Abstract
Background
Gene delivery by non-specific adsorption of non-viral vectors to protein-coated surfaces can reduce the amount of DNA required, and also increase transgene expression and the number of cells expressing the transgene. The protein on the surface mediates cell adhesion and vector immobilization, and functions to colocalize the two to enhance gene delivery. This report investigates the mechanism and specificity by which the protein coating enhances gene transfer, and determines if the protein coating targets the vector for internalization by a specific pathway.
Methods
Proteins (FBS, BSA, fibronectin, collagen I, and laminin) were dried onto culture dishes, followed by PEI/DNA complex adsorption for surface delivery. Reporter genes were employed to characterize transfection as a function of the protein identity and density. Vector immobilization was measured using radiolabeled plasmid, and internalization was quantified in the presence and absence of the endocytosis inhibitors chlorpromazine and genistein.
Results
Fibronectin coating yielded the greatest expression for PEI/DNA polyplexes, with maximal expression at intermediate protein densities. Expression in control studies with bolus delivery was independent of the protein identity. Substrate binding was independent of the protein identity; however, internalization was greatest on surfaces coated with fibronectin and collagen I. Inhibition of caveolae-mediated endocytosis reduced gene expression more than clathrin-mediated endocytosis. Similarly, inhibition of caveolae-mediated endocytosis significantly reduced the intracellular levels of DNA.
Conclusions
Fibronectin at intermediate densities mediated the highest levels of transgene expression, potentially by targeting internalization through caveolae-mediated endocytosis. Substrate modifications, such as the identity and density of proteins, provide an opportunity for modification of biomaterials for enhancing gene expression.
Keywords: gene delivery, reverse transfection, surface delivery, polyethylenimine (PEI), biomaterial, fibronectin, BSA
Introduction
Non-viral gene delivery is a promising technology that has many therapeutic and research applications such as gene therapy, tissue engineering, and functional genomics, yet this promise is unrealized due to low efficiency. Biomaterials can address some of the barriers to efficient gene transfer by preserving vector activity, localizing delivery, and overcoming vector transport limitations [1–4]. Biomaterials based approaches to gene delivery have been categorized into two mechanisms: polymeric release and substrate-mediated delivery [5]. Polymeric release involves the capture of nucleic acids within a biomaterial followed by controlled release for cellular internalization and expression [4,6–8]. A separate approach utilized in this report, termed reverse transfection [9], solid-phase delivery [10], or substrate-mediated delivery [11], immobilizes DNA vectors onto a surface that supports cell adhesion. Substrate-mediated delivery has applications other than the traditional gene delivery approaches. First, transfected cell arrays are based on the parallel delivery of DNA from a substrate, and enable high-throughput studies on gene function or activity [9,12,13]. Second, in tissue engineering, biomaterials are implanted to maintain a space for tissue formation. Cells seeded onto the material, or cells infiltrating into the material, can internalize the DNA and express the encoded gene, which could stimulate new tissue formation [14,15].
Immobilizing DNA for delivery onto a cell-adhesive substrate places DNA directly in the cell microenvironment; however, the immobilization must still allow for cell internalization. Several approaches have been employed for DNA immobilization including DNA entrapment in gelatin [9], poly-electrolyte layering of DNA [16,17], or preforming complexes followed by immobilization with specific tethering [3,11,18] or non-specific adsorption [10,19–23]. Substrate and vector properties mediate the vector-surface interactions that are determinants of binding and gene transfer [11,19,21]. Increasing surface hydrophilicity increases the quantity of complexes that bind to a surface through non-specific adsorption; whereas surfaces with an increasing amount of carboxylic acid groups produce greater gene expression [21]. A straightforward approach to manipulating the surface properties of a material involves the adsorption of proteins, such as serum, which can mediate DNA complex binding. Non-specific adsorption to serum-coated surfaces can reduce the amount of DNA required for expression, increase expression levels of transgene, and the number of cells expressing transgene [19,20] relative to bolus gene delivery [20] where bolus delivery is the typical approach to transfecting cells by adding the vector to the media above previously seeded cells. Substrate coating with serum increases the amount of DNA associated with cells, and better distributes DNA to the entire cellular population [24]. On tissue-culture polystyrene (TCPS), serum exposure results in adsorption of fibronectin that supports cell adhesion [25], and immobilization of non-viral vectors to the adsorbed proteins enhances gene transfer from the surface relative to no coating.
The protein coated on the substrate mediates the binding of both complexes and cells. Individual proteins or protein mixtures, such as serum, fibronectin, collagen, or laminin, are routinely deposited onto biomaterials to support cell adhesion [26–29]. These extracellular matrix (ECM) proteins have also been used by viruses as a means to facilitate cellular association and internalization of viral particles by colocalizing cells and the viral particles [30,31]. In addition to mediating cell attachment and the immobilization of vectors, the protein coating can potentially interface with cellular processes to direct internalization and trafficking. For example, fibronectin is internalized by a caveolin-dependent pathway [32], and thus vectors associated with fibronectin may similarly be internalized via caveolae-mediated endocytosis. The internalization pathway for these vectors influences the ultimate fate of the vector, as internalization via caveolae-mediated endocytosis may avoid the lysosome and subsequent degradation relative to internalization via clathrin-mediated endocytosis [33,34].
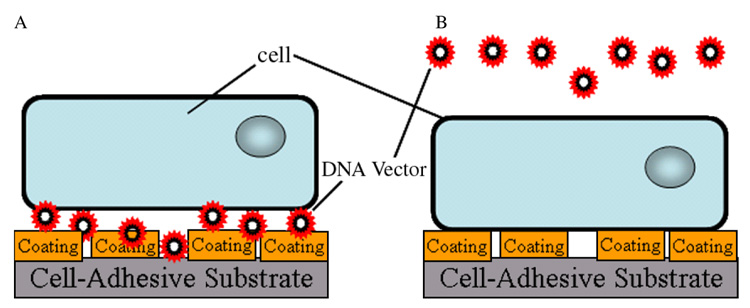
This report investigates the mechanism and specificity by which protein coatings on surfaces enhance transgene expression in substrate-mediated delivery. TCPS surfaces were coated with fetal bovine serum (FBS), bovine serum albumin (BSA) and fibronectin, which are major components of serum, and ECM components collagen and laminin. Substrate coating was followed by non-specific immobilization of polyethylenimine (PEI)/DNA polyplexes for gene expression (Figure 1A) and compared to surface coating preceding cell seeding and a bolus addition of polyplexes (Figure 1B). In addition to protein identity, differences in expression resulting from surface density were investigated for the amount of DNA bound to the substrate and amount of cell-associated DNA. For coatings with similar cell-associated DNA, differences in internalization of the complexes were investigated. Furthermore, we investigated the hypothesis that the identity of the coated protein would influence the internalization pathway. Increasing the efficiency of gene transfer using biomaterials based approaches will enhance the translation of these systems for therapeutic and research applications.
Figure 1.
Schematic of delivery system for (A) substrate-mediated delivery and (B) bolus delivery. (A) For substrate-mediated delivery, protein coatings were dried onto surfaces followed by complex immobilization for 2 h. Cells were then seeded on top of the complexes. (B) For bolus delivery, protein coatings were dried onto surfaces followed by cell seeding. Complexes were delivered as a bolus 14 h after cell seeding
Materials and methods
Materials
Plasmid DNA encoding for the enhanced green fluorescent protein and firefly luciferase (pEGFPLuc) with a cytomegalovirus (CMV) promoter was purified from bacteria culture using reagents from Qiagen (Santa Clara, CA, USA) and stored in Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, pH 7.4). Branched polyethylenimine (PEI, 25 kDa), fibronectin, and laminin were purchased from Sigma-Aldrich (St. Louis, MO, USA). PEI was dialyzed overnight with a 10 000 molecular weight (MW) cut off and lyophilized. Collagen I was purchased from BD Biosciences (San Jose, CA, USA). Fetal bovine serum (FBS) was purchased heat inactivated from Invitrogen (Carlsbad, CA, USA). Other reagents were obtained from Fisher Scientific (Fairlawn, NJ, USA) unless otherwise noted.
Substrate coating, complex formation and immobilization
Surfaces for DNA immobilization and cell seeding were prepared by adsorption of specific proteins to TCPS dishes (48- and 24-well). Initial studies compared delivery of complexes immobilized on coated substrates (Figure 1A) to bolus delivery of complexes added to cells seeded on coated substrates (Figure 1B). Subsequent studies involved complexes delivered from coated substrates. The indicated amounts of protein were diluted in phosphate-buffered saline (PBS) in 100 µL (48-well) or 200 µL (24-well) and added to each well and allowed to dry completely. Protein coatings were applied at 5 µg/cm2 or 4.7 µg/cm2 serum proteins (FBS) in PBS unless otherwise indicated. DNA complexes were formed by the addition of PEI [35] to plasmid DNA resulting in self-assembled colloidal particles. PEI/DNA complexes, termed polyplexes, were formed at an N/P ratio of 25 using 1.25 µg DNA for polyplexes to be immobilized to a 48-well dish and 2.5 µg to be immobilized to a 24-well dish. Polyplexes were formed in tris-buffered saline (TBS, pH 7.4). Polyplexes to be immobilized were formed by adding PEI solution (240 µL) dropwise to a solution containing DNA (360 µL), vortexed for 10 s and incubated for 15 min at room temperature. Complexes were immobilized on coated TCPS dishes. Preformed DNA complexes (300 µL, 48-well dish; 600 µL, 24-well dish) were incubated on the substrates for 2 h and were then washed twice with TBS. Polyplexes for bolus delivery were formed as above, but in 10% of the volume, which are typical volumes for gene delivery [35].
Cell culture and transfection
Transfection studies were performed with NIH/3T3 (ATCC, Manassas, VA, USA) cells cultured at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 1% sodium pyruvate, 1% penicillin–streptomycin, 1.5 g/L NaHCO3, and 10% heat-inactivated FBS (cDMEM). Cells were seeded at a density of 30 000 cells per well in 48-well plates. For substrate-mediated delivery, cells were seeded immediately following complex immobilization. For bolus delivery, cells were seeded on substrates 14 h prior to complex delivery. Cells were incubated with delivered vector in culture medium until transfection was analyzed, which was 24h after exposure to complexes unless otherwise indicated. Expression levels from luciferase plasmids were measured with the luciferase assay system (Promega, Madison, WI, USA). Cells were lysed with 200 µL reporter lysis buffer (Promega) and assayed for enzymatic activity. The luminometer was set for a 3 s delay with signal integration for 10 s. Luciferase activity was normalized to total cellular protein using the bicinchoninic acid (BCA) assay kit (Pierce, Rockford, IL, USA). All studies were carried out in triplicate.
Substrate binding and cellular association of DNA complexes
Quantification of plasmid DNA in the system utilized radiolabeled plasmid with α-32P-dATP. Briefly, a nick translation kit (GE Healthcare Life Sciences, Piscataway, NJ, USA) was used following the manufacturer’s protocol with minor modifications [11]. Plasmid integrity was confirmed with agarose gel electrophoresis. Radiolabeled plasmid was complexed and immobilized in 24-well dishes. The unbound DNA was determined by adding complex incubation solution and wash solution to scintillation cocktail (Biosafe II, 10 mL) for measurement on a scintillation counter. The amount of DNA immobilized was determined by subtracting the amount of unbound DNA from the DNA initially incubated on the substrate. Cell-associated DNA was determined by harvesting cells from the substrate after 24 h and adding to scintillation cock-tail. Cultured cells were washed once with PBS (300 µL) and exposed to trypsin (300 µL) for 3 min and quenched with 600 µL cDMEM. Cells were further removed from the substrate using a cell scraper. Cells were centrifuged at 500 g for 5 min and resuspended in PBS (500 µL) and added to scintillation cocktail (Biosafe II, 10 mL) for measurement on a scintillation counter.
Cellular internalization of DNA complexes
The internalization of DNA complexes was measured with a FACSscan flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with a 15 mW, 488 nm air-cooled argon-ion laser. A 530 nm bandpass filter was used to measure fluorescein-labeled plasmid DNA. Approximately 10 000 cells were analyzed per sample. For all samples, a threshold was set such that the negative control had 1% positive events (i.e. 1% of the negative control had internalized DNA). DNA was labeled with fluorescein using the Label IT nucleic acid labeling kit (Mirus, Madison, WI, USA) following the manufacturer’s protocol and complexes formed as described above for studies in 24-well dishes. Cells were harvested 24 h after exposure to DNA (as described above) and resuspended in 500 µL of 0.5% BSA and 0.1% sodium azide in PBS (PBA). Fluorescence from extracellular DNA was quenched by addition of trypan blue as described [33,36], and the mean fluorescence intensities were measured.
Inhibition of endocytosis
Cells were exposed to inhibitors of endocytosis as previously described [33] with modifications for substrate-mediated delivery. Clathrin-mediated endocytosis was inhibited with 10 µg/mL chlorpromazine [37,38] and caveolae-mediated endocytosis was inhibited with 50 µg/mL genistein [39,40]. Cells were split, counted, and diluted in inhibitor-containing media to a concentration of 215 000 cells/mL. Cells were incubated for 30 min in the media with inhibitor at 37 °C followed by seeding of 600 µL onto a protein-coated 24-well dish with immobilized DNA complexes. Inhibitors were added directly to the cell culture medium and incubated with cells during the course of the assay. In the presence of inhibitor, gene expression was analyzed 24 h after cell incubation with DNA, whereas internalization was assayed 4 h after cell incubation with DNA. Expression and internalization were measured as described above.
Statistical analysis
Results are represented by the mean and standard deviation. Experiments were performed with a minimum sample size equal to three, and replicates were performed with a similar minimum sample size. Statistical analyses were performed using a one-way analysis of variance (ANOVA) followed by Tukey-HSD in the software package JMP 4.0.4 (SAS Institute, Cary, NC). A value of p less than 0.05 was considered significant.
Results
Gene expression from coated substrates
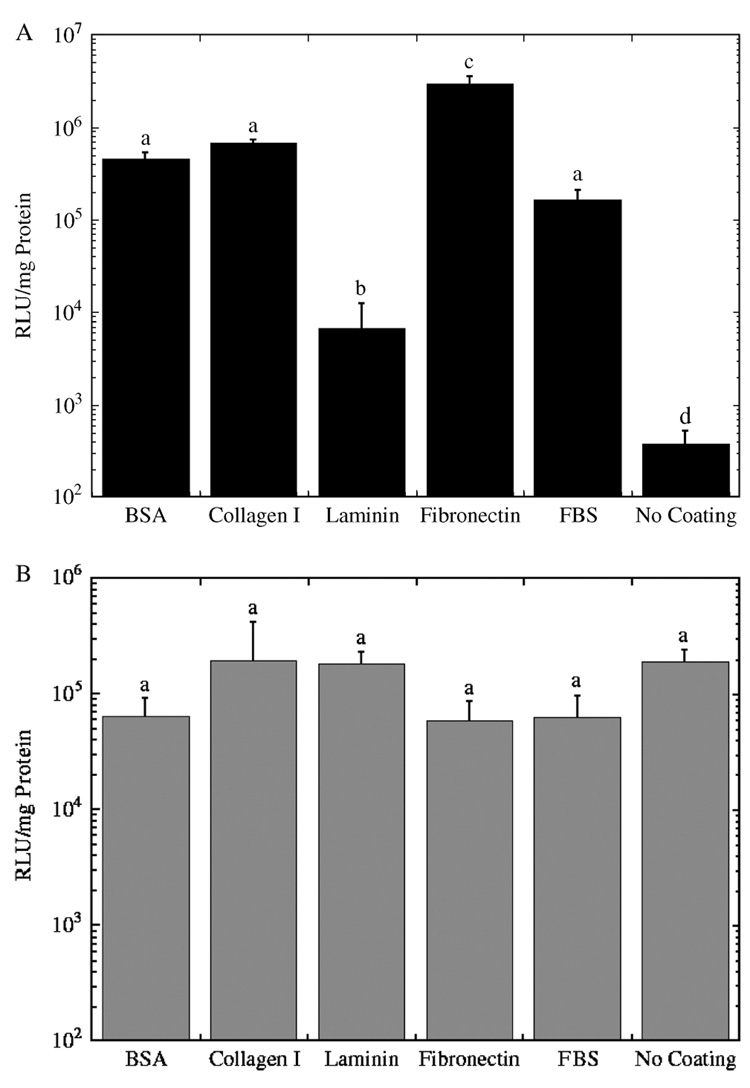
The identity of the protein deposited on the substrate influences the extent of transgene expression for substrate-mediated delivery, but has no effect for bolus delivery. Maximal expression for substrate-mediated expression was observed for PEI polyplexes delivered from a fibronectin-coated substrate (Figure 2A). Substrate coating increased expression levels over uncoated substrates 18–7800-fold. The rank order of expression levels as a function of the immobilized protein is fibronectin > collagen I = BSA = FBS > laminin > No Coating (Figure 2A; Table 1). Background levels of expression (i.e., no DNA) were negligible compared to the values obtained from the experimental conditions. The background levels provided relative light units (RLU)/mg readings between 0 and 1, which were 4 to 6 orders of magnitude less than the experimental values (104–106) (data not shown). Precoating substrates with proteins for bolus delivery yielded expression levels ranging from 1.9 × 105 to 5.8 × 104 RLU/mg protein (Figure 2B; Table 1), which was independent of the protein identity (p > 0.05), and addition of the fibronectin with complexes to preseeded cells did not enhance expression (data not shown). Coating of surfaces did not enhance expression for complex addition at the time of cell plating, or if complexes were delivered 2 h after cell plating (data not shown).
Figure 2.
Transgene expression for polyplexes delivered from (A) substrates and (B) as a bolus to NIH/3T3 cells. Luciferase expression levels 24 h after exposure of cells to polyplexes delivered at an N/P ratio of 25 with (A) coatings applied to the surface followed by complex immobilization and cell seeding and (B) coatings applied followed by cell seeding and then complex delivery as a bolus. Data is presented as an average of triplicate measurements ± standard deviation of the mean. A statistical significance with p < 0.05 is denoted for values with different letters
Table 1.
Protein coatings in substrate-mediated DNA delivery: rank order of proteins for their influence on multiple aspects of the gene transfer process
| Surface delivery | Bolus delivery | Surface-immobilized | Cell-associated | Internalized |
|---|---|---|---|---|
| Fibronectin (100%) | Collagen I (100%) | Fibronectin (100%) | Fibronectin (100%) | Fibronectin (100%); |
| No coating (99%) | FBS (97%) | BSA (89%) | Collagen I (99%) | |
| Laminin (95%) | BSA (96%) | Collagen I (85%) | ||
| BSA (33%) | Collagen I (85%) | |||
| FBS (33%) | ||||
| Fibronectin (30%) | ||||
| Collagen I (23%) | Laminin (11%) | FBS (48%) | FBS (60%) | |
| BSA (15%) | ||||
| FBS (6%) | ||||
| Laminin (0.5%) | Laminin (11%) | BSA (43%) | ||
| No coating (0%) | Laminin (2%) |
Percentages of maximum are indicated in parantheses. Differences in DNA levels between protein coatings listed in the same cell are not statistically significant.
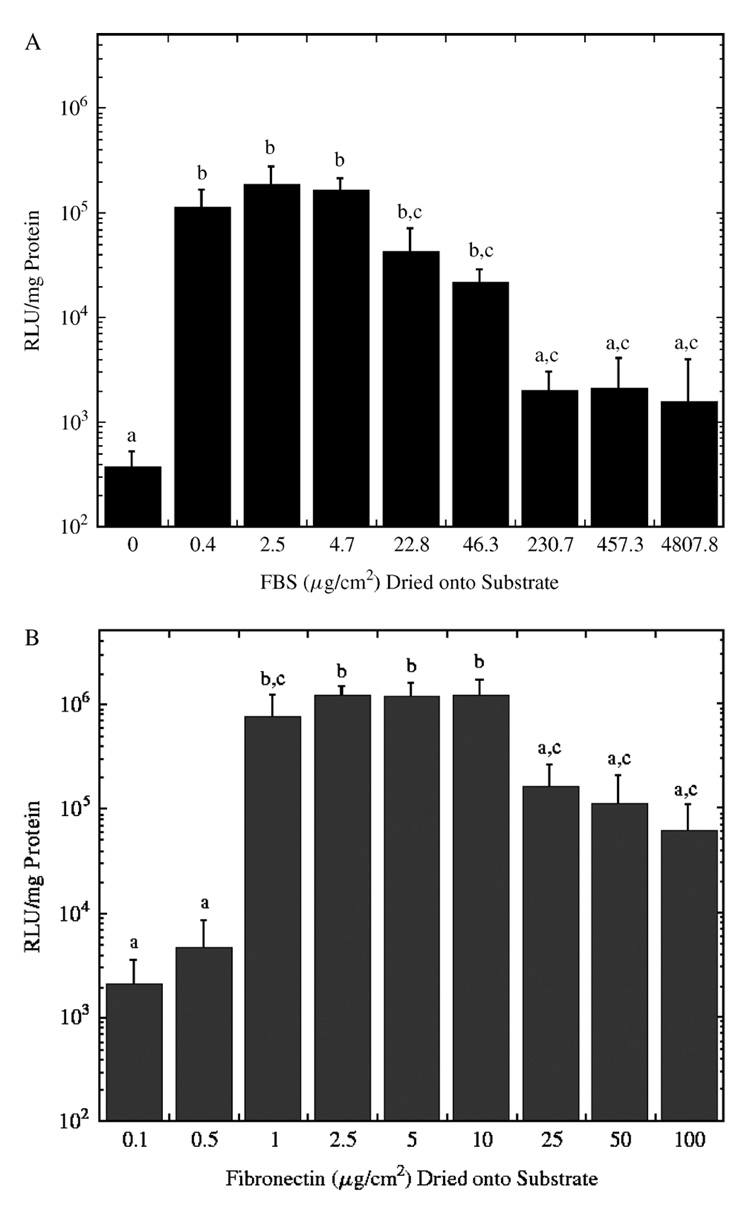
Protein surface density contributes to gene expression in surface delivery
The density of immobilized protein was subsequently investigated for effects on transgene expression. Gene expression was dependent on the amount of fibronectin or FBS adsorbed to the surface for substrate-mediated delivery. Low amounts of FBS deposited on the substrate yielded the highest levels of expression, and expression was reduced with the deposition of high levels of serum on the surface (Figure 3A). Differences in expression levels with coating more than 22.8 µg/cm2 serum proteins on the substrate were not statistically significant. Coating with 22.8 µg/cm2 serum proteins yielded 94-fold greater expression relative to 230.7 µg/cm2 serum proteins and 500-fold greater expression relative to no serum on the substrate.
Figure 3.
Luciferase expression levels for polyplexes formed at an N/P ratio of 25 and coated onto surfaces with a range of (A) serum and (B) fibronectin densities. Data is presented as an average of triplicate measurements ± standard deviation of the mean. A statistical significance with p < 0.05 is denoted for values with different letters
Expression from substrates with adsorbed fibronectin yielded the highest expression with densities ranging from 1–10 µg/cm2 (Figure 3B). This maximal expression was 19.5-fold greater than expression at higher surface densities (100 µg/cm2), and 563.5-fold greater than expression for lower densities (i.e., below 0.1 µg/cm2). Subsequent experiments investigated the mechanism by which the identity and density of adsorbed proteins affected gene expression by quantifying the amount of immobilized DNA, cellular-associated DNA, and intracellular DNA.
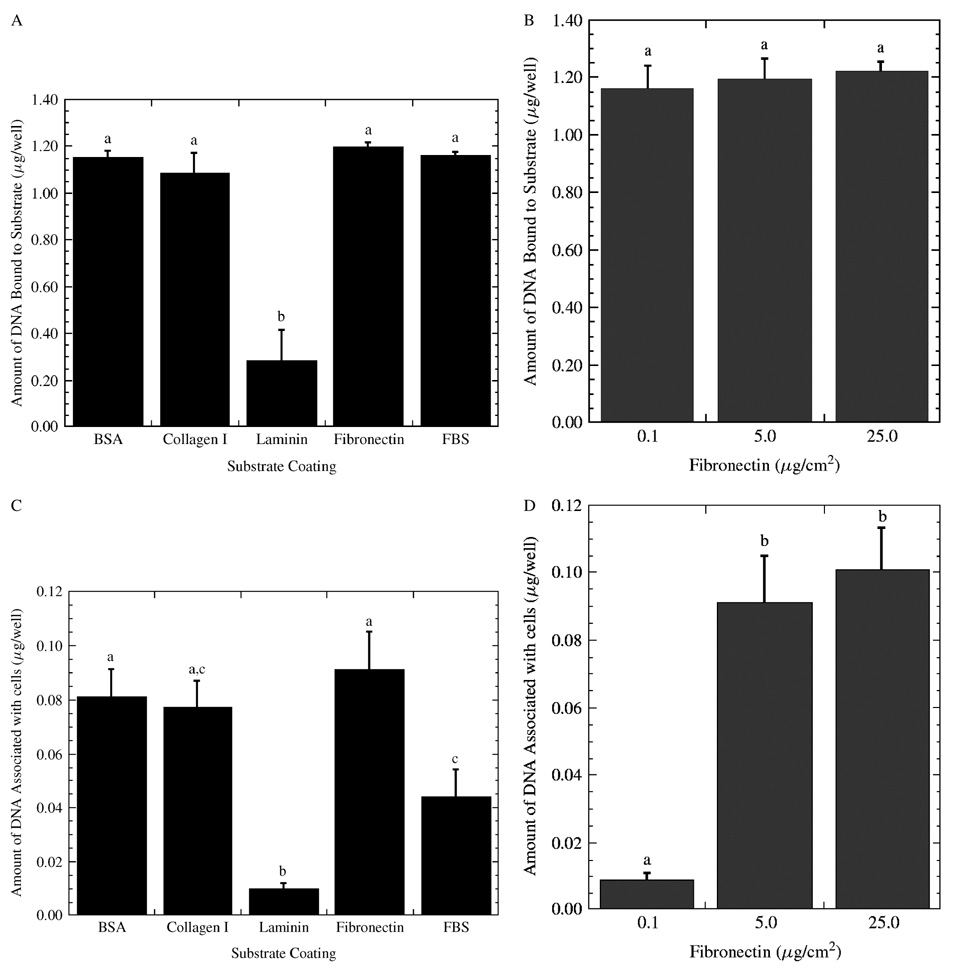
Substrate binding and cellular association of DNA
The identity of the adsorbed protein and its surface density on the substrate had no significant effect on the quantity of immobilized DNA, with the exception of laminin (Figure 4A), yet it does influence cellular quantities of DNA. The rank order of the proteins for DNA immobilization was fibronectin = FBS = BSA = collagen I > laminin (Figure 4A; Table 1), with laminin 7.3-fold less relative to the other proteins. Similarly, changing the fibronectin surface density on the surface yielded similar levels of PEI/DNA complexes bound to the substrate after immobilization (Figure 4B).
Figure 4.
Association of PEI/DNA complexes onto protein-coated substrates. The amount of DNA bound to the surfaces with (A) different protein coatings and (B) different fibronectin densities. The amount of DNA that associated with cells for delivery from substrates with (C) different protein coatings and (D) different fibronectin densities. Data is presented as an average of triplicate measurements ± standard deviation of the mean. A statistical significance with p < 0.05 is denoted for values with different letters
The identity and density of adsorbed protein affected the cell-associated DNA in a manner that did not parallel the amount immobilized. For the different proteins, the rank order based on cellular association was fibronectin = BSA = collagen I ≥ FBS > laminin (Figure 4C; Table 1). Fibronectin coating yielded 2.1- and 9.1- fold greater amounts of cell-associated DNA relative to FBS and laminin coatings. The influence of protein density was investigated with three different fibronectin densities (Figure 4D). DNA complexes delivered from substrates with fibronectin densities of 25 µg/cm2 had the highest cell-associated DNA, which was not significantly different from 5 µg/cm2 but was 10-fold higher than for densities of 0.1 µg/cm2. The amount of cell-associated DNA reflected some of the differences observed in gene expression (e.g., 0.1–10µg/cm2 fibronectin), but did not parallel the results obtained at high protein densities or the different protein identities. We thus investigated the amount of internalized complexes as a function of the protein identity and density.
Internalization of complexes from coated substrates
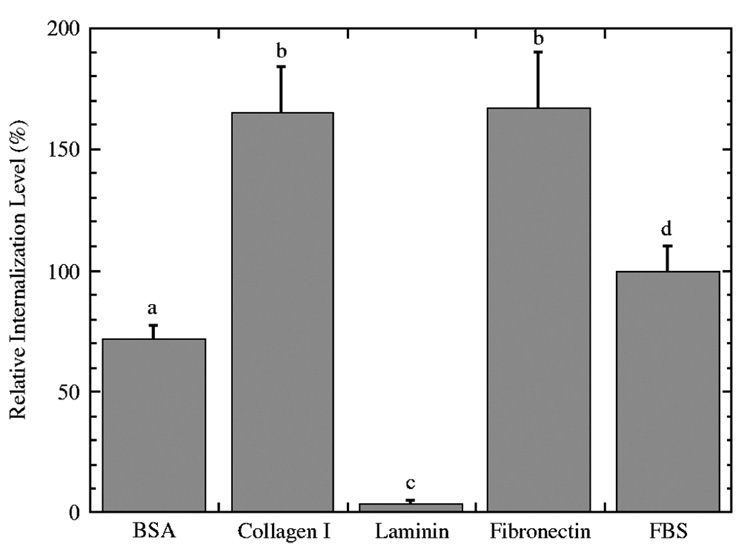
The relative amount of internalized DNA delivered from coated substrates was significantly affected by the identity of substrate coating. Relative fluorescence for each coated substrate was normalized to internalization from FBS surfaces. The rank order of the proteins with respect to the quantity of internalized DNA was fibronectin = collagen I > FBS > BSA > laminin (Figure 5; Table 1). Both BSA and laminin coatings yielded lower internalization levels relative to delivery from serum-coated substrates. FBS coating yielded 1.4- fold greater internalization relative to BSA and 27- fold greater internalization relative to laminin coatings. Fibronectin coating yielded 1.7, 2.3, and 45-fold greater internalization relative to FBS, BSA and laminin coatings, respectively. Complexes delivered from BSA and collagen I coated surfaces had similar levels of expression, yet the level of intracellular DNA was significantly greater with collagen I. Additionally, expression from surfaces with immobilized collagen I was less than for fibronectin-coated surfaces, yet internalization levels were similar. These discrepancies between the amount of intracellular DNA and the expression level suggest an effect on intracellular trafficking, and we thus investigated the pathway of endocytosis. Complex internalization at 4 °C was insignificant (data not shown).
Figure 5.
Internalization of PEI/DNA polyplexes from protein-coated substrates. Internalized fluorescence levels were normalized to fluorescence from FBS-coated substrates. Data is presented as an average of triplicate measurements ± standard deviation of the mean. A statistical significance with p < 0.05 is denoted for values with different letters
Contribution of individual endocytic pathways to gene expression from substrate-mediated delivery
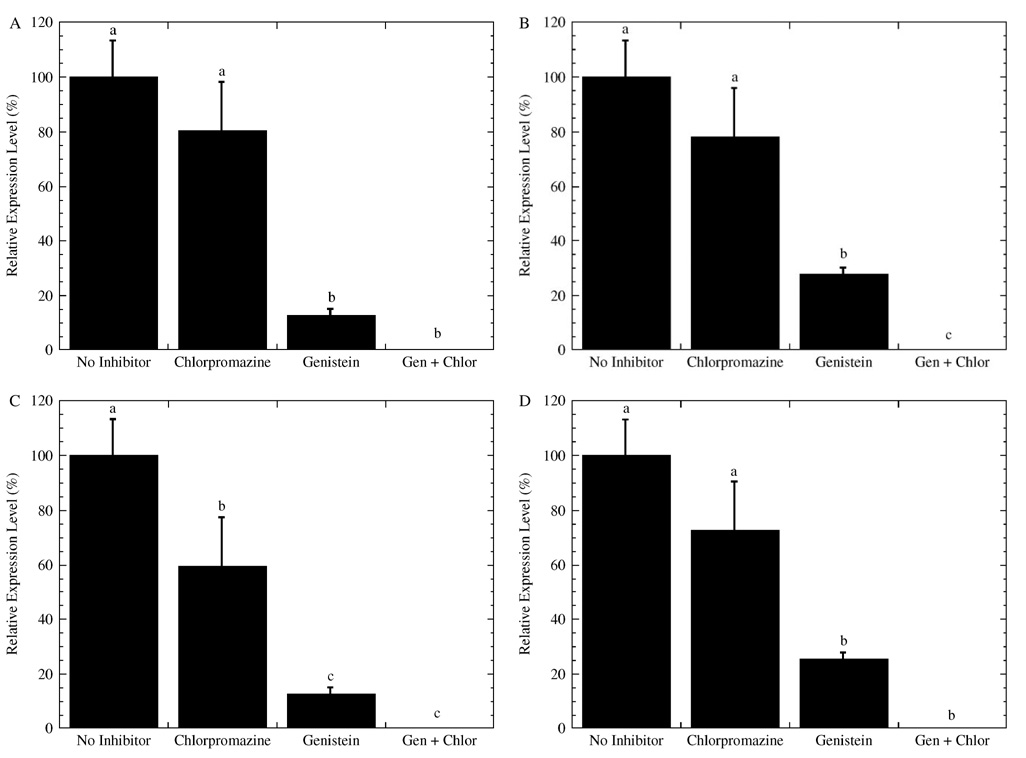
Inhibitors blocking endocytosis reduced gene expression for complexes delivered from the substrate, and inhibition of caveolae-mediated endocytosis (genistein [39,40]) yielded lower expression than inhibition of clathrin-mediated endocytosis (chlorpromazine [37,38]) (Figure 6). For all coatings, adding both chlorpromazine and genistein yielded negligible gene expression levels (Figures 6A–6D). Inhibition with chlorpromazine yielded expression levels that were 60%–80% of the no inhibitor conditions, but only fibronectin-coated substrates (Figure 6C) resulted in expression levels significantly different relative to no inhibitor conditions. Inhibition with genistein yielded expression that was less than 30% of no inhibitor conditions for all substrates. Cell treatment with genistein on BSA (Figure 6A) and fibronectin (Figure 6C) yielded relative expression levels of 13%, which was significantly less than the 27% obtained for collagen I (Figure 6B) or FBS (Figure 6D). Taken together, inhibition of caveolae-mediated endocytosis for PEI/DNA polyplexes delivered from coated substrates yielded lower expression than inhibition of clathrin-mediated endocytosis; however, BSA and fibronectin coatings were affected to a greater extent than collagen I and FBS. These differences in gene expression were subsequently investigated for the intracellular levels of DNA after exposure to the inhibitors.
Figure 6.
Gene expression from polyplexes delivered from coated substrates in the presence of endocytic inhibitors. Cells were cultured with the endocytic inhibitors chlorpromazine and genistein on substrates coated with (A) BSA, (B) collagen I, (C) fibronectin, and (D) FBS. Luciferase expression levels were normalized to non-inhibitor conditions for each substrate coating. Data is presented as an average of triplicate measurements ± standard deviation of the mean. A statistical significance with p < 0.05 is denoted for values with different letters
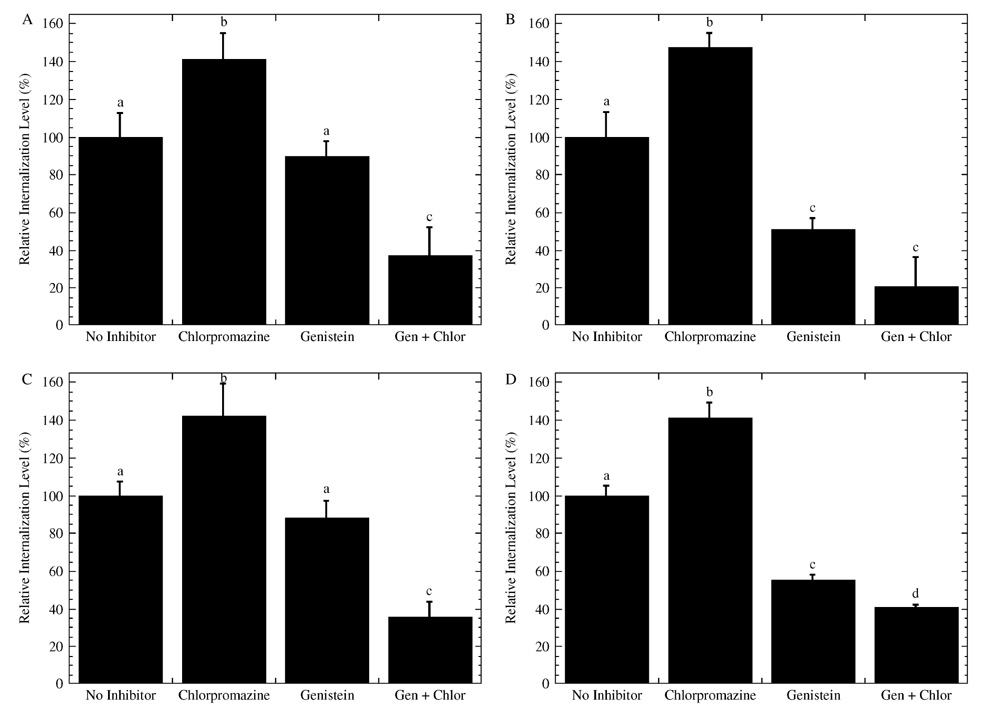
Complexes were internalized in the presence of chlorpromazine or genistein, although genistein treatment resulted in the lowest levels of intracellular DNA (Figure 7). For each coating, internalization of complexes was greatest in the presence of chlorpromazine and had internalization levels of DNA between 135% and 148% of no inhibitor control (Figures 7A–7D). In the presence of genistein, the relative internalization was 55% for collagen I (Figure 7B) and FBS (Figure 7D) coated substrates, which was less than the relative internalization on BSA (Figure 7A) and fibronectin (Figure 7C) (i.e., 88%). Inhibition with both genistein and chlorpromazine yielded similar or less internalization of complexes than inhibition with only genistein for all substrate coatings.
Figure 7.
Internalization of polyplexes delivered from coated substrates in the presence of endocytic inhibitors. Cells were cultured with the endocytic inhibitors chlorpromazine and genistein on substrates coated with (A) BSA, (B) collagen I, (C) fibronectin, and (D) FBS. Internalized fluorescence levels were normalized to fluorescence of non-inhibitor conditions for each substrate coating. Data is presented as an average of triplicate measurements ± standard deviation of the mean. A statistical significance with p < 0.05 is denoted for values with different letters
Discussion
This report investigates the mechanisms by which protein identity and density enhance gene expression for delivery by immobilization to proteins on a biomaterial surface. Substrate-mediated gene delivery involves immobilization of DNA complexes onto surfaces [3,11,19–21], and complex adsorption to immobilized proteins can enhance both gene expression and the number of transfected cells [19,20]. In this report, we demonstrate that the identity and density of the deposited protein affect gene expression in substrate-mediated delivery, but not bolus delivery. Fibronectin yielded the greatest expression for PEI/DNA polyplexes, with expression that was maximal at the intermediate protein densities examined. The protein identity did not influence substrate binding, but did affect internalization of immobilized complexes. Fibronectin and collagen I substrates had the greatest internalization. Inhibitors of endocytic pathways reduced gene expression for substrate-mediated gene delivery, with inhibition of caveolae-mediated endocytosis reducing expression more than clathrin-mediated endocytosis. Interestingly, inhibition of clathrin-mediated endocytosis increased cellular internalization of complexes; while inhibition of caveolae-mediated endocytosis and inhibition of both endocytic mechanisms yielded internalization similar or less than internalization without inhibitors.
Expression was dependent on the identity and density of immobilized protein, which results from effects on DNA immobilization, cellular association and internalization, and intracellular trafficking. The amount of DNA that immobilized on the surface and was available for expression was similar for most coatings, with the exception of laminin. For substrates with similar densities of immobilized DNA, the differences in expression thus result from either effects on cellular association, internalization, or trafficking. Generally, the protein coatings that produced higher amounts of cell-associated DNA yielded higher expression levels. Additionally, coatings that yielded higher intracellular levels of DNA had elevated expression. However, high cell association and internalization did not always lead to maximal expression, suggesting differences in intracellular trafficking.
Surface delivery of immobilized PEI/DNA complexes with inhibition of caveolae-mediated endocytosis yielded lower expression levels than inhibition of clathrin-mediated endocytosis, which is consistent with bolus delivery of complexes [33]. Particle size is a determinant of the internalization pathway for ligand-free particle beads. Beads with diameters less than 200 nm are internalized via clathrin-mediated endocytosis, and increasing bead size to 500 nm shifted internalization to caveolaemediated endocytosis [36]. The PEI/DNA complexes used herein have a diameter less than 200 nm in solution [19], which would suggest internalization directed preferentially towards clathrin pathways. For bolus delivery of complexes, genistein and chlorpromazine inhibited internalization to a similar extent [33]; however, surface delivery of complexes had the least amount of internalization in the presence of the genistein, which inhibits caveolae-mediated endocytosis, suggesting that delivery of polyplexes from coated surfaces may promote internalization by caveolae-mediated endocytosis. Chlorpromazine treatment actually increased the intracellular levels of DNA, which has similarly been observed for genistein in kidney cells and was hypothesized to result from a decrease in exocytosis [41]. Internalization of viruses, small molecules and DNA complexes through caveolaemediated endocytosis may avoid lysosomes [33,34,42], which could increase gene expression. This alternate pathway may lead to internalization of larger complexes that have a greater amount of DNA, which may be a tradeoff between the endosomal and lysosomal pathways.
Fibronectin coating yielded the greatest levels of gene expression, and complexes immobilized to fibronectin may enhance internalization and target the complexes to the caveolae. Fibronectin is a glycoprotein that supports cell adhesion through integrin receptors and mechanically integrates the cells with the extracellular matrix (ECM) [27,31]. Surface immobilization of fibronectin is thought to activate fibronectin by exposing cell-binding sites that are normally hidden with soluble fibronectin [43]. Fibronectin concentration affects the conformation of the adsorbed protein and the presentation of binding motifs to the cell [44]. Thus, at low concentrations, protein unfolding due to the increased availability of immobilization sites on the surface increases the availability of cell-binding domains and can thus promote complex internalization [44–46]. Maximal expression occurred when fibronectin was immobilized on the substrate at concentrations similar to fibronectin concentrations found in serum [25,47]. Fibronectin-coated substrates had similar levels of internalized complexes relative to collagen I coated substrates, suggesting that the complexes were trafficked through the cell more effectively on fibronectin-coated surfaces. Relative to other ECM components, fibronectin interacts with several integrin receptors on cell surfaces and has multiple synergistic sequences for receptor binding, which could contribute to the high cellular association and internalization of complexes. In particular, fibronectin colocalizes with β1 integrins and caveolin in fibrillar adhesions on the cell surface [48], and is internalized through a caveolin-1-dependent process [32]. Therefore, fibronectin coatings on the substrate may increase polyplex internalization and the propensity of complexes to internalize via caveolae-mediated endocytosis for better intracellular trafficking that avoids lysosomal degradation.
Complexes delivered from BSA-coated substrates had expression levels similar to collagen I, but had decreased levels of intracellular DNA. BSA was investigated as it is a major component of serum, and its incorporation into non-viral vectors can enhance gene expression [49–51]. For both BSA and collagen I, similar amounts of DNA bound to the surface and associated with cells. The lower internalization of complexes from BSA-coated substrates relative to collagen I suggests an enhanced intracellular trafficking for complexes on albumin. Inhibition of internalization with genistein reduced expression similar to complexes delivered from fibronectin, suggesting that complexes on BSA-coated surfaces may also prefer caveolae-mediated endocytosis. Endocytosis of albumin by kidney cells is blocked by genistein [41], and therefore may promote caveolae-mediated endocytosis to increase complex potency. Additionally, albumin incorporated into PEI nanoparticles produces particles with diameters of 300–700 nm [50]. This increase in size may influence intracellular trafficking and enhance expression [36,49–51] by targeting the complexes to the caveolae. For surface delivery, immobilized albumin could be incorporated into the immobilized PEI/DNA complexes to induce aggregation and increase the complex size, which may influence cellular trafficking.
In conclusion, substrate modifications, such as the identity and density of proteins, provide an opportunity for modification of biomaterials for enhancing gene expression. Substrate-mediated gene delivery has nontraditional applications in both applied and basic research, such as tissue engineering and transfected cell arrays. In tissue engineering contexts, specific protein coatings may enhance gene expression and provide a conducive ECM for tissue formation. Transfected cell arrays have used fibronectin to promote cell adhesion and increase gene expression while investigating gene knockdown by siRNA in parallel [23]. The surface proteins can influence extracellular events such as vector immobilization and cellular internalization, but may also influence intracellular events such as trafficking. These results support the potential to design substrates that will promote cellular association and support efficient intracellular trafficking that avoids degradation during intracellular processing. These results also suggest that materials may need to be designed based on the anticipated target cell, and the cellular response at the surface. Combining materials that enhance expression with efficient vectors will facilitate transfected cell array and tissue engineering applications.
Acknowledgements
Support was for this research was provided by grants from the National Institutes of Health (RO1 GM066830) and the National Science Foundation (BES 0092701).
References
- 1.Luo D, Saltzman WM. Enhancement of transfection by physical concentration of DNA at the cell surface. Nat Biotechnol. 2000;18:893–895. doi: 10.1038/78523. [DOI] [PubMed] [Google Scholar]
- 2.Schaffer DV, Lauffenburger DA. Optimization of cell surface binding enhances efficiency and specificity of molecular conjugate gene delivery. J Biol Chem. 1998;273:28004–28009. doi: 10.1074/jbc.273.43.28004. [DOI] [PubMed] [Google Scholar]
- 3.Segura T, Shea LD. Surface-tethered DNA complexes for enhanced gene delivery. Bioconjugate Chem. 2002;13:621–629. doi: 10.1021/bc015575f. [DOI] [PubMed] [Google Scholar]
- 4.Shea LD, et al. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 5.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Cohen-Sacks H, et al. Delivery and expression of pDNA embedded in collagen matrices. J Control Release. 2004;95:309–320. doi: 10.1016/j.jconrel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J Control Release. 2003;86:157–168. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 9.Ziauddin J, Sabatini DM. Microarrays of cells expressing defined cDNAs. Nature. 2001;411:107–110. doi: 10.1038/35075114. [DOI] [PubMed] [Google Scholar]
- 10.Bielinska AU, et al. Application of membrane-based dendrimer/DNA complexes for solid phase transfection in vitro and in vivo. Biomaterials. 2000;21:877–887. doi: 10.1016/s0142-9612(99)00229-x. [DOI] [PubMed] [Google Scholar]
- 11.Segura T, Volk MJ, Shea LD. Substrate-mediated DNA delivery: role of the cationic polymer structure and extent of modification. J Control Release. 2003;93:69–84. doi: 10.1016/j.jconrel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Bailey SN, Wu RZ, Sabatini DM. Applications of transfected cell microarrays in high-throughput drug discovery. Drug Discov Today. 2002;7 Suppl:S113–S118. doi: 10.1016/s1359-6446(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 13.Delehanty JB, Shaffer KM, Lin BC. Transfected cell microarrays for the expression of membrane-displayed single-chain antibodies. Anal Chem. 2004;76:7323–7328. doi: 10.1021/ac049259g. [DOI] [PubMed] [Google Scholar]
- 14.Houchin-Ray T, et al. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials. 2007;28:2603–2611. doi: 10.1016/j.biomaterials.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houchin-Ray T, Whittlesey KJ, Shea LD. Spatially patterned gene delivery for localized neuron survival and neurite extension. Mol Ther. 2007;15:705–712. doi: 10.1038/mt.sj.6300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jewell CM, et al. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J Control Release. 2005;106:214–223. doi: 10.1016/j.jconrel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Jewell CM, et al. Release of plasmid DNA from intravascular stents coated with ultrathin multilayered polyelectrolyte films. Biomacromolecules. 2006;7:2483–2491. doi: 10.1021/bm0604808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segura T, Chung PH, Shea LD. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26:1575–1584. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bengali Z, et al. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol Bioeng. 2005;90:290–302. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang JH, Bengali Z, Houchin TL, Shea LD. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A. 2005;77A:50–58. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pannier AK, Anderson BC, Shea LD. Substrate-mediated delivery from self-assembled monolayers: Effect of surface ionization, hydrophilicity, and patterning. Acta Biomaterialia. 2005;1:511–522. doi: 10.1016/j.actbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen H, Tan J, Saltzman WM. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat Mat. 2004;3:569–574. doi: 10.1038/nmat1179. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa T, et al. Transfection microarray of human mesenchymal stem cells and on-chip siRNA gene knockdown. J Control Release. 2004;96:227–232. doi: 10.1016/j.jconrel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Bengali Z, Shea LD. Cellular association and distribution of DNA delivered by immobilization to a culture substrate. 2006 submitted. [Google Scholar]
- 25.Steele JG, et al. Adsorption of fibronectin and vitronectin onto Primaria and tissue culture polystyrene and relationship to the mechanism of initial attachment of human vein endothelial cells and BHK-21 fibroblasts. Biomaterials. 1995;16:1057–1067. doi: 10.1016/0142-9612(95)98901-p. [DOI] [PubMed] [Google Scholar]
- 26.Buck CA, Horwitz AF. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- 27.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 28.Plow EF, et al. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 29.White DJ, et al. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol. 2004;36:1405–1410. doi: 10.1016/j.biocel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj B, Lei P, Andreadis ST. High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol Prog. 2001;17:587–596. doi: 10.1021/bp010039n. [DOI] [PubMed] [Google Scholar]
- 31.Proctor RA. Fibronectin: a brief overview of its structure, function, and physiology. Rev Infect Dis. 1987;9 Suppl 4:S317–S321. doi: 10.1093/clinids/9.supplement_4.s317. [DOI] [PubMed] [Google Scholar]
- 32.Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell. 2005;16:757–768. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 34.Bathori G, Cervenak L, Karadi I. Caveolae - an alternative endocytotic pathway for targeted drug delivery. Crit Rev Ther Drug Carrier Syst. 2004;21:67–95. doi: 10.1615/critrevtherdrugcarriersyst.v21.i2.10. [DOI] [PubMed] [Google Scholar]
- 35.Boussif O, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rejman J, et al. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sofer A, Futerman AH. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the Golgi apparatus and the subsequent elevation of cyclic AMP. J Biol Chem. 1995;270:12117–12122. doi: 10.1074/jbc.270.20.12117. [DOI] [PubMed] [Google Scholar]
- 39.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 40.Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116:1059–1071. doi: 10.1242/jcs.00327. [DOI] [PubMed] [Google Scholar]
- 41.Gekle M, et al. Albumin endocytosis in OK cells: dependence on actin and microtubules and regulation by protein kinases. Am J Physiol. 1997;272:F668–F677. doi: 10.1152/ajprenal.1997.272.5.F668. [DOI] [PubMed] [Google Scholar]
- 42.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 43.Akiyama SK, Yamada KM. Fibronectin. Adv Enzymol Relat Areas Mol Biol. 1987;59:1–57. doi: 10.1002/9780470123058.ch1. [DOI] [PubMed] [Google Scholar]
- 44.Koenig AL, Gambillara V, Grainger DW. Correlating fibronectin adsorption with endothelial cell adhesion and signaling on polymer substrates. J Biomed Mater Res A. 2003;64:20–37. doi: 10.1002/jbm.a.10316. [DOI] [PubMed] [Google Scholar]
- 45.Lewandowska K, et al. Modulation of fibronectin adhesive functions for fibroblasts and neural cells by chemically derivatized substrata. J Cell Physiol. 1989;141:334–345. doi: 10.1002/jcp.1041410215. [DOI] [PubMed] [Google Scholar]
- 46.Kowalczynska HM, et al. Fibronectin adsorption and arrangement on copolymer surfaces and their significance in cell adhesion. J Biomed Mater Res A. 2005;72:228–236. doi: 10.1002/jbm.a.30238. [DOI] [PubMed] [Google Scholar]
- 47.Hayman EG, et al. Vitronectin - a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985;160:245–258. doi: 10.1016/0014-4827(85)90173-9. [DOI] [PubMed] [Google Scholar]
- 48.Boyd ND, Chan BM, Petersen NO. Beta1 integrins are distributed in adhesion structures with fibronectin and caveolin and in coated pits. Biochem Cell Biol. 2003;81:335–348. doi: 10.1139/o03-063. [DOI] [PubMed] [Google Scholar]
- 49.Carrabino S, et al. Serum albumin enhances polyethylenimine-mediated gene delivery to human respiratory epithelial cells. J Gene Med. 2005;7:1555–1564. doi: 10.1002/jgm.799. [DOI] [PubMed] [Google Scholar]
- 50.Rhaese S, et al. Human serum albumin-polyethylenimine nanoparticles for gene delivery. J Control Release. 2003;92:199–208. doi: 10.1016/s0168-3659(03)00302-x. [DOI] [PubMed] [Google Scholar]
- 51.Simoes S, et al. Human serum albumin enhances DNA transfection by lipoplexes and confers resistance to inhibition by serum. Biochim Biophys Acta. 2000;1463:459–469. doi: 10.1016/s0005-2736(99)00238-2. [DOI] [PubMed] [Google Scholar]