Abstract
The antiarrhythmic drug amiodarone has microbicidal activity against fungi, bacteria and protozoa. In Saccharomyces cerevisiae, amiodarone triggers an immediate burst of cytosolic Ca2+, followed by cell death markers. Ca2+ transients are a common response to many forms of environmental insults and toxic compounds, including osmotic and pH shock, endoplasmic reticulum stress, and high levels of mating pheromone. Downstream signaling events involving calmodulin, calcineurin and the transcription factor Crzl are critical in mediating cell survival in response to stress. In this study we asked whether amiodarone induced Ca2+ influx was beneficial, toxic or a bystander effect unrelated to the fungicidal effect of the drug. We show that downregulation of Ca2+ channel activity in stationary phase cells correlates with increased resistance to amiodarone. In actively growing cells, extracellular Ca2+ modulated the size and shape of the Ca2+ transient and directly influenced amiodarone toxicity. Paradoxically, protection was achieved both by removal of external Ca 2+ or by adding high levels of CaCl2(10 mM) to block the drug induced Ca2+ burst. Our results support a model in which the fungicidal activity of amiodarone is mediated by Ca2+ stress, and highlight the pathway of Ca2+ mediated cell death as a promising target for antifungal drug development.
Keywords: amiodarone, calcium influx, fungicide, cell death, calcium stress
Introduction
Amiodarone [2-butyl-3-benzofuranyl-4-[2-(diethylamio)-ethoxy]-3,5-diiodophenyl-ketone hydrochloride; Fig. 1a inset]is listed as a Class III antiarrhythmic drug that prevents atrial fibrillation and ventricular tachycardia (Doggrell, 2001; Varbiro et al., 2003; Stevenson & Tedrow, 2007). It acts by blocking K+ channels and prolonging the action potential and duration of refractory periods, leading to reduced membrane excitability in all myocardial tissues. However, the therapeutic basis of action is complex, and amiodarone has also been shown to block calcium and sodium channels, β- and α-adrenergic receptors and ion exchangers (Hageluken et al., 1995; Kodama et al., 1997). Although it has few adverse cardiovascular effects, high doses of amiodarone can damage noncardiac tissues, most prominently the lungs and thyroid glands (Auer et al., 2002). Nevertheless, amiodarone remains an effective and reasonably safe agent for the restoration of sinus rhythm and has been widely prescribed for the last 30 years.
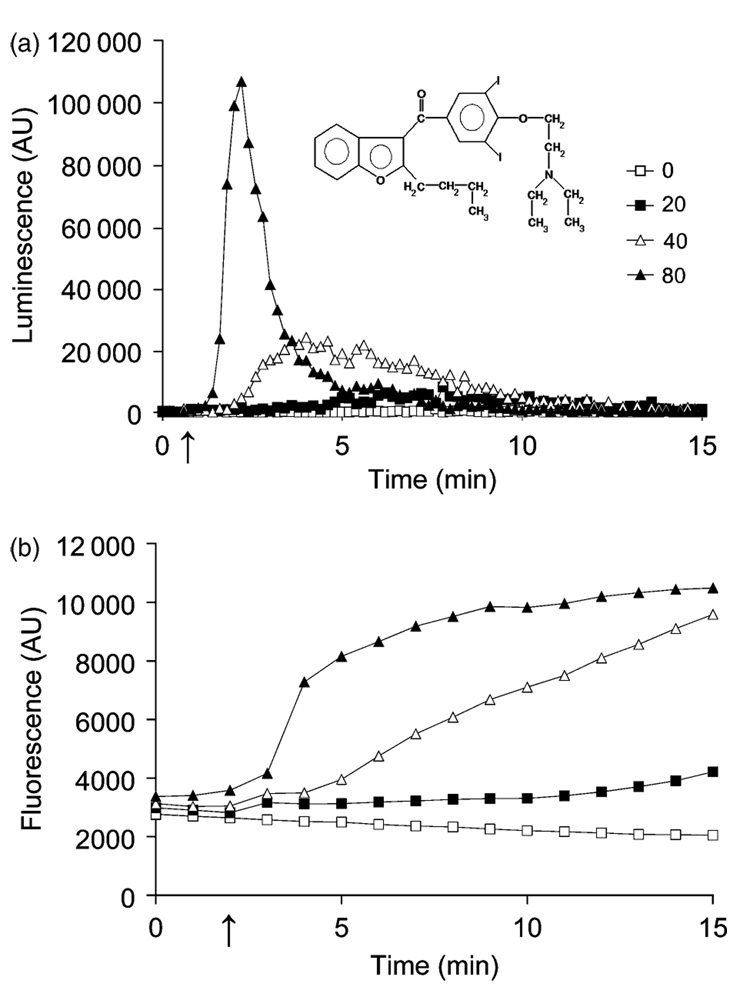
Fig. 1.
Dose dependence of amiodarone induced Ca2+ burst and toxicity. (a) Typical dose dependence of aequorin-coelenterazine luminescence upon injection (see arrow) of yeast cells (0.5 OD600 nm units) with amiodarone concentrations ranging from 0 to 80 µM. The drug was added from 300 µM amiodarone stock in water, freshly diluted from a 5 mM stock in dimethyl sulfoxide. Injected volumes ranged from 10 to 40 µL in a final volume of 150 µL. Luminescence is in arbitrary units (AU). Data are representative of at least four independent experiments. The inset shows the chemical structure of amiodarone. (b) Dose dependent increase of green fluorescence emitted from FUN-1 loaded yeast following injection (arrow) of amiodarone. Fluorescence (520 nm) is in AU. Data are averages of two replicates and are representative of four experiments.
Recently, amiodarone was shown to exhibit potent fungicidal activity against Saccharomyces cerevisiae and a range of pathogenic fungi, including Candida albicans, Cryptococcus neoformans, Fusarium oxysporum and Aspergillus nidulans (Courchesne, 2002). For example, 5 µM amiodarone completely blocked proliferation of C. neoformans. This was shown to be due to a fungicidal, rather than fungistatic effect. It appeared that Ca2+ played a role in the cytotoxic effect of amiodarone because addition of 10 mM CaCl2 protected against growth inhibition of S. cerevisiae, whereas Ca2+ chelators [0.5–2 mM l,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA)] exacerbated amiodarone toxicity (Gupta et al., 2003). Amiodarone was shown to elicit an immediate influx of Ca2+ that was monitored by a rise in the luminescence of aequorin-coelenterazine photo-protein (Courchesne & Ozturk, 2003; Gupta et al., 2003), increased uptake of 45Ca2+ (Yadav et al., 2007), and nuclear translocation of the Ca2+-regulated transcription factor Crzl-GFP (Zhang & Rao, 2007). Mutants lacking key regulators of calcium homeostasis, including the secretory pathway Ca2+ pump Pmrl, the vacuolar H+-ATPase, and Ca2+/calmodulin-activated protein phosphatase calcineurin, were shown to be hypersensitive to amiodarone, corroborating an important role for Ca2+ in the cellular mechanism of amiodarone toxicity (Gupta et al., 2003; Yadav et al., 2007; Zhang & Rao, 2007).
In S. cerevisiae, cytosolic Ca2+ entry is considered to be critical for survival under a variety of cell stresses including prolonged exposure to pheromone (Iida et al., 1990), hyper-tonic shock (Matsumoto et al., 2002), unfolded proteins (Bonilla et al., 2002), and alkaline pH (Viladevall et al., 2004). Such elevations are thought to trigger protective responses that are crucial to cell survival. For example, brief exposure to hypertonic stress was shown to trigger a Ca2+ transient that preconditioned cells to growth in high salt conditions (Matsumoto et al., 2002). Paradoxically, calcium is a double-edged sword, such that prolonged or uncontrolled elevation of cytosolic Ca2+ can lead to cell death. High levels of amiodarone (80 µM) were shown to elicit mitochondrial fragmentation and subsequent cell death, similar to the effect of pheromone and the Ca2+ ionophore A23187 (Pozniakovsky et al., 2005) and thereby implicating Ca2+ toxicity as fungicidal. However, a causative role for Ca2+ influx in amiodarone mediated cell death has not been established.
The goal of this study was to determine whether Ca2+ influx is protective, toxic or merely a bystander effect in the fungicidal activity of amiodarone. We manipulated the metabolic status of cells and composition of culture medium to modulate Ca2+ channel activity and correlated Ca2+ influx to cell viability. Our findings demonstrate that Ca2+ stress mediates yeast cell death in response to amiodarone and highlight the potential of this pathway as a target for antifungal development.
Materials and methods
Media, strains and reagents
Yeast strains were grown at 30 °C in standard synthetic complete (SC) medium (Biol0l Inc., Vista, CA) or YPD. Where specified, ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′ -tetraacetic acid (EGTA) was added from a stock solution of 200 mM in water, buffered to pH 8.8 with KOH. Stock solutions of amiodarone (Sigma, St Louis, MO) were 5 mM in dimethyl sulfoxide. Cycloheximide (Sigma) was added from a l0 mg mL−1 stock in dimethyl sulfoxide. Saccharomyces cerevisiae wild-type strain BY4742 (MATa, his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) was purchased from Invitrogen (Carlsbad, CA). Plasmid pEVPll-Aeq89 has been described (Matsumoto et al., 2002).
Aequorin assay
The procedure was modified from Gupta et al. (2003) as follows: 1 OD600 nm of overnight cultures carrying pEVP11-Aeq-89 were spun down and resuspended in 50 µL SC media containing 0.75 µg of coelenterazine (Invitrogen, Carlsbad, CA) and incubated in the dark at 30 °C for 1 h. Following incubation, the cells were washed twice with 2% glucose and resuspended to 0.5 OD600 nm mL−1 in SC media. Luminescence was quantified on a white opaque 96-well plate using a BMG FLUOStar OPTIMA™ plate reader (BMG Lab-technologies Durham, NC). Total luminescence (Lmax) was determined following lysis with 4% Triton X-100 (Fisher Scientific) in 2 M CaCl2. Free Ca2+ concentrations (in µM) were calculated as described in Gupta et al (2003). All assays performed on a microplate had 150 µL of culture well−1.
2-Chloro-4-(2,3-dihydro-3-methyl-(benzo-1,3-thiazol-2-yl)- methylidene)-1-phenylquinolinium iodide (FUN-1) assay
WT cells (1 OD600 nm) were preloaded with 4 µM FUN-1 for 1 h at 30 °C in SC medium (Millard et al., 1997). Following incubation, cells were washed twice with 2% glucose and resuspended to 0.5 0D600 nm mL−1 in SC media. Green fluorescence was quantified on a black, clear-bottomed 96-well plate using a BMG FLUOstar Optima microtiter plate reader (Excitation, 485 nm; Emission, 520 nm). Data were normalized for cell density, measured as OD600 nm units, and are averages of triplicates; figures are representative of at least two experiments. Data were organized, plotted and analyzed using BMG FLUOSTAR OPTIMA version 1.20 software (BMG Labtechnologies Durham, NC) and MICROSOFT EXCEL (Microsoft Corp., Redmond, WA) software.
Results
Dose dependence of Ca2+ burst and metabolic inactivation elicited by amiodarone
Amiodarone is a cationic amphiphilic drug (Fig. 1a inset) that is known to interact strongly with the lipid bilayer and alter membrane fluidity in model and native membranes (Antunes-Madeira et al., 1995, 2002; Rosa et al., 2000). Addition of amiodarone to yeast cells triggered the opening of plasma membrane Ca2+ channels that can be monitored by a rise in luminescence from the Ca2+ activated photo-protein aequorin-coelenterazine (Courchesne & Ozturk, 2003; Gupta et al., 2003). Here, we show that both the kinetics and amplitude of the Ca2+ signal depend on drug concentration (Fig. 1a): higher doses of amiodarone elicited both sharper and higher peaks, consistent with a concerted recruitment of more Ca2+ channels. Estimated peak values for free Ca2+, calibrated from total luminescence, increased from 2.2 µM in response to 20 µM amiodarone to 6.2 µM (40 µM amiodarone) and 12.7 µM (80 µM amiodarone). In comparison, hypertonic shock (1 M NaCl) transiently elevated cytosolic Ca2+ to c. 3 µM (Matsumoto et al., 2002) and secreted toxins from the pathogenic fungus Trichoderma elicited peak Ca2+ levels of > 7 µM in soybean cells (Navazio et al., 2007). Concomitantly, amiodarone triggered a dose-dependent loss of metabolic activity, monitored by a rise in green fluorescence of the vital stain FUN-1 (Millard et al., 1997) as seen in Fig. 1b. These results show that amiodarone toxicity correlates with the kinetics and amplitude of the Ca2+ burst.
Loss of Ca2+ channel activity correlates with resistance to amiodarone in stationary phase cells
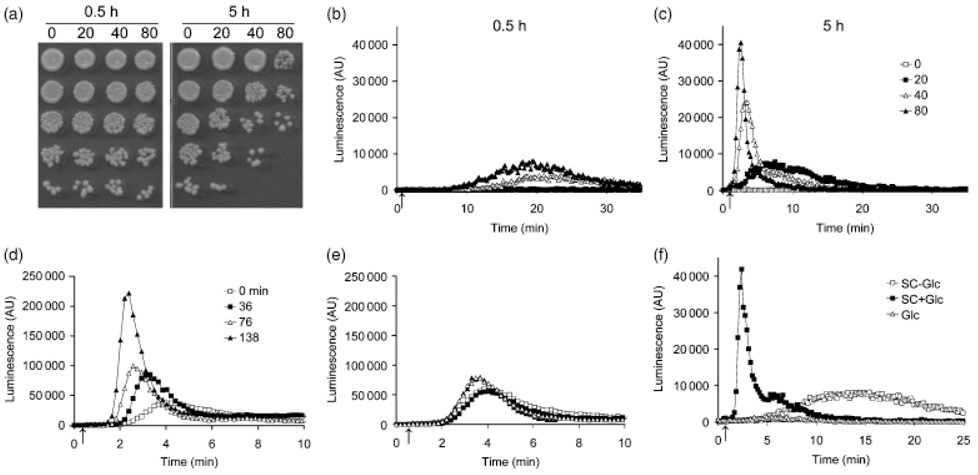
We found that the fungicidal activity of amiodarone was dependent on the metabolic status of the cells. Following overnight growth of the culture to stationary phase, yeast cells were largely resistant to amiodarone toxicity, even after transfer to fresh SC medium for 0.5 h (Fig. 2a). However, amiodarone sensitivity was regained following continued growth in fresh medium to exponential phase (Fig. 2a; 5 h). Investigation of amiodarone induced aequorin-coelenterazine luminescence revealed that stationary phase cultures that were resistant to drug toxicity also showed low levels of drug induced Ca2+ influx (Fig. 2b). In contrast, amiodarone was able to elicit large dose-dependent Ca2+ bursts in the exponential phase, drug sensitive culture (Fig. 2c). This suggested that the expression and/or activity of Ca2+ influx channels were dependent on growth phase, being down-regulated in stationary phase cells. Upon transfer of stationary phase cells to fresh medium, we observed a time-dependent increase in the size of the Ca2+ burst (Fig. 2d; 40 µM amiodarone), consistent with increasing numbers of active channels. Addition of cycloheximide completely blocked the time-dependent reappearance of Ca2+ burst, indicating a requirement for new protein synthesis (Fig. 2e). Glucose, possibly as the energy source, was necessary for the recovery of Ca2+ influx; cultures transferred to SC medium lacking glucose failed to show full activation of Ca2+ channels. However, glucose alone, in the absence of media, was insufficient for this effect (Fig. 2f). Taken together, the data show that Ca2+ channel activity is dependent on metabolic state and suggest that stationary phase cells may be more tolerant to amiodarone toxicity because of the downregulation of Ca2+ channels.
Fig. 2.
Amiodarone induced toxicity and Ca2+ influx are both dependent on metabolic state. (a) Yeast cultures were grown to stationary phase and transferred to fresh SC medium for 0.5 h (left panel) or 5 h (right panel). Viability was assessed on YPD agar after 15 min exposure to amiodarone (0–80 µM). Cells (0.5 0D600 nm units) were spotted in serial fivefold dilutions in each column, (b–c) Ca2+ influx was monitored in cultures freshly transferred (0.5 h) from stationary phase to SC medium or after 5 h. tuminescence is in arbitrary units (AU). Dose dependence of amiodarone-induced aequorin-coelenterazine luminescence is greatly diminished in stationary phase cells (b), but recovers after 5 h growth in fresh medium (c). The arrow indicates the injection point of amiodarone. Data are representative of at least two experiments, (d) Time-dependent recovery of Ca2+ influx (luminescence) following transfer to fresh SC medium. The arrow indicates injection of 40 µM amiodarone. (e) Time-dependence of Ca2+ influx (luminescence) of cultures grown as in (d), but in the presence of 50 g mL−1 cycloheximide. Data are representative of at least two separate experiments. (f) Aequorin-coelenterazine luminescence of yeast cultures grown to stationary phase and transferred to fresh media with (SC+Glc) or without (SC−Glc) 2% glucose, or 2% glucose in water (Glc) for 2.5 h before injection of 40 µM amiodarone (arrow).
Glucose removal from growing cells reduces both calcium burst and amiodarone toxicity
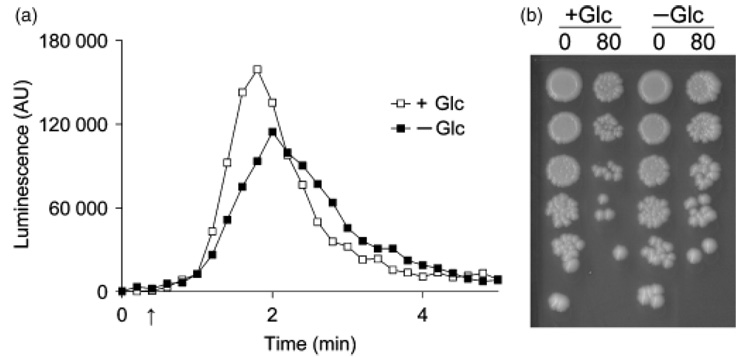
It was possible that amiodarone resistance in stationary phase yeast may have arisen due to altered metabolic response to the drug rather than a consequence of altered Ca2+ influx. Therefore, we assessed the effect of glucose removal from exponentially growing yeast. Upon removal of glucose from the medium, there was an immediate, although modest, decrease in Ca2+ influx (Fig. 3a). Remark-ably, this was accompanied by a reproducible increase in cell viability in the same culture (Fig. 3b). After treatment with amiodarone and normalization for cell density as described in Fig. 3b, cultures containing glucose were found to have significant reduction in CFU relative to cultures without glucose (43 ± 14% from four independent trials). These results suggest that even small changes in Ca2+ influx could influence amiodarone toxicity, irrespective of the metabolic status of the cells.
Fig. 3.
Glucose removal immediately diminishes amiodarone induced Ca2+ burst and toxicity. Exponentially growing yeast cultures were spun down and resuspended in fresh SC medium with (+Glc) or without (−Glc) 2% glucose, (a) Aequorin-coelenterazine luminescence was measured after injection of 80 µM amiodarone (arrow). Data are representative of four independent experiments, (b) Viability of the cultures was assessed on YPD agar immediately following luminescence measurements in (a), as described in Fig. 2a. One of two duplicate plates from this experiment is shown. Data are representative of four independent experiments.
Extracellular Ca2+ modulates amiodarone toxicity
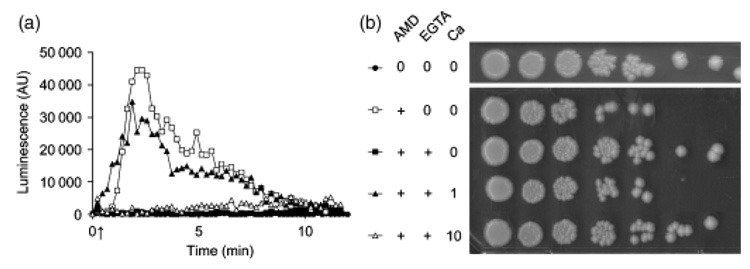
Addition of the Ca2+ chelating agent EGTA resulted in a decrease in the amplitude of the amiodarone induced Ca2+ burst, as expected, consistent with a removal of free extracellular calcium by the chelator (Fig. 4a). At 6.8 mM added EGTA, Ca2+ influx was completely abolished. We assessed the viability of cells immediately after the Ca2+ burst (Fig. 4b): increasing amounts of chelator conferred increasing protection against amiodarone induced cell death, with maximal protection observed at 6.8 mM EGTA, similar to the untreated control. We also noted that increasing amounts of EGTA resulted in a progressive delay in the time to peak, suggesting that extracellular Ca2+ influenced channel opening (Fig. 4a). To evaluate this more carefully, we added different amounts of CaCl2 to EGTA buffered medium and assessed amiodarone induced Ca2+ bursts together with cell viability. As described in Fig. 4, addition of 6.8 mM EGTA to exponentially grown cells in SC medium completely abolished Ca2+ influx and conferred protection against cell death in response to 40 µM amiodarone (Fig. 5a and b). Introduction of 1 mM CaCl2 reinstated both the Ca2+ influx peak and amiodarone sensitivity to levels similar to control (no EGTA; Fig. 5a and b). Surprisingly, higher levels of added CaCl2 actually decreased Ca2+ influx; Fig. 5a shows that addition of 10 mM CaCl2 completely inhibited influx to levels achieved with EGTA alone. Consistent with inhibition of Ca2+ influx, 10 mM CaCl2 also protected yeast cells against amiodarone toxicity (Fig. 5b). Taken together, these results support a model in which the fungicidal activity of amiodarone is mediated by Ca2+ influx.
Fig. 4.
EGTA decreases amiodarone induced Ca2+ burst and protects against toxicity, (a) tuminescence from aequorin-coelenterazine was monitored as described in Fig. 1a upon addition of 40 µM amiodarone (20 µL of stock; arrow). EGTA (1–6 µL) was added from a stock solution (200 mM, pH 8.8). No change in extracellular pH was observed upon EGTA addition. Note the decrease in amplitude and delay in Ca2+ burst with increasing EGTA (1.2, 2.3, 4.6, and 6.8 mM final concentration), (b) Viability of cultures treated in (a) was assessed on YPD agar, immediately following luminescence measurements, as described in Fig. 2a. EGTA concentrations ranged from 1.2 to 6.8 mM.
Fig. 5.
Extracellular Ca2+ modulates amiodarone induced Ca2+ burst and toxicity, (a) tuminescence from aequorin-coelenterazine was monitored as described in Fig. 1a upon addition of 40 µM amiodarone (arrow). Luminescence was abolished upon addition of 6.8 mM EGTA and recovered upon reintroduction of 1 mM CaCl2, showing a requirement for extracellular Ca2+. Higher levels of CaCl2 (10 mM) also blocked luminescence, (b) Viability of cultures treated in (a) was assessed on YPD agar, immediately following luminescence measurements, as described in Fig. 2a. Note the increased viability of cells treated with EGTA alone, and with EGTA plus 10 mM CaCl2, relative to control (no EGTA) and EGTA plus 1 mM CaCl2. Data are representative of four independent experiments.
Discussion
Ca2+ influx is a common cellular consequence of exposure to many forms of environmental stress and cytotoxic drugs. Hyper- or hypo-osmotic stress, cell wall damage, endoplasmic reticulum stress and prolonged exposure to mating pheromone all trigger the opening of calcium channels resulting in the elevation of cytosolic free Ca2+ from submicromolar baseline levels. At the plasma membrane, the Cchl/Midl complex has been implicated in Ca2+ entry following exposure to pheromones or tunicamycin (Bonilla et al., 2002). At the vacuolar membrane, the TRP-like channel Yvcl mediates Ca2+ release in response to hypertonic shock (Denis & Cyert, 2002). Following cytoplasmic Ca2+ influx, homeostasis is accomplished by an array of ATP-dependent pumps (Pmrl, Pmcl, V-ATPase) and exchangers (Vcxl) that work to sequester Ca2+ into stores or transport it out of the cell (reviewed by Ton & Rao, 2004). In S. cerevisiae, the absence of key transporters, particularly the Vma H+-pump that provides a driving force for secondary transport, and the secretory pathway Ca2+-ATPase Pmrl, results in hypersensitivity to numerous stresses and toxic drugs, including amiodarone, that are accompanied by a rise in intracellular Ca2+ (Yadav et al., 2007). The stress or drug induced influx of Ca2+ activates signaling pathways, most prominently calmodulin-calcineurin-Crzl, that mediates a cellular response which includes cell wall synthesis, cell cycle arrest and upregulation of ion homeostasis factors (Matsumoto et al., 2002; Yoshimoto et al., 2002; Miyakawa & Mizunuma, 2007; Zhang & Rao, 2007). Although not essential for viability under normal growth conditions, disruption of the calcineurin signaling pathway is lethal to both model and pathogenic fungi under stress: calcineurin mutants or cells treated with the calcineurin inhibitors cyclosporine A or FK506 rapidly lose viability in the face of cell stress, antifungal agents, and other toxic drugs (Kraus & Heitman, 2003; Steinbach et al., 2007; Zhang & Rao, 2007).
The purpose of this study was to understand whether amiodarone-induced Ca2+ influx was beneficial, deleterious or unrelated to cell viability. On the one hand, a Ca2+ transient can be viewed as a signaling event, occurring down-stream from a stress sensor, and allowing the cell to respond to its environment. Thus, the delayed influx of Ca2+, reported to occur after an hour of tunicamycin treatment (Bonilla et al., 2002), has been interpreted as a protective mechanism that is necessary for viability. In this case, deletion of the plasma membrane Cchl/Midl channel complex resulted in decreased viability in tunicamycin treated cultures. Alternatively, the opening of Ca2+ channels may simply be a physical consequence of membrane perturbation (such as mechanical or electrical). Examples might include membrane shrinking or swelling in response to osmotic changes (Denis & Cyert, 2002), or partitioning of a drug such as amiodarone into the bilayer. In such cases, calcineurin mediated signaling events would represent the cellular response to counter Ca2+ stress. If the Ca2+ stress is small, the protective response effectively preconditions cells to further insults, as was found to occur following brief exposure to high salt (Matsumoto et al., 2002). However, the Ca2+ stress can overwhelm the protective response and lead to cell death.
In this study, we show that high levels of Ca2+ entry elicited by the amphiphilic drug amiodarone correlate with cell death. Furthermore, varying the size and temporal kinetics of Ca2+ transients by altering metabolic status or extracellular Ca2+ levels, resulted in concomitant changes in amiodarone toxicity. First, we confirmed that the dose dependence of amiodarone induced Ca2+ transients correlated with loss of viability, measured by the vital stain FUN-1 or by drop tests. Second, we found that drug sensitivity was dependent on the metabolic status of the cells. Stationary phase cells were more resistant to amiodarone, up to half hour following dilution into fresh medium, and amiodarone failed to induce signifi-cant Ca2+ transients in these cultures. Over time, amiodarone sensitivity increased with concomitant increases in the amiodarone induced Ca2+ burst, strongly suggesting that cell death was downstream of Ca2+ influx. This increase in Ca2+ influx required new protein synthesis, as it was inhibited by cycloheximide. Presumably, one or more Ca2+ channel proteins are downregulated in stationary phase cells, although we cannot exclude that membrane lipid composition or other components upstream of calcium channel opening may be altered as well. The Cchl/Midl channels appear not to be involved in amiodarone mediated toxicity, since both single and double gene deletions showed no loss of amiodarone-induced Ca2+ transients (data not shown; see also, Gupta et al., 2003). This is consistent with the findings of Loukin et al., (2007) that there are functionally redundant Ca2+ entry pathways in S. cerevisiae that remain to be identified. The immediate decrease in Ca2+ influx upon glucose removal from actively growing cells may be related to the reported drop in plasma membrane potential in the absence of glucose. It has been established that H+ pump activity of Pmal is immediately downregulated in low glucose medium (Serrano, 1983; Lecchi et al., 2007). These findings raise the interesting possibility that opening of calcium channels may be triggered by changes in plasma membrane potential and warrant further investigation.
Third, we show that addition of the Ca2+ chelator EGTA to the culture medium minutes before drug addition decreased the Ca2+ burst and concomitantly increased cell viability, assessed immediately after the Ca2+ burst (Fig. 4). This contrasts with the exacerbating effect of EGTA (2 mM) on sensitivity to amiodarone, which was monitored over 24h of growth as reported earlier (Gupta et al., 2003). Calcium starvation over this period of time is harmful to cells, and likely to induce a compensatory opening of calcium channels so that the drug actually has a slightly more harmful effect under these conditions. The ability of high extracellular Ca2+ to block drug-mediated Ca2+ entry (Fig. 5) explains the paradoxical protective effect of high CaCl2 on growth toxicity of amiodarone, reported earlier (Courchesne, 2002; Gupta et al., 2003). The protective effect of high Ca2+ may be mediated by unknown membrane effects that mitigate drug toxicity or integration, by rapid desensitization via Ca2+ block of the channel pore, or by activation of calcineurin and other effectors. Taken together, our findings clearly show that the calcium burst is closely coupled to the fungicidal effect of amiodarone, although the drug may affect more than one cellular pathway. Our findings point to the importance of Ca2+ stress in mediating the cytotoxic effects of multiple environmental challenges and drugs and highlight the potential of key regulators of Ca2+ homeostasis and signaling as drug targets.
Acknowledgements
This work was supported by a Public Health Service grant from the National Institutes of Allergy and Infectious Disease (R01AI065983).
References
- Antunes-Madeira MC, Videira RA, Kluppel ML, Madeira W. Amiodarone effects on membrane organization evaluated by fluorescence polarization. Int J Cardiol. 1995;48:211–218. doi: 10.1016/0167-5273(94)02247-g. [DOI] [PubMed] [Google Scholar]
- Antunes-Madeira MC, Videira RA, Madeira VM. Cholesterol modulates amiodarone-membrane interactions in model and native membranes. Appl Biochem Biotechnol. 2002;97:23–32. doi: 10.1385/abab:97:1:23. [DOI] [PubMed] [Google Scholar]
- Auer J, Berent R, Weber T, Eber B. Thyroid hormone and arrhythmogenic activity. J Am Coll Cardiol. 2002;40:574–575. doi: 10.1016/s0735-1097(02)01995-2. author reply 575. [DOI] [PubMed] [Google Scholar]
- Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne WE. Characterization of a novel, broad-based fungicidal activity for the antiarrhythmic drug amiodarone. J Pharmacol Exp Titer. 2002;300:195–199. doi: 10.1124/jpet.300.1.195. [DOI] [PubMed] [Google Scholar]
- Courchesne WE, Ozturk S. Amiodarone induces a caffeine-inhibited, MID1-depedent rise in free cytoplasmic calcium in Saccharomyces cerevisiae. Mol Microbiol. 2003;47:223–234. doi: 10.1046/j.1365-2958.2003.03291.x. [DOI] [PubMed] [Google Scholar]
- Denis V, Cyert MS. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggrell SA. Amiodarone - waxed and waned and waxed again. Expert Opin Pharmacother. 2001;2:1877–1890. doi: 10.1517/14656566.2.11.1877. [DOI] [PubMed] [Google Scholar]
- Gupta SS, Ton VK, Beaudry V, Rulli S, Cunningham K, Rao R. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J Biol Chem. 2003;278:28831–28839. doi: 10.1074/jbc.M303300200. [DOI] [PubMed] [Google Scholar]
- Hageluken A, Nurnberg B, Harhammer R, Grunbaum L, Schunack W, Seifert R. The class III antiarrhythmic drug amiodarone directly activates pertussis toxin-sensitive G proteins. Mol Pharmacol. 1995;47:234–240. [PubMed] [Google Scholar]
- Iida H, Yagawa Y, Anraku Y. Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. A study of [Ca 2+]i in single Saccharomyces cerevisiae cells with imaging of fura-2. J Biol Chem. 1990;265:13391–13399. [PubMed] [Google Scholar]
- Kodama I, Kamiya K, Toyama J. Cellular electro-pharmacology of amiodarone. Cardiovasc Res. 1997;35:13–29. doi: 10.1016/s0008-6363(97)00114-4. [DOI] [PubMed] [Google Scholar]
- Kraus PR, Heitman J. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem Biophys Res Commun. 2003;311:1151–1157. doi: 10.1016/s0006-291x(03)01528-6. [DOI] [PubMed] [Google Scholar]
- Lecchi S, Nelson CJ, Allen KE, Swaney DL, Thompson KL, Coon JJ, Sussman MR, Slayman CW. Tandem phosphorylation of Ser-911 and Thr-912 at the carboxy terminus of yeast plasma membrane H+ -ATPase leads to glucose-dependent activation. J Biol Chem. 2007;282:35471–35481. doi: 10.1074/jbc.M706094200. [DOI] [PubMed] [Google Scholar]
- Loukin SH, Kung C, Saimi Y. Lipid perturbations sensitize osmotic down-shock activated Ca2+influx, a yeast “deletome” analysis. FASEB J. 2007;21:1813–1820. doi: 10.1096/fj.06-7898com. [DOI] [PubMed] [Google Scholar]
- Matsumoto TK, Ellsmore AJ, Cessna SG, Low PS, Pardo JM, Bressan RA, Hasegawa PM. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J Biol Chem. 2002;277:33075–33080. doi: 10.1074/jbc.M205037200. [DOI] [PubMed] [Google Scholar]
- Millard PJ, Roth BL, Thi HP, Yue ST, Haugland RP. Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl Environ Microbiol. 1997;63:2897–2905. doi: 10.1128/aem.63.7.2897-2905.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Mizunuma M. Physiological roles of calcineurin in Saccharomyces cerevisiae with special emphasis on its roles in G2/M cell-cycle regulation. Biosci Biotechnol Biochem. 2007;71:633–645. doi: 10.1271/bbb.60495. [DOI] [PubMed] [Google Scholar]
- Navazio L, Baldan B, Moscatiello R, Zuppini A, Woo SL, Mariani P, Lorito M. Calcium-mediated perception and defense responses activated in plant cells by metabolite mixtures secreted by the biocontrol fungus Trichoderma atroviride. BMC Plant Biol. 2007;7:41. doi: 10.1186/1471-2229-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozniakovsky AI, Knorre DA, Markova OV, Hyman AA, Skulachev VP, Severin FF. Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J Cell Biol. 2005;168:257–269. doi: 10.1083/jcb.200408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa SM, Antunes-Madeira MC, Matos MJ, Jurado AS, Madeira VM. Lipid composition and dynamics of cell membranes of Bacillus stearothermophilus adapted to amiodarone. Biochim Biophys Acta. 2000;1487:286–295. doi: 10.1016/s1388-1981(00)00122-0. [DOI] [PubMed] [Google Scholar]
- Serrano R. In vivo glucose activation of the yeast plasma membrane ATPase. FEBS Lett. 1983;156:11–14. doi: 10.1016/0014-5793(83)80237-3. [DOI] [PubMed] [Google Scholar]
- Steinbach WJ, Reedy JL, Cramer RA, Jr, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5:418–430. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- Stevenson WG, Tedrow U. Management of atrial fibrillation in patients with heart failure. Heart Rhythm. 2007;4:S28–S30. doi: 10.1016/j.hrthm.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Ton VK, Rao R. Functional expression of heterologous proteins in yeast: insights into Ca2+ signaling and Ca2+ -transporting ATPases. Am J Physiol Cell Physiol. 2004;287:C580–C589. doi: 10.1152/ajpcell.00135.2004. [DOI] [PubMed] [Google Scholar]
- Varbiro G, Toth A, Tapodi A, Bognar Z, Veres B, Sumegi B, Gallyas F., Jr Protective effect of amiodarone but not N-desethylamiodarone on postischemic hearts through the inhibition of mitochondrial permeability transition. J Pharmacol Exp Ther. 2003;307:615–625. doi: 10.1124/jpet.103.053553. [DOI] [PubMed] [Google Scholar]
- Viladevall L, Serrano R, Ruiz A, Domenech G, Giraldo J, Barcelo A, Arino J. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J Biol Chem. 2004;279:43614–43624. doi: 10.1074/jbc.M403606200. [DOI] [PubMed] [Google Scholar]
- Yadav J, Muend S, Zhang Y, Rao R. A phenomics approach in yeast links proton and calcium pump function in the golgi. Mol Biol Cell. 2007;18:1480–1489. doi: 10.1091/mbc.E06-11-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto H, Saltsman K, Gasch AP, et al. Genome-wide analysis of gene expression regulated by the calcineurin/Crzlp signaling pathway in Saccharomyces cerevisiae. J Biol Chem. 2002;277:31079–31088. doi: 10.1074/jbc.M202718200. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Rao R. Global disruption of cell cycle progression and nutrient response by the antifungal agent amiodarone. J Biol Chem. 2007;282:37844–37853. doi: 10.1074/jbc.M707593200. [DOI] [PubMed] [Google Scholar]