Abstract
The invasive container-dwelling mosquito Aedes albopictus (Skuse) shows modest behavioral responses to water-borne cues from predatory Corethrella appendiculata Grabham in North America. We investigate whether Ae. albopictus adjust their antipredatory responses to be proportional to size-dependent risk of predation. Fourth-instar Ae. albopictus attain a size refuge from C. appendiculata predation, and we compared the responses of second- and fourth-instar Ae. albopictus to cues from C. appendiculata predation. More vulnerable second-instar larvae showed a larger change in behavior in response to predation cues than did less vulnerable fourth-instar larvae, indicating threat-sensitive behavioral responses by Ae. albopictus.
Keywords: Aedes albopictus, Corethrella appendiculata, predator-prey, size-dependent
In aquatic systems, prey often show behavioral modifications in response to predation risk, and these modifications reduce an individual’s risk from predation. These responses to cues to predation risk typically come at a cost that can result in significant nonlethal predator impacts on the fitness of prey (Preisser et al. 2005). Because of these costs, we hypothesize that there should be behavioral responses that are proportional to the actual risk of predation. Insufficient responses to highly dangerous predators would be selected against by increased mortality, and excessive responses to minimally dangerous predators would be selected against by costs of reduced foraging, movement, and growth. Because relative size of predators and prey can influence risk (Chivers et al. 2001), we expect prey individuals of different sizes to show different responses to the same predators.
Second instars of both the invasive Aedes albopictus and the native Aedes triseriatus (Say) mosquitoes reduce their activity at the bottom of containers in the presence of water-borne predation risk cues from Corethrella appendiculata Grabham, and these changes reduce the risk of predation (Kesavaraju et al. 2007). Second-instar Ae. albopictus show these behavioral modifications only in water that has held a feeding C. appendiculata and not to a nonfeeding predator (our unpublished data). The activity of Ae. albopictus changes less than that of Ae. triseriatus, so Ae. albopictus larvae are more vulnerable to predation from Corethrella (Kesavaraju et al. 2007). Second-instar mosquitoes are more vulnerable than are larger instars to predation by fourth-instar C. appendiculata (Kesavaraju et al. 2007). Thus, second-instar Ae. albopictus should show a greater change in behavioral responses to cues from predation by C. appendiculata than should the relatively invulnerable fourth-instar Ae. albopictus.
Materials and Methods
Ae. albopictus were F1 progeny from a colony collected initially as larvae from tree holes (Indrio Road, Fort Pierce, FL) and propagated as adults in cages (Illinois State University IACUC protocol 01–2005). C. appendiculata larvae were from a laboratory colony (generation unknown) maintained since 2005 at the Florida Medical Entomology Laboratory, Vero Beach, FL. The experiment was conducted in a walk-in incubator maintained at 26°C, a photoperiod of 14:10 (L:D) h, and ≈80% humidity.
Behavior of second- and fourth-instar Ae. albopictus in control and predation treatments was video recorded using a Panasonic digital video camera (model WV-D5100, Panasonic Corporation of North America, Secaucus, NJ). The control treatment was prepared by holding 10 second-instar Ae. albopictus alone, and the predation treatment by holding 10 second-instar Ae. albopictus with three fourth-instar C. appendiculata for 5 d in 10-ml cups. Dead, eaten, and pupated treatment larvae were replaced daily.
The test larvae were hatched and then held individually in 5 ml of water in 15-ml vials. Each larva was fed 1 ml of liver powder suspension prepared by stirring 0.3 g of liver powder in 1,000 ml water on a stir plate (Juliano and Gravel 2002, Kesavaraju and Juliano 2004). A single feeding was sufficient to rear the 80 Ae. albopictus test larvae to second instar, but the 80 fourth-instar test larvae were fed every 2 d until they attained the fourth instar.
Test larvae were starved for 24 h in 10 ml of water before being transferred for behavior recording. Before transfer of test larvae, all predator and prey treatment larvae were removed from the treatment cups, leaving behind only water-borne cues (e.g., uneaten body parts, feces, dissolved chemicals) from the treatment larvae. Depending on the treatment, one second or fourth-instar Ae. albopictus was placed in each container and their behavior was recorded using a Winfast XP 2000, TV tuner card (PCI) and associated software (NuMedia Systems USA, Inc. [Leadtek], Walnut, CA) for 15 min. A video clip contained four cups, with one cup for each treatments.
Behaviors were classified into activities and positions in the experimental vessel (Juliano and Reminger 1992). Activities were 1) browsing: mouthparts in contact with the container surfaces; 2) filtering: moving via feeding motions of the mouthparts; 3) thrashing: moving with vigorous lateral flexion of the body; and 4) resting: none of the previous activities. Positions were 1) surface: siphon in contact with water surface; 2) wall: within 1 mm of the sides; 3) bottom: within 1 mm of the bottom; and 4) middle: >1 mm from the sides, bottom, and surface.
Activity and position of the test larvae were noted every 30 s for 15 min upon playback of the video clips. For each behavior, the proportion of the total number of observations (n = 30) was determined for each replicate. The number of variables per replicate was reduced by principal components analysis (PCA). Principal components (PC) with eigenvalues >1 were retained and analyzed by a multivariate analysis of variance with the PCs as response variables and the treatments, instars, and interaction as independent variables (Juliano and Gravel 2002, Kesavaraju et al. 2007). Standardized canonical coefficients (SCC) were used to interpret the relative contribution of the PCs in a significant effect (Scheiner 2001).
Results
There were three PCs with eigenvalues >1 (Table 1). A greater positive score on PC1 indicated that larvae spent more time resting at the surface and a negative score indicated that more time was spent browsing at the wall and bottom. A greater positive score on PC2 indicated that larvae spent more time thrashing in the middle and a negative score indicated more time spent in other behaviors. A greater positive score on PC3 indicated that the larva spent more time filtering in the middle and a negative score indicated more time spent in other behaviors (Table 1).
Table 1.
Rotated factor patterns of the PCA for comparing the behavioral responses of second and fourth instar Ae. albopictus
| Variable | PC1 | PC2 | PC3 |
|---|---|---|---|
| Resting | 98 | 9 | 0 |
| Browsing | −91 | −38 | −9 |
| Thrashing | 24 | 95 | −11 |
| Filtering | 2 | 8 | 99 |
| Surface | 98 | 9 | −1 |
| Wall | −85 | −30 | 1 |
| Middle | 26 | 86 | 42 |
| Bottom | −65 | −36 | −21 |
| Interpretation | Resting, surface VS browsing, wall, bottom | Thrashing, middle VS other | Filtering, middle VS other |
Values >40 are in bold.
The interaction between instar and treatment was significant. SCCs indicated that PC1 contributed most to the significant interaction, followed by PC2 and PC3 (Table 2). Multivariate contrasts revealed that all multivariate means differed significantly except second and fourth instar controls (Table 2). Most importantly second instar responses to predation cues were significantly different from fourth-instar responses to predation cues and second instars showed a much greater change in behavior than did fourth instars (Fig. 1). For all contrasts SCCs indicated that PC1 made the greatest contribution in the significant effect followed by PC2 and PC3 (Table 2).
Table 2.
Results of MANOVA and multivariate contrasts for the behavioral responses of second and fourth instar Ae. albopictus
| Variable | Numerator df | Denominator df | Pillai’s trace | P | Standardized canonical coefficients |
||
|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | |||||
| Instar | 3 | 62 | 0.311 | <0.0001 | 1.777 | 0.906 | −0.372 |
| Treatment | 3 | 62 | 0.725 | <0.0001 | 1.812 | 0.959 | −0.088 |
| Instar × treatment | 3 | 62 | 0.314 | <0.0001 | 1.757 | 0.627 | −0.499 |
| Multivariate contrasts | |||||||
| Second instar control vs. fourth instar control | 3 | 62 | 0.018 | 0.7744 | 0.034 | 0.975 | 0.465 |
| Fourth instar control vs. fourth instar predation | 3 | 62 | 0.369 | <0.0001 | 1.623 | 1.048 | 0.179 |
| Fourth instar control vs. second instar predation | 3 | 62 | 0.722 | <0.0001 | 1.817 | 0.951 | −0.172 |
| Second instar control vs. fourth instar predation | 3 | 62 | 0.332 | <0.0001 | 1.755 | 0.952 | 0.106 |
| Second instar control vs. second instar predation | 3 | 62 | 0.715 | <0.0001 | 1.844 | 0.884 | −0.215 |
| Fourth instar predation vs. second instar predation | 3 | 62 | 0.471 | <0.0001 | 1.785 | 0.774 | −0.440 |
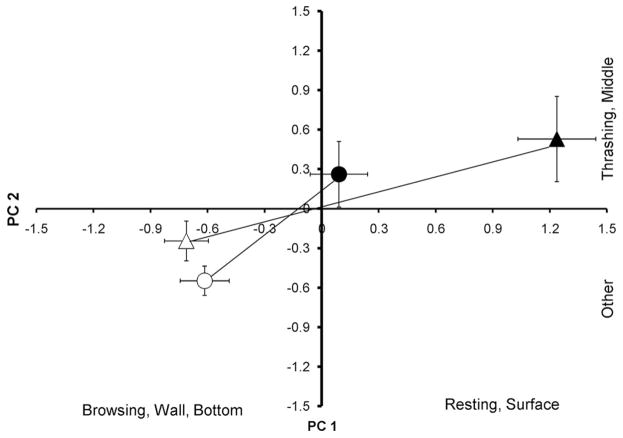
Fig. 1.
Ordination of principal components (means ± SE) between second-instar (triangles) and fourth-instar (circles) Ae. albopictus in control (open symbols) and predation (closed symbols) treatments (N = 30).
Discussion
As predicted, second-instar Ae. albopictus reduced their movement at the bottom of containers more than did fourth instar Ae. albopictus in the presence of water-borne predation cues from C. appendiculata (Fig. 1). Second-instar Ae. albopictus are highly vulnerable to predation by C. appendiculata, but fourth-instar Ae. albopictus are large enough to be relatively invulnerable to predation (Kesavaraju et al. 2007). These results support our hypothesis that behavioral responses of Ae. albopictus are proportional to the size-dependent risk of predation. In contrast, another container-dwelling mosquito, Ae. triseriatus, showed behavioral responses that were not proportional to the size-dependent threat of predation. Fourth-instar Ae. triseriatus are relatively invulnerable to predation by C. appendiculata (Kesavaraju et al. 2007), yet fourth-instar Ae. triseriatus show essentially the same response to predatory fly larvae as do second instars. Paradoxically, it is the non-native Ae. albopictus, which has no evolutionary history with C. appendiculata (although they probably encounter other Corethrella in their native range in Asia; Miyagi 1974) that shows the predicted behavioral response to size-dependent risk of predation, and not the native Ae. triseriatus, which is presumed to have an evolutionary history with C. appendiculata.
Whereas the response of Ae. albopictus seems to be targeted at a size-selective predator like C. appendiculata, the response of Ae. triseriatus may not be specific to C. appendiculata. Toxorhynchites rutilus (Coquillett) larvae, another predator of North American container-dwelling mosquitoes, are larger than C. appendiculata larvae, and all instars of Ae. albopictus and Ae. triseriatus are vulnerable to Tx. rutilus predation (Griswold and Lounibos 2005b, 2006). Unlike fourth-instar Ae. triseriatus, fourth-instar Ae. albopictus do not adopt low risk behaviors similar to the low risk behaviors shown by smaller larvae in the presence of predation risk cues from Tx. rutilus (Kesavaraju and Juliano 2004). Ae. triseriatus behavioral response to predation risk cues in all stages may represent a general response to cues that could come from multiple predators, and the response by fourth instars would thus help alleviate risk of predation from both C. appendiculata and Tx. rutilus.
The difference between responses of Ae. albopictus and Ae. triseriatus to C. appendiculata may have consequences for competitive interactions. Antipredatory behavior often comes at a cost of reduced foraging and reduced competitive ability of the prey (Lima and Dill 1990, Sih 1992). For Ae. albopictus, the main predator-induced behavioral change is increased resting and reduced browsing (Tables 1 and 2), consistent with a cost of behavioral response. Ae. albopictus is superior in resource competition to Ae. triseriatus in the absence of C. appendiculata (Livdahl and Willey 1991; Novak et al. 1993; Griswold and Lounibos 2005a, b), but in the presence of a limited number of C. appendiculata, larvae of the two mosquitoes seem to be able to coexist despite greater predation on Ae. albopictus (Griswold and Lounibos 2005a, b). Limited behavioral change by larger stages that are relatively invulnerable to the most abundant predator seems likely to contribute to maintenance of high competitive ability of Ae. albopictus even when some Corethrella are present. Adopting low risk behaviors in response to water-borne predation risk cues only in the most vulnerable stages may be optimal because such size-dependent responses do not compromise foraging ability in less vulnerable stages.
Acknowledgments
We thank M. Mathews for help with the experiments, R. L. Escher for providing us with C. appendiculata, and two referees for helpful comments. This research was supported by grants from the National Institute of Allergy and Infectious Disease (R01 [AI]-44793, Illinois State University subcontract) and National Science Foundation (DEB 0507015).
References Cited
- Chivers DP, Mirza RS, Bryer PJ, Kiesecker JM. Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can J Zool. 2001;79:867–873. [Google Scholar]
- Griswold MW, Lounibos LP. Competitive outcomes of aquatic container Diptera depend on predation and resource levels. Ann Entomol Soc Am. 2005a;98:673–681. doi: 10.1603/0013-8746(2005)098[0673:COOACD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol. Entomol. 2005b;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold M, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2006;87:987–995. doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behav Ecol. 2002;13:301–311. [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval tree-hole mosquitoes: geographic and ontogenetic differences. Oikos. 1992;63:465–467. [Google Scholar]
- Kesavaraju B, Alto BW, Lounibos LP, Juliano SA. Behavioural responses of larval container mosquitoes to a size-selective predator. Ecol Entomol. 2007;32:262–272. doi: 10.1111/j.1365-2311.2006.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68:619–640. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science (Wash, DC) 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Miyagi I. On a blood-sucking Corethrella sp. collected in Nagasaki, Japan (Diptera: Chaoboridae) Trop Med. 1974;16:89–93. [Google Scholar]
- Novak MG, Higley LG, Christianssen CA, Rowley WA. Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera, Culicidae) through replacement series experiments. Environ Entomol. 1993;22:311–318. [Google Scholar]
- Preisser EL, Bolnick DI, Benard MF. Scared to death? Behavioral effects dominate predator–prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interaction. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2. Oxford University Press; Oxford, United Kingdom: 2001. pp. 99–115. [Google Scholar]
- Sih A. Prey uncertainty and the balancing of anti-predator and feeding needs. Am Nat. 1992;139:1052–1069. [Google Scholar]