Abstract
Nucleic acid reagents, including small interfering RNA (siRNA) and plasmid DNA, are important tools for the study of mammalian cells and are promising starting points for the development of new therapeutic agents. Realizing their full potential, however, requires nucleic acid delivery reagents that are simple to prepare, effective across many mammalian cell lines, and nontoxic. We recently described the extensive surface mutagenesis of proteins in a manner that dramatically increases their net charge. Here, we report that superpositively charged green fluorescent proteins, including a variant with a theoretical net charge of +36 (+36 GFP), can penetrate a variety of mammalian cell lines. Internalization of +36 GFP depends on nonspecific electrostatic interactions with sulfated proteoglycans present on the surface of most mammalian cells. When +36 GFP is mixed with siRNA, protein–siRNA complexes ≈1.7 μm in diameter are formed. Addition of these complexes to five mammalian cell lines, including four that are resistant to cationic lipid-mediated siRNA transfection, results in potent siRNA delivery. In four of these five cell lines, siRNA transfected by +36 GFP suppresses target gene expression. We show that +36 GFP is resistant to proteolysis, is stable in the presence of serum, and extends the serum half-life of siRNA and plasmid DNA with which it is complexed. A variant of +36 GFP can mediate DNA transfection, enabling plasmid-based gene expression. These findings indicate that superpositively charged proteins can overcome some of the key limitations of currently used transfection agents.
Keywords: cell-penetrating protein, nucleic acid delivery
Commercially available cationic lipid reagents are typically used to transfect nucleic acids in mammalian cell culture. The effectiveness of these reagents, however, varies greatly by cell type. A number of cell lines, including some neuron, T cell, fibroblast, and epithelial cell lines, have demonstrated resistance to common cationic lipid transfection reagents (1–4). Alternative transfection approaches, including electroporation (5) and virus-mediated siRNA delivery (6, 7), have been used; however, these methods can be cytotoxic or perturb cellular function in unpredictable ways.
Recent efforts to address the challenge of nucleic acid delivery have resulted in a variety of nucleic acid delivery platforms. These methods include lipidoids (8), cationic polymers (9), inorganic nanoparticles (10), carbon nanotubes (11), cell-penetrating peptides (12, 13), cationic protein–antibody fusions (14, 15), and chemically modified nucleic acids (16). Each of these methods offers benefits for particular applications; in most cases, however, questions regarding cytotoxicity, ease of preparation, stability, or generality across different cell lines remain. Easily prepared reagents capable of effectively delivering nucleic acids to a variety of cell lines without significant cytotoxicity therefore are of considerable interest.
We recently described resurfacing proteins without abolishing their structure or function through the extensive mutagenesis of nonconserved, solvent-exposed residues (17). When the replacement residues are all positively or all negatively charged, the resulting “supercharged” proteins can retain their activity while gaining unusual properties such as robust resistance to aggregation and the ability to bind oppositely charged macromolecules. For example, we reported that a green fluorescent protein with a +36 net theoretical charge (+36 GFP) was highly aggregation-resistant, could retain fluorescence even after being boiled and cooled, and reversibly complexed DNA and RNA through electrostatic interactions.
A variety of cationic peptides and proteins have been observed to penetrate mammalian cells (18–24). We hypothesized that superpositively charged proteins such as +36 GFP might also associate with negatively charged components of the cell membrane in a manner that results in cell penetration. Given the ability of these proteins to bind nucleic acids reversibly, we further speculated that superpositively charged proteins may also deliver precomplexed nucleic acids, including small interfering RNA (siRNA) or plasmid DNA, into mammalian cells.
Here, we describe the cell-penetrating and nucleic acid transfection activities of superpositively charged GFP variants. We found that +36 GFP potently enters cells through sulfated peptidoglycan-mediated, actin-dependent endocytosis. When premixed with siRNA, +36 GFP forms monodisperse particles that deliver siRNA effectively and without cytotoxicity into a variety of cell lines, including several known to be resistant to cationic lipid-mediated transfection. The siRNA delivered into cells by using +36 GFP was able to effect gene silencing in four of five mammalian cell lines tested. Comparison of the siRNA transfection ability of +36 GFP with that of several synthetic peptides of comparable charge magnitude suggests that the observed mode of siRNA delivery requires features of +36 GFP that are not present among cationic peptides. When fused to an endosomolytic peptide, +36 GFP is also able to transfect plasmid DNA into several mammalian cell lines in a manner that enables plasmid-based gene expression. In addition, we observed that +36 GFP is stable in murine serum and can significantly enhance the serum stability of siRNA and plasmid DNA to which it is complexed.
Results
Mammalian Cell Penetration by Supercharged GFPs.
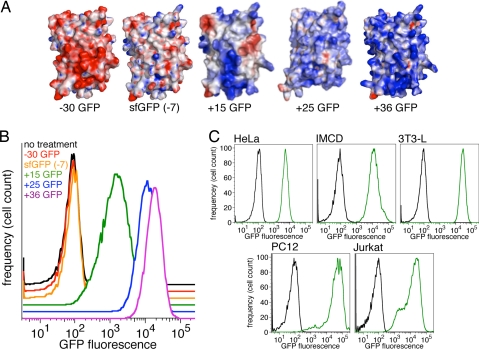
We generated and characterized a series of resurfaced variants of “superfolder GFP” (sfGFP) (25) with theoretical net charges ranging from −30 to +48 that retain fluorescence (17). The evaluation of the ability of these supercharged GFPs to penetrate mammalian cells requires a method to remove surface-bound, noninternalized GFP. We therefore confirmed that washing conditions known to remove surface-bound cationic proteins from cells (13) also effectively remove cell surface-bound superpositively charged GFP. We treated HeLa cells with +36 GFP at 4 °C, a temperature that allows +36 GFP to bind to the outside of cells but blocks internalization (see below). Cells were washed three times at 4 °C with either PBS or with PBS containing heparin and analyzed by flow cytometry for GFP fluorescence. Cells washed with PBS were found to have significant levels of GFP (presumably surface-bound), whereas cells washed with PBS containing heparin exhibited GFP fluorescence intensity very similar to that of untreated cells [Fig. S1 in the supporting information (SI) Appendix]. These observations confirmed the effectiveness of three washes with heparin at removing surface-bound superpositively charged GFP.
Next, we incubated HeLa cells with 10–500 nM sfGFP (theoretical net charge of −7), −30 GFP, +15 GFP, +25 GFP, or +36 GFP for 4 h at 37 °C (Fig. 1A). After incubation, cells were washed three times with PBS containing heparin and analyzed by flow cytometry. No detectable internalized protein was observed in cells treated with sfGFP or −30 GFP. HeLa cells treated with +25 GFP or +36 GFP, however, were found to contain high levels of internalized GFP. In contrast, cells treated with +15 GFP contained 10-fold less internalized GFP, indicating that positive-charge magnitude is an important determinant of effective cell penetration (Fig. 1B). We found that +36 GFP readily penetrates HeLa cells even at concentrations as low as 10 nM (Fig. S2 in the SI Appendix).
Fig. 1.
Supercharged GFP variants and their ability to penetrate cells. (A) Calculated electrostatic surface potentials of GFP variants used in this work, colored from −25 kT/e (dark red) to +25 kT/e (dark blue). (B) Flow cytometry analysis showing amounts of internalized GFP in HeLa cells treated with 200 nM each superpositive GFP variant and washed three times with PBS containing heparin to remove cell surface-bound GFP. (C) Flow cytometry analysis showing amounts of internalized +36 GFP (green) in HeLa, IMCD, 3T3-L, PC12, and Jurkat cells compared with untreated cells (black).
To test the generality of cell penetration by +36 GFP, we repeated these experiments by using four additional mammalian cell types: inner medullary collecting duct (IMCD) cells, 3T3-L preadipocytes, rat pheochromocytoma PC12 cells, and Jurkat T cells. Flow cytometry revealed that 200 nM +36 GFP effectively penetrates all five types of cells tested (Fig. 1C). Internalization of +36 GFP in stably adherent HeLa, IMCD, and 3T3-L cell lines was confirmed by fluorescence microscopy (see below). Real-time imaging showed that +36 GFP bound rapidly to the cell membrane of HeLa cells and was internalized within minutes as punctate foci that migrated toward the interior of the cell and consolidated into larger foci, consistent with uptake by endocytosis.
Mechanistic Probes of +36 GFP Cell Penetration.
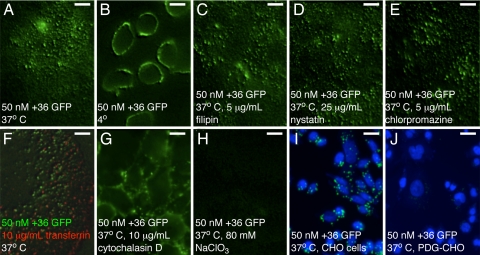
To illuminate the mechanism by which +36 GFP enters cells, we repeated the cell penetration experiments in HeLa cells under a variety of conditions that each blocks a different component of an endocytosis pathway (26, 27). Cell penetration of +36 GFP was not observed when HeLa cells were cooled to 4 °C before and during +36 GFP treatment (Fig. 2B). This result suggests that uptake of +36 GFP requires an energy-dependent process, consistent with endocytosis (13). We next evaluated the effects of 5 μg/mL filipin or 25 μg/mL nystatin, small molecules known to inhibit caveolin-dependent endocytosis. Neither inhibitor significantly altered +36 GFP internalization (Fig. 2 C and D, respectively). Treatment with chlorpromazine, a known inhibitor of clathrin-mediated endocytosis, similarly had little effect on +36 GFP cell penetration (Fig. 2E). In addition, simultaneous treatment of HeLa cells with 50 nM +36 GFP and 10 μg/mL fluorescently labeled transferrin, a protein known to be internalized in a clathrin-dependent manner (28), resulted in little GFP/transferrin colocalization (Fig. 2F). Treatment with cytochalasin D, an actin polymerization inhibitor, however, significantly decreased +36 GFP cell penetration (Fig. 2G). Taken together, these results are consistent with a model in which +36 GFP uptake proceeds through an endocytotic pathway that is energy-dependent, requires actin polymerization, and does not require clathrin or caveolin.
Fig. 2.
Mechanistic probes of +36 GFP internalization. (A) Internalization of +36 GFP in HeLa cells after coincubation for 1 h at 37 °C. (B) Inhibition of +36 GFP cell penetration in HeLa cells incubated at 4 °C for 1 h. Cells were only partially washed to enable +36 GFP to remain partially bound to the cell surface. (C and D) +36 GFP internalization under the conditions in A but in the presence of caveolin-dependent endocytosis inhibitors filipin and nystatin, respectively. (E) +36 GFP internalization under the conditions in A but in the presence of the clathrin-dependent endocytosis inhibitor chlorpromazine. (F) Localization of Alexa Fluor 647-labeled transferrin (red) and +36 GFP (green) 20 min after endocytosis. (G) Inhibition of +36 GFP internalization in HeLa cells in the presence of the actin polymerization inhibitor cytochalasin D. (H) Inhibition of +36 GFP internalization in HeLa cells treated with 80 mM sodium chlorate. (I) Internalization of +36 GFP in CHO cells incubated at 37 °C for 1 h. (J) Lack of +36 GFP internalization in PDG-CHO cells. (I and J) Cell nuclei were stained with DAPI (blue). [Scale bars, 10 μm (A–H) and 20 μm (I–J).]
Based on previous studies (29) we hypothesized that anionic cell surface proteoglycans might serve as receptors to mediate +36 GFP internalization. Indeed, 80 mM sodium chlorate, an inhibitor of the ATP sulfurylase enzyme required to biosynthesize sulfated proteoglycans (30), completely blocked +36 GFP penetration (Fig. 2H). Moreover, wild-type CHO cells (Fig. 2I), but not proteoglycan-deficient CHO cells (PGD-CHO) that lack xylosyltransferase, an enzyme required for glycosaminoglycan synthesis (Fig. 2J), efficiently internalized +36 GFP. These findings suggest that +36 GFP penetration of mammalian cells requires binding to sulfated cell surface peptidoglycans.
+36 GFP Binds siRNA and Delivers siRNA into a Variety of Mammalian Cell Lines.
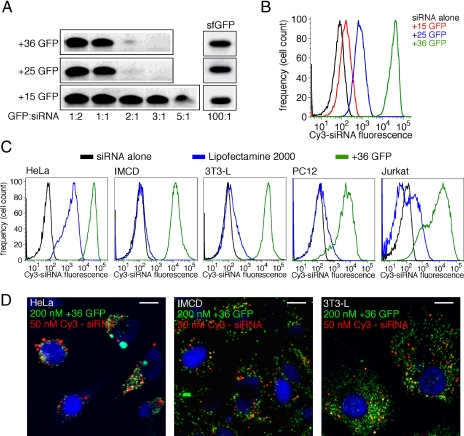
We observed the ability of superpositively charged proteins to form complexes with DNA and tRNA (17). In light of these results, we evaluated the ability of +15, +25, and +36 GFP to bind siRNA. Using a gel-shift assay (31), we observed binding of +25 and +36 GFP to siRNA with a stoichiometry of ≈2:1, whereas more than five +15 GFP proteins on average were required to complex a single siRNA molecule (Fig. 3A). In contrast, 100 eq of sfGFP did not detectably bind siRNA under the assay conditions.
Fig. 3.
Binding and transfection efficiency of siRNA by supercharged GFPs. (A) Gel-shift assay (31) to determine superpositive GFP:siRNA binding stoichiometry. Ten picomoles of siRNA was mixed with various molar ratios of each GFP for 10 min at 25 °C and then analyzed by nondenaturing PAGE. GFP–siRNA complexes were observed to remain in the loading well of the gel. (B) Flow cytometry analysis showing levels of siRNA in HeLa cells treated with a mixture of 50 nM Cy3-siRNA and 200 nM +15, +25, or +36 GFP, followed by three heparin washes to remove noninternalized GFP (Fig. S1 in the SI Appendix). (C) Flow cytometry analysis showing levels of Cy3-labeled siRNA delivered into HeLa, IMCD, 3T3-L, PC12, and Jurkat cells after incubation with a mixture of 50 nM Cy3-siRNA and either ≈2 μM Lipofectamine 2000 (blue) or 200 nM +36 GFP (green) compared with cells treated with siRNA without transfection reagent (black). (D) Fluorescence microscopy images of stably adherent cell lines (HeLa, IMCD, and 3T3-L) 24 h after a 4-h treatment with 200 nM +36 GFP and 50 nM Cy3-siRNA. Each image is an overlay of three channels: blue (DAPI stain), red (Cy3-siRNA), and green (+36 GFP); yellow indicates the colocalization of red and green. (Scale bar, 10 μm.)
Next, we examined the ability of +15, +25, and +36 GFP to deliver bound siRNA into HeLa cells. A Cy3-conjugated GAPDH siRNA (Ambion) was briefly mixed with 200 nM +36 GFP, and the resulting mixture was added to cells in serum-free medium for 4 h. After washing the cells, flow cytometry revealed that +25 and +36 GFP delivered 100- and 1,000-fold more siRNA into HeLa cells, respectively, than treatment with siRNA alone (Fig. 3B) and ≈20-fold more siRNA than was delivered with the common cationic lipid transfection reagent Lipofectamine 2000 (Fig. 3C). In contrast, +15 GFP did not efficiently transfect siRNA into HeLa cells (Fig. 3B).
In addition to HeLa cells, +36 GFP was also able to deliver efficiently siRNA in IMCD cells, 3T3-L preadipocytes, rat pheochromocytoma PC12 cells, and Jurkat T cells, four cell lines that are resistant to siRNA transfection using Lipofectamine 2000 (1–4). Treatment with Lipofectamine 2000 and Cy3-siRNA resulted in efficient siRNA delivery in HeLa cells but no significant delivery of siRNA into IMCD, 3T3-L, PC12, or Jurkat cells (Fig. 3C). Treatment of IMCD or 3T3-L cells with FuGENE 6 (Roche), a different cationic lipid transfection agent, and Cy3-siRNA also did not result in significant siRNA delivery these cells (Fig. S4 in the SI Appendix). In contrast, treatment with +36 GFP and Cy3-siRNA resulted in significant siRNA levels in all five cell lines tested (Fig. 3C) that were 20- to 200-fold higher than siRNA levels resulting from Lipofectamine 2000 treatment. Fluorescence microscopy of the adherent cell lines used in this study (HeLa, IMCD, and 3T3-L) reveal internalized Cy3-siRNA and +36 GFP in punctate foci that we presume to be endosomes (Fig. 3D). These results collectively indicate that +36 GFP can effectively deliver siRNA into a variety of mammalian cell lines, including several that are poorly transfected by commonly used cationic lipid transfection reagents.
When HeLa cells were treated with the a premixed solution containing 200 nM +36 GFP and 50 nM Cy3-siRNA in the presence of cytochalasin D or at 4 °C, no internalized GFP or Cy3 siRNA was observed (Fig. S3 in the SI Appendix). These data support a mechanism of siRNA delivery that depends on endocytosis and actin polymerization, consistent with our mechanistic studies of +36 GFP in the absence of siRNA.
Size and Cytotoxicity of +36 GFP–siRNA Complexes.
We analyzed +36 GFP–siRNA complexes by dynamic light scattering (DLS) by using stoichiometric ratios identical to those used for transfection. From a mixture containing 20 μM +36 GFP and 5 μM siRNA, we observed a fairly monodisperse population of particles with a hydrodynamic radius (Hr) of 880.6 ± 62.2 nm (Fig. S5A in the SI Appendix), consistent with microscopy data (Fig. S5B in the SI Appendix). These observations demonstrate the potential for +36 GFP to form large particles when mixed with siRNA, a phenomenon observed by previous researchers using cationic delivery reagents (12, 13).
To assess the cytotoxicity of +36 GFP–siRNA complexes, we performed MTT assays on all five cell lines 24 h after treatment with 0.2–2 μM +36 GFP and 50 nM siRNA. These assays revealed no significant apparent cytotoxicity to HeLa, IMCD, 3T3-L, PC12, or Jurkat cells (Fig. S6 in the SI Appendix).
Gene Silencing with +36 GFP-Delivered siRNA.
Although the above results demonstrate the ability of +36 GFP to deliver siRNA into a variety of mammalian cells, they do not establish the availability of this siRNA for gene silencing. To evaluate the gene suppression activity of siRNA delivered with +36 GFP, we treated HeLa cells with a solution containing 50 nM GAPDH-targeting siRNA and either ≈2 μM Lipofectamine 2000 or 200 nM +36 GFP. Cells were exposed to the siRNA transfection solution for 4 h and then grown for up to 4 days.
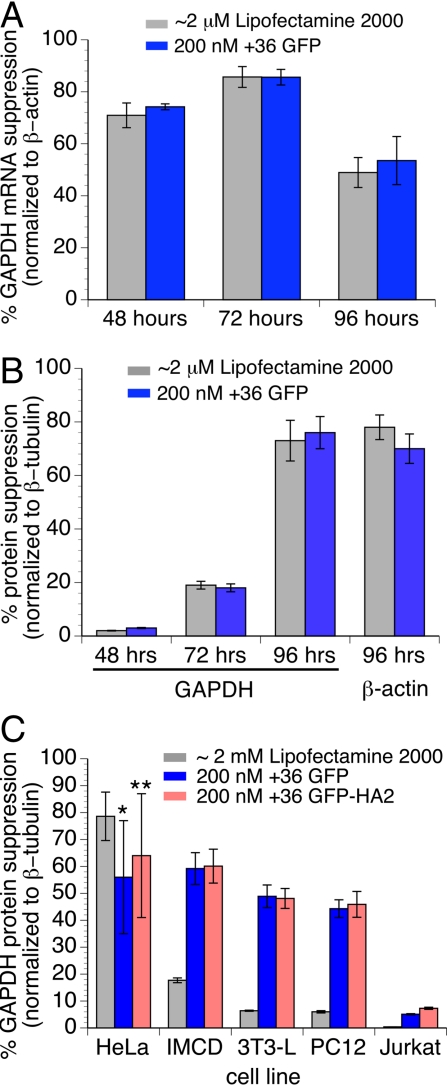
In HeLa cells, observed decreases in GAPDH mRNA and protein levels indicate that both Lipofectamine 2000 and +36 GFP mediate efficient siRNA-induced suppression of GAPDH expression with similar kinetics. GAPDH-targeting siRNA delivered with Lipofectamine 2000 or +36 GFP resulted in a ≈85% decrease in GAPDH mRNA levels after 72 h (Fig. 4A) and a ≈55–80% decrease in GAPDH levels after 96 h (Fig. 4 B and C). Similarly, delivery of β-actin-targeting siRNA with either ≈2 μM Lipofectamine 2000 or 200 nM +36 GFP resulted in a decrease in β-actin protein levels in HeLa cells of 70–78% for both transfection agents (Fig. 4B).
Fig. 4.
Suppression of GAPDH mRNA and protein levels resulting from siRNA delivery. (A) GAPDH mRNA level suppression in HeLa cells 48, 72, or 96 h after treatment with 50 nM siRNA and ≈2 μM Lipofectamine 2000, or with 50 nM siRNA and 200 nM +36 GFP, as measured by RT-quantitative PCR. Suppression levels shown are normalized to β-actin mRNA levels; 0% suppression is defined as the mRNA level in cells treated with negative control siRNA. (B) GAPDH or β-actin protein level suppression in HeLa cells 48, 72, or 96 h after treatment with siRNA and ≈2 μM Lipofectamine 2000, or with siRNA and 200 nM +36 GFP. (C) GAPDH protein level suppression in HeLa, IMCD, 3T3-L, PC12, and Jurkat cells 96 h after treatment with 50 nM siRNA and ≈2 μM Lipofectamine 2000, 200 nM +36 GFP, or 200 nM +36 GFP-HA2. Protein levels were measured by Western blotting and are normalized to β-tubulin protein levels; 0% suppression is defined as the protein level in cells treated with negative control siRNA. Values and error bars represent the mean ± SD of three independent experiments in A and five independent experiments in B and C. Independently prepared protein batches were tested (*, 5 batches; **, 3 batches).
In contrast to the efficiency of gene suppression in HeLa cells, treatment with Lipofectamine 2000 and 50 nM siRNA in IMCD, 3T3-L, PC12, and Jurkat cells effected no significant decrease in GAPDH protein levels (Fig. 4C), consistent with the resistance of these four cell lines to cationic lipid-mediated transfection (Fig. 3C) (1–4). Treatment with 200 nM +36 GFP and 50 nM siRNA, however, resulted in 44–60% suppression of GAPDH protein levels in IMCD, 3T3-L, and PC12 cells (Fig. 4C). Despite efficient siRNA delivery by +36 GFP (Fig. 3C), we observed no significant siRNA-mediated suppression of GAPDH expression in Jurkat cells (Fig. 4C). GAPDH protein suppression levels among these 5 cell lines was similar when using +36 GFP or +36 GFP-HA2, a C-terminal fusion of +36 GFP and a hemagglutinin-derived peptide that has been reported to enhance endosome degradation (32) (Fig. 4C).
Together, these results indicate that +36 GFP and +36 GFP-HA2 are capable of delivering siRNA and effecting gene silencing in a variety of mammalian cells, including some cell lines that do not exhibit gene silencing when treated with siRNA and cationic lipid-based transfection agents.
Stability of +36 GFP and Stability of RNA and DNA Complexed with +36 GFP.
In addition to generality across different mammalian cell types and low cytotoxicity, effective siRNA delivery agents should be resistant to rapid degradation and ideally should also extend siRNA lifetimes. Treatment of +36 GFP with proteinase K, a robust, broad-spectrum protease, revealed that +36 GFP exhibits significant protease resistance compared with BSA. Although no uncleaved BSA remained 1 h after proteinase K digestion, 68% of +36 GFP remained uncleaved after 1 h, and 48% remained uncleaved after 6 h (Fig. S7A in the SI Appendix). We also treated +36 GFP with murine serum at 37 °C (Fig. S7B in the SI Appendix). After 6 h, no significant degradation was observed, suggesting its potential in vivo serum stability. In comparison, when we incubated BSA in mouse serum for the same period, we observed 71% degradation after 3 h and complete degradation by 4 h.
Next, we assessed the ability of +36 GFP to protect siRNA and plasmid DNA from degradation. We treated siRNA or siRNA precomplexed with +36 GFP with murine serum at 37 °C. After 3 h, only 5.9% of the siRNA remained intact in the sample lacking +36 GFP, whereas 34% of the siRNA remained intact in the sample precomplexed with +36 GFP (Fig. S7C in the SI Appendix). Similarly, although plasmid DNA was nearly completely degraded by murine serum after 30 min at 37 °C, virtually all plasmid DNA precomplexed with +36 GFP remained intact after 30 min, and 84% of plasmid DNA was intact after 1 h (Fig. S7D in the SI Appendix). These results together indicate that +36 GFP is capable of significantly inhibiting serum-mediated siRNA and plasmid DNA degradation.
Comparison of +36 GFP with Synthetic Cationic Peptides.
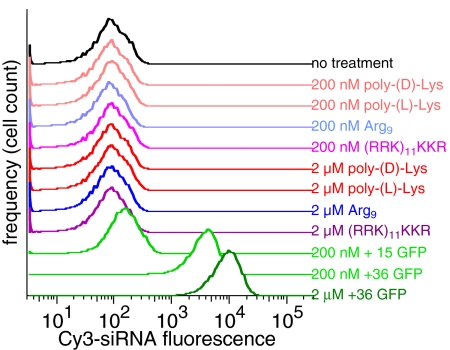
To probe the features of superpositively charged GFPs that impart their ability to deliver siRNA into cells, we compared the siRNA transfection ability of +36 GFP at 200 nM with that of a panel of synthetic cationic peptides at 200 nM or 2 μM. This panel consisted of poly-(l)-Lys (a mixture containing an average of ≈30 Lys residues per polypeptide), poly-(d)-Lys, Arg9, and a synthetic +36 peptide [(KKR)11RRK] that contains the same theoretical net charge and Lys:Arg ratio as +36 GFP. MTT assays on HeLa cells treated with these synthetic polycations indicated low cytoxicity at the concentrations used, consistent with that of superpositively charged GFPs (Fig. S6B in the SI Appendix). None of the four synthetic peptides tested, however, delivered a detectable amount of Cy3-siRNA into HeLa cells as assayed by flow cytometry, even when used at concentrations 10-fold higher than those needed for +36 GFP to effect efficient siRNA delivery or for +15 GFP to effect detectable siRNA delivery (Fig. 5).
Fig. 5.
The siRNA transfection activities of a variety of cationic synthetic peptides compared with that of +15 and +36 GFP. Flow cytometry was used to measure the levels of internalized Cy3-siRNA in HeLa cells treated for 4 h with a mixture of 50 nM Cy3-siRNA and the peptide or protein shown.
Coupled with our observation that +15 GFP exhibits low cell penetration and siRNA-binding activity compared with +25 and +36 GFP (Fig. 3 A and B), these results indicate that although GFP must be sufficiently positively charged to acquire the ability to enter cells and transfect siRNA efficiently, positive charge magnitude is not sufficient to confer transfection activity, and other features of +36 GFP lacking in the synthetic peptides tested are also required.
+36 GFP-Mediated Transfection of Plasmid DNA.
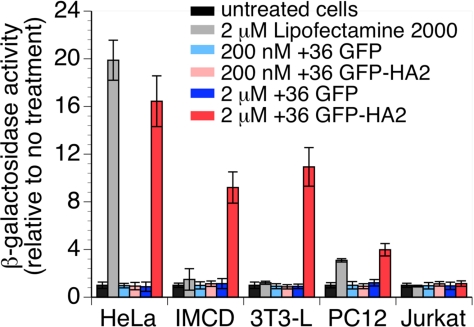
Similar to the case with siRNA, we observed by gel-shift assay that +36 GFP forms a complex with plasmid DNA (Fig. S8 in the SI Appendix). To test whether +36 GFP can deliver plasmid DNA to cells in a manner that supports plasmid-based gene expression, we treated HeLa, IMCD, 3T3-L, PC12, and Jurkat cells with a β-galactosidase expression plasmid premixed with Lipofectamine 2000, +36 GFP, or +36 GFP-HA2. After 24 h, cells were analyzed for β-galactosidase activity by using a fluorogenic substrate-based assay.
Consistent with our previous results (Figs. 3 and 4), Lipofectamine 2000 treatment resulted in significant β-galactosidase activity in HeLa cells, but only modest β-galactosidase activity in PC12 cells, and no detectable activity in any of the other three cell lines tested (Fig. 6). In contrast, plasmid transfection mediated by 2 μM +36 GFP-HA2 resulted in significant β-galactosidase activity in HeLa, IMCD, and 3T3-L cells, and modest activity in PC12 cells (Fig. 6). Interestingly, treatment with plasmid DNA and 2 μM +36 GFP did not result in detectable β-galactosidase activity (Fig. 6), suggesting that the hemagglutinin-derived peptide enhances DNA transfection or plasmid-based expression efficiency despite its lack of effect on siRNA-mediated gene silencing (Fig. 4D).
Fig. 6.
Plasmid DNA transfection into HeLa, IMCD, 3T3-L, PC12, and Jurkat cells by Lipofectamine 2000, +36 GFP, or +36 GFP-HA2. Cells were treated with 800 ng of pSV-β-galactosidase plasmid and 200 nM or 2 μM +36 GFP or +36 GFP-HA2 for 4 h. After 24 h, β-galactosidase activity was measured. Values and error bars represent the mean ± SD of three independent experiments.
These results collectively indicate that +36 GFP-HA2 is able to deliver plasmid DNA into mammalian cells, including several cell lines resistant to cationic lipid-mediated transfection, in a manner that enables plasmid-based gene expression. Higher concentrations of +36 GFP-HA2 are required to mediate plasmid DNA transfection than the amount of +36 GFP needed to induce efficient siRNA transfection.
Discussion
We have characterized the cell penetration and nucleic acid delivery properties of three superpositively charged GFP variants with net charges of +15, +25, and +36. We discovered that superpositive GFPs penetrate cells in a charge-dependent manner and that +36 GFP is capable of efficiently delivering siRNA into a variety of mammalian cell lines, including those resistant to cationic lipid-based transfection, with low cytotoxicity.
Mechanistic studies revealed that +36 GFP enters cells through a clathrin- and caveolin-independent endocytosis pathway that requires sulfated cell surface proteoglycans and actin polymerization. This delivery pathway differs from described strategies for nucleic acid delivery to eukaryotic cells that rely on cell-specific targeting to localize their nucleic acid cargo (15, 31, 33). For use in cell culture and even in certain in vivo applications, a general, non-cell type-specific approach to nucleic acid delivery may be a preferred alternative.
In four of the five cell lines tested, +36 GFP-mediated siRNA delivery induced significant suppression of gene expression. A +36 GFP-HA2 peptide fusion mediated plasmid DNA transfection in a manner that enables plasmid-based gene expression in the same four cell lines. The demonstrated ability to transfect RNA 21 bp in length and plasmid DNA >5,000 bp in length suggests that +36 GFP and its derivatives may serve as fairly general nucleic acid delivery vectors.
An important distinction between delivery methods that rely on the synthesis of covalently linked transfection agent-nucleic acid conjugates such as carbon nanotube-siRNA (11), nanoparticle-siRNA (34), TAT peptide-siRNA (35), cholesterol-siRNA (36), dynamic polyconjugate-siRNA (37), and the use of +36 GFP is that the latter simply requires mixing the protein and nucleic acid together. Moreover, the reagent described here is purified directly from bacterial cells and used without chemical cotransfectants such as exogenous calcium or chloroquine.
We reported that +36 GFP is thermodynamically almost as stable as sfGFP but unlike the latter is able to refold after boiling and cooling (17). We now report that +36 GFP exhibits resistance to proteolysis, stability in murine serum, and significant protection of complexed siRNA in murine serum. Although additional studies are needed to characterize further the potential of +36 GFP for in vivo nucleic acid delivery, these features are consistent with several of the key requirements for such an application.
Taken together, these findings describe an application of protein resurfacing: the potent delivery of nucleic acids into mammalian cells. This unusual potency (32, 38) is complemented by low cytotoxicity, stability in mammalian serum, generality across various mammalian cell types including several that resist traditional transfection methods, the ability to transfect both small RNAs and large DNA plasmids, straightforward preparation from Escherichia coli cells, and simple use by mixing with an unmodified nucleic acid of interest. These qualities collectively suggest that superpositively charged proteins merit exploration as a new class of solutions to general nucleic acid delivery problems in mammalian cells.
Materials and Methods
See the SI Appendix for descriptions of additional experimental procedures.
Cationic Lipid-Based and GFP-Based Transfections.
Transfections using Lipofectamine 2000 (Invitrogen) and FuGENE 6 (Roche) were performed following the manufacturers' protocols. Although the molecular masses of these reagents are not provided by the manufacturers, the concentration of Lipofectamine 2000 during transfection is 2 μg/mL, and assuming the molecular mass of this cationic lipid is ≤1,000 Da, the concentration is approximately ≥2 μM.
Cells were plated in a 12-well tissue culture plate at a density of 80,000 cells per well. After 12 h at 37 °C, the cells were washed with 4 °C PBS, and for HeLa, IMCD, 3T3-L, and PC12 cells the media were replaced with 500 μL of serum-free DMEM at 4 °C. Jurkat cells were transferred from the culture plate wells into individual 1.5-mL tubes, pelleted by centrifugation, and resuspended in 500 μL of serum-free RPMI medium 1640 at 4 °C.
A solution of GFP and either siRNA or plasmid DNA was mixed in 500 μL of either 4 °C DMEM (for HeLa, IMCD, 3T3-L, and PC12 cells) or 4 °C RPMI medium 1640 (for Jurkat cells). After 5 min at 25 °C, this solution was added to the cells and slightly agitated to mix. After 4 h at 37 °C, the solution was removed from the cells and replaced with 37 °C medium containing 10% FBS. GAPDH-targeting Cy3-labeled siRNA and unlabeled siRNA were purchased from Ambion. Plasmid transfections were performed by using pSV-β-galactosidase (Promega). β-Galactosidase activity was measured by using the β-Fluor assay kit (Novagen) following the manufacturer's protocol.
Supplementary Material
Acknowledgments.
We thank Sara Jones and Professor Xiaowei Zhuang (Harvard University, Cambridge, MA) for assistance with live cell imaging and for providing CHO and PGD-CHO cell lines. Alan Saghatelian, Gregory Verdine, Matthew Shair, and Paula Nunes (Harvard University) kindly provided 3T3-L, Jurkat, HeLa, and IMCD cells, respectively. This work was supported by the National Institute of General Medical Sciences/National Institutes of Health Grant R01 GM 065400 and by the Howard Hughes Medical Institute. J.J.C. is supported by a National Science Foundation Graduate Student Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807883106/DCSupplemental.
References
- 1.Carlotti F, et al. Lentiviral vectors efficiently transduce quiescent mature 3T3–L1 adipocytes. Mol Ther. 2004;9:209–217. doi: 10.1016/j.ymthe.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Ma H, et al. Non-classical nuclear localization signal peptides for high efficiency lipofection of primary neurons and neuronal cell lines. Neuroscience. 2002;112:1–5. doi: 10.1016/s0306-4522(02)00044-1. [DOI] [PubMed] [Google Scholar]
- 3.McManus MT, et al. Small interfering RNA-mediated gene silencing in T lymphocytes. J Immunol. 2002;169:5754–5760. doi: 10.4049/jimmunol.169.10.5754. [DOI] [PubMed] [Google Scholar]
- 4.Strait KA, Stricklett PK, Kohan JL, Miller MB, Kohan DE. Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol. 2007;293:F601–F606. doi: 10.1152/ajprenal.00085.2007. [DOI] [PubMed] [Google Scholar]
- 5.Jantsch J, et al. Small interfering RNA (siRNA) delivery into murine bone marrow-derived dendritic cells by electroporation. J Immunol Methods. 2008;337:71–77. doi: 10.1016/j.jim.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 7.Stewart SA, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akinc A, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segura T, Hubbell JA. Synthesis and in vitro characterization of an ABC triblock copolymer for siRNA delivery. Bioconjug Chem. 2007;18:736–745. doi: 10.1021/bc060284y. [DOI] [PubMed] [Google Scholar]
- 10.Sokolova V, Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew Chem Int Ed Engl. 2008;47:1382–1395. doi: 10.1002/anie.200703039. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Winters M, Holodniy M, Dai H. siRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angew Chem Int Ed Engl. 2007;46:2023–2027. doi: 10.1002/anie.200604295. [DOI] [PubMed] [Google Scholar]
- 12.Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: Tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62:1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv Drug Deliv Rev. 2008;60:530–536. doi: 10.1016/j.addr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peer D, Zhu P, Carman CV, Lieberman J, Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc Natl Acad Sci USA. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song E, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 16.Krutzfeldt J, et al. Silencing of microRNAs in vivo with “antagomirs.”. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence MS, Phillips KJ, Liu DR. Supercharging proteins can impart unusual resilience. J Am Chem Soc. 2007;129:10110–10112. doi: 10.1021/ja071641y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 19.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 20.Thoren PE, Persson D, Karlsson M, Norden B. The antennapedia peptide penetratin translocates across lipid bilayers: The first direct observation. FEBS Lett. 2000;482:265–268. doi: 10.1016/s0014-5793(00)02072-x. [DOI] [PubMed] [Google Scholar]
- 21.Daniels DS, Schepartz A. Intrinsically cell-permeable miniature proteins based on a minimal cationic PPII motif. J Am Chem Soc. 2007;129:14578–14579. doi: 10.1021/ja0772445. [DOI] [PubMed] [Google Scholar]
- 22.Smith BA, et al. Minimally cationic cell-permeable miniature proteins via α-helical arginine display. J Am Chem Soc. 2008;130:2948–2949. doi: 10.1021/ja800074v. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs SM, Raines RT. Arginine grafting to endow cell permeability. ACS Chem Biol. 2007;2:167–170. doi: 10.1021/cb600429k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs SM, Rutkoski TJ, Kung VM, Groeschl RT, Raines RT. Increasing the potency of a cytotoxin with an arginine graft. Protein Eng Des Sel. 2007;20:505–509. doi: 10.1093/protein/gzm051. [DOI] [PubMed] [Google Scholar]
- 25.Pedelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- 26.Payne CK, Jones SA, Chen C, Zhuang X. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic. 2007;8:389–401. doi: 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veldhoen S, Laufer SD, Trampe A, Restle T. Cellular delivery of small interfering RNA by a non-covalently attached cell-penetrating peptide: quantitative analysis of uptake and biological effect. Nucleic Acids Res. 2006;34:6561–6573. doi: 10.1093/nar/gkl941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cells. Biochemistry. 2004;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeuerle PA, Huttner WB. Chlorate: A potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986;141:870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- 31.Kumar P, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg P, El-Andaloussi S, Sütlü T, Johansson H, Langel Ü. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB J. 2007;21:2664–2671. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso ALC, et al. siRNA delivery by a transferrin-associated lipid-based vector: A non-viral strategy to mediate gene silencing. J Gene Med. 2007;9:170–183. doi: 10.1002/jgm.1006. [DOI] [PubMed] [Google Scholar]
- 34.Rosi N, Giljohann D, Thaxton C, Lytton-Jean A, Mirkin C. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 35.Fisher A, Ye D, Sergueev D, Fisher M, Juliano R. Evaluating the specificity of antisense oligonucleotide conjugates. J Biol Chem. 2002;277:22980–22984. doi: 10.1074/jbc.M203347200. [DOI] [PubMed] [Google Scholar]
- 36.Soutschek J, Akinc A, Bramlage B, Charisse K, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 37.Rozema D, Lewis D, Wakefield D, Wong S, Wolff J. Dynamic polyconjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci USA. 2007;104:12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshayes S, Simeoni F, Morris MC, Divita G, Heitz F. Peptide-mediated delivery of nucleic acids into mammalian cells. Methods Mol Biol. 2007;386:299–308. doi: 10.1007/978-1-59745-430-8_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.