Abstract
The highly selective delta opioid agonist, SNC80, elicits dopamine-related behaviors including locomotor stimulation and conditioned place-preference. In contrast, it has been reported that SNC80 fails to promote dopamine efflux from the striatum of freely moving rats. However, SNC80 does enhance behavioral responses to the stimulants, amphetamine and cocaine, suggesting an interaction between delta opioids and psychostimulants. Since the increase in locomotor activity elicited by amphetamine and related stimulants acting at the dopamine transporter is associated with increases in extracellular concentrations of dopamine within the striatum, we hypothesized that SNC80 enhances this activity by potentiating the overflow of dopamine through the transporter. To test this hypothesis, striatal preparations from Sprague-Dawley rats were assayed for dopamine efflux in response to amphetamine challenge. SNC80 was given either in vivo or in vitro directly to rat striatal tissue, prior to in vitro amphetamine challenge. Both in vivo and in vitro administration of SNC80 enhanced amphetamine-mediated dopamine efflux in a concentration- and time-dependent manner. However, SNC80 in either treatment paradigm produced no stimulation of dopamine efflux in the absence of amphetamine. The effect of SNC80 on amphetamine-mediated dopamine overflow, but not the effect of amphetamine alone, was blocked by the delta selective antagonist, naltrindole and was also observed with other delta agonists. The results of this study demonstrate that even though SNC80 does not stimulate dopamine efflux alone, it is able to augment amphetamine-mediated dopamine efflux through a delta opioid receptor mediated action locally in the striatum.
Keywords: SNC80, amphetamine, delta opioid, dopamine, striatum, naltrindole
1. Introduction
Ligands that selectively activate the delta opioid receptor include peptidic enkephalin derivatives, such as DPDPE ([D-Pen2,D-Pen5]-enkephalin]) and nonpeptidic agonists, as exemplified by SNC80. In general, agonists of both classes elicit positive reinforcing behaviors. In rodents, DPDPE has been reported to stimulate locomotor activity, induce condition place-preference, promote self-administration and decrease brain-stimulation reward threshold intensities (Shippenberg et al., 1987; Meyer and Meyer, 1993; Devine and Wise, 1994; Duvauchelle et al., 1996; Suzuki et al., 1996). Similarly, SNC80 has been demonstrated to elicit conditioned place-preference and stimulate locomotor activity in rodents (Longoni et al., 1998; Spina et al., 1998).
Both the peptidic delta opioid agonist, [D-Pen2,L-Pen5]-enkephalin, and SNC80 produced cocaine-like discriminative effects in rodents and primates, possibly indicating that delta opioid agonists share mutual stimulatory effects with cocaine (Ukai et al., 1993; Negus et al., 1998). Indeed, in rats dopamine D1 receptor antagonists inhibited DPDPE-and SNC80-induced condition place preference (Suzuki et al., 1996; Longoni et al., 1998) and both D1 and D2 antagonist treatment decreased SNC80-mediated locomotor activity (Spina et al., 1998). Studies also suggest there are interactions between delta opioids and psychostimulants. For example, DPDPE increased cocaine-induced locomotor activity in rats (Waddell and Holtzman, 1998) and SNC80, in combination with cocaine, resulted in leftward shifts in the discriminative stimulus effects of cocaine in primates (Negus et al., 1998; Rowlett and Spealman, 1998). SNC80 has also been shown to enhance the locomotor stimulating effects of amphetamines and other psychomotor stimulants which act at the dopamine transporter (Mori et al., 2006; Jutkiewicz et al., 2008).
There is evidence that DPDPE and other delta peptide agonists can induce the efflux of dopamine in the striatum at doses similar to those which mediate rewarding behaviors, although studies disagree. Some reports indicate peptidic delta agonists elicit dopamine overflow exclusively in the caudate-putamen (Lubetzki et al., 1982; Petit et al., 1986) or the nucleus accumbens (Spanagel et al., 1990; Manzanares et al., 1993), while other reports fail to demonstrate an effect of DPDPE in either area (Mulder et al., 1989; Pentney and Gratton, 1991). These discrepancies could be due to the lack of delta opioid selectivity of DPDPE which produces mu opioid receptor-mediated antinoception in delta opioid knockout mice (Scherrer et al., 2004), whereas SNC80 is greater than 800-fold selective for the delta opioid receptor (Calderon et al., 1997). Indeed, SNC80 failed to induce increases in extracellular dopamine in either the nucleus accumbens or caudate-putamen as measured by in vivo microdialysis (Longoni et al., 1998) at doses that elicited locomotor stimulation and condition place-preference (Longoni et al., 1998; Spina et al., 1998). Thus a working hypothesis is that activation of the delta opioid receptor stimulates locomotor activity, promotes place-preference, and enhances stimulant-induced behaviors by altering dopamine neurotransmission through a mechanism other than direct modulation of dopamine efflux. The study of the interaction between delta opioids and stimulants could therefore lead to a greater understanding of drug-seeking behaviors.
The current studies examine the hypothesis that SNC80, as well as other delta opioid agonists, promote the activity of stimulants that act at the dopamine transporter by increasing their ability to efflux dopamine. The ability of both in vivo and in vitro delta opioids to increase amphetamine-mediated dopamine efflux from rat striatal preparations were evaluated in the present study. The results demonstrate that delta opioid receptor activation enhances the magnitude of amphetamine-mediated dopamine efflux from striatal preparations without having any effect on dopamine efflux in the absence of amphetamine. Portions of this paper were previously presented in abstract form (Bosse et al., 2006).
2. Methods
2.1. Drugs
SNC80 ((+)-4-[(α-R*)-α-((2S*,5S*)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide), DPDPE ([D-Pen2,5]-enkephalin), TAN-67 (2-methyl-4aα-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12aα-octahydro-quinolino[2,3,3,-g]isoquinoline), oxymorphindole (OMI), naltrindole hydrochloride (NTI), and amphetamine sulfate were obtained from the Narcotic Drug and Opioid Peptide Basic Research Center at the University of Michigan (Ann Arbor, MI). All compounds were dissolved in sterile water, except SNC80 that was dissolved in 8% 1 M HCl solution. In assays employing in vivo exposure to SNC80 or NTI, the drugs were administered by subcutaneous (s.c.) injection. For the in vitro studies, the drugs were diluted from stock solutions with Krebs-Ringer buffer (KRB) (125 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 1.2 mM CaCl2, 1.2 mM KH2PO4, 10 mM glucose, 24.9 mM NaHCO3, and 0.25 mM ascorbic acid; oxygenated with 95% O2/5% CO2 for 1h before use with a final pH of 7.4, adjusted with NaOH).
2.2. Animals
Male Sprague Dawley rats (250–350 g) were obtained from Harlan Sprague Dawley (Indianapolis, IN) and housed in groups of two or three animals. All animals were fed on a standard laboratory diet and kept on a 12h light/dark cycle, with lights on a 6:30 A.M., at a temperature of 21°C. Studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the U.S. National Institutes of Health. The experimental protocols were approved by the University of Michigan Committee on the Use and Care of Animals.
2.3. Striatal tissue and P2 synaptosomal preparation
Rats were sacrificed by decapitation and a coronal slice of the brain, approximately 3.0 mm thick, rostral to the anterior commissure (Bregma −0.30) was obtained using a brain-cutting block on ice as described by Heffner et al. (1980). The striatum, both dorsal and ventral, was dissected from the caudal surface of the slice and prepared for the release assay by using a razor blade to chop the tissue into approximately 1-mm3 pieces, which was divided for duplicate measures. Wet tissue weight (35 ± 1.6 mg per sample) was measured in pre-weighed boats containing ice-cold KRB.
To prepare synaptosomes, striatal tissue was homogenized on ice in 10 volumes of ice-cold homogenization buffer comprising 0.32 M sucrose, 4 mM HEPES, and a protease inhibitor cocktail tablet (Complete Mini; Roche Diagnostics, Mannheim, Germany) with a final pH of 7.4. Homogenate fractions were centrifuged at 1000 × g for 10 min at 4°C. The pellet was washed, and the combined supernatants were centrifuged at 15,000 × g for 30 min at 4°C. The P2 fraction was resuspended in 600 µl of ice-cold modified KRB containing 30 mM HEPES instead of KH2PO4. Protein content was analyzed by the method of Bradford (1976).
2.4. Dopamine efflux assay
Striatal or P2 synaptosomal preparations from vehicle or SNC80 pretreated rats (1, 10, or 32 mg/kg, s.c.) were transferred onto Whatman GF/B glass-fiber filters (Maidstone, England) in the appropriate chambers of a Brandel superfusion apparatus (Brandel SF-12; Gaithersburg, MD). Superfusion chambers were maintained at 37°C, and 37°C KRB was perfused through the chambers at a rate of 100 µl/min and collected in 5 min fractions into vials containing 25 µl of internal standard solution (0.05 M HClO4, 4.55 mM dihydroxybenzylamine (DHBA), 1 M metabisulfate, and 0.1 M EDTA). Generally, preparations were perfused with KRB or KRB containing either SNC80 (1, 3, 10, or 100 µM), DPDPE (10 µM), TAN-67 (10 µM), or OMI (10 µM) for 30 min (fractions 1–6), KRB containing amphetamine sulfate (3, 10, 30, 100, or 1000 µM) was perfused through the sample during the 5 min collection of fraction 7. The amphetamine-containing buffer was replaced with fresh KRB and fraction collection was continued for an additional 40 min (fractions 8–15). For antagonist studies, NTI was administered either in vivo (1 mg/kg, s.c.) or in vitro (10 nM) at times indicated in Figure 3.2. Results were not corrected for the time taken for perfused drug to reach the striatal preparations. Fractions were filtered through 0.22 µm PVDF syringe filters (Whatman; Florham Park, NJ) and stored at −80°C to be measured within a month for dopamine content by HPLC with electrochemical detection. Samples (50 µl) were injected onto an electrochemical detector (Waters; Franklin, MA, Model 2465) through which mobile phase (60 mM NaH2PO4, 30 mM citric acid, 0.1 mM EDTA, 0.021 mM sodium dodecyl sulphate, and 2 mM NaCl in 25% methanol/75% HPLC-grade water, apparent pH of 3.3) was delivered at 0.8 ml/min. Dopamine was separated on a Waters Symmetry (reverse phase, C-18, 3.5µm) column and measured using a glassy carbon working electrode with a potential of +0.6 V against an Ag/AgCl reference electrode; sensitivity was set at 200 pA/V. The chromatograms resulting from sample runs were analyzed for peak area using Breeze software (Waters). Dopamine peaks were analyzed based on the ratio of the peak area of dopamine to the peak area of the internal standard DHBA and dopamine levels determined against a standard curve (6 points over the range 12.5 pg to 2500 pg) then converted to pmol dopamine per mg wet tissue weight.
2.5. Ligand-binding assays
Crude striatal homogenates were made from striatal tissue isolated as previously described of rats treated with either vehicle or SNC80 (10 mg/kg, s.c.) 3h prior to sacrifice. The tissue was suspended in ice-cold 50 mM Tris-HCl buffer (pH 7.4) and homogenized using a Tissue Tearor (Biospec Products; Bartlesville, OK) for 15 s at setting 2 and then 15 s at setting 1. The resulting lysates were then homogenized using a Dounce homogenizer and stored in aliquots at −80°C. Protein concentration was determined by the method of Bradford (1976). Striatal lysates (50 – 64 µg) were incubated for 2 h with shaking at 25°C with varying concentrations (0.5 – 28 nM) of [3H]-DPDPE in 40 ml of 50 mM Tris-HCl buffer (pH 7.4). Non-specific binding was defined with 1 µM NTI. The contents of the tubes were rapidly vaccum-filtered through GF/C filters (Whatman; Florham Park, NJ) presoaked in 0.1% solution of polyethyleneimine using a Brandel harvester (MLR-24; Gaithersburg, MD) and rinsed with ice-cold Tris-HCl buffer three times. Radioactivity retained on the filters was determined by liquid scintillation counting on a Wallac 1450 MicroBeta counter (PerkinElmer; Waltham, MA).
2.6. Data and Statistical analyses
All data were analyzed using either GraphPad Prism 4 software (San Diego, CA) or Systat Sigma Stat 3.5 (San Jose, CA). All release data were for eight samples from striatal tissue harvested from four rats and expressed as n = 4 in duplicate. Dopamine efflux was plotted as the amount of endogenous dopamine in pmol/mg wet weight of tissue collected in each fraction. In some figures, dopamine release curves were presented as area under curve (AUC) generated from GraphPad Prism 4 software using the trapezoidal method. The AUC was computed from the dopamine release curves plotted as the cumulative measurement of dopamine efflux over a 45-min period (fractions 7–15) after subtracting each pre-amphetamine baseline (0.033 ± 0.016 pmol/mg wet weight) and expressed in arbitrary units. Potency (EC50) values were calculated from individual amphetamine concentration-effect curves (Fig. 1f and 4f) which were calculated with no constraints using fixed slope sigmoidal dose response curve analysis in GraphPad Prism 4. Data are expressed as means ± SEM and compared for statistical significance using a Student’s t-test. Statistical significance was assessed for other experiments using two-way analysis of variance (ANOVA) with either Bonferroni (Fig. 1, 2b, 4, and 5a) or Tukey’s (Fig. 2a and 3) multiple comparison adjustment for differences across treatment groups. A three-way ANOVA with Tukey’s post hoc test was used for analysis of the effect of calcium ions (Fig. 6). Both the data from the in vitro SNC80 concentration response (Fig. 5b and c) and comparison of other delta agonist on amphetamine-mediated dopamine release (Fig. 5) were analyzed using one-way ANOVA with post-test Tukey’s multiple comparison. Ligand saturation binding data were analyzed using a one-site saturation binding equation in GraphPad Prism 4. Binding data are presented as means ± SEM and significance determined by a Student’s t-test from three separate experiments in duplicate, each performed with lysates from separate rats. For all tests, significance was set at p < 0.05.
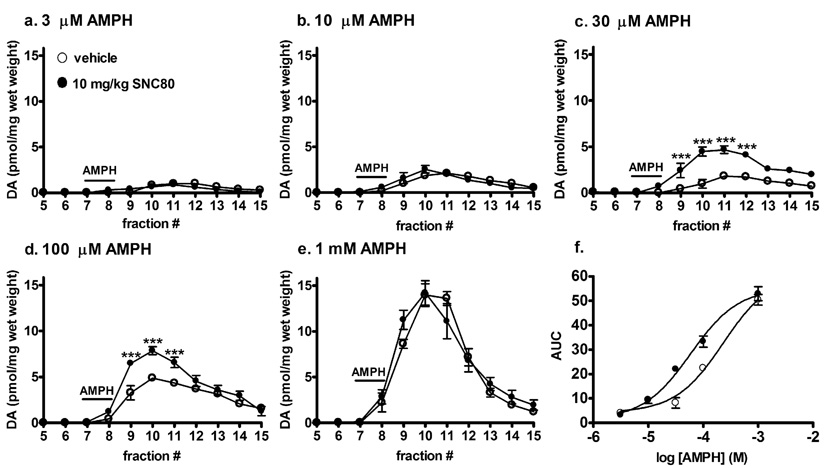
Fig. 1.
Effect of in vivo SNC80 on amphetamine-mediated dopamine efflux from striatal tissue. Striatal preparations from either vehicle or 10 mg/kg SNC80 treated rats (s.c.) were perfused with a. 3 µM, b. 10 µM, c. 30 µM, d. 100 µM or e. 1 mM amphetamine sulfate for 5 min at fraction 7 as indicated by the line labeled AMPH. Fractions were collected at 5 min intervals for an additional 40 min. Dopamine content of the fractions was measured by HPLC-electrochemical detection as described in the Methods, and reported as pmol of dopamine per mg wet weight ± S.E.M (n = 4 in duplicate). f. The concentration-response curve of amphetamine-mediated dopamine efflux was plotted using AUC values obtained from graphs a.–e. Statistical significance was determined by two-way ANOVA with Bonferroni multiple comparison analysis. ***p < 0.001 compared to vehicle treated animals.
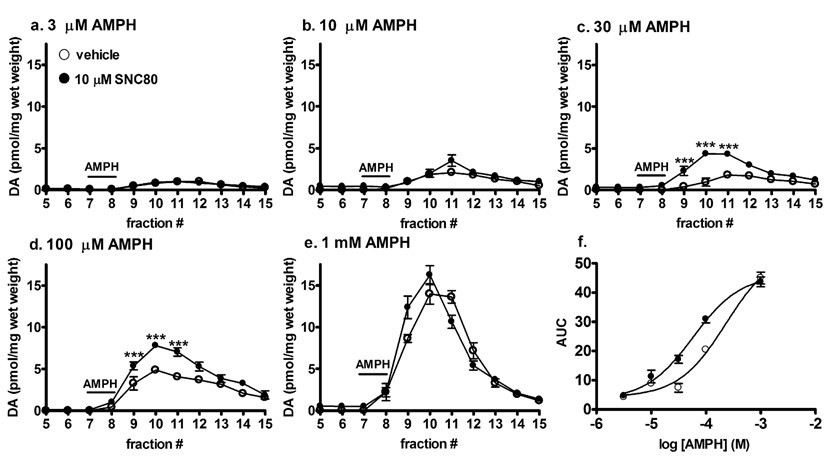
Fig. 4.
Effect of in vitro SNC80 pretreatment of striatal tissue from naïve rats on amphetamine-induced dopamine efflux. Striatal preparations were perfused with either vehicle or 10 µM SNC80 for 30 min, after which either a. 3 µM, b. 10 µM, c. 30 µM, d. 100 µM or e. 1 mM amphetamine sulfate was administered for 5 min at fraction 7 as indicated by the line labeled AMPH. Fractions were collected at 5 min intervals for an additional 40 min. Dopamine content of the fractions was measured by HPLC-electrochemical detection as described in the Methods and reported as pmol of dopamine per mg wet weight ± S.E.M (n = 4 in duplicate). f. The concentration-response curve of amphetamine-mediated dopamine efflux was plotted using AUC values obtained from graphs a.–e. Statistical significance was determined by two-way ANOVA with Bonferroni multiple comparison analysis. ***p < 0.001 compared to vehicle.
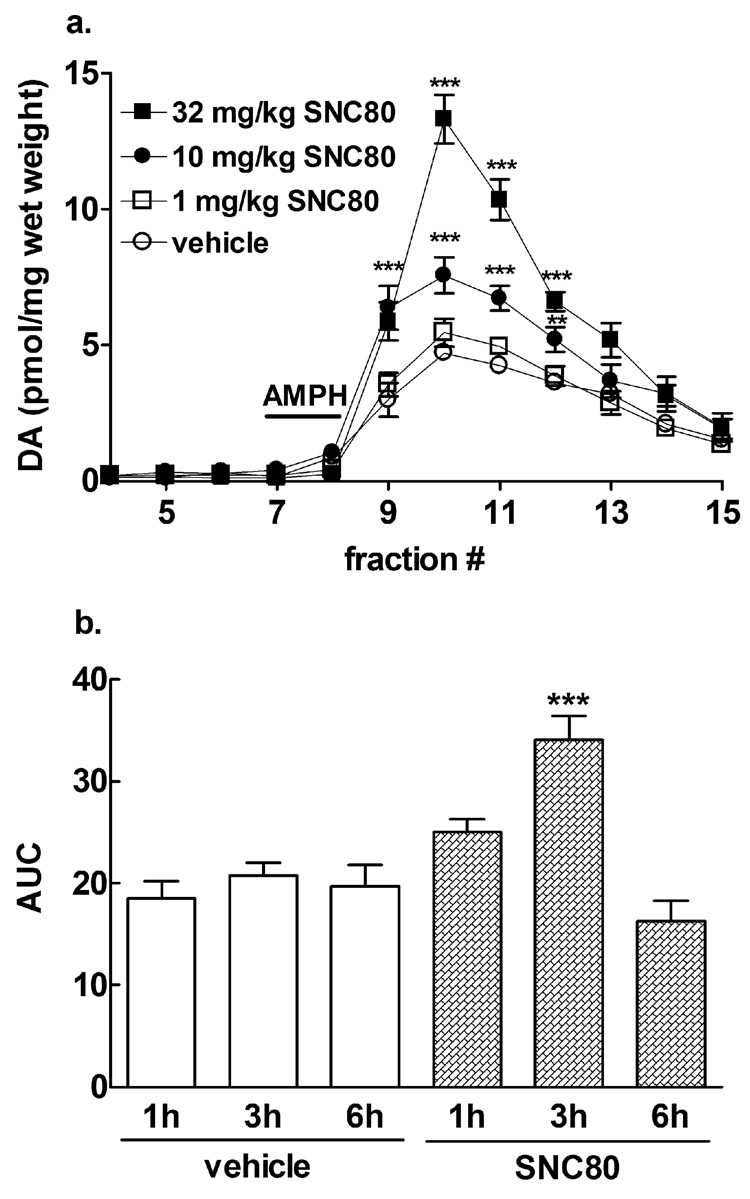
Fig. 2.
Enhancement of amphetamine-mediated dopamine efflux following in vivo SNC80 is dose- and time-dependent. Male Sprague-Dawley rats were injected (s.c.) with: a. either vehicle or doses of 1 mg/kg, 10 mg/kg, 32 mg/kg SNC80 or b. either vehicle or 10 mg/kg SNC80 (s.c.) for one, three or six h prior to the preparation of striatal tissue. The striatal preparations from all the groups (n = 4 in duplicate) were perfused with 100 µM amphetamine sulfate for 5 min during fraction 7 as indicated by the line labeled as AMPH. Dopamine content was measured by HPLC-electrochemical detection and reported in a. as pmol of dopamine per mg wet weight ± S.E.M. and in b. as AUC (± S.E.M.). Statistical significance was determined by two-way ANOVA with Tukey’s (a) or Bonferroni (b) multiple comparison analysis. ***p < 0.001, **p < 0.01 compared to vehicle-treated animals.
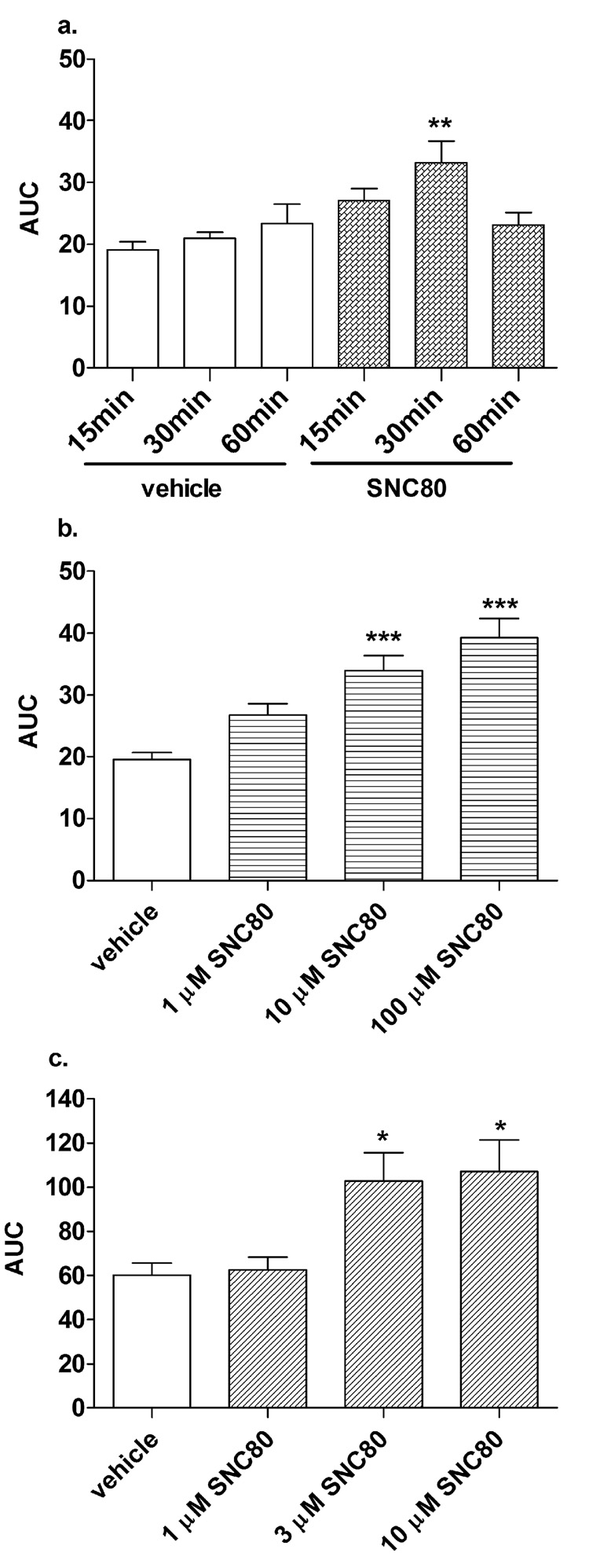
Fig. 5.
In vitro SNC80 potentiates amphetamine-induced dopamine efflux when administered directly to striatal preparations or P2 striatal synaptosomes from naïve rats. Striatal slices were perfused with: a. either vehicle or 10 µM SNC80 for 15, 30 or 60 min prior to amphetamine or b. 1 µM, 10 µM, or 100 µM SNC80 for 30 min before amphetamine. c. P2 synaptosomal fractions were perfused with 1µM, 3 µM, or 10 µM SNC80 for 30 min before amphetamine. All treatments received 100 µM amphetamine sulfate for 5 min and SNC80 administration was discontinued during the addition of amphetamine and subsequent perfusates. Samples were collected at 5 min intervals for an additional 40 min. Dopamine content of the fractions was measured by HPLC-electrochemical detection as described in the Methods and plotted AUC (± S.E.M.) as measured from the release curves (fractions 4–15) of each treatment group (n = 4 in duplicate). Statistical significance was determined in a. by two-way ANOVA with Bonferroni multiple comparison analysis and in b. by one-way ANOVA with Tukey’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001 compared to vehicle.
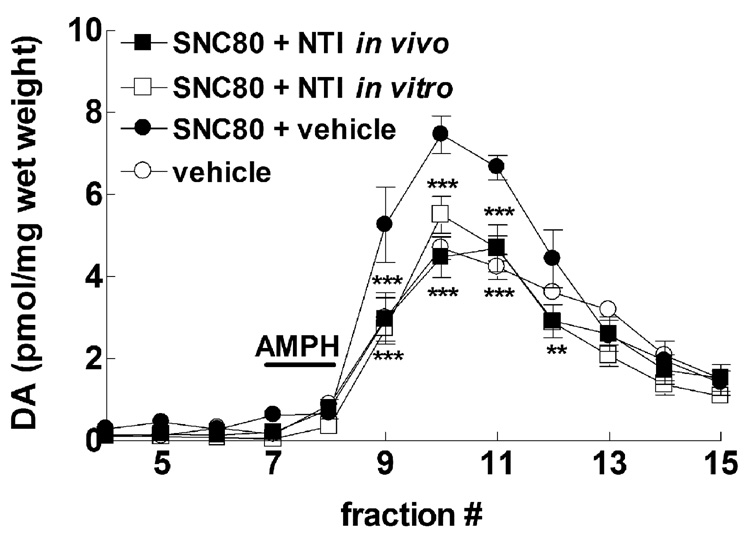
Fig. 3.
In vivo and in vitro administration of the delta selective antagonist NTI diminishes the ability of in vivo SNC80 to enhance amphetamine-mediated dopamine efflux. Male Sprague-Dawley rats were injected (s.c.) with vehicle alone, 10 mg/kg SNC80 alone, or 1.0 mg/kg NTI 30 min after SNC80 administration (in vivo NTI). Striatal preparations were made from all groups 3h after the vehicle or SNC80 injections, and perfused with buffer for 30 min before challenge with 100 µM amphetamine sulfate for 5 min. Some preparations from SNC80 treated rats were perfused with 10 nM NTI for 30 min prior to amphetamine challenge (in vitro NTI). The dopamine content of the fractions from all groups were assessed by HPLC-electrochemical detection, as described in the Methods, and reported as pmol of dopamine per mg wet weight ± S.E.M (n = 4 in duplicate). Statistical significance for values was determined by two-way ANOVA with Tukey’s multiple comparison analysis. **p < 0.01, *** p < 0.001 compared to SNC80 + vehicle.
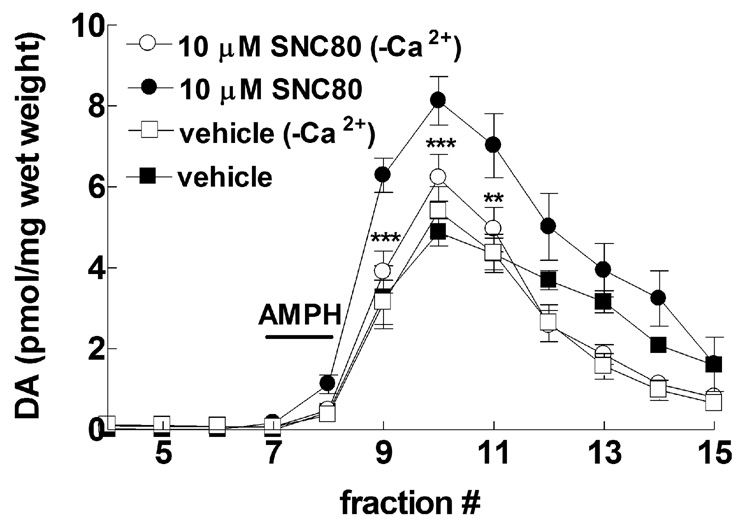
Fig. 6.
Effect of extracellular calcium on SNC80-potentiation of amphetamine-induced dopamine efflux. Striatal preparations from naïve rats were treated with 10 µM SNC80, 30 min prior to a 5 min perfusion with 100 µM amphetamine, in the presence or absence of calcium ions in the perfusion buffer. Fractions were collected at 5 min intervals for an additional 40 min. Dopamine content of the fractions was measured by HPLC-electrochemical detection as described in the Methods. The data was graphed as pmol of dopamine per mg wet weight ± S.E.M (n = 4 in duplicate) and statistical significance was determined by three-way ANOVA with Tukey’s multiple comparison analysis **p < 0.01, ***p < 0.001 compared to buffer containing calcium ions.
3. Results
3.1. Effect of in vivo SNC80 on amphetamine-mediated dopamine efflux from rat striatal tissue measured in vitro
SNC80 produced a marked increase in amphetamine-mediated behaviors, peaking 3 h after administration (Jutkiewicz et al., 2008). Striatal preparations from rats treated 3 h previously with SNC80 (10 mg/kg, s.c.) were evaluated for the amount of dopamine released in response to different concentrations of amphetamine sulfate. Amphetamine perfused through striatal preparations from naïve, vehicle-treated rats produced a concentration-dependent increase in dopamine overflow (Fig. 1 a–e). Compared to vehicle-treated rats, SNC80 had no affect on dopamine efflux prior to the addition of amphetamine, but significantly enhanced the amount of dopamine released in vitro by 30 µM (F(11,120) = 9.06, p < 0.0001) and 100 µM (F(11,120) = 6.22, p < 0.0001) amphetamine, with significant increases at fractions 9, 10, and 11 (Fig. 1, c and d). This produced a significant 4-fold leftward shift in the EC50 for amphetamine-mediated dopamine efflux (p < 0.01) from 253.9 ± 54.2 µM in the absence of SNC80 to 66.8 ± 12.8 µM after SNC80 treatment (Fig. 1f).
To establish a dose effect relationship for the ability of SNC80 to enhance amphetamine-mediated dopamine efflux, varying doses of SNC80 were administered to rats 3 h before striatal preparations were made (Fig. 2a). As before, no dose of SNC80 alone affected basal levels of in vitro dopamine overflow from striatal tissue. However, the amount of dopamine efflux elicited by 100 µM amphetamine sulfate was dose-dependently enhanced by pretreatment with either 10 mg/kg (F(33,231) = 15.58, p < 0.01) or 32 mg/kg (F(33,231) = 15.58, p < 0.001) SNC80. Although 32 mg/kg SNC80 elicited an even greater enhancement of amphetamine-mediated dopamine efflux compared to 10 mg/kg SNC80 (F(33,231) = 15.58, p < 0.05), this dose of SNC80 can produce brief, non-lethal convulsions not seen with administration of 10 mg/kg SNC80 (Broom et al., 2002). Thus, 10 mg/kg SNC80 was used in subsequent experiments to minimize the confounding effects of convulsant activity. When given for different pretreatment times, 10 mg/kg SNC80 significantly potentiated amphetamine-mediated dopamine overflow after 3 h (F(2,37) = 10.90, p < 0.001), but not after a 1 h or 6 h pretreatment (Fig 2b).
Although a 3 h pretreatment with SNC80 also produced the greatest enhancement of amphetamine-mediated behaviors in vivo (Jutkiewicz et al., 2008), this long time course does not correspond with the more rapid onset of behavioral effects seen with administration of SNC80 alone. For example, SNC80 induces locomotor activity immediately after systemic administration (Spina et al., 1998; Jutkiewicz et al., 2008). This may suggest the effect of SNC80 on amphetamine-mediated dopamine efflux is due to induction of a persistent change rather than a direct acute action of SNC80. To address this question, the effect of SNC80 on amphetamine-mediated dopamine overflow was evaluated in the presence of the delta antagonist, naltrindole (NTI) and by direct addition of SNC80 to striatal preparations.
3.2. Effect of the delta selective antagonist, naltrindole (NTI), on in vivo SNC80 enhancement of amphetamine-mediated dopamine efflux measured in vitro
To confirm that the effect of SNC80 to enhance amphetamine-mediated dopamine efflux was a delta opioid receptor mediated effect, rats were treated with 10 mg/kg SNC80 followed by 1 mg/kg NTI 30 min later (Fig 3). Striatal preparations were made 3 h after the initial SNC80 injection. NTI administered in vivo completely blocked the SNC80 enhancement of dopamine overflow elicited by 100 µM amphetamine sulfate in vitro (F(33,231) = 5.14, p < 0.01). This same treatment paradigm with NTI attenuated the ability of SNC80 to enhance amphetamine-mediated locomotor stimulation in rats (Jutkiewicz et al., 2008).
This finding establishes that delta opioid receptor activation is necessary for SNC80 to enhance amphetamine-mediated dopamine efflux. However, it does not clarify whether the SNC80 is still present in the striatal preparations after the 3 h pretreatment, or if there is a persistent change following the SNC80 administration that leads to enhanced amphetamine-mediated dopamine efflux. Consequently, striatal preparations from rats pretreated 3 h previously with 10 mg/kg SNC80 were perfused with 10 nM NTI in vitro for 30 min before addition of amphetamine (Fig. 3). NTI added directly to the striatal preparations was able to fully block the enhancement of amphetamine-mediated dopamine efflux observed with SNC80 administered systemically (F(33,231) = 5.14, p < 0.001), suggesting SNC80 is acting acutely at delta opioid receptors in the striatum, even 3 h after in vivo administration. NTI administered in vivo to vehicle treated rats or in vitro to preparations from vehicle treated rats, had no effect on amphetamine-mediated dopamine efflux alone (data not shown). To confirm that SNC80 was present in striatal tissue 3 h after systemic administration, saturation binding of the delta agonist [3H]-DPDPE was measured in striatal lysates from rats treated with either vehicle or 10 mg/kg SNC80 (s.c.). In lysates from vehicle treated rats, [3H]-DPDPE bound with a Bmax of 183 ± 18.5 fmol/mg protein and Kd of 7.2 ± 1.1 nM. Alternatively, [3H]-DPDPE afforded a Bmax of 215 ± 53.7 fmol/mg protein and Kd of 23.9 ± 2.1 nM in lysates from SNC80 treated rats. Thus, while there was no change in the Bmax (p = 0.55) for [3H]-DPDPE binding, the Kd was significant lowered (p < 0.001) in lysates from SNC80 treated rats, indicating SNC80 was binding to delta opioid receptors 3 h after in vivo administration.
3.3. Effect of SNC80 added directly to striatal preparations and P2 synaptosomal fractions on amphetamine-mediated dopamine efflux
Striatal preparations from naïve rats were treated with 10 µM SNC80 for 30 min immediately before perfusion with various concentrations of amphetamine (Fig. 4 a–e). The amount of dopamine overflow elicited by 30 µM (F(11,120) = 8.01, p < 0.0001) and 100 µM (F(11,120) = 5.77, p < 0.0001) amphetamine was enhanced in the SNC80 treated tissues in fractions 9, 10, and 11. A significant leftward shift in the concentration-response curve of amphetamine-mediated dopamine efflux was observed following in vitro SNC80, to a similar degree (4-fold) as seen with in vivo treatment (p < 0.01) [amphetamine EC50: vehicle, 251.0 ± 47.8 µM; SNC80, 62.6 ± 9.36 µM] (Fig. 4f). Direct in vitro treatment of SNC80 alone to striatal slices produced no effect on dopamine overflow in the absence of amphetamine.
A small increase in amphetamine-mediated dopamine efflux in the striatal preparations with in vitro 10 µM SNC80 was seen after a 15 min pretreatment with a significant effect at 30 min when analyzed using two-way ANOVA (F(2,29) = 1.94, p < 0.01). The effect was diminished after 60 min (Fig. 5a). Using the 30 min pretreatment paradigm, 1, 10, and 100 µM SNC80 produced a concentration-dependent promotion of amphetamine-induced dopamine efflux (F(3,19) = 15.27, p < 0.001). Both the 10 µM and 100 µM concentrations of SNC80 significantly increased the amphetamine response (p < 0.001) (Fig. 5b).
SNC80 modulation of amphetamine-induced dopamine efflux was also measured in striatal P2 synaptosomal fractions (Fig. 5c). The synaptosomal preparations were pretreated for 30 min with increasing concentrations of SNC80 before perfusion with a submaximal 3 µM concentration of amphetamine sulfate, since these preparations were more sensitive than the slices to amphetamine-elicited dopamine efflux (F(3,19) = 4.67, p < 0.05). The amount of endogenous dopamine overflow in response to amphetamine treatment (19.28 pmol/mg protein) in the striatal P2 synaptosomal preparations was increased to the same extent after pretreatment with either 3 µM (30.59 pmol dopamine/mg protein) or 10 µM SNC80 (31.28 pmol dopamine/mg protein) (p < 0.05). SNC80 treatment alone in synaptosomes did not affect dopamine overflow in the absence of amphetamine (data not shown).
3.4. Effect of in vitro SNC80 on amphetamine-mediated dopamine release is attenuated by removal of extracellular calcium
Although thus far SNC80 has been shown to locally modulate dopamine efflux from striatal preparations and synaptosomes, a majority of delta opioid receptors have been shown to be localized on neuronal terminals apposed to neurons expressing dopamine transporter (Svingos et al., 1999). To confirm if SNC80 modulates amphetamine-mediated dopamine efflux indirectly through regulating presynaptic transmission of an unidentified neurotransmitter(s), calcium ions were removed from the Krebs-Ringer buffer to inhibit exocytotic neurotransmitter release. The removal of Ca2+ from the Ringer and its replacement with Mg2+ had no effect on the level of basal or amphetamine-induced dopamine release compared to control conditions (Fig. 6), suggesting no major toxicity to the system. Previous in vivo (Carboni et al., 1989) and in vitro (Kantor and Gnegy, 1998) studies have also demonstrated that the transport-mediated release of dopamine by amphetamine is independent of extracellular calcium. However, in the absence of calcium ions, a 30 min pretreatment with 10 µM SNC80 failed to augment dopamine efflux elicited by 100 µM amphetamine (F(11,336) = 2.38, p < 0.001), with significant decreases compared to SNC80 treatment alone at fractions 9, 10, and 11 (Fig 6).
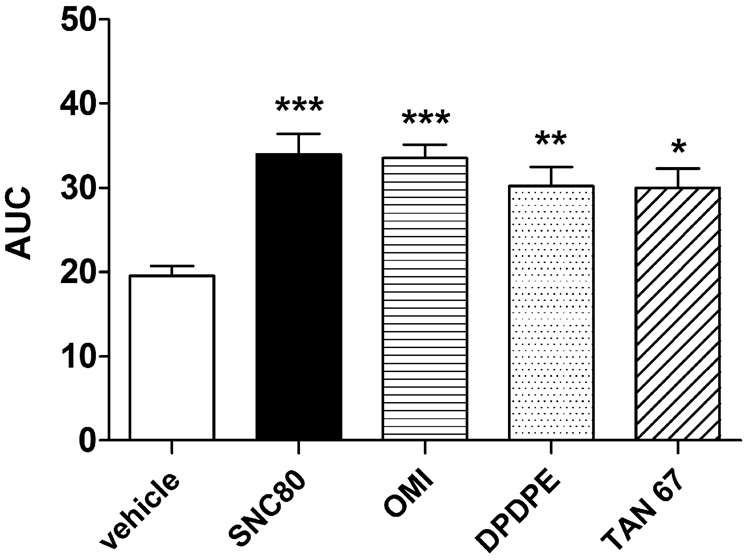
3.5. Effects of other delta agonists on in vitro amphetamine-mediated dopamine efflux
Striatal preparations from naïve rats were treated in vitro with a 10 µM concentration of either the peptidic, DPDPE, or the nonpetidic, TAN-67 or oxymorphindole (OMI), agonists of the delta opioid receptor for 30 min prior to 100 µM amphetamine. Fig. 7 demonstrates that all the delta agonists significantly enhanced amphetamine-induced dopamine release compared to vehicle (F(4,26) = 8.11, p < 0.001). SNC80 (p < 0.001) produced the greatest percentage (± S.E.M.) increase of amphetamine-mediated dopamine efflux over vehicle at 74 ± 12 % (p < 0.001), followed by OMI at 72 ± 8 % (p < 0.001), DPDPE at 55 ± 11 % (p < 0.01), and TAN-67 at 53 ± 11 % (p < 0.05). None of the delta agonists had an effect on dopamine efflux in the absence of amphetamine (data not shown).
Fig. 7.
Comparison of the effects of the delta opioid agonists SNC80, DPDPE, TAN-67, and OMI on amphetamine-mediated dopamine efflux. A 10 µM concentration of each agonist was perfused through rat striatal preparations for 30 min before 100 µM amphetamine challenge for 5 min. Fractions were collected at 5 min intervals for an additional 40 min. Dopamine content of the fractions was measured by HPLCelectrochemical detection as described in the Methods and plotted AUC (± S.E.M.) as measured from the release curves (fractions 4–15) of each treatment group (n = 4 in duplicate). Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001 compared to vehicle.
4. Discussion
The present findings demonstrate that the highly selective non-peptide delta agonist SNC80 enhances striatal amphetamine-mediated dopamine efflux by increasing the potency of amphetamine to efflux dopamine, as well as the total amount of dopamine released. In contrast, SNC80 alone did not increase dopamine overflow. The use of isolated striatal tissue indicates that this effect is intrinsic to the striatum, rather than the result of an action on dopaminergic cell bodies in midbrain regions. The SNC80-induced enhancement of amphetamine-mediated dopamine release was observed after either in vivo or in vitro treatment, was concentration- and time-dependent, reversed by NTI, and required extracellular calcium. The findings were obtained using in vitro superfusion, which allows for the measurement of the dynamics of dopamine efflux, while minimizing the complications of re-uptake, enzymatic degradation, and feedback regulation. This method cannot be directly extrapolated to the effect of SNC80 on amphetamine-mediated dopamine overflow in vivo. Nonetheless, the overall findings of this study support and provide an explanation for the ability of SNC80 to promote behaviors mediated by stimulants that act at the dopamine transporter, but not agents that act directly at dopamine receptors (Mori et al., 2006; Negus et al., 1998; Rowlett and Spealman, 1998; Waddell and Holtzman, 1998; Jutkiewicz et al, 2008). Moreover, the results support the findings of Longoni et al. (1998) that systemic administration of SNC80 failed to increase dopamine efflux in the striatum as measured by in vivo microdialysis.
The concentrations of amphetamine employed in the current study were selected as effective from response curves of dopamine release. These concentrations of amphetamine likely release dopamine from both cytoplasmic and vesicular pools (Liang and Rutledge, 1982). Although it is difficult to relate the concentrations of amphetamine used in this study to doses used in vivo, findings indicate that animals and humans will self-administer extremely high doses of amphetamine (Seiden and Sabol, 1993). For example, rats can self-administer up to 43 mM amphetamine locally in the nucleus accumbens as often as 70 times an hour (Hoebel et al., 1983).
The potentiation of amphetamine-induced dopamine efflux with SNC80 requires activation of the delta opioid receptor, since the effect was dose-dependent following both in vivo and in vitro exposure to SNC80, and was completely blocked by the delta selective antagonist NTI. Effects of endogenous enkephalins acting at delta opioid receptors in intact systems have been demonstrated to contribute to stimulant-mediated behaviors (Jones and Holtzman, 1992; Menkens et al., 1992) and dopamine release (Schad et al., 1996). However, in the present study NTI had no effect alone on amphetamine-mediated dopamine efflux, probably indicating a lack of endogenous enkephalinergic tone in isolated striatal tissue. The action of SNC80 to enhance dopamine efflux evoked by amphetamine was, however, a direct acute response to delta opioid receptor occupancy rather than any possible long-term neural changes induced by SNC80, since it was relatively rapid in onset in vitro and prevented by NTI added to slices 3 h after in vivo administration of SNC80. Indeed, the binding affinity (Kd) of the delta-opioid ligand [3H]-DPDPE was reduced in brain homogenates prepared from rats systemically treated 3 h previously with SNC80, confirming that even after 3 h retained SNC80 was competitively binding to delta opioid receptors. This is supported by a previous study showing brain levels of SNC80 remained elevated for at least 3 h following s.c. injection (Jutkiewicz et al., 2005a). Therefore, while the slower time course following in vivo administration of SNC80 to augment amphetamine-mediated dopamine efflux matches the time course for SNC80 enhancement of amphetamine-mediated locomotor activity (Jutkiewicz et al., 2008), it likely allows for achievement of sufficient levels of SNC80 to survive preparation and perfusion of striatal tissue. Consequently, this does not represent a true time course of SNC80 action. The lack of effect of a 6 h pretreatment with SNC80 on amphetamine-mediated dopamine efflux is probably due to clearance of the administered SNC80 from the brain. Alternatively, tolerance may be occurring since the behavioral effects of SNC80 to promote locomotor activity alone, as well as enhance amphetamine-mediated locomotor activity are subject to profound tolerance (Jutkiewicz et al., 2008). The lack of effectiveness of SNC80 after a 60 min perfusion in vitro would agree with the rapid development of desensitization and/or downregulation of the delta opioid receptor (Lecoq et al, 2004).
The ability to enhance amphetamine-mediated dopamine efflux was not solely a property of SNC80, since this effect was also observed with the structurally-unrelated non-peptidic agonists, TAN-67 and OMI, as well as the peptidic ligand, DPDPE. Surprisingly, the ability of the various delta agonists to promote amphetamine-mediated dopamine efflux did not correlate with the rank order of efficacy of these compounds to activate G-proteins in brain slices from the same strain of rat (Jutkiewicz et al., 2005b). Thus OMI, a low efficacy delta agonist, elicited a similar enhancement of amphetamine-mediated dopamine overflow as the high efficacy agonist SNC80, suggesting the striatal delta opioid system has a high receptor reserve for enhancing dopamine efflux. Alternatively, there may be agonist-specific signaling downstream of these receptors (Lecoq et al, 2004), such that G-protein activation is not directly correlated with amphetamine-mediated dopamine release.
Like SNC80, neither OMI, TAN-67, or DPDPE alone elicited endogenous dopamine efflux from striatal slices. While this finding corresponds with the observations of Longoni et al. (1998) using in vivo microdialysis to study SNC80, it is in contrast to other in vivo microdialysis studies which demonstrate an ability of peptide delta agonists to directly release dopamine (Spanagel et al., 1990; Devine et al., 1993). However, in vivo the delta peptides are likely acting in the ventral tegmental area to promote dopamine efflux from projections to the striatum. In support of this, direct administration of DPDPE into the striatum only enhanced impulse-dependent dopamine release (Pentney and Gratton, 1991), although a direct effect of the peptidic delta agonist DTLET (Tyr-DThr-Gly-Phe-Leu-Thr) to release newly synthesized [3H]dopamine, rather than stored dopamine, has been shown in striatal slices (Petit et al., 1986).
Our finding that SNC80 enhances amphetamine-mediated dopamine efflux provides an in vitro explanation for the ability of this delta opioid agonist to sensitize rats to the locomotor stimulating properties of amphetamines (Mori et al., 2006; Jutkiewicz et al., 2008). However, the observation that SNC80 and the other delta ligands alone do not evoke dopamine efflux fails to explain why SNC80 increases locomotor activity in its own right. Systemically administered SNC80 may be acting at delta opioid receptors distant from the striatum to promote these effects, for instance, there is dense expression of delta opioid receptors in midbrain regions such as the ventral tegmental area that sends dopamine projections to the striatum (Mansour et al. 1995). On the other hand this is not supported by a microdialysis study in which extracellular concentrations of dopamine were unchanged in either the dorsal or ventral striatum following systemic SNC80 administration (Longoni et al., 1998).
The present results are important for an in vitro understanding the basis for the behavioral interaction between psychostimulants and SNC80, but they do not elucidate the molecular mechanism through which this occurs, though it must be intrinsic to the striatum. Certainly, delta opioid receptors are densely located in the caudate putamen and nucleus accumbens (Mansour et al., 1995; Svingos et al., 1999) and have been identified on striatal dopaminergic nerve terminals (Trovero et al.. 1990). An increase in extracellular dopamine elicited by amphetamine is attributed to both an increase in outward transport of dopamine, as well as inhibition of reuptake of dopamine through the transporter. The lack of effect of DPDPE on [3H]dopamine uptake in either the caudate-putamen or nucleus accumbens (Das et al., 1994), indicates a mechanism involving outward transport. Delta opioid agonists have been reported to elevate intracellular calcium leading to the recruitment of calcium-dependent second messenger systems, including protein kinase C (Tang et al., 1994), which have been implicated in regulation of outward transport of dopamine through the dopamine transporter (Gnegy, 2003). Alternatively, delta opioids may modulate amphetamine-induced dopamine efflux through an indirect mechanism, perhaps involving striatal glutamate signaling at (RS)-α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) and/or N-methyl-D-aspartate (NMDA) receptors (Dourmap and Costentin, 1994) which leads to calcium-dependent exocytotic release of dopamine (Wang, 1991). Certainly, delta opioid receptors have been identified on corticostriatal glutamatergic nerve endings, and DPDPE has been shown to promote glutamate efflux in the striatum (Billet et al., 2004). This suggestion is in agreement with the requirement for extracellular calcium ions for SNC80-mediated promotion of amphetamine-induced dopamine release, which would be needed for both the release of glutamate, and the ability of glutamate to induce overflow of dopamine.
In conclusion, the current data demonstrate that SNC80 and other delta opioid agonists have the ability to potentiate the action of amphetamine to efflux dopamine from superfused striatal tissue. This intrinsic striatal interaction may contribute to the documented behavioral interactions between the delta opioid system and amphetamine and other stimulants acting at the dopamine transporter.
Acknowledgements
This study was supported by the National Institute of Drug Abuse Grants R01 DA04087, R01 DA11697 and F31 DA019728. Additional support for KEB and EMJ was provided by T32 DA07267 and T32 DA07268 respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Billet F, Dourmap N, Costentin J. Involvement of corticostriatal glutamatergic terminals in striatal dopamine release elicited by stimulation of delta-opioid receptors. Eur. J. Neurosci. 2004;20:2629–2638. doi: 10.1111/j.1460-9568.2004.03723.x. [DOI] [PubMed] [Google Scholar]
- Bosse KE, Jutkiewicz EM, Gnegy ME, Traynor JR. The selective delta opioid agonist, SNC80, increases amphetamine-mediated release of dopamine. San Francisco, CA: Experimental Biology-ASPET; 2006. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacol. 2002;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Calderon SN, Rice KC, Rothman RB, Porreca F, Flippen-Anderson JL, Kayakiri H, Xu H, Becketts K, Smith LE, Bilsky EJ, Davis P, Horvath R. Probes for narcotic receptor mediated phenomena. 23. Synthesis, opioid receptor binding, and bioassay of the highly selective delta agonist (+)-4-[(α-R*)-α-((2S*,5S*)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,Ndiethylbenzamide (SNC80) and related novel nonpeptide delta opioid receptor ligands. J. Med. Chem. 1997;40:695–704. doi: 10.1021/jm960319n. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentration preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Das D, Rogers J, Michael-Titus AT. Comparative study of the effects of mu, delta, and kappa opioid agonists on 3H-dopamine uptake in rat striatum and nucleus accumbens. Neuropharmacology. 1994;33:221–226. doi: 10.1016/0028-3908(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J. Neurosci. 1994;14:1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmap N, Costentin J. Involvement of glutamate receptor in the striatal enkephalin-induced dopamine release. Eur. J. Pharmacol. 1994;253:R9–R11. doi: 10.1016/0014-2999(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Fleming SM, Kornetsky C. Involvement of delta- and mu-opioid receptors in the potentiation of brain-stimulation reward. Eur. J. Pharmacol. 1996;316:137–143. doi: 10.1016/s0014-2999(96)00674-7. [DOI] [PubMed] [Google Scholar]
- Gnegy ME. The effect of phosphorylation on amphetamine-mediated outward transport. Eur. J. Pharmacol. 2003;479:83–91. doi: 10.1016/j.ejphar.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacol. Biochem. Behav. 1980;13:453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacol. (Berl.) 1983;81:158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Jones DNC, Holtzman SG. Interaction between opioid antagonists and amphetamine: evidence for mediation by central delta opioid receptors. J. Pharmacol. Exp. Ther. 1992;227:229–237. [PubMed] [Google Scholar]
- Jutkiewicz EM, Chang KJ, Rice KC, Woods JH. Characterization of a novel nonpeptidic delta-opioid agonist analog DPI-287. San Diego, CA: Experimental Biology-ASPET; 2005a. [Google Scholar]
- Jutkiewicz EM, Walker NP, Folk JE, Rice KC, Portoghese PS, Woods JH, Traynor JR. Comparison of peptidic and nonpeptidic delta-opioid agonists on guanosine 5’-O-(3-[35S]thio)triphosphate ([35S]GTPgammaS) binding in brain slices from Sprague-Dawley rats. J. Pharmacol. Exp. Ther. 2005b;312:1314–1320. doi: 10.1124/jpet.104.078741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Woods JH. The delta-opioid receptor agonist SNC80 synergistically enhances the locomotor-activating effects of some psychomotor stimulants, but not direct dopamine agonists in rats. J. Pharmacol. Exp. Ther. 2008;324:714–724. doi: 10.1124/jpet.107.123844. [DOI] [PubMed] [Google Scholar]
- Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J. Pharmacol. Exp. Ther. 1998;284:592–598. [PubMed] [Google Scholar]
- Lecoq I, Marie N, Jauzac P, Allouche S. Different regulation of human deltaopioid receptors by SNC-80 [(+)-4-[(α-R*)-α-((2S*,5S*)-4-allyl-2,5-dimethyl-1- piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide] and endogenous enkephalins. J. Pharmacol. Exp. Ther. 2004;310:666–677. doi: 10.1124/jpet.103.063958. [DOI] [PubMed] [Google Scholar]
- Longoni R, Cadoni C, Mulas A, Di Chiara G, Spina L. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC 80: 2. Place-preference and brain microdialysis studies in rats. Behav. Pharmacol. 1998;9:9–14. [PubMed] [Google Scholar]
- Lubetzki C, Chesselet MF, Glowinski J. Modulation of dopamine release in rat striatal slices by delta opiate agonist. J. Pharmacol. Exp. Ther. 1982;222:435–440. [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Durham RA, Lookingland KJ, Moore KE. Delta-opioid receptor-mediated regulation of central dopaminergic neurons in the rat. Eur. J. Pharmacol. 1993;249:107–112. doi: 10.1016/0014-2999(93)90668-8. [DOI] [PubMed] [Google Scholar]
- Menkens K, Bilsky EJ, Wild KD, Portoghese PS, Reid LD, Porreca F. Cocaine place preference is blocked by the delta-opioid receptor antagonist, naltrindole. Eur. J. Pharmacol. 1992;219:345–346. doi: 10.1016/0014-2999(92)90319-y. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Meyer ME. Behavioral effects of opioid peptide agonists DAMGO, DPDPE, and DAKLI on locomotor activities. Pharmacol. Biochem. Behav. 1993;45:315–320. doi: 10.1016/0091-3057(93)90245-o. [DOI] [PubMed] [Google Scholar]
- Mori T, Ito S, Kita T, Narita M, Suzuki T, Sawaguchi T. Effects of mu-, delta-, and kappa-opioid receptor agonists on methamphetamine-induced self-injurious behavior in mice. Eur. J. Pharmacol. 2006;532:81–87. doi: 10.1016/j.ejphar.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Mulder AH, Wardeh G, Hogenboom F, Frankhuyzen AL. Kappa- and delta-opioid receptor agonists differentially inhibit striatal dopamine and acetylcholine release. Nature. 1984;308:278–280. doi: 10.1038/308278a0. [DOI] [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J. Pharmacol. Exp. Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- Pentney RJ, Gratton A. Effects of local delta and mu opioid receptor activation on basal and stimulated dopamine release in striatum and nucleus accumbens of rat: an in vivo electrochemical study. Neuroscience. 1991;45:95–102. doi: 10.1016/0306-4522(91)90106-x. [DOI] [PubMed] [Google Scholar]
- Petit F, Hamon M, Fournie-Zaluski MC, Roques BP, Glowinski J. Further evidence for a role of delta-opioid receptors in the presynaptic regulation of newly synthesized dopamine release. Eur. J. Pharmacol. 1986;126:1–9. doi: 10.1016/0014-2999(86)90731-4. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Spealman RD. Opioid enhancement of the discriminative stimulus effects of cocaine: evidence for involvement of mu and delta opioid receptors. Psychopharmacol. (Berl.) 1998;140:217–224. doi: 10.1007/s002130050760. [DOI] [PubMed] [Google Scholar]
- Schad CA, Justice JB, Jr, Holtzman SG. Differential effects of delta- and mu-opioid receptor antagonists on the amphetamine-induced increase in extracellular dopamine in striatum and nucleus accumbens. J. Neurochem. 1996;67:2292–2299. doi: 10.1046/j.1471-4159.1996.67062292.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur. J. Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE. Amphetamine: effects on catecholamine systems and behavior. Annu. Rev. Pharmacol. Toxicol. 1993;32:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Motivational properties of opioids: evidence that an activation of delta-receptors mediates reinforcement processes. Brain Res. 1987;436:234–239. doi: 10.1016/0006-8993(87)91667-2. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J. Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spina L, Longoni R, Mulas A, Chang KL, Di Chiara G. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC80: 1. Locomotion, rearing, and stereotypies in intact rats. Behav. Pharmacol. 1998;9:1–8. [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Ikeda H, Misawa M, Nagase H. The effects of dopamine D1 and D2 receptor antagonists on the rewarding effects of delta 1 and delta 2 opioid receptor agonists in mice. Psychopharmacology (Berl.) 1996;124:211–218. doi: 10.1007/BF02246659. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Clarke CL, Pickel VM. Localization of the delta-opioid receptor and dopamine transporter in the nucleus accumbens shell: implication for opiate and psychostimulant cross-sensitization. Synapse. 1999;34:1–10. doi: 10.1002/(SICI)1098-2396(199910)34:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tang T, Kiang JG, Cox BM. Opioids acting through delta receptors elicit a transient increase in the intracellular free calcium concentration in dorsal root ganglion-neuroblastoma hybrid ND8-47 cells. J. Pharmacol. Exp. Ther. 1994;270:40–46. [PubMed] [Google Scholar]
- Trovero F, Herve D, Desban M, Glowinski J, Tassin JP. Striatal opiate mu-receptors are not located on dopamine nerve endings in the rat. Neuroscience. 1990;39:313–321. doi: 10.1016/0306-4522(90)90270-e. [DOI] [PubMed] [Google Scholar]
- Ukai M, Mori E, Kameyama T. Cocaine-like discriminative stimulus properties of the delta-selective opioid receptor agonist, [D-Pen2,L-Pen5]enkephalin, in the rat. Eur. J. Pharmacol. 1993;231:143–144. doi: 10.1016/0014-2999(93)90696-f. [DOI] [PubMed] [Google Scholar]
- Waddell AB, Holtzman SG. Modulation of cocaine-induced motor activity in the rat by opioid receptor agonists. Behav. Pharmacol. 1998;9:397–407. doi: 10.1097/00008877-199809000-00003. [DOI] [PubMed] [Google Scholar]
- Wang JK. Presynaptic glutamate receptors modulate dopamine release from striatal synaptosomes. J. Neurochem. 1991;57:819–822. doi: 10.1111/j.1471-4159.1991.tb08224.x. [DOI] [PubMed] [Google Scholar]