Abstract
The copper-catalyzed insertions of nitriles into the Si–C bonds of silacyclopropenes provide azasilacyclopentadienes, which can be converted to allylic amines after reduction and protodesilylation. The enamine functionality of azasilacyclopentadienes also participates in 1,4-addition reactions and undergoes a hydroboration and oxidation sequence to form an allylic 1,2-amino alcohol.
Allylic amines are useful intermediates in organic synthesis, and a number of methods have been developed for preparing these compounds.1 Because the insertion of carbonyl compounds into silacyclopropenes provides a method for the synthesis of allylic alcohols,2 we considered that reactions of silacyclopropenes with C–N multiple bonds could lead to a synthesis of allylic amines. Although the photochemical reactions of nitriles with a silacyclopropene have been reported,3,4 the insertion products underwent further reactions in modest yields, and applications of these reactions in synthesis were not described.3 In this Letter, we report the copper-catalyzed insertions of nitriles into the Si–C bonds of silacyclopropenes to form azasilacyclopentadienes. These compounds can be functionalized by reductions, 1,4-additions, and hydroborations to form allylic amines and allylic amino alcohols.5
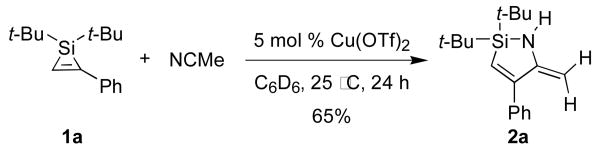
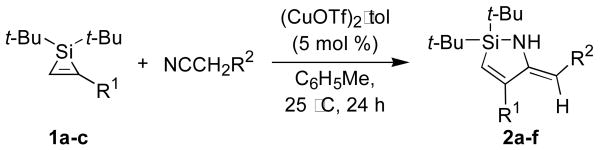
Copper salts proved to be efficient catalysts for the insertion of nitriles into silacyclopropenes. When a 1:1 mixture of acetonitrile and silacyclopropene 1a in C6D6 was treated with 5 mol % of Cu(OTf)2, silacyclopropene 1a disappeared over 24 hours, and enamine 2a was formed as a single regioisomer (Scheme 1). Preference for the 1,2-insertion product, which was confirmed by a 1H-1H NOESY experiment, is consistent with the regioselectivity of insertions of carbonyl compounds into silacyclopropenes and silacyclopropanes.2,6 The imine tautomer of 2a was not observed in the product mixure or at any time during the transformation. A subsequent catalyst screen for the acetonitrile insertion of silacyclopropene 1a showed that Cu(OTf)2 and (CuOTf)2·tol were more efficient catalysts than CuBr2 or CuI. When the transformation was performed on a one mmol scale, (CuOTf)2·tol gave higher yields than Cu(OTf)2 (Table 1, entry 1).
Scheme 1.
Insertion of acetonitrile into the Si–C bond of 1a.
Table 1.
Insertions of nitriles into monosubstituted silacyclopropenes.
 | |||
|---|---|---|---|
| entry | R1 | R2 | product, % yield |
| 1 | Ph | H | 2a, 82 |
| 2 | Ph | Me | 2b, 86 |
| 3 | Ph | Ph | 2c, 84 |
| 4 | Ph | CH2OTBDMS | 2d, 83 |
| 5a | SiMe3 | Ph | 2e, 65 |
| 6 | X | Ph | 2f, 82 |
Cu(OTf)2 was used as a catalyst. X = CH(Ph)(OSiEt3).
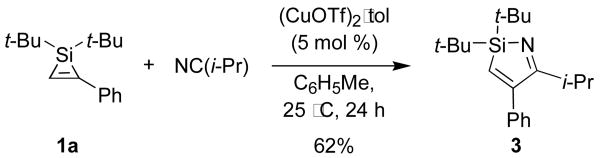
The insertion of a nitrile into a monosubstituted silacyclopropene was general for a number of nitriles and silacyclopropenes (Table 1). Nitriles with alkyl, aryl, and silyloxy groups (Table 1, entries 1-4) and silacyclopropenes with silyl- and silyloxy substituents (Table 1, entries 5-6) were tolerated by the reaction conditions.7 In all cases, the 1,2-insertion product and the Z-enamine were observed exclusively.8 The imine tautomer of the azasilacyclopentadiene was only observed for the insertion of isobutyronitrile into the Si–C bond of silacyclopropene 1a (Scheme 2). The formation of the enamine tautomer in this case may be disfavored because it would be destablized by interactions between the resulting isopropylidene group and the phenyl substituent.
Scheme 2.
Insertion of isobutyronitrile into 1a.
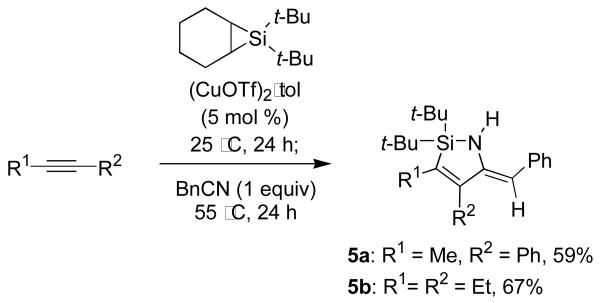
The copper-catalyzed insertion of nitriles was also successful for disubstituted silacyclopropenes (Table 2). These transformations required mild heating but proceeded smoothly with (CuOTf)2·tol as a catalyst. As with the analogous reactions of monosubstituted silacyclopropenes, a number of functional groups were tolerated. Consistent with the results described in Table 1, insertions into disubstituted silacyclopropenes favored the 1,2-regioisomer and Z-enamine products, although the regioselectivity of these insertions was lower than for the monosubstituted silacyclopropene reactions. Only a small degredation in regioselectivity was observed for 1-phenylpropyne-derived silacyclopropene 4a, but a further decrease in regioselectivity was observed for the silyloxy-substituted silacyclopropene 4c. These results suggest that steric effects are not the only factor contributing to regioselectivity.
Table 2.
Insertions of nitriles into disubstituted silacyclopropenes.
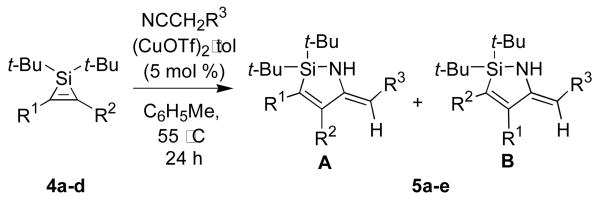 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | A:B | product, % yield |
| 1 | Me | Ph | Ph | 10:1 | 5a, 85 |
| 2 | Et | Et | Ph | na | 5b, 86 |
| 3 | Me | X | Me | 3:2 | 5c, 96 |
| 4 | Me | X | Ph | 3:1 | 5d, 83 |
| 5 | n-Bu | OTIPS | Ph | >10:1 | 5e, 85 |
X = CH(i-Pr)(OTIPS)
A two-step, one-flask synthesis of azasilacyclopentadienes was developed to avoid the isolation of air-sensitive silacyclopropenes. With internal alkenes, a single metal salt, Cu(OTf)2, was employed to catalyze both silylene transfer and insertion, as shown in Scheme 3.9 With terminal alkynes, Ag3PO4 was employed for silylene transfer,2 and, after filtration of the reaction mixture through glass fiber filter paper, copper salts were added to catalyze the insertion of the nitrile. 10
Scheme 3.
Two-Step, One-Flask, Single Catalyst Synthesis of Azasilacyclopentadienes
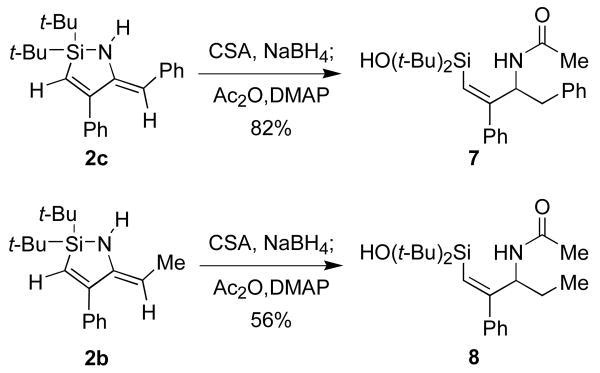
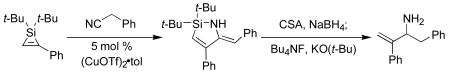
Reduction of the enamine moiety of the nitrile insertion products served as the first step of a synthesis of allylic amines. Although metal-catalyzed hydrogenation reduced both the alkene and enamine functional groups,10 the enamine functionality could be reduced selectivity by NaBH4 in the presence of camphorsulfonic acid (CSA).11 After aqueous workup, hydrolysis of the Si–N bond occurred to form the aminosilanols 6a-e (Table 3). The unpurified products of these transformations were isolated in high yields and were of sufficient purity (>90% as estimated by 1H NMR spectroscopy) to use in subsequent transformations. The purification of these compounds by chromatography, however, was challenging because of their amphiphilic nature.12 Protection of the amino group of the products was investigated to facilitate purification of the reduction products, but the success of this approach was substrate-dependent (Scheme 4).
Table 3.
Reduction of azasilacyclopentadienes.
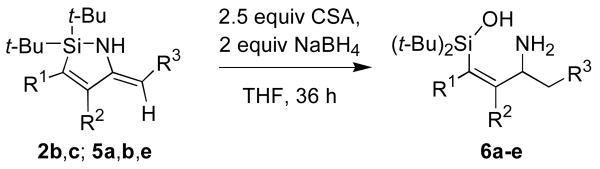 | ||||
|---|---|---|---|---|
| entry | R1 | R2 | R3 | product, % yielda |
| 1 | H | Ph | Ph | 6a, 90 (59) |
| 2 | H | Ph | Me | 6b, 95 (60) |
| 3 | Me | Ph | Ph | 6c, 80 (32) |
| 4 | Et | Et | Ph | 6d, 82 |
| 5 | n-Bu | OTIPS | Ph | 6e, 88 |
Isolated yields of unpurified products are reported. Yields after chromatography are shown in parentheses.
Scheme 4.
Enamine reduction and amine protection.
Once conditions for the reduction of azasilacyclopentadienes had been determined, the protodesilylation of allylic aminosilanols was investigated. Although protodesilylation under acidic conditions was not successful, treatment of 6a with KO(t-Bu) and Bu4NF in a mixture of DMSO and THF (4:1)4,5 provided the desired allylic amine 9a. This procedure was general for the synthesis of a range of allylic amines and amides (Table 4).
Table 4.
Protodesilylations of allylic aminosilanols.
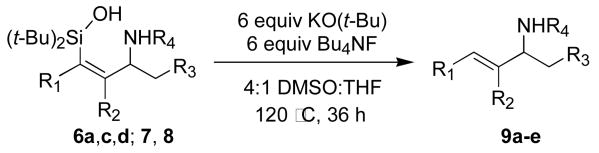 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | R4 | % yield |
| 1 | H | Ph | Ph | H | 9a, 74 |
| 2 | H | Ph | Ph | COMe | 9b, 55 |
| 3 | H | Ph | Me | COMe | 9c, 74 |
| 4 | Et | Et | Ph | H | 9d, 58 |
| 5 | Me | Ph | Ph | H | 9e, 54 |
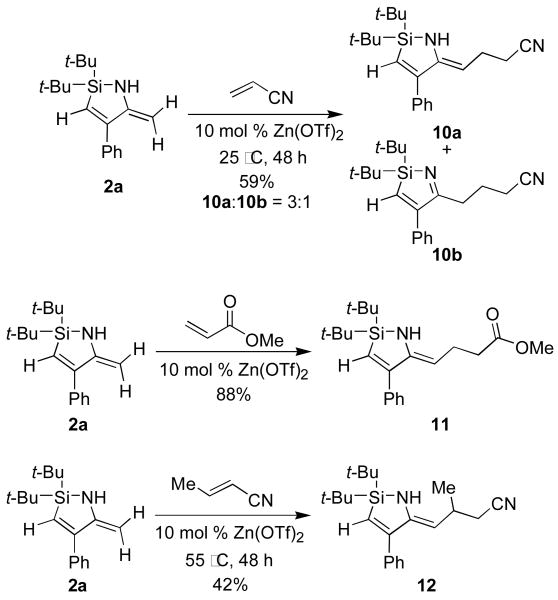
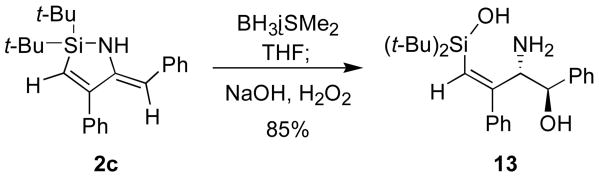
The enamine functionality of the azasilacyclopentadiene provided a handle to functionalize the nitrile insertion products. 1,4-Additions13 of azasilacyclopentadiene 2a to acrylonitrile gave a tautomeric mixture of the addition product 10, but additions to methyl acrylate and crotononitrile were selective for the enamine tautomer (Scheme 5). Hydroboration of azasilacyclopentadiene 12c followed by oxidation with NaOH and H2O2 provided allylic amino alcohol 13 as a single stereoisomer.14,15 These transformations showed that azasilacyclopentadienes can be functionalized at the enamine position to provide highly substituted allylic amine derivatives.
Scheme 5.
1,4-Addition Reactions of 2a.
In summary, a procedure for the synthesis of azasilacyclopentadienes has been developed based on insertions of nitriles into the Si–C bonds of silacyclopropenes. The synthetic utility of these reactions has been demonstrated by conversions of the products to allylic amines and allylic amino alcohols. The azasilacyclopentadiene insertion products were also shown to undergo 1,4-addition reactions as well as a hydroboration and oxidation procedure to form an allylic amino alcohol.
Supplementary Material
Scheme 6.
Hydroboration and Oxidation of 2c.
Acknowledgments
This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health (GM-54909). L. L. A. thanks the National Institutes of Health for a postdoctoral fellowship (GM-57688). K. A. W. thanks Amgen and Lilly for awards to support research. We would like to thank Dr. John Greaves and Ms. Shirin Sorooshian (UCI) for assistance with mass spectrometry, and Dr. Phil Dennison (UCI) for help with NMR spectroscopy.
Footnotes
Supporting Information Available Complete experimental procedures and product characterization. This information is available free of charge via the internet at http://www.pubs.acs.org.
References
- 1.For reviews that include methods for the synthesis of allylic amines, see: Johannsen M, Jørgensen KA. Chem Rev. 1998;98:1698–1708.Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2943. doi: 10.1021/cr020027w.Overman LE, Carpenter NE. Org React. 2005;66:1–107. For examples of different methods for allylic amine synthesis, see: Wipf P, Kendall C, Stephenson CRJ. J Am Chem Soc. 2003;125:761–768. doi: 10.1021/ja028092a.Anderson CE, Overman LE. J Am Chem Soc. 2003;125:12412–12413. doi: 10.1021/ja037086r.Yamashita Y, Gopalarathnam A, Hartwig JF. J Am Chem Soc. 2007;129:7508–7509. doi: 10.1021/ja0730718.Ngai MY, Barchuk A, Krische M. J Am Chem Soc. 2007;129:12644–12645. doi: 10.1021/ja075438e.Lalic G, Krinsky JL, Bergman RG. J Am Chem Soc. 2008;130:4459–4465. doi: 10.1021/ja7106096.Kinder RE, Zhang Z, Widenhoefer RA. Org Lett. 2008;10:3157–3159. doi: 10.1021/ol8010858.
- 2.Clark TB, Woerpel KA. J Am Chem Soc. 2004;126:9522–9523. doi: 10.1021/ja047498f. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai H, Kamiyama Y, Nakadaira Y. J Chem Soc, Chem Commun. 1978:80–81. [Google Scholar]
- 4.For a related insertion of an imine, see: Seyferth D, Duncan DP, Shannon ML. Organometallics. 1984;3:579–583.
- 5.For the synthesis of an allylic amine via a silaaziridine intermediate, see: Nevárez Z, Woerpel KA. Org Lett. 2007;9:3773–3776. doi: 10.1021/ol701424a.
- 6.a Franz AK, Woerpel KA. J Am Chem Soc. 1999;121:949–957. [Google Scholar]; b Franz AK, Woerpel KA. Angew Chem Int Ed. 2000;39:4295–4299. doi: 10.1002/1521-3773(20001201)39:23<4295::AID-ANIE4295>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.All of the insertion products shown in Table 1 and Scheme 2 were isolated and purified by a hexane extraction from an acetonitrile solution of the reaction mixture in an inert atmosphere glovebox. This procedure allowed the products to be separated from the copper catalyst and avoided hydrolysis of the Si–N bonds of these sensitive products. Additional confirmation of structure was obtained for the corresponding hydrolysis products; details are provided as Supporting Information.
- 8.The enamine stereochemistry was determined by 1H-1H NOESY experiments.
- 9.For transition metal-mediated reductive coupling reactions of alkynes and imines, see: Wipf P, Kendall C, Stephenson CRJ. J Am Chem Soc. 2003;125:76–768.Barchuk A, Ngai MY, Krische MJ. J Am Chem Soc. 2007;129:8432–8433. doi: 10.1021/ja073018j.Ngai MY, Barchuk A, Krische M. J Am Chem Soc. 2007;129:12644–12645. doi: 10.1021/ja075438e.
- 10.Details are provided as Supporting Information.
- 11.For recent examples of reductive amination under acidic conditions, see: Reddy PS, Kanjilal S, Sunitha S, Prasad RBN. Tetrahedron Lett. 2007;48:8807–8810.Heydari A, Arefi A, Esfandyari M. J Mol Catal A: Chem. 2007;274:169–172.
- 12.A purified sample of 6a was resubjected to chromatography conditions, and only 78% of the pure material was recovered. This observation suggested that a similar loss in yield occurred during the initial purification and accounted for the discrepancy between the unpurified and purified yields shown in Table 3. Attempts to solve the purification problems by buffering the eluant with Et3N or using silanized silica gel or alumina did not improve the purification.
- 13.For examples of additions of enamines to acrylonitrile and methyl acrylate, see: de Jeso B, Pommier JC. J Chem Soc, Chem Commun. 1977:565–566.Jones RCF, Hirst SC. Tetrahedron Lett. 1989;30:5361–5364.Pfau M, Ughetto-Monfrin J. Tetrahedron. 1979;35:1899–1904.Fourtinon M, de Jeso B, Pommier JC. J Organomet Chem. 1985;289:239–246.
- 14.For examples of enamine hydroboration, see: Butler DN, Soloway AH. J Am Chem Soc. 1966;88:484–487.Goralski CT, Hasha DL, Nicholson LW, Zakett D, Fisher GB, Singaram B. Tetrahedron Lett. 1994;35:3251–3254.
- 15.The allylic amino alcohol was isolated in 85% yield with >90% purity. This compound was of sufficient purity to be used in subsequent reactions. Purification by chromatography provided a 44% yield of the allylic amino alcohol.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.