Abstract
Biomaterials can potentially enhance the delivery of viral and nonviral vectors for both basic science and clinical applications. Vectors typically consist of nucleic acids (DNA, RNA) packaged with proteins, lipids, or cationic polymers, which facilitate cellular internalization and trafficking. These vectors can associate with biomaterials that support cell adhesion, a process we term substrate-mediated delivery. Substrate immobilization localizes the DNA and the delivery vector to the cellular microenvironment. The interaction between the vector and substrate must be appropriately balanced to mediate immobilization, yet allow for cellular internalization. Balancing the binding between the biomaterial and the vector is dependent upon the surface chemistries of the material and the vector, which can be designed to provide both specific (e.g., biotin–avidin, the strongest known noncovalent interaction between a protein and its ligand) and nonspecific (e.g., van der Waals) interactions. In this review, we describe the biomaterial and vector properties that mediate binding and gene transfer, identify potential applications, and present opportunities for further development.
Keywords: biomaterials, gene therapy, plasmid delivery, reverse transfection, solid-phase delivery, tissue engineering, transfected cell arrays
Introduction
Gene delivery has tremendous potential for use therapeutically, such as in gene therapy and tissue engineering, and in research applications, such as functional genomics. A critical factor limiting the development of these applications is the inefficiency of gene transfer. Successful gene transfer requires delivery of the nucleic acid (e.g., DNA, RNA) to the target tissue or cell population without being degraded, followed by internalization by the cell, escape from the endosome into the cytoplasm, transport into the nucleus for transcription, and ultimately protein production (Figure 1). Gene delivery vectors have been developed to enhance the efficiency of gene transfer to the target cells. Vectors consist of nucleic acids packaged by proteins, lipids, or polymers, which produce small particles that protect against degradation, and are less negatively charged relative to the nucleic acid. The small size of the particles promotes internalization, and the properties of the protein, lipid, or polymer can facilitate intracellular trafficking. Viral and nonviral vectors target many of the intracellular barriers in gene transfer, whereas biomaterial-based delivery addresses extracellular barriers to enhance gene transfer.1
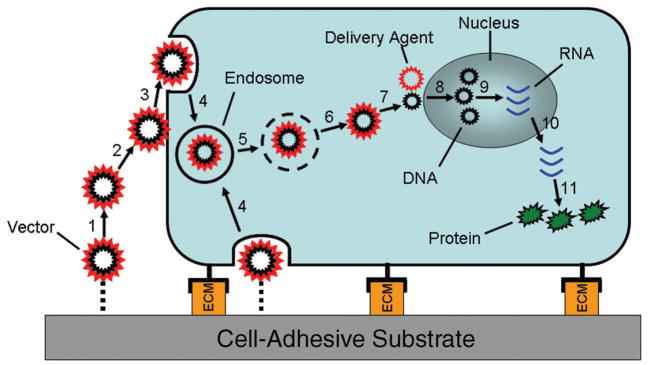
Figure 1.
Steps leading to gene expression: (1) Release of vector from substrate, (2) association of vector with cell membrane, (3) endocytosis, (4) formation of early endosome, (5) transport in late endosome, (6) escape from endosome, (7) transport to nucleus and dissociation of delivery agent, (8) entry into nucleus, (9) transcription into RNA, (10) transport of RNA to cytoplasm, and (11) translation of RNA into protein.
Biomaterials can enhance gene transfer by maintaining consistent levels of the vector in the microenvironment of the cell and reducing the amount of DNA required, which can decrease cell toxicity.2 Traditionally, delivery of vectors by injection or inhalation, known as systemic or bolus delivery, leads to the presence of vector in the target cell population for a short time prior to clearance, aggregation, or degradation. Bolus addition of viral and nonviral vectors to cultured cells leads to only a fraction of the vector being internalized by the cell population,3,4 and systems that can enhance vector transport to the cell surface can improve gene transfer. Biomaterials may be able to overcome these mass transport limitations, with delivery systems categorized according to two basic mechanisms: polymeric release and substrate-mediated delivery. For polymeric release, DNA is encapsulated within the material and slowly released. Alternatively, substrate-mediated delivery involves immobilization of either viral or nonviral vectors to a surface that supports cell adhesion. Substrate immobilization places the vector directly in the cellular microenvironment to reduce the amount of DNA required, preventing aggregation and distributing the DNA homogeneously among the cell population; it can potentially be used to spatially regulate gene transfer.5,6 In this review, we briefly describe the properties of viral and nonviral vectors, present the strategies for immobilizing DNA to a biomaterial, and identify opportunities for biomaterials development to promote binding and enhance gene transfer.
Vector Properties
Viral vectors provide the most efficient gene transfer among gene delivery vehicles. They can provide stable expression of the delivered gene and can target specific cell types; however, viruses have safety concerns for use in vivo. The common types of viruses used for gene delivery include retroviruses (which deliver RNA), adenoviruses (which deliver transiently expressing double-stranded DNA), and adeno-associated viruses (AAVs, which deliver single-stranded stably expressing DNA). Viral particles range in size from 25 nm (AAVs) to 60–100 nm (adenoviruses and retroviruses). Many viral vectors have evolved to infect cells and, therefore, provide an established mechanism to exploit for the delivery of foreign DNA. Viruses consist of genetic material surrounded by envelope proteins that determine the surface properties. To facilitate internalization, the virus surface can mediate binding to specific cell–surface receptors or to extra-cellular matrix molecules, which provides a natural corollary for substrate-mediated delivery.
Nonviral vectors are attractive for their safety profiles and the ability to control their chemical and physical properties, yet they yield lower efficiencies of gene transfer relative to viral vectors. Plasmids, which are circular DNA molecules, self-assemble with cationic lipids or polymers to form complexes. Complexes of cationic polymers such as polyethylenimine (PEI) have mean diameters ranging from 100 nm to 1 μm, and zeta-potentials ranging from −14 mV to 21 mV.2 Complexes formed with cationic lipids typically have mean diameters ranging from 200 nm to 1100 nm, with zeta-potentials that depend upon the cationic lipid.2 The quantity of cationic lipid or polymer determines the properties of the complex; however, increasing the amount of lipid or polymer leads to cytotoxicity. Nonviral vectors for gene delivery facilitate internalization and intracellular trafficking of nucleic acids, and the ability to manipulate the properties of the complex is attractive for surface-based delivery.
Substrate-Mediated Delivery
Materials that balance immobilization and release can increase the number of transfected cells and achieve similar levels of transgene expression with a decreased quantity of DNA relative to bolus DNA delivery. The material must support cell adhesion and maintain the vector at the surface but allow for cellular internalization. Materials that strongly immobilize vectors may limit cellular internalization, but weakly immobilized vectors will not be retained on the surface for presentation to cells. Many materials support cell adhesion, either through the incorporation of specific adhesion molecules or through the adsorption of extracellular matrix proteins,7,8 and vectors can be immobilized using similar approaches onto tissue-culture polystyrene or natural and synthetic polymers.
The properties of the vector can regulate both the number of transfected cells and the extent of transgene expression.7 The immobilization of complexes prevents the aggregation of nonviral vectors,6 and research has shown that small complexes lead to a greater number of cells expressing protein, while large complexes yield higher total levels of protein production.7 Vector immobilization combined with surface patterning provides the necessary tools to spatially regulate gene transfer.
Immobilization on Biomaterials
Vector immobilization to the biomaterial surface occurs through a combination of nonspecific and specific interactions that can be regulated through the design of the material and the vector. The mechanisms of vector immobilization and the subsequent gene transfer efficiency are described in the following sections.
Nonspecific Immobilization
The nonspecific immobilization of viral and nonviral vectors to biomaterials is determined by molecular-scale interactions such as electrostatic, van der Waals, and hydrophobic interactions. Many materials support the adsorption of proteins to the surface (reviewed in Reference 8), and delivery vectors can adsorb directly to the substrate or to the proteins that are coating the surface. Based on protein adsorption,9 vector adsorption to biomaterials may be characterized by (1) changes in the hydration of the surface and vector, (2) charge interactions between the vector and the surface, (3) structural rearrangement of the adsorbing vector, and (4) the solution properties from which the complexes adsorb. Conformational changes in the vector may contribute to irreversible binding that limits cellular uptake, while hydrophilic substrates, which generally result in reversible interactions for proteins, may facilitate cellular internalization.2
Surface immobilization typically employs either sequential deposition of DNA or cationic lipids and polymers, or the adsorption of preformed DNA complexes. Initial approaches immobilized DNA at the surface by entrapment within gelatin, followed by the addition of the transfection reagent.10 Several cationic polymers, such as poly(L-lysine) (PLL) and PEI, are routinely used to coat tissue-culture surfaces for cell adhesion. Following this model, the transfection reagent has been initially adsorbed to the surface, followed by addition of the DNA.11 This latter approach can be extended to the adsorption of multilayer films that gradually erode to expose the DNA for transfection.12 Alternatively, the vectors can be formed in solution and subsequently immobilized to the substrate,6 which mimics virus binding to the extracellular matrix.13
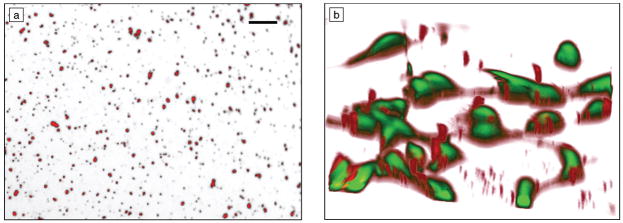
Transgene expression by nonspecific immobilization of preformed complexes is dependent on the properties of the vector and substrate. Plasmid can be precipitated with calcium phosphate and immobilized to a surface, with maximum densities of 10 μg/cm2.14 These immobilized DNA nanoparticles were relatively stable on the substrate, and required 20 times more DNA to achieve expression levels similar to delivery as a bolus. Alternatively, DNA complexes formed with either cationic polymers or cationic lipids have been adsorbed for gene delivery, with similar levels of transgene expression using quantities similar to or less than that delivered as a bolus.2 Complexes adsorbed to the substrate and were homogeneously distributed across the surface (Figure 2a). The quantity of immobilized complexes depended upon the charge and size of the DNA complexes. Transgene expression was observed on all substrates; however, the extent of transgene expression and the number of transfected cells were enhanced by pre-coating the substrate with serum proteins. This surface coating enabled homogeneously distributed complexes to redistribute to the cell surface (Figure 2b).
Figure 2.
(a) Polyethylenimine–DNA complexes homogenously distributed across a polystyrene substrate. Scale bar is 10 μm. (b) Redistribution of complexes to cell surface on a serum-coated substrate. Cells (green) have an average width of ~15–20 μm.
Surface coatings can be applied to implantable biomaterials, regulating the interactions between the vector and substrate for enhanced gene transfer. A coating of poly(lactide-co-glycolide) (PLG) and collagen membranes with phosphatidyl glycerol (PG) (1–5%) supports the binding of complexes formed with polyamidoamine dendrimers and yields more efficient transfection than surfaces without the coating.15 Coating stents with phosphoryl-choline mixed with a plasmid encoding for vascular endothelial growth factor (VEGF), a protein that promotes vascularization, yielded significant re-endothelialization compared with uncoated stents.16
Specific Immobilization
Biomaterials can be chemically modified for the specific binding of viral and nonviral vectors. In this approach, the vectors are formed prior to immobilization and contain functional groups on the surface that are complementary to the biomaterial. For nonviral vectors, the functional groups are attached to or incorporated within the cationic lipids or cationic polymers used for complexation. Upon complexation, a fraction of these functional groups will be available on the exterior of the particle for immobilization. Similarly, viral particles can be genetically engineered with specific sequences for binding or chemically modified after formation (reviewed in Reference 17). Although the functional groups provide specific interactions between the biomaterial and vector, nonspecific interactions contribute to vector immobilization.7 Cellular internalization can occur through breaking of the linkage between the complex and substrate, degradation of the substrate, or disruption of the complex to allow for release.
The specific binding of avidin-modified materials to biotinylated vectors has allowed researchers to identify several key design parameters for specific immobilization strategies. PLL and PEI have been modified with biotin residues and complexed with plasmid DNA followed by immobilization to NeutrAvidin-modified substrates (NeutrAvidin is an avidin derivative).6,7,18 Increasing the number of biotin groups in the complex, either through increasing the number of biotin residues per polymer or increasing the percentage of biotinylated polymers in the complex, increased the binding of the complexes to the substrate; however, maximal transfection in vitro was achieved when only a small fraction of the polymer forming the complex contained biotin. Maximal binding occurs when there is a high affinity of the complex for the substrate, but this high affinity reduces transfection.18 Hydrogel scaffolds composed of hyaluronic acid (HA), a degradable polysaccharide useful for wound healing, have been designed to capture biotinylated complexes that can release as the gel degrades.7
Alternatively, viruses have been modified with biotin for immobilization to a substrate. Adenoviral particles were biotinylated and immobilized to a collagen gel, resulting in localized transgene expression with transduction levels comparable to those of free viruses.19 The methods for modifying viruses must be controlled to avoid inactivation of the viruses.17
Biomaterials have also been covalently modified with antibodies, which bind a specific viral epitope, the specific site where antibodies bind, to mediate the immobilization of unmodified viruses. Collagen gels modified with IgG, one of the major classes of antibodies specific for the adenovirus hexon, result in adenovirus binding to the substrate.5 Implantation of the gel with adenovirus in a porcine right ventricle localized cellular expression as compared with direct injection.5 This strategy has been applied to delivery from collagen-coated stainless steel stents20 and platinum microcoils.21
Applications and Opportunities
Biomaterials that provide controlled and efficient gene transfer can extend the current utility of gene delivery in basic science research and provide opportunities for use in functional genomics. As mentioned earlier, substrate immobilization can increase the extent of transgene expression and the number of transfected cells while reducing the amount of DNA required, which can decrease toxicity in cell types that are difficult to transfect.1,2 Extending the delivery system to various cell types would significantly expand the number of potential applications. Coated polystyrene substrates and self-assembled monolayers of alkanethiols have most recently been employed for their ability to support cell adhesion, control surface chemistry, and transfect multiple cell types.22–24 Immobilization of DNA to specific regions of the substrate can provide for spatially controlled gene expression, which is the basis for the creation of transfected cell arrays for high-throughput functional genomics studies. Localized gene expression on an array can correlate expression or repression of a gene with a functional cell response and can be applied in the discovery of therapeutic strategies.10,25,26 Precisely controlling the substrate chemistry, architecture, and patterning using tools from the field of nanotechnology may enhance the delivery efficiency and control gene transfer, which would increase applications to research, diagnostics, and therapies.
Biocompatible materials for efficient gene delivery in vivo will enhance the current clinical applications of gene therapy and will provide novel opportunities in fields such as tissue engineering. In vivo gene delivery is being investigated for multiple diseases, such as cancer and hemophilia, but is often limited by the efficiency of gene transfer. Biomaterials may be able to enhance the safety and efficacy of vector delivery to increase the number of cells expressing the transgene and the level and duration of transgene expression.
Biomaterials-based delivery has recently been extended to tissue engineering, where scaffolds provide structural support for cell adhesion and subsequent tissue formation. Gene delivery from the scaffold provides a versatile approach to stimulate cellular processes in tissue regeneration. Implantable biomaterials with immobilized vectors can provide a means to locally express tissue-inductive factors and thus maintain therapeutic concentrations for extended times. The immobilization of genes on biomaterials has been used in gene delivery from stainless steel stents,5,16,20 platinum microcoils,21 and polyurethane heart valve cusps.27
The ability to spatially pattern gene expression will be important to regenerating complex tissue structures such as vascular and neural networks, which require controlling the placement of molecular signals.28 Genes have been delivered from a topographically patterned HA hydrogel, resulting in patterned expression in cells oriented in the grooves of the gel.7 Substrate-mediated delivery may be broadly applicable to a variety of implantable materials, both natural and synthetic, to develop novel clinical therapies.
Conclusions
Gene delivery from the surface of a material is a valuable approach to enhancing gene delivery by overcoming transport limitations, reducing degradation of delivered particles, enhancing effective concentration of vectors, and localizing gene delivery. The interaction between the substrate and vector requires an appropriate balance to maintain the DNA at the surface while allowing for cellular internalization. Surface immobilization of genes has applications to basic research, tissue engineering, and functional genomics. The nanoscale control of chemical and physical properties of biomaterial substrates, combined with the development of strategies to regulate vector immobilization and release, will enable a variety of novel applications.
Acknowledgments
Support for this work was provided in part by grants from the U.S. National Institutes of Health (RO1GM066830) and the National Science Foundation (BES0092701). We apologize to all of the scientists whose work we could not cite due to space restrictions.
References
- 1.Luo D, Saltzman WM. Nat Biotechnol. 2000;18:893. doi: 10.1038/78523. [DOI] [PubMed] [Google Scholar]
- 2.Bengali Z, Pannier AK, Segura T, Anderson BC, Jang JH, Mustoe TA, Shea LD. Biotechnol Bioeng. 2005;90:290. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng WC, Haselton FR, Giorgio TD. J Biol Chem. 1997;272:25641. doi: 10.1074/jbc.272.41.25641. [DOI] [PubMed] [Google Scholar]
- 4.Varga CM, Hong K, Lauffen-burger DA. Mol Ther. 2001;4:438. doi: 10.1006/mthe.2001.0475. [DOI] [PubMed] [Google Scholar]
- 5.Levy RJ, Song C, Tallapragada S, DeFelice S, Hinson JT, Vyavahare N, Connolly J, Ryan K, Li Q. Gene Ther. 2001;8:659. doi: 10.1038/sj.gt.3301452. [DOI] [PubMed] [Google Scholar]
- 6.Segura T, Shea LD. Bioconjugate Chem. 2002;13:621. doi: 10.1021/bc015575f. [DOI] [PubMed] [Google Scholar]
- 7.Segura T, Chung PH, Shea LD. Biomaterials. 2005;26:1575. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Tissue Eng. 2005;11:1. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 9.Norde W, Lyklema J. J Biomater Sci, Polym Ed. 1991;2:183. doi: 10.1080/09205063.1991.9756659. [DOI] [PubMed] [Google Scholar]
- 10.Ziauddin J, Sabatini DM. Nature. 2001;411:107. doi: 10.1038/35075114. [DOI] [PubMed] [Google Scholar]
- 11.Chang FH, Lee CH, Chen MT, Kuo CC, Chiang YL, Hang CY, Roffler S. Nucleic Acids Res. 2004;32:33. doi: 10.1093/nar/gnh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JT, Chua LS, Lynn DM. Langmuir. 2004;20:8015. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj B, Lei P, Andreadis ST. Biotechnol Prog. 2001;17:587. doi: 10.1021/bp010039n. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Tan J, Saltzman WM. Nat Mater. 2004;3:569. doi: 10.1038/nmat1179. [DOI] [PubMed] [Google Scholar]
- 15.Bielinska AU, Yen A, Wu HL, Zahos KM, Sun R, Weiner ND, Baker JR, Jr, Roessler BJ. Biomaterials. 2000;21:877. doi: 10.1016/s0142-9612(99)00229-x. [DOI] [PubMed] [Google Scholar]
- 16.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, Tietz AB, Kirchmair R, Silver M, Curry C, Wecker A, Yoon YS, Heidenreich R, Hanley A, Kearney M, Tio FO, Kuenzler P, Isner JM, Losordo DW. Circulation. 2003;110:36. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 17.Barry MA, Campos SK, Ghosh D, Adams KE, Mok H, Mercier GT, Parrott MB. Expert Opin Biol Ther. 2003;3:925. doi: 10.1517/14712598.3.6.925. [DOI] [PubMed] [Google Scholar]
- 18.Segura T, Volk MJ, Shea LD. J Controlled Release. 2003;93:69. doi: 10.1016/j.jconrel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Pandori M, Hobson D, Sano T. Virology. 2002;299:204. doi: 10.1006/viro.2002.1510. [DOI] [PubMed] [Google Scholar]
- 20.Klugherz BD, Song C, DeFelice S, Cui X, Lu Z, Connolly J, Hinson JT, Wilensky RL, Levy RJ. Hum Gene Ther. 2002;13:443. doi: 10.1089/10430340252792576. [DOI] [PubMed] [Google Scholar]
- 21.Abrahams JM, Song C, DeFelice S, Grady MS, Diamond SL, Levy RJ. Stroke. 2002;33:1376. doi: 10.1161/01.str.0000014327.03964.c0. [DOI] [PubMed] [Google Scholar]
- 22.Honma K, Ochiya T, Nagahara S, Sano A, Yamamoto H, Hirai K, Aso Y, Terada M. Biochem Biophys Res Commun. 2001;289:1075. doi: 10.1006/bbrc.2001.6133. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa T, Uchimura E, Kishi M, Funeriu DP, Miyake M, Miyake J. J Controlled Release. 2004;96:227. doi: 10.1016/j.jconrel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi F, Kato K, Iwata H. Biochim Biophys Acta–General Subjects. 2004;1672:138. doi: 10.1016/j.bbagen.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Michiels F, van Es H, van Rompaey L, Merchiers P, Francken B, Pittois K, van der Schueren J, Brys R, Vandersmissen J, Beirinckx F, Herman S, Dokic K, Klaassen H, Narinx E, Hagers A, Laenen W, Piest I, Pavliska H, Rombout Y, Langemeijer E, Ma L, Schipper C, Raeymaeker MD, Schweicher S, Jans M, van Beeck K, Tsang IR, van de Stolpe O, Tomme P, Arts GJ, Donker J. Nat Biotechnol. 2002;20:1154. doi: 10.1038/nbt746. [DOI] [PubMed] [Google Scholar]
- 26.Delehanty JB, Shaffer KM, Lin BC. Anal Chem. 2004;76:7323. doi: 10.1021/ac049259g. [DOI] [PubMed] [Google Scholar]
- 27.Stachelek SJ, Song C, Alferiev I, DeFelice S, Cui X, Connolly JM, Bianco RW, Levy RJ. Gene Ther. 2004;11:15. doi: 10.1038/sj.gt.3302129. [DOI] [PubMed] [Google Scholar]
- 28.Saltzman WM, Olbricht WL. Nat Rev Drug Discov. 2002;1:177. [Google Scholar]