Abstract
The aryl hydrocarbon receptor (AhR) is a basic-helix-loop-helix transcription factor that binds halogenated aromatic hydrocarbons, polycyclic aromatic hydrocarbons, and endogenous compounds. We previously reported that AhR null (Ahr−/−) transgenic adenocarcinoma of the mouse prostate (TRAMP) mice on a C57BL/6J background develop prostate tumors with much greater frequency than AhR wild-type (Ahr+/+) TRAMP mice, suggesting that the AhR has tumor suppressor properties. Because AhR signaling pathway inactivation increased susceptibility to prostate tumorigenesis, we tested the hypothesis that a selective AhR modulator (SAhRM), 6-methyl-1,3,8-trichlorodibenzofuran (6-MCDF), can protect against prostate tumorigenesis. TRAMP mice on the standard C57BL/6J × FVB genetic background were fed 0, 10, or 40 mg 6-MCDF/kg diet beginning at 8 weeks of age. Tumor incidence, pelvic lymph node metastasis, and serum vascular endothelial growth factor (VEGF) concentrations were determined at 140 days of age. Prostate tumor incidence and size were not significantly reduced in mice fed 6-MCDF. However, the frequency of pelvic lymph node metastasis was reduced 5-fold in mice fed the 40 mg 6-MCDF/kg diet. Serum VEGF concentrations were also reduced by 6-MCDF treatment, particularly in mice without prostate tumors, and 6-MCDF was shown to act directly on cultured prostates to inhibit VEGF secretion. Together, these results suggest that 6-MCDF inhibits metastasis, in part, by inhibiting prostatic VEGF production prior to tumor formation. This is the first report that 6-MCDF can confer protection against prostate cancer in vivo.
Keywords: TRAMP Mice, Prostate Cancer, VEGF, 6-MCDF, Selective Aryl Hydrocarbon Receptor Modulator, Metastasis Inhibition
1. INTRODUCTION
Prostate cancer and dioxin
Prostate cancer is the most common non-cutaneous cancer diagnosed annually and the second leading cause of cancer death in American men [1]. There is considerable speculation that exposure to environmental pollutants may increase susceptibility to prostate cancer. One group of chemicals that is believed to have an association between exposure and cancer are dioxins, including the prototype 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The Institute of Medicine has found “limited or suggestive evidence” of an association between Agent Orange (a herbicide contaminated with TCDD) exposure and human prostate cancer susceptibility [2]. It was further reported that US Air Force veterans with the greatest serum dioxin concentrations had the greatest prostate cancer risk [3]. Another study reported no overall increased risk of prostate cancer in the Agent Orange-exposed group but that prostate cancer was associated with high TCDD exposure [4]. And a recent study of more than 13,000 veterans found that Agent Orange-exposed men had a increased incidence of prostate cancer, developed prostate cancer at an earlier age, and had more aggressive prostate cancer than did unexposed veterans [5].
In mice, we recently identified an association between exposure to TCDD during early critical stages of prostate development and altered prostate histology later in life. In utero and lactational (IUL) TCDD exposure not only resulted in ventral prostate agenesis but also in a greater incidence of cribriform structures in dorsolateral prostates of senescent C57BL/6J mice [6]. Cribriform structures are hyperplastic lesions found in aging rats and mice, and are often considered to be pre-cancerous lesions in mouse strains susceptible to prostate carcinogenesis [7, 8]. However, C57BL/6J mice are not naturally susceptible to prostate cancer. The implications that early developmental exposure to TCDD may increase the prevalence of lesions associated with greater prostate cancer susceptibility in mice supports the epidemiological evidence that TCDD exposure may also increase prostate cancer risk, although epidemiology studies only correlated adult TCDD exposure with prostate cancer risk.
TCDD binds to the aryl hydrocarbon receptor (AhR), a basic-helix-loop-helix/Per-Arnt-Sim ligand-activated transcription factor. Upon ligand binding, the TCDD/AhR complex translocates to the nucleus and dimerizes with the AhR nuclear translocator (ARNT). The AhR-ARNT complex alters the transcription of a number of genes containing dioxin response elements [9, 10]. Using mice lacking the AhR (Ahr−/−), we demonstrated that effects of IUL TCDD exposure on the mouse prostate, including ventral prostate agenesis, were AhR dependent [11]. Dorsolateral and anterior prostate lobe weights in untreated AhR null mice were also reduced compared to prostates from wild-type littermates at various ages [11]. Even though androgen-dependent prostate lobe weights were reduced, circulating testosterone concentrations were unaltered [11]. This suggested that the AhR signaling pathway, in the absence of TCDD, is in some way involved in normal development of these two prostate lobes.
Role of Ahr in the TRAMP mouse model of prostate cancer
A commonly utilized model for experimental prostate cancer research is the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse [12–16]. The TRAMP model uses the minimal rat probasin gene promoter to drive expression of simian virus 40 (SV40) large T and small t antigens in the prostate epithelium in a hormonally and developmentally regulated manner. TRAMP mice characteristically express the T antigen oncoprotein by 8 weeks of age and develop distinct pathology as mild to severe hyperplasia precedes focal carcinoma. Further cytologic and immunohistochemical characterization revealed that the TRAMP tumor consisted of chromogranin A immunopositive and/or synaptophysin immunopositive neuroendocrine cells and that the architecture of these tumors resembled that of poorly differentiated prostate carcinoma [7, 17]. These fast growing neuroendocrine tumors soon lose their localization in the prostate and tumor cells metastasize to adjacent pelvic lymph nodes as early as 12 weeks of age.
When the effect of AhR was investigated in a C57BL/6J TRAMP mouse model of prostate carcinogenesis, diffuse prostatic epithelial hyperplasia characteristic of the TRAMP model was observed in all mice by 105 days of age. Yet Ahr+/+ TRAMP mice rarely developed prostate tumors, while Ahr−/− TRAMP mice did so with much greater frequency [18]. Quantitative RT-PCR and immunohistochemical analysis indicated that the tumors expressed molecular markers indicative of a neuroendocrine phenotype, suggesting that the Ahr regulated prostate carcinogenesis by inhibiting development of neuroendocrine tumors [18]. These results suggest that the Ahr has neuroendocrine tumor suppressor properties in the C57BL/6J TRAMP model.
Selective AhR modulators as candidates for prostate cancer prevention
Since mice lacking the Ahr were more susceptible to prostate carcinogenesis, we hypothesized that moderate activation of the AhR might confer protection. A 1999 study reporting that testosterone-induced cell proliferation in LNCaP prostate cancer cells was inhibited by TCDD [19] supports this hypothesis. While adverse effects of AhR activation by TCDD would negate any potential therapeutic value, less potent AhR ligands have emerged as potential candidates for protection against certain cancers. These selective AhR modulators (SAhRMs) bind to the AhR, but do so with little of the toxicity caused by TCDD exposure [20–22]. SAhRMs are being developed for the treatment of breast cancer and other hormone-dependent cancers [23–25]. The focus of the present study is on 6-methyl-1,3,8-trichlorodibenzofuran (6-MCDF), a SAhRM whose structure is similar to that of the prototypical AhR agonist TCDD. 6-MCDF inhibited carcinogen-induced mammary tumor growth in rats [21, 22, 26], and inhibited pancreatic cancer cell proliferation in vitro [27]. The finding that 6-MCDF could also inhibit LNCaP prostate cancer cell growth in vitro [28] prompted the present study, which is the first to investigate whether 6-MCDF can inhibit prostate cancer development in vivo. We utilized the TRAMP mouse model to investigate effects of this SAhRM on prostate neuroendocrine tumorigenesis and on metastasis to the adjacent lymph nodes.
2. MATERIALS AND METHODS
2.1 Transgenic mice
All experiments were conducted in accordance with University of Wisconsin Animal Care and Use Committee guidelines and the NIH Guide for the Care and Use of Laboratory Animals. Mice were housed in clear plastic cages with heat-treated chipped aspen bedding in rooms maintained at 24 ± 1°C and with 12 h light and dark cycles. C57BL/6-Tg(TRAMP)8247Ng/J (TRAMP) mice originating from Jackson Laboratory (Bar Harbor, ME) were obtained from Dr. George Wilding (Department of Medicine, University of Wisconsin, Madison, WI). TRAMP male mice homozygous for the probasin-driven SV40 Tag were mated with FVB wild-type females (Harlan, Indianapolis, IN) to obtain TRAMP F1 offspring. TRAMP genotyping measured transgene DNA by quantitative real-time LightCycler PCR using primers described by Greenberg et al. [12], with cytokeratin 8 primers [11] as the loading control. Mice were weaned at 21 days of age, and a maximum of four males were housed in one microisolation cage until they were used. Mice were fed 5015 Mouse Diet (PMI Nutrition International, Brentwood, MO) except during 6-MCDF treatment. Feed and tap water were available ad libitum.
2.2 Study design and 6-MCDF supplementation
6-MCDF (>99% purity) was synthesized as previously described [29]. Study diets were generated by dissolving 6-MCDF in hexane (Fisher Scientific, Pittsburgh, PA; 4 mg/ml) before stirring into rodent chow (powdered Harlan Teklad 5008 rodent chow, Madison, WI) at a final concentration of 10 g/kg. The mixture was allowed to dry overnight to ensure that all hexane had evaporated. The initial stock diet was diluted to the maximum 6-MCDF concentration investigated (100 mg 6-MCDF/kg diet), mixed by tumbling in glass jars for one hour, and then stirred by hand. This process was repeated three times. Each diet was serially diluted to generate diets containing progressively lower 6-MCDF concentrations.
A preliminary study was utilized to determine dietary 6-MCDF concentrations that did not result in overt toxicity, reduce daily feed consumption or body weight gain, or affect reproductive tract weights. Five adult male FVB mice per group were housed individually and fed 0, 1, 16, 40 or 100 mg 6-MCDF/kg diet in glass feeding cups (Allentown Caging, Allentown, PA) for one week. These dietary 6-MCDF concentrations were selected to approximate the daily exposure previously shown to protect against mammary tumorigenesis in rats [22]. Feed consumption, body weights, and overall health were monitored daily. Reproductive tract weights were measured one week after 6-MCDF exposure, as well as weights of liver and thymus, typical sensitive target organs of TCDD [30].
For the 6-MCDF tumorigenesis and metastasis study, 8-week-old C57BL/6-Tg(TRAMP)8247Ng/J × FVB male mice were randomly assigned to 0, 10, or 40 mg 6-MCDF/kg diet, concentrations that were well tolerated in the range-finding experiment. Litter independence was maintained in that only one pup per litter was utilized for each diet. Mice were maintained on test diet until 20 weeks of age, at which time the experiment was terminated. Mice euthanized prior to 140 days due to excessive tumor burden were included as prostate tumor positive for the incidence data. Animals were investigated at 20 weeks of age as this was the earliest age we identified that the Ahr significantly reduced prostate tumor incidence in C57BL/6-Tg(TRAMP)8247Ng/J mice [18]. For the 12 week duration, 6-MCDF feed and tap water were available ad libitum.
At 20 weeks of age, animals were euthanized by CO2 overdose, and investigated for grossly evident tumors. Ventral, dorsolateral, and anterior prostates and/or prostate tumors were removed and weighed. One half of each prostate (i.e., the left or right lobe) was frozen in liquid nitrogen. The other half was fixed overnight in Bouin’s, paraffin embedded, sectioned (5 μm) and stained with hematoxylin (Fisher Scientific) and eosin (Sigma-Aldrich, St. Louis, MO) for histologic analysis by light microscopy [17]. Whenever possible, tumors were separated from surrounding intact prostate lobes, then bisected so that half could be processed for histological analysis and the other half frozen. If necessary, weights from samples collected from mice euthanized prior to 140 days were included in the 140-day analysis. Pelvic lymph nodes were also monitored for gross tumor metastasis. Routine histopathologic analysis, large T antigen immunohistochemistry, and chromogranin A immunohistochemistry were conducted as previously described [18] to confirm neuroendocrine cell infiltration into the lymph nodes. Blood was collected by cardiac puncture, and serum was separated by centrifugation at 2800 × g, then stored at −80°C until analysis.
2.3 Serum VEGF analysis
Serum VEGF concentrations were measured using an ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. Briefly, samples (diluted 5-fold as indicated) and appropriate standards were added to a microtiter plate coated with an affinity-purified polyclonal antibody specific for mouse VEGF and incubated for 2 h at room temperature. After unbound sample was removed by a series of washes, the plate was incubated with an enzyme-linked polyclonal antibody specific for mouse VEGF. Unbound antibody-enzyme reagent was washed from wells prior to incubation with substrate solution. Absorbance was measured at 450 nm with correction wavelength set at 540 nm.
2.4 Characterization of the direct effects of 6-MCDF on VEGF secretion from TRAMP prostate organ cultures
At 140 days of age, tumor-free dorsolateral prostates were removed from TRAMP mice fed the control diet and placed in ice-cold Hanks’ balanced salt solution (Sigma-Aldrich) containing antibiotic/antimycotic (Gibco Invitrogen Corporation, Grand Island, NY). Dorsolateral prostates were minced into small (~2–4 mg) pieces. Two pieces from the each prostate were processed for histological analysis, while remaining pieces from the same prostate were transferred to a 24-well culture plate with 1 ml Dulbecco’s Modified Eagle’s medium:F12 (with L-glutamine, Mediatech, Inc., Herndon, VA) culture medium (1:1, v:v) containing 10% charcoal-dextran-stripped fetal bovine serum (Fisher Scientific), antibiotic/antimycotic, insulin/tranferrin/selenium (Gibco Invitrogen), 10 nM 5α-dihydrotestosterone (DHT, Sigma-Aldrich), and either 0, 100, or 1000 nM 6-MCDF for three days. Each prostate contributed duplicate pieces to each of the three groups. Growth medium was changed every 24 h, and medium from the last 24 h incubation was used for VEGF analysis. After three days in culture, dorsolateral prostate pieces were weighed so that VEGF concentrations could be normalized to individual sample weight. After incubation, prostate pieces were fixed, sectioned, and stained for histology. VEGF concentrations from samples cultured with 6-MCDF were compared to concentrations from pieces taken from same dorsolateral prostate incubated with vehicle, thereby allowing each prostate to serve as its own control.
2.5 Statistical analysis
Results were analyzed for statistical significance using SigmaStat software (Jandel Scientific, San Rafael, CA). Analysis of variance (ANOVA) was conducted on parametric data that passed Levene’s test for homogeneity of variance and were normally distributed. Kruskal-Wallis one way analysis of variance on ranks was utilized for comparisons without normal distribution. If a significant effect was found, the least significant difference test was used to determine which group(s) differed from the appropriate control group. Tumor and metastasis incidence data were compared using Fisher’s exact test. Values of p < 0.05 were considered statistically significant.
3. RESULTS
3.1 Determination of 6-MCDF concentrations in the diet that did not alter weights of reproductive organs, liver, or thymus
An initial experiment was conducted to determine concentrations of 6-MCDF that would not influence weights of androgen-dependent reproductive organs. This minimized the likelihood that possible effects of 6-MCDF on tumorigenesis could be caused simply by increasing or decreasing androgen-dependent transgene expression. Likewise, it was important to determine 6-MCDF concentrations that would not alter weights of organs typically associated with the toxicity of a full AhR agonist like TCDD. In FVB mice fed graded concentrations of 6-MCDF, none of the diets altered daily feed intake or body weight gain during the one week of 6-MCDF exposure (data not shown). Thymus, seminal vesicle, and individual prostate lobe weights were not altered by exposure to any of the diets containing 6-MCDF (Table 1). However, liver weights were reduced in mice fed the 100 mg 6-MCDF/kg diet. Thus, 40 mg 6-MCDF/kg diet was selected as the maximum dietary concentration to investigate effects on TRAMP prostate tumorigenesis and metastasis.
Table 1.
Effect of One Week Dietary Exposure to Graded Concentrations of 6-MCDF on Relative Organ Weights in Adult FVB Mice1
| 6-MCDF Concentration (mg/kg diet) | |||||
|---|---|---|---|---|---|
| Organ | 0 | 1 | 16 | 40 | 100 |
| Ventral Prostate | 0.41 ± 0.07 | 0.39 ± 0.06 | 0.32 ± 0.05 | 0.35 ± 0.02 | 0.41 ± 0.10 |
| Dorsolateral Prostate | 0.59 ± 0.08 | 0.61 ± 0.07 | 0.55 ± 0.04 | 0.56 ± 0.03 | 0.65 ± 0.04 |
| Anterior Prostate | 1.35 ± 0.12 | 1.51 ± 0.12 | 1.29 ± 0.07 | 1.39 ± 0.14 | 1.41 ± 0.09 |
| Seminal Vesicle | 8.83 ± 0.17 | 8.78 ± 0.40 | 8.37 ± 0.45 | 9.41 ± 0.35 | 8.53 ± 0.62 |
| Thymus | 1.16 ± 0.09 | 1.20 ± 0.12 | 1.31 ± 0.13 | 1.12 ± 0.08 | 1.03 ± 0.12 |
| Liver | 52.0 ± 0.60 | 51.4 ± 1.12 | 49.2 ± 0.97 | 49.8 ± 1.01 | 48.2 ± 0.86* |
mg organ/g body weight (mean ± SEM, n = 5 mice/group)
p < 0.05 compared to 0 mg 6-MCDF/kg diet (control)
3.2 No significant reductions in prostate tumor incidence or size in TRAMP mice fed 6-MCDF
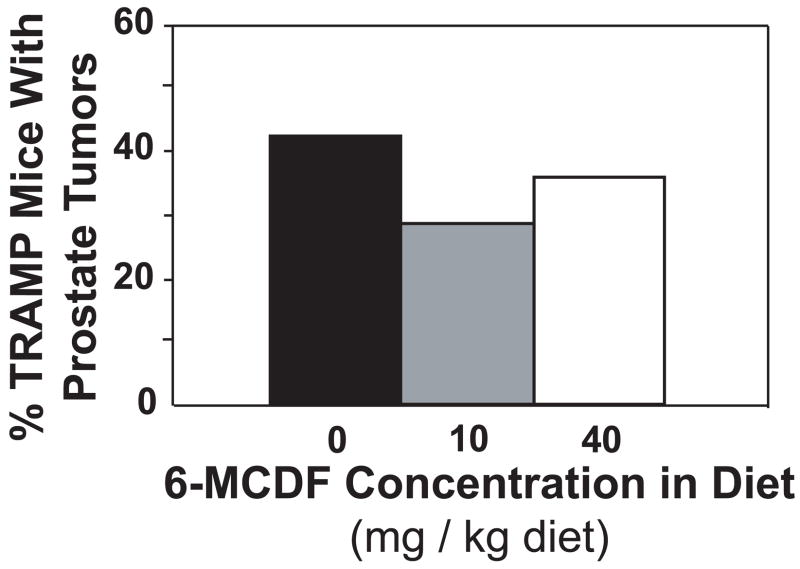
Body weights were not reduced in TRAMP mice fed 10 or 40 mg 6-MCDF/kg diet starting at 56 days of age (Table 2). After 12 weeks on the diet, mice were euthanized and prostate tumors were evaluated. Prostate tumors were found in 28 of the 73 mice used in this experiment, but no mouse had more than a single prostate tumor. Prostate tumor incidence at 140 days of age was not significantly reduced in TRAMP mice fed either diet containing 6-MCDF (Fig. 1).
Table 2.
Effect of 6-MCDF in the Diet on Body, Prostate or Prostate Tumor Weights in 140-Day-Old TRAMP Mice1
| 6-MCDF (mg/kg Diet) | Body Weight (g) | Prostate Weight (g)2 | Prostate Tumor Weight (g) |
|---|---|---|---|
| 0 | 37.5 ± 0.8 (45)3 | 0.14 ± 0.01 (26) | 4.19 ± 0.67 (19) |
| 10 | 35.3 ± 0.8 (14) | 0.14 ± 0.01 (10) | 3.97 ± 2.19 (4) |
| 40 | 36.6 ± 0.8 (14) | 0.13 ± 0.01 (9) | 1.93 ± 1.64 (5) |
Mice were fed 0, 10 or 40 mg 6-MCDF/kg diet from 8 to 20 weeks of age. The number of mice per group is shown in parenthesis. All weights are mean ± SEM.
Combined ventral, dorsolateral, and anterior prostate weights in TRAMP mice without prostate tumors.
N value of observation.
Fig. 1.
Percentage of 140-day-old TRAMP mice fed 0, 10 or 40 mg 6-MCDF/kg diet with macroscopic prostate tumors. Tumor incidence was determined in TRAMP mice fed 6-MCDF in the diet from 8–20 weeks of age. The numbers of mice fed each diet are shown in Table 2.
In TRAMP mice that did not have prostate tumors, combined ventral, dorsolateral, and anterior prostate weights were not altered by exposure to 6-MCDF (Table 2). When present, mean prostate tumor weights were reduced by 54% in TRAMP mice fed 40 mg 6-MCDF/kg diet, although this reduction was not statistically significant (p = 0.16). Tumor weights varied widely in each 6-MCDF treatment group.
3.3 Histological characterization of microscopic prostate lesions in TRAMP mice fed 6-MCDF
Microscopic analysis of prostate sections stained with hematoxylin and eosin was carried out at 140 days of age. Dorsolateral prostates from all mice had diffuse epithelial hyperplasia characteristic of the TRAMP model (results not shown). Thus, development of prostatic hyperplasia was not inhibited by exposure to 6-MCDF. The percentage of mice with histological regions indicative of prostate tumors closely paralleled the incidence of macroscopic prostate tumors reported in Fig. 1. Consistent with induction of histological alterations resulting from transgene action in all mice, immunohistochemical localization of the SV40 large T antigen to epithelial cells lining prostatic ducts and in prostate tumor cells was not altered by exposure to 6-MCDF (results not shown).
3.4 Reduced frequency of pelvic lymph node metastasis in TRAMP mice fed 6-MCDF
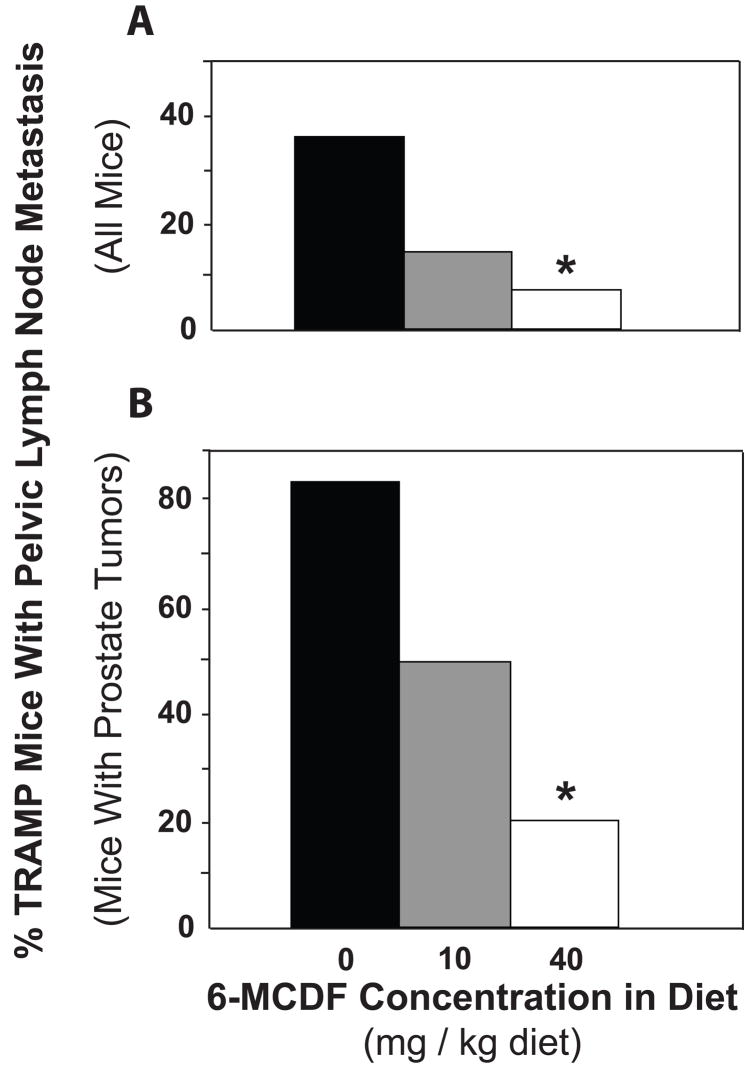
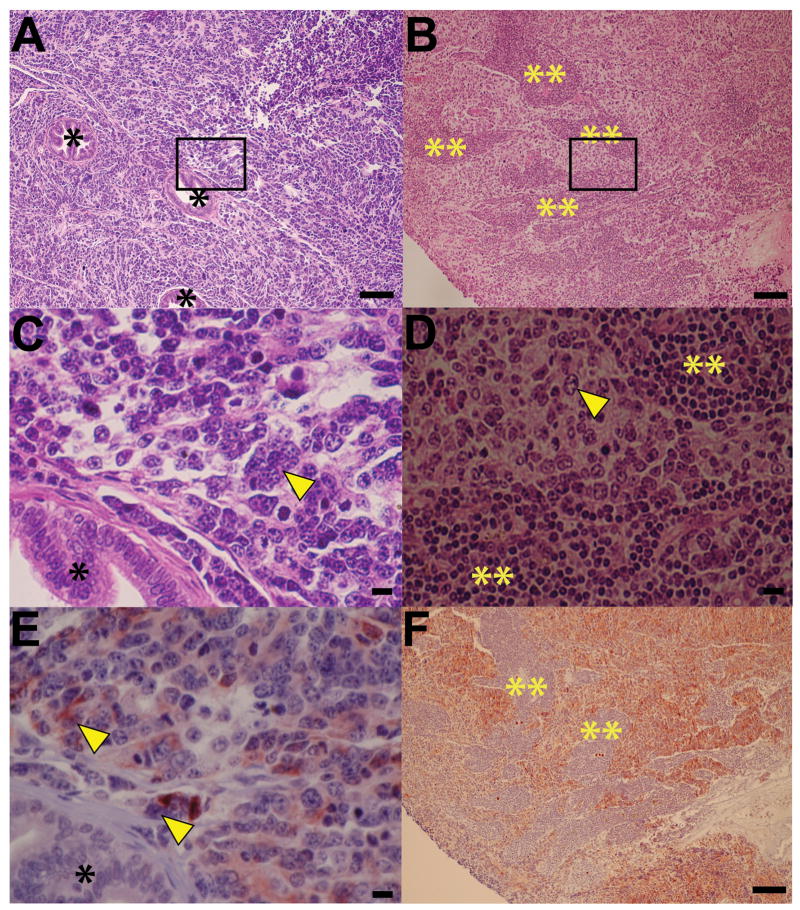
The incidence of pelvic lymph node metastasis at 20 weeks of age was reduced from 36% in TRAMP mice fed control diet to 14% in those fed the 10 mg 6-MCDF/kg diet and 7% in those fed the 40 mg/kg diet (Fig. 2A). Among mice with prostate tumors, the incidence of lymph node metastasis declined from 84% (control diet) to 50% (10 mg/kg diet) to 20% (40 mg/kg diet; Fig. 2B). The effects of the 10 mg 6-MCDF/kg diet were not statistically significant while the reductions in metastasis caused by the 40 mg 6-MCDF/kg diet were statistically significant. All pelvic lymph nodes identified by visual inspection as containing metastases were characterized by infiltration of neuroendocrine cells with their classical salt-and-pepper chromatin morphology (Fig. 3B and 3D) and chromogranin A immunoreactivity (Fig. 3F). Most likely these neuroendocrine cells originated from prostate confined TRAMP tumors, where similar characteristic neuroendocrine cells were readily identified (Fig. 3A, 3C and 3E).
Fig. 2.
Effect of exposure to 0, 10 or 40 mg 6-MCDF/kg diet starting at 8 weeks of age on the incidence of gross pelvic lymph node metastases in 140-day-old TRAMP mice. At necropsy, pelvic lymph nodes were visually inspected for metastasis, which was confirmed by chromogranin A immunohistochemistry (see Fig. 3). In all mice (A), and in only tumor-bearing mice (B), fewer TRAMP mice fed 40 mg 6-MCDF/kg diet had pelvic lymph node metastasis than TRAMP mice not fed 6-MCDF (* p < 0.05, Fisher’s exact test). The numbers of mice studied are shown in Table 2.
Fig. 3.
Representative neuroendocrine characterization of prostate-confined TRAMP tumors and adjacent enlarged pelvic lymph nodes. Prostate tumors and grossly identifiable pelvic lymph nodes were harvested from 140-day-old TRAMP mice fed 0, 10 or 40 mg 6-MCDF/kg diet starting at 8 weeks of age. Tissues were fixed, paraffin-embedded, and sectioned for histopathology. Histology of a representative prostate tumor from a TRAMP mouse fed 0 mg 6-MCDF/kg diet (A and C, higher magnification) and of its enlarged pelvic lymph node (B and D, higher magnification) is shown after hematoxylin and eosin staining. Cells with characteristic neuroendocrine salt-and-pepper nuclei [46] are visible (arrowheads) in both the prostate tumor (C) and pelvic lymph node (D). Localization of the neuroendocrine differentiation marker, chromogranin A, was assessed using standard immunohistochemical techniques (E, prostate tumor and F, lymph node). Note that large reddish brown chromogranin A IHC positive cells (E, arrow heads) with characteristic neuroendocrine salt-and-pepper nuclei are abundant in the prostate tumor, and that chromogranin A IHC positive cells are abundant in the pelvic lymph node (F). Regions of prostate luminal epithelia hyperplasia (*) in prostate tumor and lymphocytes (**) in the lymph node are chromogranin A IHC negative. The horizontal bar in each panel represents either 100 μm (A, B and F) or 10 μm (C, D and E).
3.5 Serum VEGF concentrations in TRAMP mice fed 6-MCDF
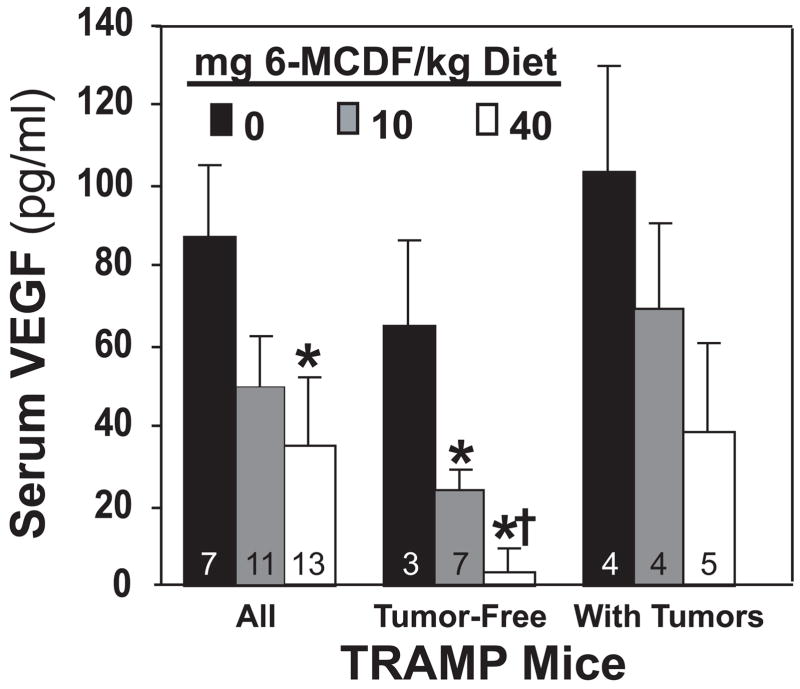
When serum from all TRAMP mice were compared, VEGF concentrations were reduced in 20-week-old mice fed the 40 mg 6-MCDF/kg diet starting at 8 weeks of age (Fig. 4). TRAMP mice without tumors that were fed either 10 or 40 mg 6-MCDF/kg diet had reduced serum VEGF concentrations compared to tumor-free TRAMP mice not fed 6-MCDF. However, there were no significant effects of 6-MCDF on serum VEGF levels in mice that had prostate tumors.
Fig. 4.
Serum VEGF concentrations in 140-day-old TRAMP mice fed 0, 10 or 40 mg 6-MCDF/kg diet starting at 8 weeks of age. VEGF concentrations were determined using a commercial ELISA kit on serum collected at 140 days of age. Separate comparisons were also made for tumor-free sera and for sera from mice with prostate tumors. Values are the mean ± SEM with the number of samples shown in each bar except for † n = 8. * significantly different from control TRAMP mice (p < 0.05).
When the relationship between serum VEGF concentrations and disease progression was examined without regard to 6-MCDF treatment, VEGF concentrations progressively increased as a function of both increasing prostate tumor weight and total pelvic lymph node metastasis weight (not shown).
3.6 Direct inhibition of VEGF production in prostates cultured in the presence of 6-MCDF
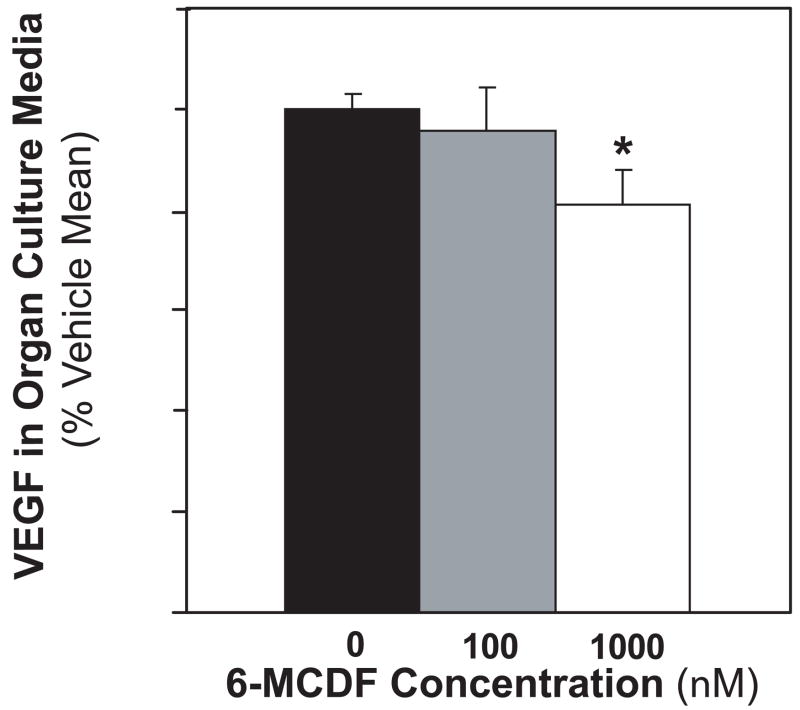
To determine if reduced VEGF concentrations in the serum of mice fed 6-MCDF could potentially be explained by direct effects on the prostate, VEGF secretion was investigated in minced control 140-day-old TRAMP mouse dorsolateral prostates cultured in the presence of 0, 100 or 1000 nM 6-MCDF. All dorsolateral prostates at this age that have not yet developed macroscopic tumors have diffuse epithelia hyperplasia. Addition of 6-MCDF to tumor-free TRAMP prostate cultures reduced VEGF secretion into the culture media (Fig. 5). There were no grossly evident morphological alterations or overt signs of tissue apoptosis in samples exposed to 6-MCDF in the culture media that could account for reduced VEGF production. These results demonstrate that 6-MCDF is capable of acting directly on the TRAMP mouse prostate to inhibit VEGF production.
Fig. 5.
Direct inhibition of VEGF secretion by TRAMP mouse dorsolateral prostates cultured in the presence of 6-MCDF. Pieces from tumor-free 140-day-old TRAMP dorsolateral prostates were minced and incubated for three days in organ culture media containing 0, 100 or 1000 nM 6-MCDF. For each mouse, pieces taken from the same prostate were cultured in duplicate for each concentration of 6-MCDF. Prostate histology was determined for two pieces not selected for organ culture, and confirmed after 3 day organ culture on individual prostate pieces. VEGF concentrations relative to weight of each cultured prostate piece were determined in the culture media by ELISA after the final 24 hr period. For each mouse prostate, VEGF levels were expressed as a percentage of the mean for the pieces taken from the same prostate cultured without 6-MCDF. Values are the mean ± SEM for 0 (n = 15), 100 (n = 12) or 1000 nM (n = 15) 6-MCDF in TRAMP mice. * indicates a significant difference compared to vehicle group (p < 0.05).
DISCUSSION
The AhR is best known for regulating biological responses to environmental chemicals including polycyclic aromatic hydrocarbons and dioxins [9, 10]. Studies with Ahr−/− mice have implicated the AhR in normal developmental processes [30–33], including those for the anterior and dorsolateral prostate [11]. We subsequently demonstrated that the Ahr also has tumor suppressor activity, in that Ahr−/− TRAMP mice developed prostate tumors with far greater frequency than Ahr+/+ TRAMP mice [18]. The present study was designed to test the hypothesis that 6-MCDF, a SAhRM structurally very similar to the prototypical AhR agonist TCDD, can protect against prostate cancer in vivo.
Although 6-MCDF had previously been found to inhibit LNCaP prostate cancer cell proliferation in vitro [28], it had no detectable protective effect in vivo with respect to the percentage of mice with prostate tumors. Prostate tumor weights tended to be smaller in mice fed 6-MCDF than in those fed the control diet, yet the difference was not statistically significant. Whether the lack of any major protective effect in the in vivo versus the in vitro experiment was due to smaller effective 6-MCDF concentrations in vivo, differences between the types of cancer cells in these two model systems, or to other effects remains unknown.
Despite the lack of any statistically significant effect of 6-MCDF on tumors within the prostate itself, the key finding from this study is that 6-MCDF greatly inhibited metastasis from the prostate to the pelvic lymph nodes. TRAMP mice fed the high dose of 6-MCDF were five times less likely to have such metastases than those fed the control diet. Among TRAMP mice with prostate tumors, 16 of 19 fed the control diet had pelvic lymph node metastases whereas 2 of 4 fed the low dose and only 1 of 5 fed the high dose of 6-MCDF had lymph node metastases. This is the first report that 6-MCDF can confer protection against prostate cancer in vivo. Although both doses appeared to protect against lymph node metastasis, this effect was not statistically significant at the lower dose (p = 0.12 when all mice were considered; p = 0.19 for mice with prostate tumors). Whether 10 mg 6-MCDF/kg diet is capable of significantly inhibiting metastasis would require more mice than the 14 that were fed this concentration in the present experiment. Additional experiments would also be needed to determine how long the protection against metastasis persists, and if 6-MCDF protects against metastasis to sites other than the pelvic lymph nodes.
In our initial characterization of the effects of the Ahr on prostate carcinogenesis, the prostate tumors had molecular evidence of a neuroendocrine phenotype [18], suggesting that the Ahr regulates prostate tumorigenesis through regulation of neuroendocrine differentiation. Prostatic neuroendocrine cells are typically androgen-insensitive [34], and produce growth stimulatory factors including VEGF [35, 36], one of the key regulators of prostate tumor vascularization, growth and metastasis [37]. VEGF expression has been correlated with disease progression in men with prostate cancer [38, 39], and with tumor progression and angiogenesis in TRAMP mice [14]. It was therefore somewhat surprising that serum VEGF concentrations were reduced in TRAMP mice fed 6-MCDF whereas prostate tumor incidence was not. Yet mice with large prostate tumors and pelvic lymph node metastases generally had high serum VEGF concentrations, regardless of 6-MCDF treatment. In addition, both serum VEGF concentrations and the incidence of pelvic lymph node metastasis were significantly reduced by 6-MCDF treatment. The greatest reductions in serum VEGF concentrations occurred in mice without prostate tumors, suggesting that 6-MCDF inhibits metastasis, in part, by reducing VEGF production prior to tumor formation.
To more directly determine whether 6-MCDF regulation of prostate tumor growth and metastasis related to VEGF can be attributed to a direct action on the prostate rather than due to a peripheral action in other organs, we utilized a prostate organ culture technique to measure VEGF production in response to direct exposure to 6-MCDF. Using prostates at 140 days of age with diffuse epithelial hyperplasia and 6-MCDF concentrations commonly used in studies on other cell types, we demonstrated that 6-MCDF could act directly on the tumor-free prostate to inhibit VEGF secretion into culture media. The percent inhibition was smaller than the reduction in serum VEGF concentrations seen in 6-MCDF-fed mice, however. In addition, it is not known how the 6-MCDF concentrations used in vitro compare with 6-MCDF concentrations in vivo because there are no published in vivo 6-MCDF concentration data. Nevertheless, these results suggest that at least some of the chemoprotective effects of 6-MCDF in TRAMP mice related to VEGF can be attributed to direct effects on the prostate, and that chemoprotection is not necessarily dependent on actions that occur in other organs.
6-MCDF was found to inhibit androgen-dependent reporter gene activity in LNCaP prostate cancer cells [28], suggesting that it has antiandrogenic activity. Yet 6-MCDF treatment in the present study caused no reduction in accessory sex organ weights in either the pilot study or the main experiment. Furthermore, 6-MCDF treatment caused no detectable inhibition of either androgen-dependent large T antigen expression or androgen-dependent initiation of prostate histology characteristic of the TRAMP model. It is therefore unlikely that the effects of 6-MCDF in the present study were due to an inhibition of androgen signaling.
The AhR can interact with estrogen receptors through cross-talk mechanisms [40]. 6-MCDF and related SAhRMs have been shown to possess anti-estrogenic properties in rat uterus [41], and have proven to be effective chemopreventive agents for mammary carcinogenesis in rats [21, 22, 26]. In addition to AhR binding, 6-MCDF can also bind to estrogen receptor α and activate estrogen-dependent signaling in an AhR-independent manner [42, 43]. Both estrogenic and antiestrogenic compounds have been previously shown to effectively reduce prostate cancer incidence in TRAMP mice [44, 45]. Thus, inhibition of prostate tumor metastasis to the adjoining pelvic lymph nodes by 6-MCDF may be due in part to selective AhR modulation and in part to a direct modulation of estrogen receptor signaling. 6-MCDF may also be acting via effects on other signaling pathways as well.
In conclusion, we expand our previous findings that the Ahr protects against prostate carcinogenesis in untreated C57BL/6J TRAMP mice [18] by demonstrating chemoprevention by a SAhRM in the standard TRAMP mouse model. More specifically, we show that 6-MCDF reduces the incidence of prostate tumor pelvic lymph node metastasis. 6-MCDF also was found to decrease serum concentrations of VEGF, a known regulator of prostate tumor progression and metastasis [14, 37–39], and this regulation plausibly occurred through a direct action of 6-MCDF on the TRAMP mouse prostate.
This was the initial in vivo experiment to determine if 6-MCDF can protect against prostate cancer, consequently, it was not designed to determine which processes that influence metastasis (e.g., extracellular matrix proteolysis, cell migration, evasion of host-immune cells, etc.) were affected. Similarly, additional research is needed to determine whether 6-MCDF is capable of inhibiting metastasis in other experimental prostate cancer models, and whether 6-MCDF can inhibit the metastasis of neuroendocrine cells from cancers in other organs.
Acknowledgments
Portions of this work were supported by National Cancer Institute grant CA095751 and National Institutes of Health grants ES01332 and ES12352. Wayne Fritz was supported by the Molecular and Environmental Toxicology Postdoctoral Training Grant number T32 ES007015 from the NIEHS, NIH. The authors thank Dr. Terry Oberley, Pathology Department, School of Medicine, University of Wisconsin, for assistance with histological characterization of TRAMP prostates. We thank Heather Hardin for assistance with histopathology and immunohistochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Veterans and Agent Orange: Update 2006. Washington, D.C: National Academies Press; 2007. [Google Scholar]
- 3.Akhtar FZ, Garabrant DH, Ketchum NS, Michalek JE. Cancer in US Air Force veterans of the Vietnam War. J Occup Environ Med. 2004;46:123–36. doi: 10.1097/01.jom.0000111603.84316.0f. [DOI] [PubMed] [Google Scholar]
- 4.Pavuk M, Michalek JE, Ketchum NS. Prostate cancer in US Air Force veterans of the Vietnam war. J Expo Sci Environ Epidemiol. 2006;16:184–90. doi: 10.1038/sj.jea.7500448. [DOI] [PubMed] [Google Scholar]
- 5.Chamie K, deVere White RW, Lee D, Ok J-H, Ellison LM. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008;113:2464–70. doi: 10.1002/cncr.23695. [DOI] [PubMed] [Google Scholar]
- 6.Fritz WA, Lin T-M, Moore RW, Cooke PS, Peterson RE. In utero and lactational 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) exposure: Effects on the prostate and its response to castration in senescent C57BL/6J mice. Toxicol Sci. 2005;86:387–95. doi: 10.1093/toxsci/kfi189. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–37. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 8.Shain SA, McCullough B, Segaloff A. Spontaneous adenocarcinomas of the ventral prostate of aged A × C rats. J Natl Cancer Inst. 1975;55:177–80. doi: 10.1093/jnci/55.1.177. [DOI] [PubMed] [Google Scholar]
- 9.Poland A, Knutson JC. 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Lin T-M, Ko K, Moore RW, Simanainen U, Oberley TD, Peterson RE. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;68:479–87. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–102. [PubMed] [Google Scholar]
- 14.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–91. [PubMed] [Google Scholar]
- 15.Hsu CX, Ross BD, Chrisp CE, Derrow SZ, Charles LG, Pienta KJ, et al. Longitudinal cohort analysis of lethal prostate cancer progression in transgenic mice. J Urol. 1998;160:1500–5. [PubMed] [Google Scholar]
- 16.Huss WJ, Hanrahan CF, Barrios RJ, Simons JW, Greenberg NM. Angiogenesis and prostate cancer: Identification of a molecular progression switch. Cancer Res. 2001;61:2736–43. [PubMed] [Google Scholar]
- 17.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 18.Fritz WA, Lin T-M, Peterson RE. The aryl hydrocarbon receptor (AhR) inhibits prostate cancer progression in the TRAMP mouse. Carcinogenesis. 2007;28:497–505. doi: 10.1093/carcin/bgl179. [DOI] [PubMed] [Google Scholar]
- 19.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Cross-talk between 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and testosterone signal transduction pathways in LNCaP prostate cancer cells. Biochem Biophys Res Commun. 1999;256:462–8. doi: 10.1006/bbrc.1999.0367. [DOI] [PubMed] [Google Scholar]
- 20.Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–9. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- 21.McDougal A, Sethi Gupta M, Ramamoorthy K, Sun G, Safe SH. Inhibition of carcinogen-induced rat mammary tumor growth and other estrogen-dependent responses by symmetrical dihalo-substituted analogs of diindolylmethane. Cancer Lett. 2000;151:169–79. doi: 10.1016/s0304-3835(99)00406-1. [DOI] [PubMed] [Google Scholar]
- 22.McDougal A, Wilson C, Safe S. Inhibition of 7, 12-dimethylbenz[a]anthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonists. Cancer Lett. 1997;120:53–63. doi: 10.1016/s0304-3835(97)00299-1. [DOI] [PubMed] [Google Scholar]
- 23.Safe S, Qin C, McDougal A. Development of selective aryl hydrocarbon receptor modulators for treatment of breast cancer. Expert Opin Investig Drugs. 1999;8:1385–96. doi: 10.1517/13543784.8.9.1385. [DOI] [PubMed] [Google Scholar]
- 24.Safe S, McDougal A, Gupta MS, Ramamoorthy K. Selective Ah receptor modulators (SAhRMs): Progress towards development of a new class of inhibitors of breast cancer growth. J Women’s Cancer. 2001;3:37–45. [Google Scholar]
- 25.Safe S, McDougal A. Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers. Int J Oncol. 2002;20:1123–8. [PubMed] [Google Scholar]
- 26.McDougal A, Wormke M, Calvin J, Safe S. Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective aryl hydrocarbon receptor modulator. Cancer Res. 2001;61:3902–7. [PubMed] [Google Scholar]
- 27.Koliopanos A, Kleeff J, Xiao Y, Safe S, Zimmermann A, Büchler MW, et al. Increased arylhydrocarbon receptor expression offers a potential therapeutic target for pancreatic cancer. Oncogene. 2002;21:6059–70. doi: 10.1038/sj.onc.1205633. [DOI] [PubMed] [Google Scholar]
- 28.Morrow D, Qin C, Smith R, III, Safe S. Aryl hydrocarbon receptor-mediated inhibition of LNCaP prostate cancer cell growth and hormone-induced transactivation. J Steroid Biochem Mol Biol. 2004;88:27–36. doi: 10.1016/j.jsbmb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Astroff B, Zacharewski T, Safe S, Arlotto MP, Parkinson A, Thomas P, Levin W. 6-Methyl-1, 3, 8-trichlorodibenzofuran as a 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin antagonist: inhibition of the induction of rat cytochrome P-450 isozymes and related monooxygenase activities. Mol Pharmacol. 1988;33:231–6. [PubMed] [Google Scholar]
- 30.Lin TM, Ko K, Moore RW, Buchanan DL, Cooke PS, Peterson RE. Role of the aryl hydrocarbon receptor in the development of control and 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-exposed male mice. J Toxicol Environ Health A. 2001;64:327–42. doi: 10.1080/152873901316981312. [DOI] [PubMed] [Google Scholar]
- 31.Benedict JC, Lin T-M, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56:382–8. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–6. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krijnen JL, Janssen PJ, Ruizeveld de Winter JA, van Krimpen H, Schroder FH, van der Kwast TH. Do neuroendocrine cells in human prostate cancer express androgen receptor? Histochemistry. 1993;100:393–8. doi: 10.1007/BF00268938. [DOI] [PubMed] [Google Scholar]
- 35.Harper ME, Glynne-Jones E, Goddard L, Thurston VJ, Griffiths K. Vascular endothelial growth factor (VEGF) expression in prostatic tumours and its relationship to neuroendocrine cells. Br J Cancer. 1996;74:910–6. doi: 10.1038/bjc.1996.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borre M, Nerstrom B, Overgaard J. Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin Cancer Res. 2000;6:1882–90. [PubMed] [Google Scholar]
- 37.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 38.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, et al. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: in situ and in vitro expression of VEGF by human prostate cancer cells. J Urol. 1997;157:2329–33. [PubMed] [Google Scholar]
- 39.Jones A, Fujiyama C, Turner K, Fuggle S, Cranston D, Bicknell R, et al. Elevated serum vascular endothelial growth factor in patients with hormone-escaped prostate cancer. BJU Int. 2000;85:276–80. doi: 10.1046/j.1464-410x.2000.00432.x. [DOI] [PubMed] [Google Scholar]
- 40.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor α cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–16. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 41.Dickerson R, Keller LH, Safe S. Alkyl polychlorinated dibenzofurans and related compounds as antiestrogens in the female rat uterus: structure-activity studies. Toxicol Appl Pharmacol. 1995;135:287–98. doi: 10.1006/taap.1995.1235. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Abdelrahim M, Khan S, Ariazi E, Jordan VC, Safe S. Aryl hydrocarbon receptor agonists directly activate estrogen receptor α in MCF-7 breast cancer cells. Biol Chem. 2006;387:1209–13. doi: 10.1515/BC.2006.149. [DOI] [PubMed] [Google Scholar]
- 43.Pearce ST, Liu H, Radhakrishnan I, Abdelrahim M, Safe S, Jordan VC. Interaction of the aryl hydrocarbon receptor ligand 6-methyl-1, 3, 8-trichlorodibenzofuran with estrogen receptor α. Cancer Res. 2004;64:2889–97. doi: 10.1158/0008-5472.can-03-1770. [DOI] [PubMed] [Google Scholar]
- 44.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–82. [PubMed] [Google Scholar]
- 45.Raghow S, Hooshdaran MZ, Katiyar S, Steiner MS. Toremifene prevents prostate cancer in the transgenic adenocarcinoma of mouse prostate model. Cancer Res. 2002;62:1370–6. [PubMed] [Google Scholar]
- 46.Chetty R, Weinreb I. Gastric neuroendocrine carcinoma arising from heterotopic pancreatic tissue. J Clin Pathol. 2004;57:314–7. doi: 10.1136/jcp.2003.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]