Abstract
Argument structure, as in the participant roles entailed within the lexical representation of verbs, affects verb processing. Recent neuroimaging studies show that when verbs are heard or read, the posterior temporoparietal region shows increased activation for verbs with greater versus lesser argument structure complexity, usually bilaterally. In addition, patients with agrammatic aphasia show verb production deficits, graded based on argument structure complexity. In the present study, we used fMRI to examine the neural correlates of verb production in overt action naming conditions. In addition, we tested the differential effects of naming when verbs were presented dynamically in video segments versus statically in line drawings. Results showed increased neuronal activity associated with production of transitive as compared to intransitive verbs not only in posterior regions, but also in left inferior frontal cortex. We also found significantly greater activation for transitive versus intransitive action naming for videos compared to pictures in the right inferior and superior parietal cortices, areas associated with object manipulation. These findings indicate that verbs with greater argument structure density engender graded activation of both anterior and posterior portions of the language network and support verb naming deficit patterns reported in lesion studies. In addition, the similar findings derived under video and static picture naming conditions provide validity for using videos in neuroimaging studies, which are more naturalistic and perhaps ecologically valid than using static pictures to investigate action naming.
1 Introduction
Verbs differ with regard to the number and type of arguments (participant roles) they require. For example, verbs can be intransitive (requiring only an external argument, e.g., Zoë slept); transitive (requiring a subject and an object, e.g., Victor kicked the ball), or ditransitive (requiring a subject, direct object, and indirect object, e.g., Zack gave Lucy a present). Several studies with agrammatic Broca’s aphasic patients have shown that verb production becomes more difficult as the number of arguments entailed within the verb’s representation increases. This has been shown across languages, including English (Kemmerer & Tranel, 2000; Kim & Thompson, 2000, 2004; Thompson, Lange, Schneider, & Shapiro, 1997), Dutch (Jonkers, 2000; Jonkers & Bastiaanse, 1996, 1998), German (de Bleser & Kauschke, 2003), Hungarian (Kiss, 2000), and Italian (Luzzatti & Chierchia, 2002). Furthermore, this effect has been demonstrated across a range of tasks, including narrative speech (Thompson, Shapiro, Li, & Schendel, 1994), sentence production (Thompson et al., 1997), confrontation naming of verbs as singletons (Thompson et al., 1997; Kim & Thompson, 2000, 2004), and classification of verbs based on argument structure (Kim & Thompson, 2000, 2004). Also, verbs with even greater argument structure complexity, i.e., verbs such as know, which entail a sentential complement in their representation, present difficulty for Broca’s aphasic patients (Thompson et al., 1997), and intransitive unaccusative verbs such as melt, and amuse-type verbs of psychological state (psych-verbs), which involve complex syntactic and/or semantic computations, are more difficult to produce than intransitive unergatives such as sleep, and admire-type psych-verbs which do not (Bastiaanse & Van Zonneveld, 2005; Lee & Thompson, 2004; Thompson, 2003; Thompson & Lee, submitted).
These data from agrammatic Broca’s aphasic patients suggest a role for anterior perisylvian cortex in verb argument structure processing. However, other data suggest that posterior perisylvian cortex is also crucial for such processing. In several studies, Wernicke’s aphasic patients with posterior perisylvian lesions have demonstrated an inability to process aspects of argument structure information. McCann and Edwards (2002), for example, found that patients with Wernicke’s aphasia did not detect argument structure violations (e.g., John gives a car), an ability that is spared in Broca’s aphasic patients with anterior lesions (Kim & Thompson, 2000). Shapiro and Levine (1990) also found that during on-line sentence processing, Wernicke’s (but not Broca’s) aphasic patients showed a lack of sensitivity to argument structure. Neuroimaging studies with non-aphasic participants show a similar pattern. In an fMRI study using sentence grammaticality judgments, Ben-Shachar, Hendler, Kahn, Ben-Bashat, and Grodzinsky (2003) found increased left posterior superior temporal sulcus activation as a function of verb argument structure complexity. Similarly, Thompson, Bonakdarpour, Fix, Blumenfeld, Parrish, Gitelman and Mesulam (2007), using fMRI to investigate verbs with one, two, and three arguments in a lexical decision task, found activation in the posterior perisylvian cortex (inferior parietal lobule), graded based on the number of arguments. Shetreet, Palti, Friedmann and Hadar (2007) did not find activation in language areas as a function of verb argument structure complexity (only in left and right precuneus), however, activation as a function of the number of subcategorization and thematic role options allowed by verbs was found in the left superior temporal gyrus as well as in left inferior frontal gyrus.
Notably, all of these studies implicating involvement of the posterior perisylvian regions for verb argument structure processing have used receptive tasks: grammaticality judgment (McCann & Edwards, 2002; Ben-Shachar et al., 2003), anomaly detection (Shetreet et al., 2007), cross-modal lexical decision (Shapiro & Levine, 1990), and visual lexical decision (Thompson et al., 2007). Thus, these studies required participants to process argument structure, but not to produce verbs of differing argument structure, as in the aforementioned studies with agrammatic Broca’s aphasic patients. The purpose of the present study was to examine the neural correlates of verb naming, testing for effects of argument structure.
In addition, we investigated the role of the mode of presentation mode of verbs to ascertain whether or not action verbs, presented in video segments, elicited the same activation patterns as when presented in line-drawings. Most studies have used pictures or words as stimuli to examine verb processing or production, but pictures are by definition static, even if they depict dynamic events. This may add a degree of unnaturalness compared to the use of verbs in daily communication outside of laboratory conditions, particularly when the pictures are line drawings. Furthermore, in investigations of neuronal activation patterns correlated with verb processing compared to noun processing, or focused on the processing or retrieval of specific verb features or types, differences in activation patterns may be quite subtle. A more dynamic mode of stimulus representation, closer to the nature of action verbs themselves, may evoke stronger neuronal responses in verb elicitation tasks.
There are indications that for some speakers, action naming may improve under conditions where stimuli are presented in a dynamic mode. Druks and Shallice (2000) presented a case study of a patient who was severely impaired in picture naming of both actions and objects, while naming to definition was relatively preserved. Action naming based on real gestural performances (not on video, but live, by the experimenter), as well as naming of actions performed directly on the patient’s own body (for example, comb, pinch) proved to be significantly better than action naming based on picture stimuli. This patient, therefore, appears to have been helped by the strengthened semantic context and information offered by the more dynamic presentations. Even more semantic context was offered to twenty mild anomic patients in a study by Pashek and Tomkins (2002), who presented short videos for controntation naming of actions and objects, versus video segments shown in their context, for a condition called ‘video narration’. Whereas both conditions show actions in a dynamic mode, word retrieval was better in video narration, in which the lexical targets were embedded in a context.
Results from several other studies suggest that verb elicitation is more effective using video presentation than using picture presentation. D’Honincthun and Pillon (2005) reported a patient with frontotemporal dementia who showed a difference between verb and noun naming under picture naming conditions, with performance on verbs significantly worse that on nouns. However, when the patient was presented with the same verbs and nouns in video format, naming difficulty virtually disappeared.
In a crosslinguistic study on verb use, Naigles, Eisenberg, Kako, Highter and McGraw (1998) asked English and Spanish speakers to narrate scenes using picture stimuli, finding that English speakers used more manner-of-motion verbs (e.g., run), whereas Spanish speakers used more path-of-motion verbs (e.g., salir ‘exit’). This effect was subsequently replicated using video stimuli, however, the effects were stronger: the language specific verb type rates (manner for English speakers, path for Spanish speakers) were much higher in the video experiment as compared to the experiment using picture stimuli.
In another set of studies, Davidoff and Masterson (1995) performed two lexical retrieval experiments with children between 3 and 5.5 years of age, one using picture naming and the other using video naming. The primary purpose of their experiments was to examine patterns of noun, intransitive and transitive verb production. Results showed that nouns and transitive verbs were produced with greater accuracy than intransitive verbs in both experiments. In addition, inspection of their data showed differences in performance in the two experiments, which were not explicitly discussed by the authors: comparing the results from pictures and videos, the video stimuli elicited more correct responses for both intransitive verbs (57% in picture naming, versus 81% in video naming) and transitive verbs (70% in picture naming, versus 93% in video naming).
By contrast, there are also studies that have not found a difference between picture naming and videos. Jensen (2000) reports a case study of a patient with specific verb retrieval problems, whose action naming based on 3-5 second video fragments was no better than his pictured action naming. In a group study with eleven chronic aphasic speakers, with various types of aphasia, Berndt, Mitchum, Haendiges and Sandson (1997) did not find a difference between naming of actions presented on videotape and naming of actions in static pictures. Berndt, Haendiges and Wozniak (1997) present a case study of a severely anomic patient who shows no difference between naming to 30 picture and 25 video stimuli. In both modalities, verb naming was better than object naming. Lu, Crosson, Nadeau, Heilman, Gonzalez-Rothi, Raymer, Gilmore, Bauer and Roper (2002) compared action and object naming in videos, in nonaphasic speakers who had undergone left or right anterior temporal lobectomy. In both groups, they found that actions proved to be more difficult to name than objects, based on 20 second video stimuli. This pattern was reversed in picture naming control tasks, but with different stimuli, which makes it difficult to compare the results. Recently, Tranel, Manzel, Asp and Kemmerer (2008) compared dynamic action naming (based on 3-5 second videos) to static (picture) action naming and found a strong correlation between naming accuracy on the two tasks in a group of 71 brain-damaged, though nonaphasic, participants.
With respect to all such studies, it must be noted that it is very difficult to accurately match the stimuli presented as pictures and in dynamic mode. Not all verbs lend themselves for both presentation modes. In addition, dynamic action presentations may not only strengthen, or reinforce, the verb semantics accessed in lexical retrieval, but they may also introduce more noise to the visual scene. The inherent temporal constraint may have its downside as well. Most actions lead up to a ‘peak moment’, and whereas picture stimuli generally present a freeze of this moment in time, dynamic stimuli can only show that moment for as long as it lasts. Perhaps depending on such factors, the mode of stimulus presentation for action naming/training affects individuals differently, particularly in case of different types of brain damage and resulting aphasia.
Even though we would expect healthy adults without language impairment to perform at ceiling on simple verb elicitation tasks in either elicitation condition, differences may be expected in the strength or extent of neuronal activation patterns under the two conditions. The robustness of activation patterns is particularly important in studies on fine-grained distinctions within higher cognitive functions, such as verb argument structure processing. Using an optimal presentation mode for verb retrieval is even more relevant in language-impaired populations, both from the perspective of elicitation accuracy and strength of neuronal activation patterns. In addition, videos are more naturalistic, and therefore may afford greater ecological validity as compared to static pictures, another variable relevant to language-impaired individuals.
In the present study, we used event-related fMRI to examine intransitive (one-argument) and transitive (two-argument) verb naming in two conditions: one using static picture stimuli and the other using dynamic video segments of action verbs. We queried whether or not argument structure effects would be found in production, as they are in verb processing experiments, and whether these effects would be constrained to posterior perisylvian tissue or also include anterior cortical regions. We also questioned differences between the neuronal correlates of verb naming using picture versus video stimuli. Based on lesion studies and other data discussed above we expected transitive verbs to engender greater neural activation compared to intranstive verbs in both the static and video conditions. Further, we anticipated that graded argument structure effects would be seen in the inferior frontal region for production. Finally, we postulated that these effects would be greater in the video as compared to the static picture condition.
2 Methods
2.1 Participants
Eleven monolingual native speakers of English (7 females), between the ages of 20 and 36 (mean age 24) participated in the study. All were right-handed and had normal or corrected-to-normal vision. Participants gave their written informed consent and were compensated for their participation.
2.2 Materials
Stimuli consisted of pictures and videos of 10 intransitive and 10 transitive verbs (see appendix). These verbs were chosen on the basis of their imageability and their ‘filmability’, such that the verbs would not require use of extensive props and could be filmed in the laboratory. All intransitive verbs had agentive subjects; no unaccusative verbs with patients as subjects (e.g., fall) were used. All verbs had strong argument structure preferences, so no verbs took additional, optional arguments (e.g., eat, which can be transitive or intransitive, or throw, which takes an optional indirect object in John threw me the ball). The two lists of 10 intransitive and 10 transitive verbs were matched for the log10 lemma frequency per million from the CELEX English Lexical Database (t(18) = 1.01, p = 0.33; Baayen, Piepenbrock, & van Rijn, 1993).
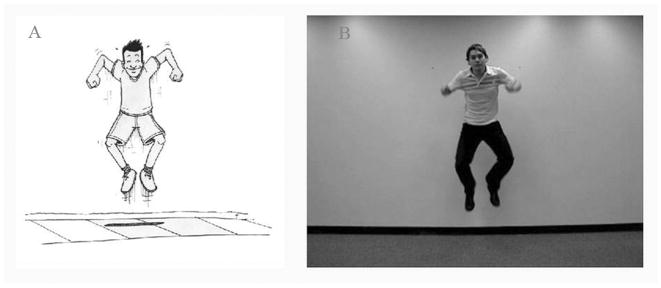
Picture stimuli for the 20 verbs, in the form of black-on-white line drawings, also were developed (Fig. 1a). Line drawings were chosen as these are our standard tools for noun and verb elicitation, and our research question sprang from our interest in whether naming from videos would yield stronger functional activation effects than naming using our usual elicitation method. The 20 videos were filmed with a Sony digital camera, against a white background (Fig. 2a). The same male actor played the agent of the action in all clips and was assisted by a female actor for transitive verbs that required an animate theme (kiss, pinch, tickle, hug, push). Pictures and videos were matched for complexity (i.e. the number and type of objects in the scene), as well as for the direction of agency for transitive verbs (i.e., always left to right). Each clip was edited to a duration of exactly 2000 ms.
FIGURE 1.
Example of a picture stimulus (A) and a still from a video stimulus (B) for the target verb jump.
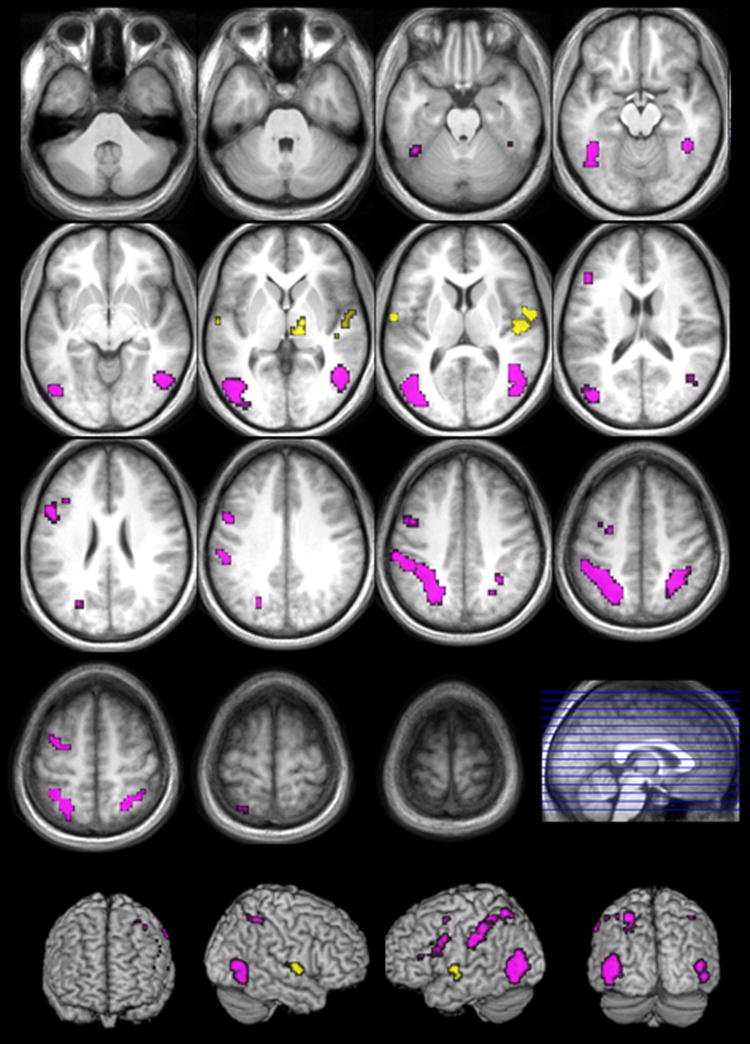
FIGURE 2.
Activation for the contrasts intransitive verb naming > transitive verb naming (yellow) and transitive verb naming >intransitive verb naming (violet) (FDR corrected; p>.05; k>10).
Pictures and videos were normed by asking 16 volunteers to name the portrayed action. These volunteers were unfamiliar with the experiment and did not further participate in the fMRI part of the study. Each verb was correctly elicited with both videos and pictures at least 90% of the time and all cases of error were verb substitutions of the same argument structure type (e.g., grin for smile).
2.3 Procedure
In a single-word response, participants overtly named the actions represented in the pictures and videos. These were presented using Superlab 4.0.1 (Cedrus Corp., Phoenix, AZ) and projected onto a non-magnetic screen behind the MRI scanner, which participants could see comfortably via a mirror placed on the head coil. In order to facilitate the auditory monitoring of participants’ responses over the scanner noise, a plastic tube was placed close to their mouths, and connected to the microphone in the scanner.
Using an event-related design, the stimuli were presented in pseudorandom order, i.e. pictures and videos showing the same action were never shown in sequence. Both videos and pictures were presented for 2000 ms.. Interstimulus invervals were jittered, with a duration of minimally 8 and maximally 10 seconds. A centrally placed fixation cross was presented between stimuli. Data were collected in three runs, each consisting of the complete list of stimuli, in varied orders.
Two researchers simultaneously monitored and scored participants’ overt responses online. Responses were clearly audible over the scanner noise via the scanner intercom system.
2.4 fMRI data acquisition and analysis
Scanning was carried out on a Siemens 3T TIM Trio scanner. A T1-weighted anatomical scan was obtained at the start of each protocol with the following parameters: TR = 2300 ms; TE = 3.36 ms; flip angle = 9°; image matrix = 224 × 256; FOV = 256; voxel size = 1 × 1 × 1 mm. During the experimental runs, functional volumes with BOLD contrast were obtained using gradient echo-planar imaging sequences (TR = 2000 ms; TE = 30 ms; flip angle = 80°; matrix size = 64 × 64; FOV = 220 mm; voxel size = 3.44 × 3.44 × 3 mm; 33 slices). To allow for image saturation, the first 6 volumes of each run were discarded.
Data preprocessing and statistical analysis was performed with SPM5 (http://www.fil.ion.ucl.ac.uk/spm) running in a Matlab 7.2.0.232 (R2006a) environment (the MathWorks, Navick, MA) on a Dell Optiplex GX620 pc. Functional scans were corrected for slice-acquisition timing and realigned to a mean functional volume. The anatomical volume was coregistered to the mean image and normalized to the Montreal Neurological Institute (MNI) 152-subject template brain (ICBM, NIH P-20 project), reslicing the volumes at a resolution of 3×3×3 mm. The functional volumes were then normalized using the same transformation and were spatially smoothed using a 6 mm (full-width, half-maximum) isotropic Gaussian kernel. Effects of global signal were removed from the functional time-series using the method described by Macey, Macey, Kumar & Harper (2004).
In the first-level analysis, a high-pass filter of 256 seconds was used to eliminate scanner drift. For each run, six movement parameters obtained during pre-processing were entered as regressors, to co-vary out effects correlated with head movement in the scanner. Individual participants’ summary activation maps for the four main effects (intransitive pictures, transitive pictures, intransitive videos and transitive videos) were entered into a second-level within-subject 2×2 analysis of variance (ANOVA) with presentation mode and argument structure as variables with two levels each.
Second-level statistics were first evaluated at a voxelwise significance threshold of p<.05, corrected for multiple comparisons per false discovery rate (FDR; Benjamini & Hochberg 1995; Genovese, Lazar & Nichols 2002), with a minimum cluster size of 10 contiguous voxels (270 mm3). A follow-up analysis evaluated the data at a clusterwise significance threshold of p<.05, corrected based on spatial extent, with all voxels in the cluster meeting an uncorrected voxel-wise significance threshold of p<.001. The latter analysis was to identify clusters of activation that should be taken into account based on their size, even if individual voxel activation within the cluster did not meet our more stringent voxel-wise threshold. Directional hypotheses were examined through t-tests, inclusively masked by t-tests of the positive main effects of the subtracted conditions (uncorrected for multiple comparisons, p < .1), to ensure that reported activation differences between conditions were not driven by negative BOLD responses (whether these be interpreted as ‘deactivation’ or not). Comparisons of argument-number effects between the two presentation modes were examined through t-tests of the interactions between argument structure and presentation mode, inclusively masked by t-tests of the relevant mode-specific contrasts (uncorrected for multiple comparisons, p<.1). This analysis revealed the effects of argument structure that were present only, or significantly stronger, in one of the two presentation modes.
3 Results
3.1 Behavioral results
Errors on the verb naming task, less than 5% of total responses, consisted of omissions and substitutions of verbs with a different argument structure than the target verb, with the majority being omissions, which were evenly distributed across modalities and verb types. Because of this even distribution and the low number of errors it was decided not to remove these trials from the dataset.
3.2 Argument structure effects
MNI coordinates and t-values of local maxima, along with cluster sizes and descriptions of cluster extent in terms of anatomical regions and Brodmann’s areas are given in Tables 1-5. Within the tables, clusters are ordered by maximal t-value.
TABLE 1.
Brodmann’s areas, MNI coordinates, cluster size and maximal t-values for local maxima in the contrast transitive verb naming > intransitive naming of actions (FDR corrected; p<.05; k>10).
| R/L | Activation Peak | Cluster Extent | BA | x | y | z | cluster size | t-Max |
|---|---|---|---|---|---|---|---|---|
| L | Superior Parietal Lobule | Supramarginal Gyrus, Precuneus, Inferior Parietal Lobule, Postcentral Gyrus | 1, 2, 7, 40 | -30 | -54 | 57 | 429 | 9.76 |
| R | Superior Parietal Lobule | Precuneus, Inferior Parietal Lobule | 7, 40 | 30 | -51 | 57 | 118 | 7.85 |
| L | Middle Temporal Gyrus | Inferior Occipital Gyrus, Inferior Temporal Gyrus, Middle Occipital Gyrus | 19, 37, 39 | -51 | -66 | 3 | 233 | 6.18 |
| L | Inferior Temporal Gyrus | Fusiform Gyrus, Middle Occipital Gyrus | 37 | -42 | -48 | -18 | 52 | 6.02 |
| R | Middle Temporal Gyrus | Inferior Temporal Gyrus, Middle Occipital Gyrus, Superior Temporal Gyrus | 19, 22, 37, 39 | 45 | -54 | 3 | 183 | 5.58 |
| L | Inferior Frontal Gyrus | Middle Frontal Gyrus | 45, 46 | -48 | 27 | 18 | 17 | 5.02 |
| R | Fusiform Gyrus | 37 | 42 | -45 | -15 | 15 | 4.15 | |
| L | Precentral Gyrus | Inferior Frontal Gyrus, Middle Frontal Gyrus | 6, 9, 44 | -51 | 6 | 30 | 64 | 4.05 |
| L | Superior Frontal Gyrus | Middle Frontal Gyrus | 6 | -27 | -6 | 60 | 31 | 4.00 |
TABLE 5.
Brodmann’s areas, MNI coordinates, cluster size and maximal t-values for local maxima in the contrast video naming > picture naming of actions (FDR corrected; p>.05; k>10).
| R/L | Activation Peak | Cluster Extent | BA | x | y | z | cluster size | t-Max |
|---|---|---|---|---|---|---|---|---|
| R | Middle Temporal Gyrus | Inferior Temporal Gyrus, Insula, Middle Occipital Gyrus, Superior Temporal Gyrus, Inferor Parietal Gyrus, Postcentral Gyrus | 13, 19, 21, 22, 29, 37, 39, 40, 41, 42 | 45 | -66 | 3 | 614 | 9.98 |
| L | Middle Temporal Gyrus | Middle Occipital Gyrus | 19, 37, 39 | -42 | -69 | 9 | 126 | 7.24 |
| L | Middle Temporal Gyrus | Insula, Superior Temporal Gyrus, Supramarginal Gyrus, Inferior Parietal Lobule | 13, 21, 22, 29, 39, 40, 42 | -51 | -48 | 12 | 223 | 6.79 |
| R | Postcentral Gyrus | Precentral Gyrus | 1, 2, 3, 4, 6 | 60 | -21 | 42 | 20 | 5.81 |
| R | Precentral Gyrus | Middle Frontal Gyrus, Inferior Frontal Gyrus | 6, 8, 9 | 42 | 0 | 42 | 131 | 5.71 |
| R | Calcarine Sulcus | Posterior Cingulate Cortex, Parahippocampal Gyrus, Lingual Gyrus | 18, 19, 30, 36 | 18 | -54 | 9 | 98 | 5.06 |
| R | Inferior Frontal Gyrus | Precentral Gyrus | 44, 45 | 54 | 15 | 21 | 74 | 4.77 |
| L | Lingual Gyrus | Parahippocampal Gyrus, Posterior Cingulate Gyrus | 18, 19, 30 | -18 | -48 | -6 | 28 | 4.30 |
| R | Superior Occipital Gyrus | Precuneus | 7, 19 | 21 | -78 | 36 | 37 | 4.15 |
Across presentation modes, transitive verb naming compared to intransitive verb naming correlated with significantly more activation in a large bilateral network of neuronal clusters, with more extensive activity in the LH (see Table 1 & Figure 2). There was bilateral activation in fusiform gyrus (BA 37), middle occipital gyrus (BA 19), posterior middle temporal and angular gyrus (BA 39), and supramarginal gyrus (BA 40), as well as parietal activation (BA 7), including the precuneus. In the LH, this activation extended to the inferior occipital gyrus (BA 37) and the parietal activation (BA 7) extended more superiorly. The LH also showed activation in the postcentral (BAs 1, 2) and precentral gyri (BA 6), middle frontal gyrus (BAs 6, 9, 46) and in the triangular and opercular parts of the inferior frontal gyrus (BAs 44 and 45).
Intransitive verb naming compared to transitive verb naming was associated with increased activation in mostly right lateralized temporal and subcortical structures (see Table 2 & Figure 2). These clusters of activation include the RH thalamus, precentral gyrus and superior temporal gyrus, with activation deep into the sylvian fissure and encompassing Heschl’s gyrus as well as the insula. In the LH, there was activation in posterior superior temporal gyrus (BAs 22, 42), and this activation extends medially into Heschl’s gyrus.
TABLE 2.
Brodmann’s areas, MNI coordinates, cluster size and maximal t-values for local maxima in the contrast intransitive verb naming > transitive naming of actions (FDR corrected; p<.05; k>10).
| R/L | Activation Peak | Cluster Extent | BA | x | y | z | cluster size | t-Max |
|---|---|---|---|---|---|---|---|---|
| R | Heschl’s Gyrus | Superior Temporal Gyrus, Transverse Temporal Gyrus, Insula, Precentral Gyrus | 6, 13, 22, 41, 42, 43 | 57 | -3 | 6 | 66 | 5.18 |
| L | Superior Temporal Gyrus | Heschl’s Gyrus, Middle Temporal Gyrus | 22, 42 | -60 | -6 | 6 | 10 | 4.50 |
| R | Thalamus | n/a | 15 | -12 | 3 | 20 | 4.22 |
3.3 Argument structure effects: movies vs. pictures
When analyzed separately by presentation mode, there was no increased activation associated with intransitive verb naming, compared to transitive verb naming, that reached the statistical threshold. By contrast, transitive verb naming was associated with increased activation in both conditions, largely in areas within those revealed by the same contrast in the combined presentation modes, as described above.
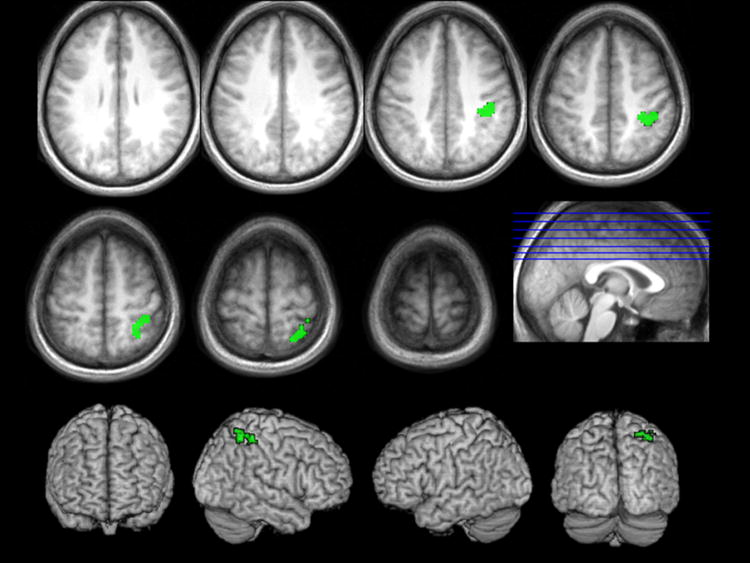
In order to examine whether activation correlated with differences in argument structure of the verbs was stronger in one or the other of the two presentation modes, we masked the t-tests of the positive and negative interactions between argument structure and presentation mode with the relevant mode-specific argument-structure effects. No voxels in either interaction survived our voxel-wise threshold. However, the positive interaction masked inclusively with the contrast of transitive videos > intransitive videos, did yield a large cluster of contiguous voxels (k=90), activation of which survived an uncorrected voxel-wise threshold of p<.001. Activation in this cluster was significant at a cluster threshold of p<.05, corrected based on spatial extent, in the RH superior and inferior parietal areas (BA 7), including the supramarginal gyrus (BA 40), and extending into postcentral gyrus (BAs 2, 3, 5) (see Table 3 & Figure 3).
TABLE 3.
Brodmann’s areas, MNI coordinates, cluster size and maximal t-value for the local maximum in the contrast transitive verb naming > intransitive naming of actions, for all areas where this activation was stronger in the video naming condition than in the picture naming condition (cluster-level correction, based on cluster extent; p>.05; voxel-level uncorrected threshold p<.0001).
| R/L | Activation Peak | Cluster Extent | BA | x | y | z | cluster size | t-Max |
|---|---|---|---|---|---|---|---|---|
| R | Postcentral Gyrus | Inferior Parietal Lobule, Supramarginal Gyrus, Superior Parietal Lobule | 2, 3, 5, 7, 40 | 42 | -30 | 42 | 90 | 4.61 |
FIGURE 3.
Stronger activation patterns for the transitive verb naming > intransitive verb naming contrast within the video naming condition (green) (cluster-level correction, based on extent; p<.05; voxel-wise uncorrected threshold p<.0001).
3.4 Pictures vs. videos
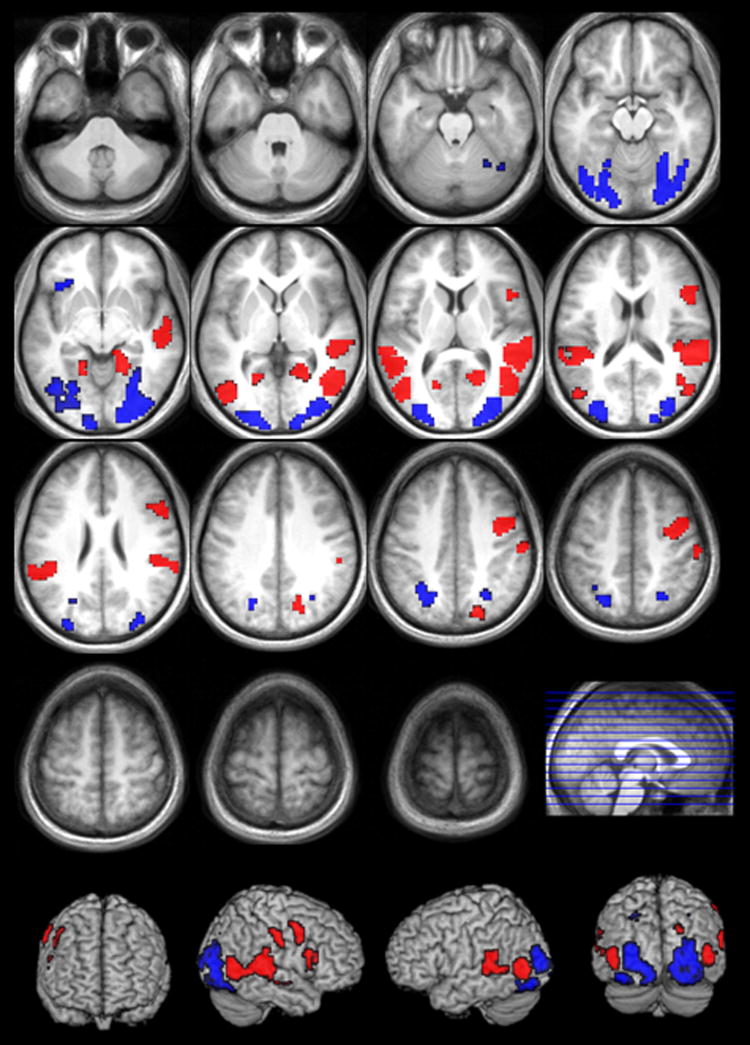
Naming verbs from pictures, compared to naming verbs from videos, correlated primarily with increased bilateral posterior activation (BAs 7, 19, 18, 17 and 37), including superior parietal cortex, inferior, middle and superior occipital gyri, posterior middle temporal gyrus, lingual gyrus, fusiform gyrus, cuneus and precuneus (see Table 4 & Figure 4). In the RH, activation extended into the parahippocampal gyrus. In the LH, there was additional prefrontal activation, specifically medially in inferior orbitofrontal cortex (BA 47), extending to the anterior insula.
TABLE 4.
Brodmann’s areas, MNI coordinates, cluster size and maximal t-values for local maxima in the contrast picture naming > video naming of actions (FDR corrected; p>.05; k>10).
| R/L | Activation Peak | Cluster Extent | BA | x | y | z | cluster size | t-Max |
|---|---|---|---|---|---|---|---|---|
| R | Middle Occipital Gyrus | Fusiform Gyrus, Inferior Occipital Gyrus, Parahippocampal Gyrus, Lingual Gyrus, Middle Temporal Gyrus, Superior Occipital Gyrus, Cuneus | 17, 18, 19, 37 | 33 | -84 | 9 | 596 | 7.57 |
| L | Fusiform Gyrus | Middle Occipital Gyrus, Inferior Occipital Gyrus, Lingual Gyrus, Middle Temporal Gyrus, Superior Occipital Gyrus, Cuneus | 17, 18, 19, 37 | -24 | -78 | -12 | 417 | 5.99 |
| L | Superior Occipital Gyrus | Precuneus, Inferior Parietal Lobule, Superior Parietal Lobule | 7, 40 | -24 | -69 | 33 | 80 | 4.87 |
| R | Superior Parietal Lobule | Precuneus | 7 | 27 | -63 | 51 | 29 | 4.11 |
| L | Inferior Frontal Gyrus | 47 | -39 | 21 | -9 | 13 | 3.76 |
FIGURE 4.
Activation for the contrasts pictures>videos (blue) and videos>pictures (red) (FDR corrected; p>.05; k>10).
Activation correlated with video naming compared to picture naming also included the visual association cortex, but more anteriorly and medially (see Table 5 & Figure 4). Bilaterally, there was activation in middle and superior temporal cortex (BAs 37, 21, 22, 42), supramarginal and angular gyrus (BAs 39, 40), inferior parietal cortex (BA7) and middle occipital gyrus (BAs 18, 19), and also in the insula (BA 13), the posterior cingulate cortex, and the parahippocampal and lingual gyri. Other activation was lateralized specifically to the RH, viz. activation in inferior frontal gyrus (BAs 44, 45), middle frontal and precentral cortex (BAs 6, 8, 9), postcentral cortex (BAs 1, 2, 3, 4), superior occipital cortex (BA 19) and precuneus (BA 7). Subcortically, activation extended to the calcarine sulcus in the RH.
4 Discussion
4.1 Accuracy
As expected, our healthy adult participants, who had no language impairment, performed well on the simple naming task. Substitutions of verbs with a different argument structure than that of target verbs were very few and most errors consisted of omissions.
4.2 General effects of argument structure
Areas that show increased activation during transitive verb naming, relative to intransitive verb naming, first of all include classically identified language areas, such as Broca’s area (BA 44, 45), supramarginal and angular gyri, with a LH domination. The general greater extent of the activation pattern for this contrast, compared to the opposite contrast described above, is not surprising. Transitive verbs are linguistically more complex, and their processing has been shown to be impaired more than that of intransitive verbs in agrammatic Broca’s aphasia, as discussed above.
Notably, there was increased activation in a large temporo-parieto-occipital area for transtitive verb naming, compared to intransitive verb naming. The angular gyrus (BA 39) is associated with lexical selection (Kemeny, Xu, Park, Hosey, Wettig and Braun, 2006), and more generally considered to be a multimodal semantic integration area (Geschwind 1965; Vigneau, Beaucousin, Hervé, Duffau, Crivello, Houdé, Mazoyer and Tzourio-Mazoyer, 2006). As in the present study, Thompson et al. (2007) found BA 39 particularly active in the processing of verbs with more complex argument structure. The precuneus, which was bilaterally activated, has been related to anomia recovery in chronic aphasia (Fridriksson 2007), as well as with processing of the number of verb complements (Shetreet et al. 2007).
Recruitment of anterior regions of the perisylvian language network in the representation of verb argument structure complexity was not observed in earlier studies (Ben-Shachar et al. 2003; Shetreet et al. 2007; Thompson et al. 2007). However, as pointed out in the Introduction, these studies examined verb processing rather than production, using receptive tasks which required lexical access and/or syntactic parsing, but no overt or covert naming. This observation that anterior regions are associated with increases in the number of verb arguments is consistent with the vast range of data indicating that agrammatic Broca’s aphasic patients’ verb production ability decreases as the number of verb arguments increases (Kemmerer & Tranel, 2000; Kim & Thompson, 2000, 2004; Thompson et al. 1997; Jonkers, 2000; Jonkers & Bastiaanse, 1996, 1998; De Bleser & Kauschke, 2003; Kiss, 2000; Luzzatti & Chierchia, 2002) and provides converging evidence that the anterior perisylvian cortex is crucial for naming verbs, with activation increasing with the number of verb arguments. Likewise, posterior perisylvian cortex is crucial for lexical access to verbs and verb arguments, with activation increasing as the number of verb arguments increases.
We also found activation along both ventral and dorsal visual pathways. Ventral activation (BAs 19, 37) is associated with object recognition and representation (Moore & Price 1999; Goodale & Milner, 1992), while more dorsal activation (parietal, BA 7) is associated with action recognition, as well as with locating objects in space (Goodale & Milner, 1992; Silveri, Perri and Cappa, 2003; Shmuelof & Zohary, 2005). According to Binkofski, Buccino, Stephan, Rizzolatti, Seitz and Freund (1999; see also Burton, Sinclair and McLaren, 2007), the frontal operculum (BA 44) and the anterior part of the intraparietal sulcus are functionally connected and play a part in object manipulation. As in other studies, the activation observed presently shows that these areas do not only respond to the actual physical manipulation of objects, but also to observation of such manipulations by others (Buccino, Binkofski, Fink, Fadiga, Fogassi, Gallese, Seitz, Zilles, Rizzolatti and Freund, 2001; Perani, Cappa, Tettamanti, Rosa, Scifo, Miozzo, Basso and Fazio, 2003; for an overview, see Jacob and Jeannerod, 2003).
Related to this, one shortcoming of the present study is that we did not match the transitive action and the intransitive action stimuli for the number of depicted objects. Thus, the transitive stimuli (e.g. kick, which shows a ball) contained more objects than the intransitive stimuli (e.g. jump), overall. Therefore, some of the increased activation shown for transitive action naming over intransitive action naming was likely driven by the visual ‘object-bias’ in the transitive actions. However, our results replicate earlier studies examining verb argument structure complexity, e.g. Ben-Shachar et al. (2003) and Thompson et al. (2007), in which visual (picture) stimuli were not used. Thus, we conclude that the visual complexity of the stimuli alone is likely not responsible for the differential activation found between the two verb types. We also point out that although it is possible to match argument-structure conditions for the number of depicted objects in visual stimuli, it is impossible to match for the objects that are actually manipulated in the actions. By definition, no external arguments are manipulated in intransitive actions. This leads to the general question of whether the representations for objects and their manipulations can indeed be separated from the representations of transitive actions. Such an incorporation may well form the basis for more complex lexical representations of transitive actions (compared to intransitive actions), correlated with increased neuronal activation in verb retrieval and naming.
Similarly, the stimuli in the present study were not matched for body-part associations, the transitive verbs mostly being ‘hand’ actions and the intransitive verbs being ‘foot/leg’ and ‘face’ actions. Such embodiment information may well be part of the lexical representation of action words (Pulvermüller, 2005). In a follow-up experiment, we are currently investigating this embodiment hypothesis directly, with stimuli that are matched for argument-structure, but the body-part associations cannot be disentangled in the data presented here.
In contrast to previous research examining argument structure effects (Ben-Shachar et al., 2003; Thompson et al., 2007), and contrary to our hypothesis, we found activation for intransitive over transitive verbs in the current experiment. The activation of bilateral primary auditory cortex, seems to suggest auditory processing, with an associated role for the RH thalamic activation, but the videos and pictures were presented without sound, so we can offer no explanation for this finding. The superior temporal activation has been related to argument structure processing (Ben-Shachar et al., 2003; Thompson et al., 2007) as well as to the related functions of animacy processing and the detection of agency (Grewe, Bornkessel-Schlesewsky, Zysset, Wiese, Von Cramon and Schlesewsky, 2007). The same temporal areas have also been related to the association of sounds or images with meaning (Vigneau et al. 2006), more in line with the classical interpretation of the role of Wernicke’s area in lexical retrieval.
It is likely that an agency detection process is different for presentations depicting only a single person, as in the intransitive condition, and presentations in which one agent actually performs an action on a patient/theme (animate or inanimate), as in the transitive condition. One might argue that only in the second condition is it necessary to ‘detect’ who is the agent of the performed action (and then particularly in the case of two animate arguments). On the other hand, it is also possible that, although agency detection is a straightforward process in the transitive condition (two potential actors – choose one), it is less straightforward in the intransitive condition under circumstances in which intransitive actions are presented intermixed with transitive actions, as in the present experiment. In this case, subjects may actually be looking for something that is not there, viz. a second argument, and this might underlie the increased activation observed in the intransitive action condition in areas associated with argument structure processing and agency detection. Beyond such speculation, however, we have at this point no satisfactory interpretation of the increased activation yielded by the contrast of intransitive over transitive verb naming.
4.3 Comparison of argument structure effects in picture naming and video naming
The only statistical difference between the activation patterns associated with the two presentation modes was found for the contrast of transitive verb naming over intransitive verb naming in video presentation. Stronger effects in the video naming condition were anticipated, based on the behavioral data differences between verb elicitation through pictures and videos reported earlier (D’Honincthun & Pillon, 2005; Naigles et al., 1998; Davidoff & Masterson, 1995). Particularly, there was increased RH activation in the inferior parietal areas associated with transitive action processing (Buccino et al., 2001), including the multimodal association cortex in the supramarginal gyrus (BA 40). The superior parietal activation followed the intraparietal sulcus anteriorly to the somatosensory cortex of the postcentral gyrus. As noted above, such activation may reflect object manipulation (Burton et al., 2007; Shmuelof & Zohari, 2005).
4.4 General effects of presentation mode
Picture naming was correlated with stronger activation in primary visual cortex and temporo-occipital and parieto-occiptial visual association cortex, than video naming. An account for this effect may be found in the visuo-spatial attention that is automatically drawn towards the moving elements in the videos, and the absence of such movement in the pictures. The pictures may thus require more visual scanning to determine the action, whereas detection of the action is more direct in the videos.
Following from the studies discussed (D’Honincthun & Pillon, 2005; Naigles et al., 1998; Davidoff & Masterson, 1995), it is likely that relatively more strategic effort is involved in naming pictures than naming depicted actions. Supporting this, the LH inferior orbitofrontal activation may reflect recruitment of the prefrontal association network involved in modulatory control over many different cognitive demands (Duncan & Owen 2000). Gazzale, Rissman, Cooney, Rutman, Seibert, Clapp and D’Esposito (2007) have shown functional connectivity between visual association cortex and the RH homologue of our activation cluster, as part of a network exerting a top-down attention-dependent modulatory influence on activation in the visual association cortex. In fact, other studies have found increased activation in occipital visuoperceptual areas, similar to what was found here, associated with perceptual processing in the recognition of unfamiliar items (Simons, Graham, Owen, Patterson and Hodges, 2001). These findings support our hypothesis that naming actions from picture representations (particularly line drawings) is less ‘natural’ than naming verbs from dynamic presentations. The former require extra processing resources, which comes with associated neuronal activation that is of no direct relevance to lexical retrieval, but which does affect baseline activations and may therefore mask more interesting patterns of activation associated with lexical retrieval.
The activation correlated with video naming over picture naming is also generally bilateral, but in more superior areas it is lateralized to the right hemisphere. The activation around the superior temporal sulcus has been related to the perception of moving stimuli (Allison, Puce, & McCarthy, 2000; Puce & Perrett, 2003). BAs 39 and 40 are associated with spatial orientation as well as semantic representation, while BA 21 is also involved in the processing of visual information.
We also observed activation for videos over pictures in areas that are typically associated with linguistic processing, including Wernicke’s area (BA 22), with its known role in semantic processing and lexical retrieval. So, although the video condition evokes activation in areas reflecting task-related processing as well, the effort associated with this type of processing is different from that associated with picture naming and may be more automatic. Importantly, naming videos of actions does not mask out activation in areas known to be associated with linguistic processing and semantic retrieval.
4.5 The neural representation of action naming
An important question is whether the observed activations actually reflected lexical processing, or whether they reflect more directly the processing/observation of the actions performed, therefore being sensitive to various visual characteristics of the performance. This is a question that ultimately needs to be addressed in separate studies, where patterns are compared across different modalities beside the visual modality used here. Here, our specific interest was in comparing action naming and argument structure effects obtained by means of our traditional presentation mode (line drawings) to effects obtained with a seemingly more naturalistic and dynamical presentation mode (videos).
Some of the parietal activation found in the present study appears to be task-related and specific to the presentation mode, e.g. associated with perception of movement, or object manipulation. However, lesion studies do show that aphasic speakers with posterior lesions, often including parietal and supramarginal cortex, are likely to have problems with production and/or processing of verbs with complex argument structure (Shapiro & Levine 1990; McCann & Edwards 2000). This suggests a more pivotal role for these areas in lexical retrieval, than a mere indirect and task-related involvement. In fact, it is conceivable that there is a direct relation between the visuospatial, functional and lexical representations (see also Jeannerod, 1997).
In this light, activation of the RH frontal operculum (BA 44) for video naming over picture naming, together with the observed frontal motor and premotor and the inferior parietal activation around the posterior parietal operculum, is suggestive of recruitment of the mirror neuron system (Rizzolatti, Fadiga, Matelli, Bettinardi, Paulesu, Perani and Fazio, 1996), through which certain neurons not only play a role in the execution of specific actions, but also in the observation of those same actions. The contrast of transitive over intransitive action naming also reveals activation that fits in the network associated with the human mirror neuron system, including inferior frontal and precentral cortex and the rostral part of the inferior parietal lobule (for an overview, see Rizzolatti & Craighero 2004). Buccino et al. (2001) showed that intransitive action observation only activated the frontal parts of this mirror network, while transitive actions were associated with additional inferior parietal activation. Possibly, recognition of the action to be goal-directed, involving an object (or a person, in some of our stimuli), makes the difference here. Along with the inferior parietal activation, we presently find activation in more superior parietal cortex, associated with the spatial localization of objects.
5 Conclusion
We conclude that the relative linguistic complexity of transitive verbs over intransitive verbs is reflected in greater neuronal activity in a network that encompasses traditional left-hemisphere perisylvian language areas. The absence of differences in Broca’s area activation in earlier studies of argument structure complexity is likely to be task-related, as this area may well be particularly involved in a network that subserves the production of verbs, as opposed to their processing.
Between picture and video presentations, the activated networks for the argument structure differences are fairly similar, but videos show stronger activation in RH inferior and superior parietal cortex for transitive verbs, i.e. a region that has been associated with transitive action processing as well as with object manipulation. This finding suggests that it is possible to use the relatively naturalistic elicitation method of video naming in the investigation of the neural representation of verbs and verb complexity. Based on independent considerations of stimulus naturalness and naming accuracy, this may make video presentation preferable to picture presentation as an elicitation method in such studies, particularly with speakers who have particular difficulty with action naming.
The present results, combined with results from earlier studies, suggest that various features of specific verbs, including the fact that they require external objects or arguments, or that they involve certain types of motion, are part of the lexical representation of these verbs, both in an abstract, conceptual sense, as in a physical, neuronal sense. Given this, it is of interest to find out to what extent this information is hard-wired into the neural representation of verbs, or action concepts. The present study was not designed to answer these specific questions, and therefore has limited scope over it. However, the issue warrants further research, as it bears directly on the question of the neural representation of linguistic complexity, its hierarchical nature, and the way it affects language processing and production after stroke.
Acknowledgments
This research was supported by NIH grant R01-DC007213-01 to C.K. Thompson. The authors wish to thank Kyla Garibaldi, Ellyn Riley, Keli Rulf and Anthony Shook for their assistance with stimulus preparation and data collection.
Appendix
List of verb stimuli used in the fMRI naming task
| Intransitive verbs | |
| 1 | cry |
| 2 | laugh |
| 3 | jump |
| 4 | crawl |
| 5 | sit |
| 6 | kneel |
| 7 | sleep |
| 8 | wink |
| 9 | cough |
| 10 | smile |
| Transitive verbs | |
| 11 | kiss |
| 12 | stir |
| 13 | cut |
| 14 | pull |
| 15 | pinch |
| 16 | squeeze |
| 17 | tickle |
| 18 | kick |
| 19 | hug |
| 20 | shove |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX lexical database (Release 1) [CD-ROM] Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania [Distributor]; 1993. [Google Scholar]
- Bastiaanse R, van Zonneveld R. Sentence production with verbs of alternating transitivity in agrammatic Broca’s aphasia. Journal of Neurolinguistics. 2005;18:57–66. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57:289–300. [Google Scholar]
- Ben-Shachar M, Hendler T, Kahn I, Ben-Bashat D, Grodzinsky Y. The neural reality of syntactic transformations: Evidence from functional magnetic resonance imaging. Psychological Science. 2003;14:433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Haendiges AN, Wozniak MA. Verb retrieval and sentence processing: dissociation of an established symptom association. Cortex. 1997;33(1):99–114. doi: 10.1016/s0010-9452(97)80007-x. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN, Sandson J. Verb retrieval in aphasia. 1. Characterizing single word impairments. Brain and Language. 1997;56(1):68–106. doi: 10.1006/brln.1997.1727. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Stephan KM, Rizzolatti G, Seitz RJ, Freund H-J. A parieto-premotor network for object manipulation: Evidence from neuroimaging. Experimental Brain Research. 1999;128:210–213. doi: 10.1007/s002210050838. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund H-J. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neuroscience. 2001;13:400–404. [PubMed] [Google Scholar]
- Buckner RL, Corbetta M, Schatz J, Raichle ME, Petersen SE. Preserved speech abilities and compensation following prefrontal damage. Proceedings of the National Academy of Sciences of the United States of America (PNAS) 1996;93:1249–1253. doi: 10.1073/pnas.93.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, McLaren DG. Cortical network for vibrotactile attention: A fMRI study. Human Brain Mapping. 2007 doi: 10.1002/hbm.20384. published online 27 March 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff J, Masterson J. The development of picture naming: Differences between verbs and nouns. Journal of Neurolinguistics. 1995;9:69–83. [Google Scholar]
- de Bleser R, Kauschke C. Acquisition and loss of nouns and verbs: Parallel or divergent patterns? Journal of Neurolinguistics. 2003;16:213–229. [Google Scholar]
- d’Honincthun P, Pillon A. Why verbs could be more demanding of executive resources than nouns: Insight from a case study of a fv-FTD patient. Brain and Language. 2005;95:36–37. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Druks J, Shallice T. Selective preservation of naming from description and the “Restricted Preverbal Message”. Brain and Language. 2000;72(2):100–128. doi: 10.1006/brln.1999.2165. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Moser D, Bonilha L, Morrow-Odoma KL, Shawa H, Fridriksson A, Baylis GC, Rorden C. Neural correlates of phonological and semantic-based anomia treatment in aphasia. Neuropsychologia. 2007;45:1812–1822. doi: 10.1016/j.neuropsychologia.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzale A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D’Esposito M. Functional Interactions between Prefrontal and Visual Association Cortex Contribute to Top-Down Modulation of Visual Processing. Cerebral Cortex. 2007;17:i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C, Lazar N, Nichols T. Thresholding of statistical maps in neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Goodale, Milner Separate pathways for perception and action. Trends in Neuroscience. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grewe T, Bornkessel-Schlesewsky I, Zysset S, Wiese R, von Cramon DY, Schlesewsky M. The role of the posterior superior temporal sulcus in the processing of unmarked transitivity. NeuroImage. 2007;35:343–352. doi: 10.1016/j.neuroimage.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Jacob P, Jeannerod M. Ways of seeing : the scope and limits of visual cognition. Oxford; New York: Oxford University Press; 2003. [Google Scholar]
- Jeannerod M. The cognitive neuroscience of action. Oxford, UK; Cambridge, Mass: Blackwell; 1997. [Google Scholar]
- Jensen LR. Canonical structure without access to verbs? Aphasiology. 2000;14(8):827–850. [Google Scholar]
- Jonkers R. Verb-finding problems in Broca’s aphasics. In: Bastiaanse R, Grodzinsky Y, editors. Grammatical disorders in aphasia: A neurolinguistic perspective. London: Whurr; 2000. pp. 105–122. [Google Scholar]
- Jonkers R, Bastiaanse R. The influence of instrumentality and transitivity on action naming in Broca’s and anomic aphasia. Brain and Language. 1996;55:37–39. doi: 10.1016/j.bandl.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Jonkers R, Bastiaanse R. How selective are selective word class deficits? Two case studies of action and object naming. Aphasiology. 1998;3:245–256. [Google Scholar]
- Kemeny S, Xu J, Park GH, Hosey LA, Wettig CM, Braun AR. Temporal dissociation of early lexical access and articulation using a delayed naming task – An fMRI study. Cerebral Cortex. 2006;16:587–595. doi: 10.1093/cercor/bhj006. [DOI] [PubMed] [Google Scholar]
- Kemmerer D. Action verbs, argument structure constructions, and the mirror neuron system. In: Arbib M, editor. Action to language via the mirror neuron system. Cambridge: Cambridge University Press; 2006. pp. 347–373. [Google Scholar]
- Kemmerer D, Tranel D. Verb retrieval in brain-damaged subjects: 1. Analysis of stimulus, lexical, and conceptual factors. Brain and Language. 2000;73:347–392. doi: 10.1006/brln.2000.2311. [DOI] [PubMed] [Google Scholar]
- Kim M, Thompson CK. Patterns of comprehension and production of nouns and verbs in agrammatism: Implications for lexical organization. Brain and Language. 2000;74:1–25. doi: 10.1006/brln.2000.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Thompson CK. Verb deficits in Alzheimer’s disease and agrammatism: Implications for lexical organization. Brain and Language. 2004;88:1–20. doi: 10.1016/s0093-934x(03)00147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss K. Effect of verb complexity on agrammatic aphasics’ sentence production. In: Bastiaanse R, Grodzinsky Y, editors. Grammatical disorders in aphasia: A neurolinguistic perspective. London: Whurr; 2000. pp. 152–170. [Google Scholar]
- Lee M, Thompson CK. Agrammatic aphasic production and comprehension of unaccusative verbs in sentence contexts. Journal of Neurolinguistics. 2004;17:315–330. doi: 10.1016/S0911-6044(03)00062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Crosson B, Nadeau SE, Heilman KM, Gonzalez-Rothi LJ, Raymer A, et al. Category-specific naming deficits for objects and actions: semantic attribute and grammatical role hypotheses. Neuropsychologia. 2002;40(9):1608–1621. doi: 10.1016/s0028-3932(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Macey P, Macey K, Kumar R, Harper R. A method for removal of global effects from fMRI time series. NeuroImage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- McCann C, Edwards S. Verb problems in fluent aphasia. Brain and Language. 2002;83(1):42–44. [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. NeuroImage. 1999;10:181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Naigles LR, Eisenberg AR, Kako ET, Highter M, McGraw N. Speaking of motion: verb use in English and Spanish. Language and Cognitive Processes. 1998;13(5):521–549. [Google Scholar]
- Pashek GV, Tompkins CA. Context and word class influences on lexical retrieval in aphasia. Aphasiology. 2002;16(3):261–286. [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, Basso A, Fazio F. A fMRI study of work retrieval in aphasia. Brain and Language. 2003;85:357–368. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6(7):576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. In: Frith C, Wolpert D, editors. The neuroscience of social interaction. Oxford: Oxford University Press; 2003. pp. 1–22. [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F. Localization of grasp representation in humans by PET: 1. Observation versus execution. Experimental Brain Research. 1996;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Levine B. Verb processing during sentence comprehension in aphasia. Brain and Language. 1990;38:21–47. doi: 10.1016/0093-934x(90)90100-u. [DOI] [PubMed] [Google Scholar]
- Shetreet E, Palti D, Friedmann N, Hadar U. Cortical representation of verb processing in sentence comprehension: Number of complements, subcategorization and thematic frames. Cerebral Cortex. 2007;17(8):1958–69. doi: 10.1093/cercor/bhl105. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. Dissociation between ventral and dorsal fMRI activation during object and action recognition. Neuron. 2005;47:457–470. doi: 10.1016/j.neuron.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Simons JS, Graham KS, Owen AM, Patterson K, Hodges JR. Perceptual and semantic components of memory for objects and faces: A PET study. Journal of Cognitive Neuroscience. 2001;13(4):430–443. doi: 10.1162/08989290152001862. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Perri R, Cappa A. Grammatical class effects in brain-damaged patients: functional locus of noun and verb deficit. Brian and Language. 2003;85:49–66. doi: 10.1016/s0093-934x(02)00504-7. [DOI] [PubMed] [Google Scholar]
- Thompson CK. Unaccusative verb production in agrammatic aphasia: The argument structure complexity hypothesis. Journal of Neurolinguistics. 2003;16:151–167. doi: 10.1016/S0911-6044(02)00014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix S, Blumenfeld H, Parrish T, Gitelman D, Mesulam M-M. Neural correlates of verb argument structure processing: An fMRI study. Journal of Cognitive Neuroscience. 2007;19:1753–1767. doi: 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C, Lange K, Schneider S, Shapiro L. Agrammatic and non-brain-damaged subjects’ verb and verb argument structure production. Aphasiology. 1997;11:473–490. [Google Scholar]
- Thompson C, Shapiro L, Li L, Schendel L. Analysis of verbs and verb argument structure: A method for quantification of aphasic language production. In: Lemme P, editor. Clinical aphasiology. Vol. 23. Austin, TX: Pro-Ed; 1994. pp. 121–140. [Google Scholar]
- Tranel D, Manzel K, Asp E, Kemmerer D. Naming dynamic and static actions: neuropsychological evidence. Journal of Physiology Paris. 2008;102(13):80–94. doi: 10.1016/j.jphysparis.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]