining recognition by the LACK-reactive hybridomas, LMR16.D2 and LMR17.1D12 (Mougneau et al., 1995). Therefore, we introduced single conservative and non-conservative substitutions modifying each amino acid along the 161–172 determinant within the 161–175 LACK peptide, and tested their stimulatory capacity in comparison to the wild type peptide (listed in the 2.2 section of Material and Methods). The following T cell systems were screened: i) a LACK 161–175/I-Ad-specific hybridoma, LMR 17 (BV4AV8 TCR), ii) splenocytes from the naive LACK-BV4 single chain TCR transgenic mice (LACK-BV4 TCR derived from the LMR 17 hybrid (Malherbe et al., 2000); and, iii) two LACK-specific T cell lines generated from the LACK-BV4 transgenic mice (see Materials and Methods). Hence, our T cell-readouts always use the LACK-BV4 TCR, but the lines and the naïve transgenic splenocytes may display variable alpha chains. Therefore, any similarities in proliferative responses across all T cell populations to a particular APL might either be the result of similar binding properties to the MHC or parallel peptide influences on the BV4 TCR. On the other hand, distinct T cell responses to an APL could reflect unique TCR specificities for peptide residue alterations at Vα-TCR contact sites.
In Figure 1, we compiled the proliferative results of all BV4 LACK-reactive T cell systems to each APL and classified the response as either strong, agonist, weak or non-agonist in comparison to the wild type (w/t) LACK peptide (please refer to Material and Methods, 2.6 section). Two observations were apparent: 1) non-conservative substitutions at positions 161, 162 and 172, were stimulatory for all T cells tested; and, 2) substitutions at 164/165 and 167 induced the largest range of effects from strong to non-agonist. It is known that T cell clones readily accept non-conservative substitutions at flanking regions, and rarely accept substitutions at TCR contacts (Huseby et al., 2005; Reay et al., 1994). This information led us to propose a likely LACK 161–175/I-Ad peptide register which places the LACK peptide amino acid 163-E in MHC pocket 1, 166-I in pocket 4, 168-V in pocket 6, and 171-S in pocket 9 (Figure 1). Thus, the stimulatory effects obtained with all non-conservative substitutions at positions 161, 162 and 172, are predicted to lie outside the core binding pockets. Moreover, the varying effects with substitutions at 164/165 and 167 (greater than 100 fold differences in stimulation) are predicted to be P2/P3 and P5 TCRα contacts, suggesting variability of TCR alpha chains amongst the cellular readouts. Indeed, structural and functional analysis of the TCR/pMHC interface shows that the P2/P3 and P5 peptide residues are preferentially contacted by the Vα CDR3, while the P5 and P7/P8 residues are predominately contacted by the Vβ CDR3 (Dai et al., 2008; Hennecke et al., 2000; Reinherz et al., 1999; Rudolph et al., 2006; Sant’Angelo et al., 1996).
This register is also consistent with other observations made by several groups: Locksley’s analysis of the LACK 164H TCR contact residue (Pingel et al., 1999), Grey and colleagues’ studies on the proposed 6 amino acid-core motif consisting of small and frequently hydrophobic residues from pocket 4 to pocket 9 for I-Ad (Sette et al., 1989; Sette et al., 1988; Sette et al., 1987), and Wilson’s crystal structure of the I-Ad molecule in complex with the well-studied HA or OVA peptide ligands (Scott et al., 1998). Indeed, the P4-P9 I-Ad peptide binding motif is retained with our LACK register (IVVSGS) and the bulky tryptophan residue is positioned at P-10, outside the shallow and narrow binding groove of I-Ad. Furthermore, the putative LACK peptide I-Ad pocket residues are in agreement with those of MYO 108–118 (England et al., 1995; Sette et al., 1988). Therefore, the most parsimonious register is depicted in Figure 1.
3.2. T cell recognition of LACK analogs
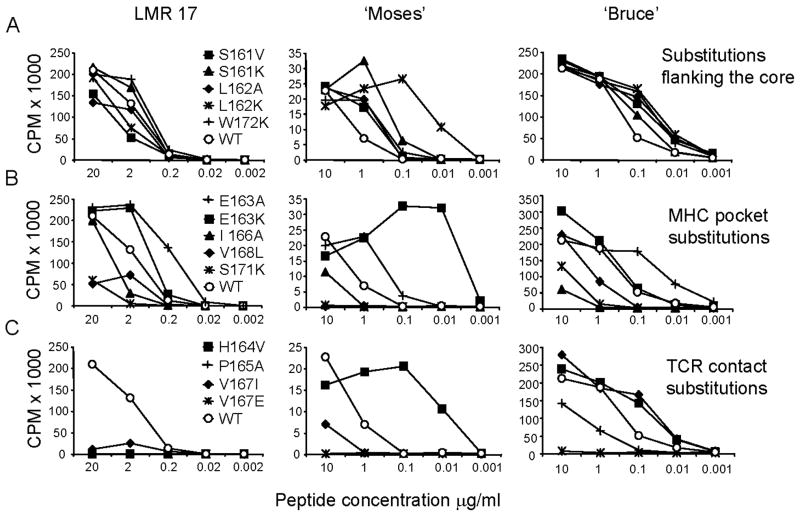
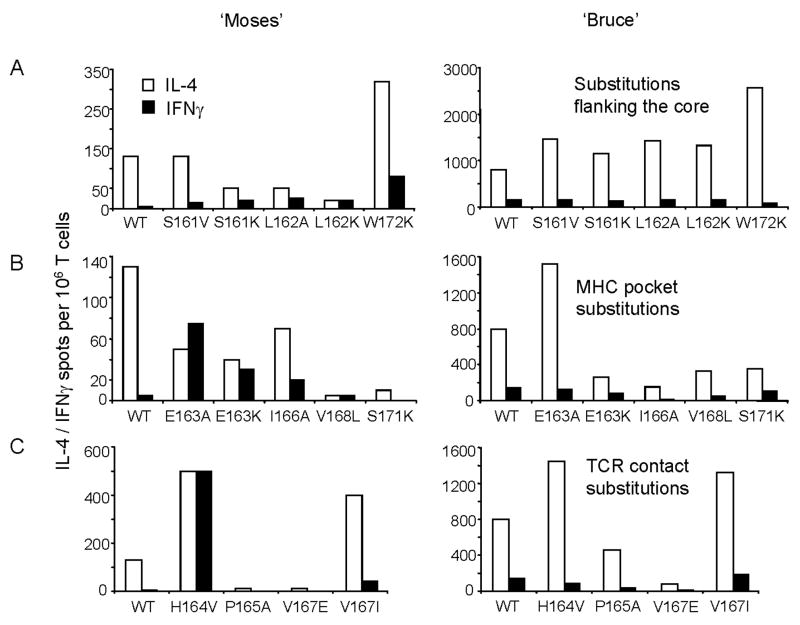
Two T cell lines, “Bruce” and “Moses”, were generated by stimulation with LACK p161–175 under limiting dilution conditions from LACK-BV4 transgenic mice, and the effects of APLs were examined. As demonstrated in Figure 2-A (middle and right panels), most substitutions in residues 161, 162 and 172 (flanking the proposed register, filled symbols), enhanced stimulation in comparison to the wild type peptide (empty circles) across most peptide concentrations tested for LMR17 and “Moses”, while for “Bruce”, enhancement over wild type stimulation was observed only at 0.1 μg/ml. However, for LMR17 (Fig. 2A, left panel) as well as for naïve LACK-BV4 splenocytes, (shown in Figure 4-A, left panel), flanking APLs behaved mainly as agonists in relation to the w/t LACK peptide. Thus, the fine specificity of a given TCR dictates the relative contribution of flanking residues to stabilizing the TCR/pMHC interface (Moudgil et al., 1998; Sercarz and Maverakis, 2003).
Figure 2. T cell proliferative responses to different categories of LACK APLs.
A. APLs with alterations flanking the core region (aa 161, 162 and 172); B. APLs with changes within potential MHC contacts (aa 163, 166, 168 and 171); and, C. APLs with residue substitutions at putative TCR contact sites (aa 164, 167, 169 and 170). Representative peptide dose (μg/ml) response curves of a LACK-reactive hybridoma, LMR 17 (left column), and T cell lines “Moses” and “Bruce” (middle and right columns respectively) to the wild type LACK 161–175 peptide (empty circles: ○), or various analogs (filled symbols) tested for all panels. IL-2 secretion by LMR 17 was monitored by the proliferation of HT-2 cells. Cellular proliferation was monitored by 3H-thymidine incorporation 3 days post-stimulation with peptides and antigen presenting cells (see Material and Methods). Cells were harvested and radiation was detected with scintillation fluid and plotted as counts per minute (CPM). One representative experiment is shown of the 3 performed.
Figure 4. LACK APLs can modulate the IFNγ and IL-4 cytokine response of Th0 naïve LACK-BV4 transgenic T cells, with heightened sensitivity at TCR contact sites.
A. APLs with alterations flanking the core region (aa 161, 162 and 172); B. APLs with changes within potential MHC contacts (aa 163, 166, 168 and 171); and, C. APLs with residue substitutions at putative TCR contact sites (aa 164, 167, 169 and 170). Proliferative responses of naïve LACK-BV4 TCR transgenic splenocytes to the w/t LACK 161–175 peptide (empty circles: ○), or various analogs (filled symbols) for all left panels. The frequency of cytokine producing cells among naïve LACK-BV4 transgenic cells in response to w/t LACK or analogs by ELISA spot (all right panels). Bars in white represent IL-4 spots per 106 splenocytes, bars in black represent IFNγ spots per 106 splenocytes (the standard deviation of duplicate wells was less than 10%.). One representative of 3 experiments is depicted.
In Figure 2-B, the stimulatory effects of APLs possessing substitutions in MHC-pockets (positions 163, 166, 168 and 171) are displayed. I-Ad has only one large pocket, P1, which accommodates larger amino acids or charged residues that only partially fill it, with no particular residue preference (Scott et al., 1998). However, we found that substituting glutamate for alanine or lysine (E163A/K) within MHC pocket 1 was not only tolerated but served to enhance stimulation in all T cell systems (Figure 2B, 4B). Other relevant features of I-Ad include a β bulge on the floor of the peptide-binding groove, and shallow P4 and P9 pockets. Thus, peptides with small, uncharged residues are selectively chosen for I-Ad binding to avoid steric clashes in the narrow P4-P9 center of the binding groove (Scott et al., 1998; Sette et al., 1987). Indeed, our LACK lysine analogs (P165K, V168K and S169K) are mostly weak or non-agonists (Figure 1, 4), and introduction of large residues within the P4 (I166E) or P9 (S171K) pockets were not accepted in all T cell systems tested (Figure 1, 2B). In contrast, there were consistently enhanced proliferative responses to the conservative alterations I166L and V168A (Figure 1, summary). These conservative substitutions take place in the shallow P4 and P6 I-Ad pockets, and can explain their minimal effects on TCR stimulation and MHC binding.
In Figure 2-C, we demonstrate the marked effects of peptide amino acid changes at TCR contact sites. Unlike those APLs with substitutions at flanking regions or within MHC pockets, which exhibited similar stimulatory capacities among the different T cell systems (never differing more than 10 fold in the half maximal response when normalized to the w/t peptide, Figure 1), residue alterations at Vα-TCR contact sites elicited the greatest variation in stimulatory capacity, ranging from strong to agonist or non-agonist potencies. For example, at position 2, H164V was a strong agonist for the “Moses” cell line and, to a lesser extent, for “Bruce” (enhancement over WT was only observed at 0.1μg/ml), but completely failed to stimulate the clone LMR17 (Figure 2C). Similarly, at position 5, V167I behaved as a strong agonist for “Bruce”, but a weak agonist for “Moses” and LMR17 (Figure 2C), indicating that unique TCRα chains and/or CDR3αs are utilized by each of these LACK-reactive T cell lines. It was also interesting to observe that most substitutions at residue 167 were weakly or not tolerated by all T cell systems, and S169V and G170S or G170L APLs failed to induce a proliferative response (summarized in Fig. 1). The damaging effect of these substitutions are likely due to peptide influences on the same BV-TCR, which is known to contact the P5 and the C-terminal P7/8 peptide residues (Dai et al., 2008; Hennecke et al., 2000; Reinherz et al., 1999; Rudolph et al., 2006; Sant’Angelo et al., 1996).
In summary, a general trend emerged for the tested APLs. Lysines in flanking regions usually enhanced T cell activation while APLs with charged residues such as K or E in positions 3–9 were either weak or non-agonists. We also found that MHC-peptide binding pockets tolerated APLs with several semi-conservative and conservative substitutions, while putative TCR contacts were less permissive, as expected.
3.3. Cytokine patterns in Th2-committed T cell lines can be altered by APLs
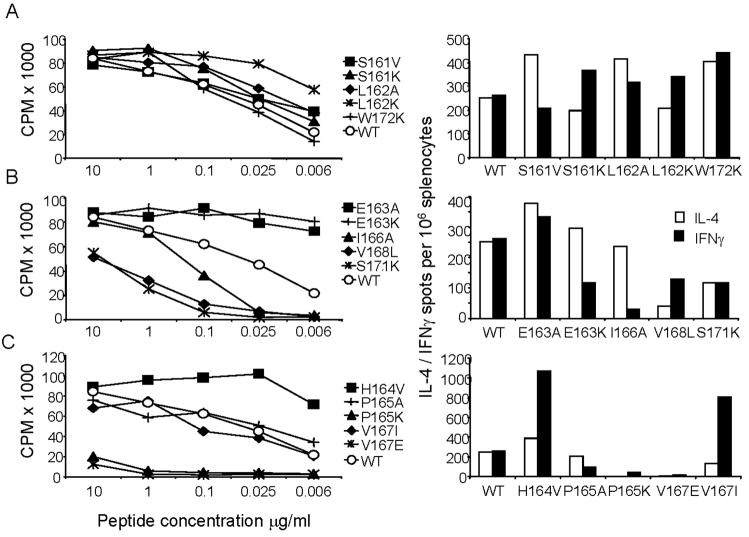
Upon in vitro stimulation with the LACK 161–175 w/t peptide, both T cell lines displayed a Th2 phenotype, with the frequency of IL-4-producing cells outnumbering IFNγ-producing cells by 8 to 13 fold. In general, the T cell line “Bruce” displayed a higher sensitivity to peptide stimulation (Figure 3) and a higher frequency of cytokine-producing cells (10 times more than “Moses”). For the “Bruce” line, the tested APLs could boost IL-4 production, but none was capable of enhancing IFNγ production, regardless of the level of proliferative activity it induced (shown in Figure 3). APLs with flanking residue substitutions (Fig 3A, right panel) and Vα-TCR substitutions H164V and V167I (Fig 3C, right panel) greatly expanded IL-4-producing cells, while APLs in Vβ-contact regions or in MHC pockets, decreased IL-4 production. In contrast, the “Moses” T cell line displayed 10 times fewer cytokine-producing cells in comparison to “Bruce”, but certain APLs led to a clear phenotypic change from Th2 to Th0 or Th1 (Figure 3, left panels). Strong agonist APLs with changes at the amino terminal residues 161 to 164 induced a moderate increase in IFNγ and downregulation of IL-4, while the carboxy-terminal flanking APL W172K, enhanced both cytokines. Interestingly, changing the Vα-TCR contact, H164V (Figure 3C, left), induced a Th0 phenotype with the frequency of IL-4-producing cells increasing 4 times, and IFNγ producing cells expanding 100-fold! Another conservative substitution for the putative Vα contact in position 5, V167I, enhanced both cytokines but maintained a Th2 profile. For substitutions in MHC pockets 1 and 4 (Figure 3B, left), APLs generally increased IFNγ production and down-modulated IL-4, while substitutions at pockets 6 (V168L) and 9 (S171K) were less tolerant, as evidenced by lowered cytokine production. Clearly, altering the peptide ligand at different positions has unique functional consequences and indicates that potential stabilization of the MHC-peptide-TCR complex by lysines within flanking regions or alanine/lysine substitutions at MHC pocket 1 favors IFNγ production, while APLs with certain changes at putative TCR V alpha contacts (positions 164 and 167) can provoke the greatest amount of cytokine secretion.
Figure 3. Residue substitutions located at the flanks or within the core determinant of LACK can promote IFNγ secretion of a Th2 LACK-reactive T cell line.
A. APLs with alterations flanking the core region (aa 161, 162 and 172); B. APLs with changes within potential MHC contacts (aa 163, 166, 168 and 171); and, C. APLs with residue substitutions at putative TCR contact sites (aa 164, 167, 169 and 170). Th2 LACK-specific T cell lines generated from LACK-BV4 TCR transgenic mice, “Moses” (left) and “Bruce” (right), were monitored for the frequency of cytokine secreting cells per million cells: IL-4 (white bars) and IFNγ (black bars) in response to WT peptide (first set of bars in each figure) or LACK APLs by ELISA spot (see Material and Methods). This experiment was performed 3 or more times; the standard deviation of duplicate wells was less than 10%.
3.4. The effect of LACK APLs on naïve BV4-LACK transgenic T cells
T cells from BV4-LACK single chain TCR transgenic BALB/c mice predominately use a restricted LACK-Vβ4 TCR (60–90% of total CD4 T cells) and furthermore possess an endogenous variable repertoire of V alphas (Malherbe et al., 2000). In vitro stimulation with APLs provides an opportunity to study the priming effects of analogs on naïve T cells, as well as their proliferation and cytokine profiles in comparison to the LACK w/t peptide. Naïve splenocytes expanded vigorously when stimulated in vitro with the LACK 161–175 w/t, with stimulation indexes of 8 at the lowest tested dilution (6 ng/ml). Similar frequencies of IFNγ and IL-4 producing cells were detected in response to the WT peptide, representing a Th0 phenotype (Figure 4, right panels). Most substitutions at amino terminal residues 161–164 (spanning flanking, MHC and TCR contacts) resulted in stronger proliferative responses (Figure 4, left panels). Flanking residues with lysine substitutions (S161K, L162K and W172K) considerably enhanced the frequency of IFNγ-producing cells (Figure 4-A, right panels). Substitutions within MHC pocket 1, E163A or E163K induced strong proliferation, but elicited distinct cytokine profiles: E163K reduced IFNγ secretion, but E163A enhanced both IL-4 and IFNγ production in comparison to the w/t peptide. The same preference for hydrophobic residues in pockets 4 and 6 and the damaging lysine effect in pocket 9 that was observed with the T cell lines (displayed in Figures 2B and 3B), was also observed with the naïve Vβ4- transgenic T cells (Figure 4B). For APLs with changes at putative TCR contact sites (Figure 4C), it was interesting to observe that H164V doubled IL-4 secretion and quadrupled IFNγ production, achieving an IFNγ/IL-4 ratio of 2.5! P165K was a weak agonist, but a Th1 inducer, while P165A was an agonist and a Th2 inducer. Carboxy-terminal TCR contacts at positions S169 and G170 did not tolerate any of the APLs tested and the putative TCR P5 contact APL V167I was the only stimulatory APL among many tested (alanine, glutamate and phenylalanine – all of which gave weak responses). Importantly, V167I induced a 3.5-fold increase of IFNγ producing cells and lowered IL-4 production by ~50%, leading to a Th1/Th2 ratio of 8.5! Due to the percentage of BV4 LACK-reactive CD4 T cells in these transgenic mice (50 to 80%) combined with all possible V alphas, a greater flexibility in the T cell response to our APL library was expected. Nonetheless, many of the APLs had similar immune deviation characteristics across our T cell systems. Thus, the effect of priming with these APLs in animals with a semi-restricted LACK-BV4 repertoire was studied in an attempt to understand general principles for the use of APLs as immune modulatory agents.
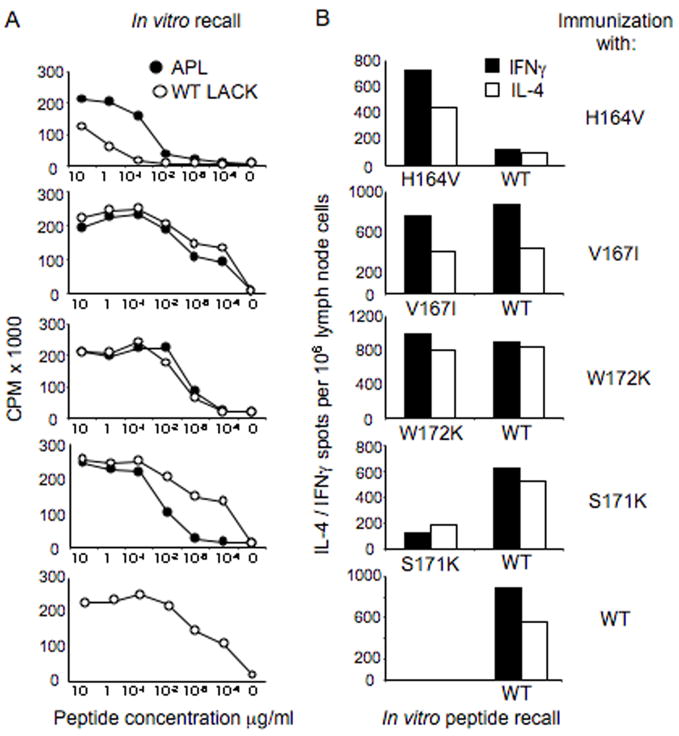
3.5. APLs as vaccine candidates: priming with APLs and the secondary recall response to the wild type LACK peptide
To investigate the effects of APL priming, we immunized LACK-BV4 transgenic mice with selected peptide analogs with substitutions that flanked or spanned the immunogenic core, the majority of which had the ability to induce IFNγ production in the previous T cell systems studied. Mice immunized with the wild type LACK peptide served as control. We used low amounts of peptide (500 ng of peptide/mouse) emulsified in IFA as an adjuvant, thus avoiding the induction of polarizing cytokine environments that are typically induced by most immunization regimens (i.e. CFA or alum). In Figure 5, the proliferative responses (left panels) and the frequency of cytokine-producing cells by ELISA spot (right panels) of draining lymph node cells from animals immunized subcutaneously with LACK analogs (or the wild type LACK, bottom panels) are shown; the responses were recalled in vitro with either the immunizing analog or the w/t LACK peptide. LACK wild type peptide immunization elicited strong proliferative and cytokine recall responses to the LACK peptide in vitro, with a slightly higher number of IFNγ secreting cells was observed (Th1/Th2 ratio ~1.5). It was interesting to observe that immunization with analog H164V, a putative TCRα-contact and a significant Th1 inducer in the previous in vitro assays, expanded a repertoire of T cells which recognized the wild type LACK peptide as a weak agonist in both proliferation and cytokine production assays. In contrast, immunization with 2 APLs that displayed agonistic capacities for naïve transgenic LACK-BV4 T cells, V167I (a putative beta chain contact) and W172K (a flanking residue, which increased IFNγ in 2 systems tested) induced a repertoire of cells which recognized both the wild type LACK peptide and the immunizing analog, with similar proliferative response and Th1/Th2 phenotype (Figure 5 middle panels) and expanded an immune response similar to that given by immunization with the wild type LACK peptide- bottom panels). Interestingly, immunization with the weak agonist S171K (with an alteration in MHC pocket 9), gave rise to T cells that preferentially recognized the wild type peptide, as evidenced by both greater cytokine and proliferative recall responses when compared to the immunizing APL. In fact, the recall response was very sensitive to w/t LACK peptide concentrations (1 ~ 0.1 ng), with a slight Th1 bias (10% greater IFNγ secreting cells over IL-4 producers).
Figure 5. Immunization with LACK APLs in LACK-BV4 transgenic mice alters the recall response to the wild type LACK peptide.
A. Proliferative response of draining lymph node cells from LACK-BV4 TCR transgenic mice immunized with either peptide analogs or with LACK wild type peptide and recalled in vitro against both the immunizing (black symbols) and the wild type peptide (white symbols). B. Frequency of IFNγ (black bars) and IL-4 producing cells (white bars) from immunized mice following in vitro recall with w/t LACK peptide or immunizing analogs; the standard deviation of duplicate wells was less than 10%. One representative experiment of 2 is displayed.
In summary, APLs that conservatively retained the immunogenic core (i.e. the TCR contact sites: P2/3 P5 P7/8) were able to prime for a T cell response reactive to the wild type peptide, but a non-conservative substitution at a TCR contact site (H164V) generated a reduced response to the wild type peptide. Thus, if the desired outcome is to dampen or antagonize the T cell response to a particular peptide epitope, then screening APLs with altered TCR contacts is recommended. However, if immune deviation is desired, a screen focusing on peptide analogs with substitutions at flanking regions or MHC contact sites might better be attempted when considering APLs for vaccine use.
4. Discussion and conclusion
Immunotherapy by altered peptide ligands for a variety of disease applications autoimmunity, allergy and cancers, have been extensively studied in the past 15 years in in vitro studies, in vivo murine models and even tested in clinical trials to induce protective phenotypes using peptide analogs. The potentially therapeutic approaches have mainly focused on the antagonistic capacity of the APLs, especially for aberrant responses mediated by CD4 or CD8 T cells in autoimmunity, allergy or transplantation (Borbulevych et al., 2005; Douat-Casassus et al., 2007; Kinnunen et al., 2007; Myers et al., 2007). Indeed, prior studies in the LACK system using analogs were focused on antagonistic substitutions of the TCR contact H164 residue. However, although antagonistic peptides can be generated on a clone by clone basis (Pingel et al., 1999), peptide antagonism often fails against a diverse TCR repertoire, as suggested by the inability of H164N mutant parasites to prevent disease in BALB/c mice (Kelly and Locksley, 2004). Given, the apparent LACK-restricted repertoire of the ensuing T cell response to Leishmania, we decided to revisit this issue and dissect the fine specificity of the T cell response against the immunodominant LACK epitope, in the hope of defining APLs with the ability to skew Th2 responses in a Th1 direction.
To our knowledge, this is the first study that narrows the core of the immune-dominant region of LACK to residues 163–171, and demonstrates the role of core and flanking residues in establishing the nature of the Th1/Th2 responses. The assumptions we made in defining the core (163–171) played an important part in assigning residues to both TCR and MHC pockets. Another important contribution of this study is the determination of the Vα-TCR peptide contacts and their ability to influence T cell phenotype and reactivity, with changes at position 2 (residue 164) and 5 (residue 167), (Figures 2, 3 and 4). We also demonstrate the ability to either initiate a Th1 or a Th2 cytokine response in naïve T cells through the use of specific APLs (Figure 4). Likewise, APLs could deviate a Th2 T cell line toward a Th1 phenotype, as demonstrated with E163A or H164V in Figure 3B. According to a publication by the Glaichenhaus group, L. major infection in LACK BV4 single chain transgenic mice of different backgrounds (BALB/c or B10.D2) expanded T cells with various affinities to LACK/IAd, which utilized different AV TCR gene segments (Malherbe et al., 2000). In our study, we have not investigated this possibility when comparing Moses and Bruce, nor when following the T cell response after immunization with different agonists in the LACK BV4 transgenic mice (Figure 5). Nonetheless, in accordance with Malherbe’s data, our results indicate that differences in the TCR alpha chain, whether encoded by unique AV genes and/or CDR3 sequences, do play a role in the TCR recognition of LACK, as evidenced by the variety of recall responses in our four T cells systems to the TCR-contact LACK analogs (Figures 1–4).
We also outline guidelines for the use of APLs as vaccine candidates for murine leishmaniasis as well as other models. In the aforementioned study by Malherbe, evidence suggests that the Th2 outcome in susceptible BALB/c mice is tightly linked to the selection of T cell clones possessing TCRs with low affinity for the LACK peptide (Malherbe et al., 2000; Malherbe et al., 2004). We reason that the easiest way to elicit a high affinity response to the wild type peptide is through residue alterations within MHC pockets or at positions that flank the immunogenic core.
Indeed, in our system it was common to observe enhancement of the proliferative responses and cytokine production with substitutions that flanked the core (Figures 3 and 4), revealing APL candidates with potential for immune deviation to the wild type peptide. This aspect has been discussed in previous work by others, with a few interesting results from Vignali’s group describing the most immunogenic peptide-flanking residues (PFRs) as the ones that can form salt bridges or hydrogen bonds instead of favoring hydrophobic interactions (Arnold et al., 2002). Contrary to MHC class I molecules that bind fixed-length peptides, the MHC class II has the property of allowing longer peptides to bind; PFRs are outside the MHC anchor groove but within the MHC binding groove, which is open at both ends. The PFR contribution, especially at position −1, is very important for peptide stability (Nelson et al., 1992). Using the model antigen, hen egg lysozyme (HEL), Vignali’s group has also described the immunogenic role of the carboxy-termini PFR of the codominant epitope (HEL)52–61 for H-2Ak. The majority of the T cell repertoire that expanded upon immunization with (HEL)48–63 required P10 and P11 (62W and 63W) for strong responses; thus, upon immunization with the longer (48–63) or the core peptide (52–61), recalls to HEL were higher after priming with the longer peptide 48–63, containing PFRs (Carson et al., 1997). Unquestionably, flanking residues can play an important role in determining the Th1/Th2 ratio in the response to the primary peptide determinant; however, predicting which substitutions will yield such a change is poorly understood, and will require further experimental validation. Here we describe that lysine residues outside the core are important as modulators to favor IFNγ responses from naïve BV4-LACK transgenic T cells (Figure 4). Immune deviation can also be achieved by modulating MHC contact peptide residues. This has been shown in several systems, where generating peptides with higher or lower affinity for the MHC enhanced/decreased or modified immune responses caused by the natural ligand (Kumar et al., 1995; Pfeiffer et al., 1995; Murray, 1998; Schountz et al., 1996; Borbulevych et al., 2005; Anderson et al., 2006; Tang et al., 2007).
In conclusion, we have defined the immunodominant LACK peptide core and binding register for I-Ad and a few APLs with differential agonistic effectiveness for LACK-reactive T cells (summarized in Figure 1). Moreover, we learned that certain residue substitutions in the APL are capable of modifying the phenotype of these T cells with potential application in the disease-inducing Th2 profile observed in BALB/c mice infected with Leishmania major. One of the most notable findings is that single residue substitutions at MHC or TCR contacts, even at areas flanking the determinant core, can have a significant impact on the T cell cytokine response ratio. However, priming with APLs that expand Th1 cells ex-vivo, does not ensure a polyspecific (or cross-reactive) recall response to the wild type peptide present in the infective parasite. Immunization with analogs such as S171K or V167I, that conservatively retain most of the immunogenic core, could be advantageous in expanding a repertoire of T cells with higher affinity to the w/t peptide, with the desired endpoint of a Th1 response. In future work, we plan to employ tests using APLs with other flanking residues or for a few selected core residues, as well as to combine favorable alterations at different residues to examine the effects of APL immunization upon recall with the w/t LACK peptide and in the in vivo model of murine leishmaniasis.
Acknowledgments
This work was supported by an NIH grant R0-1-42396 to E. Sercarz. We would like to thank Dr. Nicolas Glaichenhaus, Institut National de la Santé et de la Recherche Médicale - Nice, France, for the hybridomas and the BV4 single chain TCR-LACK transgenic mice. We would like to thank Dr. Yang Dai for careful reading and criticizing the mauscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RP, van Heel DA, Tye-Din JA, Jewell DP, Hill AV. Antagonists and non-toxic variants of the dominant wheat gliadin T cell epitope in coeliac disease. Gut. 2006;55:485–91. doi: 10.1136/gut.2005.064550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold PY, La Gruta NL, Miller T, Vignali KM, Adams PS, Woodland DL, Vignali DA. The majority of immunogenic epitopes generate CD4+ T cells that are dependent on MHC class II-bound peptide-flanking residues. J Immunol. 2002;169:739–49. doi: 10.4049/jimmunol.169.2.739. [DOI] [PubMed] [Google Scholar]
- Borbulevych OY, Baxter TK, Yu Z, Restifo NP, Baker BM. Increased immunogenicity of an anchor-modified tumor-associated antigen is due to the enhanced stability of the peptide/MHC complex: implications for vaccine design. J Immunol. 2005;174:4812–20. doi: 10.4049/jimmunol.174.8.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RT, Vignali KM, Woodland DL, Vignali DA. T cell receptor recognition of MHC class II-bound peptide flanking residues enhances immunogenicity and results in altered TCR V region usage. Immunity. 1997;7:387–99. doi: 10.1016/s1074-7613(00)80360-x. [DOI] [PubMed] [Google Scholar]
- Dai S, Huseby ES, Rubtsova K, Scott-Browne J, Crawford F, Macdonald WA, Marrack P, Kappler JW. Crossreactive T Cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity. 2008;28:324–34. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douat-Casassus C, Marchand-Geneste N, Diez E, Gervois N, Jotereau F, Quideau S. Synthetic anticancer vaccine candidates: rational design of antigenic peptide mimetics that activate tumor-specific T-cells. J Med Chem. 2007;50:1598–609. doi: 10.1021/jm0613368. [DOI] [PubMed] [Google Scholar]
- England RD, Kullberg MC, Cornette JL, Berzofsky JA. Molecular analysis of a heteroclitic T cell response to the immunodominant epitope of sperm whale myoglobin. Implications for peptide partial agonists. J Immunol. 1995;155:4295–306. [PubMed] [Google Scholar]
- Feinerman O, Germain RN, Altan-Bonnet G. Quantitative challenges in understanding ligand discrimination by alphabeta T cells. Mol Immunol. 2008;45:619–31. doi: 10.1016/j.molimm.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaglia CR, Pedersen B, Hitt M, Burdin N, Sercarz EE, Graham FL, Gauldie J, Braciak TA. A single intramuscular injection with an adenovirus-expressing IL-12 protects BALB/c mice against Leishmania major infection, while treatment with an IL-4-expressing vector increases disease susceptibility in B10.D2 mice. J Immunol. 1999;162:753–60. [PubMed] [Google Scholar]
- Hennecke J, Carfi A, Wiley DC. Structure of a covalently stabilized complex of a human alphabeta T-cell receptor, influenza HA peptide and MHC class II molecule, HLA-DR1. Embo J. 2000;19:5611–24. doi: 10.1093/emboj/19.21.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, Arendse B, Schwegmann A, Myburgh E, Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. J Immunol. 2006;176:1115–21. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- Hunt DF, Michel H, Dickinson TA, Shabanowitz J, Cox AL, Sakaguchi K, Appella E, Grey HM, Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992;256:1817–20. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–60. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–3. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Locksley RM. The Leishmania major LACK antigen with an immunodominant epitope at amino acids 156–173 is not required for early Th2 development in BALB/c mice. Infect Immun. 2004;72:6924–31. doi: 10.1128/IAI.72.12.6924-6931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen T, Jutila K, Kwok WW, Rytkonen-Nissinen M, Immonen A, Saarelainen S, Narvanen A, Taivainen A, Virtanen T. Potential of an altered peptide ligand of lipocalin allergen Bos d 2 for peptide immunotherapy. J Allergy Clin Immunol. 2007;119:965–72. doi: 10.1016/j.jaci.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon gamma by T cells. Proc Natl Acad Sci U S A. 1995;92:9510–4. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe L, Filippi C, Julia V, Foucras G, Moro M, Appel H, Wucherpfennig K, Guery JC, Glaichenhaus N. Selective activation and expansion of high-affinity CD4+ T cells in resistant mice upon infection with Leishmania major. Immunity. 2000;13:771–82. doi: 10.1016/s1074-7613(00)00075-3. [DOI] [PubMed] [Google Scholar]
- Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal Selection of Helper T cells is Determined by an Affinity Threshold with No Further Skewing of TCR Binding Properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Moudgil KD, Sercarz EE, Grewal IS. Modulation of the immunogenicity of antigenic determinants by their flanking residues. Immunol Today. 1998;19:217–20. doi: 10.1016/s0167-5699(97)01233-4. [DOI] [PubMed] [Google Scholar]
- Mougneau E, Altare F, Wakil AE, Zheng S, Coppola T, Wang ZE, Waldmann R, Locksley RM, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–6. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- Murray JS. How the MHC selects Th1/Th2 immunity. Immunol Today. 1998;19:157–63. doi: 10.1016/s0167-5699(97)01237-1. [DOI] [PubMed] [Google Scholar]
- Murray JS, Ferrandis-Edwards D, Wolfe CJ, Schountz T. Major histocompatibility complex regulation of T helper functions mapped to a peptide C terminus that controls ligand density. Eur J Immunol. 1994;24:2337–44. doi: 10.1002/eji.1830241012. [DOI] [PubMed] [Google Scholar]
- Myers LK, Tang B, Rosioniec EF, Stuart JM, Kang AH. An altered peptide ligand of type II collagen suppresses autoimmune arthritis. Crit Rev Immunol. 2007;27:345–56. doi: 10.1615/critrevimmunol.v27.i4.40. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Roof RW, McCourt DW, Unanue ER. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci U S A. 1992;89:7380–3. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181:1569–74. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel S, Launois P, Fowell DJ, Turck CW, Southwood S, Sette A, Glaichenhaus N, Louis JA, Locksley RM. Altered ligands reveal limited plasticity in the T cell response to a pathogenic epitope. J Exp Med. 1999;189:1111–20. doi: 10.1084/jem.189.7.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay PA, Kantor RM, Davis MM. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93–103) J Immunol. 1994;152:3946–57. [PubMed] [Google Scholar]
- Reiner SL, Wang ZE, Hatam F, Scott P, Locksley RM. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993;259:1457–60. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- Reinherz EL, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey RE, Smolyar A, Hare B, Zhang R, Joachimiak A, Chang HC, Wagner G, Wang J. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science. 1999;286:1913–21. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Sant’Angelo DB, Waterbury G, Preston-Hurlburt P, Yoon ST, Medzhitov R, Hong SC, Janeway CA., Jr The specificity and orientation of a TCR to its peptide-MHC class II ligands. Immunity. 1996;4:367–76. doi: 10.1016/s1074-7613(00)80250-2. [DOI] [PubMed] [Google Scholar]
- Schountz T, Kasselman JP, Martinson FA, Brown L, Murray JS. MHC genotype controls the capacity of ligand density to switch T helper (Th)-1/Th-2 priming in vivo. J Immunol. 1996;157:3893–901. [PubMed] [Google Scholar]
- Scott CA, Peterson PA, Teyton L, Wilson IA. Crystal structures of two I-Ad-peptide complexes reveal that high affinity can be achieved without large anchor residues. Immunity. 1998;8:319–29. doi: 10.1016/s1074-7613(00)80537-3. [DOI] [PubMed] [Google Scholar]
- Sercarz EE, Maverakis E. MHC-guided processing: binding of large antigen fragments. Nature Reviews Immunology. 2003;3:621–29. doi: 10.1038/nri1149. [DOI] [PubMed] [Google Scholar]
- Sette A, Adorini L, Appella E, Colon SM, Miles C, Tanaka S, Ehrhardt C, Doria G, Nagy ZA, Buus S, et al. Structural requirements for the interaction between peptide antigens and I-Ed molecules. J Immunol. 1989;143:3289–94. [PubMed] [Google Scholar]
- Sette A, Buus S, Colon S, Miles C, Grey HM. I-Ad-binding peptides derived from unrelated protein antigens share a common structural motif. J Immunol. 1988;141:45–8. [PubMed] [Google Scholar]
- Sette A, Buus S, Colon S, Smith JA, Miles C, Grey HM. Structural characteristics of an antigen required for its interaction with Ia and recognition by T cells. Nature. 1987;328:395–9. doi: 10.1038/328395a0. [DOI] [PubMed] [Google Scholar]
- Tang Y, Lin Z, Ni B, Wei J, Han J, Wang H, Wu Y. An altered peptide ligand for naive cytotoxic T lymphocyte epitope of TRP-2(180–188) enhanced immunogenicity. Cancer Immunol Immunother. 2007;56:319–29. doi: 10.1007/s00262-006-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Allen PM, Celada F, Cohen IR, De Boer R, Garcia KC, Goldstein B, Greenspan R, Hafler D, Hodgkin P, Huseby ES, Krakauer DC, Nemazee D, Perelson AS, Pinilla C, Strong RK, Sercarz EE. Polyspecificity of T cell and B cell receptor recognition. Semin Immunol. 2007;19:216–24. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]