Abstract
Many human solid tumors express MHC II molecules, and proteins normally localized to melanosomes give rise to MHC II restricted epitopes in melanoma. However, the pathways by which this occurs have not been defined. We analyzed the processing of one such epitope, gp10044-59, derived from gp100/Pmel17. In melanomas that have down-regulated components of the melanosomal pathway, but constitutively express HLA-DR*0401, the majority of gp100 is sorted to LAMP-1hi/MHC II+ late endosomes. Using mutant gp100 molecules with altered intracellular trafficking, we demonstrate that endosomal localization is necessary for gp10044-59 presentation. By depletion of the AP2 adaptor protein using siRNA, we demonstrate that gp100 protein internalized from the plasma membrane to such endosomes is a major source for gp10044-59 epitope production. Gp100 trapped in early endosomes gives rise to epitopes that are indistinguishable from those produced in late endosomes but their production is less sensitive to inhibition of lysosomal proteases. In melanomas containing melanosomes, gp100 is underrepresented in late endosomes, and accumulates in stage II melanosomes devoid of MHC II molecules. Gp10044-59 presentation is dramatically reduced, and processing occurs entirely in early endosomes / stage I melanosomes. This suggests that melanosomes are inefficient antigen processing compartments. Thus, melanoma de-differentiation may be accompanied by increased presentation of MHC II restricted epitopes from gp100 and other melanosome-localized proteins, leading to enhanced immune recognition.
Keywords: Human, Antigens/peptides/epitopes, MHC, Antigen presentation/processing, Tumor immunity
INTRODUCTION
Melanoma immunotherapy strategies developed over the last several years have been focused on cytotoxic CD8 T cells that recognize defined peptide antigens presented by MHC I molecules on tumor cells and widely recognized by T cells from patients (1, 2). Melanocyte differentiation proteins (MDPs)5 represent a majority of these shared melanoma antigens. Several groups have also identified MHC II-associated peptide epitopes derived from MDPs (3-9), and some of these have more recently been included in vaccination regimens (1, 10). While many melanoma cells express MHC II molecules constitutively or in response to IFNγ induction (11-13), the extent to which they present MHC II-associated epitopes derived from endogenously synthesized MDPs and the pathways leading to this presentation are not well understood. IFNγ treatment of melanoma cells results in down-regulation of MDP expression (14), J. Fortini and MSM, unpublished data) and there appears to be an inverse correlation between expression of MHC II molecules and MDPs (15). It remains unclear whether this also prevents presentation of MDP-derived epitopes in MHC II+ melanoma cells. Alternatively, if presentation occurs, the common intracellular compartment where MHC II molecules and melanosomal proteins meet for antigen processing remains to be identified.
Pigmented melanoma cells contain conventional endosomes as well as the melanosomes that synthesize and store the pigment melanin (16). In contrast to melanosomes, which are distinct organelles, the compartments in which MHC II processing occurs correspond to conventional endosomes and lysosomes, modified by expression of MHC II, Ii and HLA-DM (17). Despite the fact that melanosomes share a number of characteristics with conventional endosomes (18), their MHC II processing abilities have not been investigated. In addition, many melanomas lose their ability to synthesize pigment and no longer contain identifiable melanosomes (19, 20). These changes are partially due to down-regulation of expression of MDPs and other components of the intracellular machinery involved in maintaining the identity of melanosomes within the endocytic pathway (21, 22). Given that MDPs expressed in non-melanocytic cells localize to conventional late endosomes (23-25), it is conceivable that their intracellular localization will also be perturbed in melanoma cells that display this de-differentiated phenotype.
Gp100 (also called Pmel17 or Silver) is an MDP that plays a critical role in melanosome formation (26), and is also a tumor antigen expressed by more than 75% of human melanomas (27). Gp100 expressed endogenously in both melanoma and non-melanoma cells is processed for presentation of multiple epitopes by MHC II molecules (7, 28). This suggests that gp100 is targeted to MHC II processing compartments via an intracellular pathway. In melanocytes and pigmented melanoma gp100 is localized to the most immature (stage I and II) melanosomes and poorly represented in late endosomes and lysosomes (16). During transit through stage I melanosomes, the lumenal domain of gp100 is cleaved by a proprotein convertase into 26kDa and 70kDa fragments (24, 29). The latter can be further processed to a 34-38kDa molecule (30), and forms the fibrillar scaffolding characteristic of stage II melanosomes and on which melanin is deposited (29). In contrast, gp100 accumulates in conventional late endosomes and lysosomes when expressed in non-melanocytic cells (24). This targeting is mediated by sequences encoded in the gp100 lumenal domain, the removal of which results in predominant retention of the protein in early endosomes (31, 32). Two alternative mechanisms for gp100 intracellular sorting have been proposed: one directly from the trans-Golgi network, the other indirectly via endocytosis from the plasma membrane (33-35). Thus, the cell biology of this system offers a unique opportunity to investigate the compartments with class II processing capabilities in melanomas and the impact of sub-cellular trafficking of an intact membrane protein on its processing for antigen presentation.
Two distinct antigen processing pathways have been defined that enable presentation of a broad range of peptides derived from exogenous proteins entering endocytic compartments. In the classical pathway, epitopes require the proteolytic processing capacity of the highly acidic late endocytic compartments, where nascent class II molecules exchange CLIP for antigenic peptide (36). The presentation of such epitopes is sensitive to depletion of newly synthesized MHC II molecules through the use of protein synthesis inhibitors and ablation of Ii and HLA-DM functions (37, 38). In the alternative pathway, epitopes are generated within mildly proteolytic conditions of early endosomes and are loaded on mature MHC II molecules recycling through this compartment, independently of Ii and HLA-DM (37, 39-42). Which of these pathways is involved in processing of endogenously synthesized gp100 protein leading to MHC II presentation in melanoma cells has not been fully investigated.
In the current work we investigated the mechanisms for endogenous presentation of the HLA-DR*0401- restricted gp10044-59 epitope. We used melanoma cells that express MHC II molecules constitutively and gp100 mutants targeted to early or late endosomes to identify the endosomal compartments with roles in generating this epitope and the processing requirements for its presentation. By expressing MHC II molecules in pigmented melanoma cells, we also investigated the processing capabilities of melanosomes. Finally, we established that pigmented melanoma cells displayed lower levels of gp100 epitope compared with their de-differentiated counterparts that do not contain melanosomes. Our results emphasize that the presentation of gp100 epitopes by MHC II molecules is influenced by its unique cell biology, and that the alteration of this biology during malignant transformation modulates its recognition by the immune system.
MATERIAL AND METHODS
Melanoma cell lines and transfectants
The non-pigmented melanoma cell lines DM331 (gp100neg) (43) and 1102mel (gp100+) (gift of Suzanne L. Topalian, National Cancer Institute), both express HLA-DR*0401 constitutively. The pigmented melanoma cell lines 1011mel and MNT-1 (16) expresses all MDPs but not MHC II molecules. DM331, 1011, and MNT-1 all fail to express significant amounts of the invariant chain. DM331 cells transfected to express tyrosinase (DM331-tyrosinase) have been previously described (44). Melanoma cells were grown in RPMI-1640 (Invitrogen) supplemented with 5% heat-inactivated fetal bovine serum (Valley Biologicals) and 2 mM glutamine.
The wild-type gp100 gene and the DEL, ΔPKD, and ΔRPT gp100 mutants (31) were subcloned in pcDNA3.0 (Invitrogen Life Technologies) and transfected in DM331 melanoma using Fugene6 (Roche Diagnostics) or a Nucleofection system (Amaxa Biosystems). The high efficiency of the latter system facilitated experiments with long timeframes or requiring co-transfection of multiple gene constructs. A bulk stable line expressing wild-type gp100 (DM331-GPs) or short term transfected lines were obtained by culturing with 300μg/ml Zeocin (Invitrogen Life Technologies). HLA-DRB1*0401 α and β chain expression constructs (gift of Jack Gorski, Medical College of Wisconsin) were used for transient transfection of the HLA-DR-negative melanomas 1011mel and MNT1. Expression of gp100 was measured by intracellular staining after permeabilization with BD Perm/wash (BD Pharmingen) using the gp100 specific Abs HMB45 or HMB50 (Lab Vision Corp) and Phycoerythrin (PE)-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories). Expression of HLA-DRB1*0401 was measured by surface staining using the mAb L243 (45) or the HLA-DR4- specific Ab NFLD.D10 (46). Data were acquired on a flow cytometer and analyzed using CellQuest software (BD Pharmingen).
HLA-DR*0401 + gp10044-59 specific CD4 T cell lines
Human CD4 T cell lines specific for HLA-DR*0401 + gp10044-59 (6) were stimulated with a combination of irradiated (10000 rads) HLA-DR*0401+ BLCL, gp10044-59-expressing tumor cells (1102mel), and non-matched PBMC in T cell culture media. Cells were fed after 5 days using fresh medium supplemented with 20U/ml IL-2 and used in antigen presentation assays after another 2-3 days.
Murine CD4 T cells specific for HLA-DR*0401 + human gp10044-59 were generated in DR4-IE transgenic mice as previously described (6). For antigen presentation assays, T cells were used 6 or 7 days after restimulation.
Confocal microscopy
Cells were grown on coverslips, washed with PBS, fixed for 20 min at room temperature in 4% paraformaldehyde in PBS, and permeabilized with 200μl BD Perm/wash (BD Pharmingen) on ice. Cells were incubated for 1 h with Abs specific for gp100 (HMB45 or HMB50), HLA-DR (L243) or lysosome-associated membrane protein 1 (LAMP1) (BD Pharmingen). These primary Abs were detected with Alexa Fluor 488 anti-mouse IgG1 and Alexa Fluor 594 anti-mouse IgG2 conjugates (Molecular Probes). In some experiments, late endosomes/ lysosomes were stained by incubating cells for 60 min at 37°C with 60μg/ml Lysotracker–Alexa Fluor 594 (Molecular Probes) resuspended in phenol red free- HBSS. Early endocytic compartments were labeled by incubation with 40μg/ml Transferrin-Alexa Fluor 594 (Molecular Probes) for 10 min at 37°C. Cells were washed with PBS containing 5% serum, and fixed, permeabilized and stained with gp100-specific Abs as described above.
To examine internalization of gp100, cells were incubated with HMB50 Ab for 30 min at 4°C, and then shifted to 37°C for 90 min in the presence of Alexa Fluor 594- conjugated Lysotracker. After fixation and permeabilization, cells were stained with Alexa Fluor 488 donkey anti-mouse secondary Ab. To investigate the intracellular accumulation of newly formed peptide-MHC II complexes, DM331 cells transfected with mock or AP2-specific siRNA 3 days earlier were exposed to Brefeldin A at a final concentration of 3 μg/ml in complete medium for 12 h. Cells were washed three times in PBS and chased for 0 or 4½ h at 37°C before fixation in 4% paraformaldehyde. Mature MHC II molecules were detected with L243 mAb.
Samples were mounted on glass slides with Vectashield (Vector Laboratories), visualized using an Olympus confocal microscope, and processed with Adobe Photoshop 7.0.
RNA-mediated interference
Expression of the μ2-subunit of the AP2 adaptor protein was blocked by transfecting cells twice at 4 or 72 h intervals with the small interfering RNA (siRNA) duplexes (Qiagen) to the sequence GUGGAUGCCUUUCGGGUCA using Oligofectamine (Invitrogen) (20nM siRNA/ transfection). An irrelevant siRNA duplex was used as control. Cells were analyzed for depletion of AP2 at 48–72 h after the second round of transfection using flow cytometry or immunofluorescence microscopy.
T cell assays
CD4 T cells (5 × 104) were incubated with 5 × 104 melanoma cells in U-bottom 96-well plates (Costar) for 16 h. Culture supernatants were assayed using ELISA kits for murine IFN-γ or GM-CSF (eBiosciences) or human IFN-γ (Endogen). The data presented are average of duplicate wells, with error bars indicating SDs. To assess the ability of inhibitors to block endogenous Ag presentation, cell surface HLA-DR molecules were denatured by incubating melanoma cells in mild acid buffer (47) at room temperature for 3 min. Acid-stripped cells were incubated with drugs (100μM chloroquine, 0.5mM leupeptin, 100μM primaquine) for 16 h, fixed for 10 min on ice with 1% paraformaldehyde, and then incubated with CD4 T cells for 16 h prior to analysis of cell supernatants by ELISA. Doses of inhibitors were chosen because they showed maximal inhibition of presentation without affecting cell viability.
Western blots
Cell pellets were solubilized in 10 mM Tris-HCl, pH 7.5, 0.5% deoxycholate, 1% Igepal, 5 mM EDTA, 4 mM PMSF, 10 μg/ml aprotinin, 10 μM pepstatin A, 10 μg/ml leupeptin, and 100 μM iodoacetamide. Where indicated, melanoma cells were lysed using premixed M-PER (Pierce), which allows recovery of gp100 from stage II melanosomes. After centrifugation at 21,000 × g for 30 min, supernatants were separated by SDS-PAGE on 8–16% Tris-glycine gels (Invitrogen) and transferred to Immobilon-P membranes (Millipore). Blots were blocked in 10% (w/v) nonfat dry milk in PBS with 0.05% Tween 20 and incubated with first Abs diluted in the same buffer plus 2% nonfat dry milk for 2 h at room temperature (αPep13h) or overnight at 4°C (HMB45). Blots were incubated with horseradish peroxidase-linked anti-rabbit (αPep13h) or anti-mouse (HMB45) whole Abs (Jackson Laboratories) and detected by ECL (Amersham Biosciences).
RESULTS
Gp100 accumulates in melanosomes of pigmented melanoma and in conventional endosomes of melanoma that do not express other MDPs
We investigated the intracellular localization of gp100 and MHC II molecules in melanoma cells displaying different pigmentation phenotypes. The non-pigmented melanoma cell line 1102mel expresses gp100 but not Tyrp1, and tyrosinase in these cells is retained in the endoplasmic reticulum (ER) due to a genetic polymorphism (VR and VHE, unpublished data). The non-pigmented DM331 fails to express tyrosinase, gp100, Tyrp1 or MART-1 (43). Both of these cell lines constitutively express HLA-DRB1*0401. The pigmented melanoma 1011mel expresses all of these MDPs but not MHC II molecules. To analyze the relationship between MHC II molecules, gp100, and melanosomes, 1011mel was transfected to express HLA-DR*0401 (1011mel-DR4), while DM331 was stably transfected with a plasmid encoding gp100 (DM331-GPs). Using laser scanning confocal microscopy and HMB50, an Ab that detects gp100 in both stage I and II melanosomes, we found a marked segregation between gp100 and LAMP-1, a marker of endosomes and lysosomes in 1011mel-DR4 cells (Fig. 1). This is consistent with previous work (16, 48) that has demonstrated in pigmented melanomas, gp100 is principally localized to stage I and II melanosomes and excluded from conventional endosomes. Using HMB45, an Ab that recognizes a proteolyzed fragment of gp100 characteristic of stage II melanosomes (30), we observed a similar segregation between gp100+ and MHC II+ vesicles. In contrast, there was significant co-localization of gp100+ with both LAMP-1 and MHC II in intracellular vesicles in non-pigmented DM331 and 1102mel melanomas (Fig. 1). This indicates that, in these de-differentiated cells, gp100 molecules are routed to conventional late endosomal compartments, including those where MHC II molecules also reside.
Figure 1. Gp100 localizes in melanosomes of pigmented melanoma, and in conventional endosomes of non-pigmented melanoma.
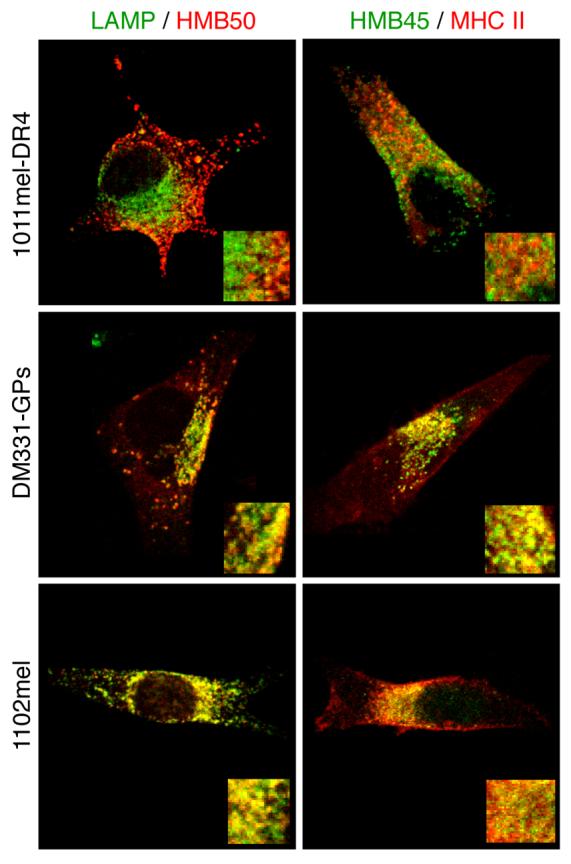
Pigmented 1011mel-DR4 and non-pigmented 1102mel and DM331-GPs melanoma cells were fixed, permeabilized and stained for LAMP-1 and gp100 (HMB50) (left panels) or HLA-DR (L243) and gp100 (HMB45) (right panels), and analyzed by laser scanning confocal microscopy. Insets: 3X magnification of the same field.
Access of gp100 to the endosomal pathway is required for human gp10044-59 epitope production and MHC II presentation
MHC II-restricted epitopes from different endogenous proteins have been shown to arise from proteolytic processing either by the proteasome in the cytoplasm or by proteases in endocytic compartments (49-51). To test which of these was involved in processing of the HLA-DR*0401-restricted gp100 epitope, gp10044-59, surface MHC molecules of non-pigmented 1102mel and DM331-GPs cells were first denatured by mild acid buffer treatment, and then new MHC-peptide complexes were allowed to form for 6 h in the presence of lactacystin, a specific proteasome inhibitor, or chloroquine, which neutralizes endosomal pH, and inhibits resident cathepsins. Lactacystin did not inhibit gp10044-59 presentation to gp10044-59 specific CD4 T cells (Fig. 2A,B), although it stabilized two gp100 degradation intermediates in the cytosol (not shown) and, as expected, abrogated presentation of Tyr369, a proteasome-dependent (52, 53) HLA-A*0201-restricted epitope derived from tyrosinase (Fig. 2C). In contrast, whereas presentation of Tyr369 by HLA-A*0201 was not affected by chloroquine, this inhibitor blocked gp10044-59 epitope presentation, indicating that its formation required the functions of acidified endosomes.
Figure 2. Gp10044-59 processing requires endosomal proteolysis.
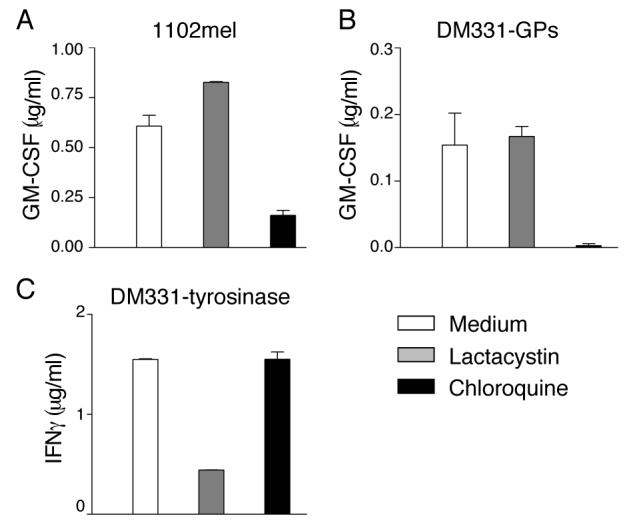
1102mel (A) DM331-GPs (B), and DM331-tyrosinase (C) cell lines were acid-stripped to remove existing MHC-peptide complexes and then incubated in presence or absence of lactacystin or chloroquine for 6 h. Following fixation with paraformaldehyde, cells were incubated with (A, B) gp10044-59-specific murine CD4 T cells and epitope presentation evaluated based on GM-CSF secretion, or (C) Tyr369 specific CD8 T cells and epitope presentation evaluated based on IFNγ secretion.
To test whether gp100 localization to endosomal compartments was essential for MHC II presentation of gp10044-59, we used a gp100 mutant, DEL, in which both the trans-membrane and cytoplasmic domains were deleted. DEL expressed in DM331 cells showed no vesicular distribution and failed to localize to LAMP-1+ compartments (Fig. 3A). The range of expression of DEL was very broad in these transient transfectants, and the median MFI was about 2/3 that of cells expressing wild-type gp100 (Fig 3B). However, these cells failed to present the gp10044-59 epitope to any significant extent (Fig. 3C). Thus, the presentation of gp10044-59 strongly correlates with the presence of full-length gp100 protein in late endosomes and lysosomes.
Figure 3. Gp10044-59 presentation correlates with gp100 protein localization in the endosomal pathway.
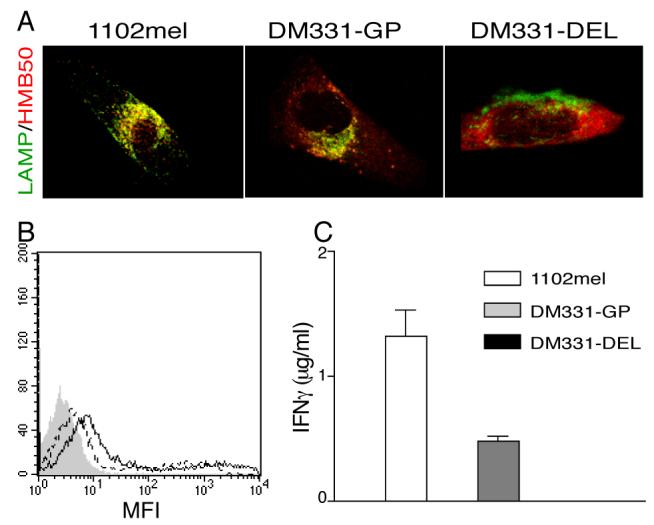
A, 1102mel, and DM331 cells 5 days post- transfection with wild- type (GP) or DEL mutant gp100 were fixed, permeabilized, stained for LAMP-1 and gp100 (HMB50), and analyzed by laser scanning confocal microscopy. B, Expression of gp100 in DM331-GP (solid line) and DM331-DEL (dotted line) was analyzed by intracellular staining using HMB50 Ab and flow cytometry. Filled peak represents isotype control staining. C, Gp10044-59-specific CD4 T cells were incubated with 1102mel, DM331-GP and DM331-DEL. Epitope presentation was evaluated based on IFN-γ secretion by the T cells, measured by ELISA.
Gp10044-59 epitope can be produced in both early and late endosomal compartments
Distinct MHC II- restricted epitopes are produced in either early or late endosomes based on differences in protease content, loading on mature or newly synthesized or mature MHC II molecules or the presence or absence of HLA-DM (37, 41, 54, 55). To investigate whether gp10044-59 epitope could be produced in early endosomes, we used another gp100 mutant, ΔPKD, which lacks amino acids 243-293 in the lumenal domain and accumulates in early endosomes (31). In DM331 transfectants, WT gp100 co-localized with Lysotracker, a marker of late endosomes, but not with transferrin internalized for 10 minutes, an early endosomal marker (Fig. 4A). Conversely, the ΔPKD mutant co-localized partially with internalized transferrin, and was largely excluded from late endosomes. Nonetheless, DM331 cells expressing ΔPKD were efficiently recognized by gp10044-59 specific T cells (Fig. 4B).
Figure 4. Gp10044-59 epitope can be produced both in early and late endosomes of DM331 melanoma.

A, DM331 cells were transfected with GP, ΔPKD or ΔRPT. Five days later, they were incubated in medium containing Transferrin-Alexa Fluor 594 for 10 minutes or Lysotracker- Alexa Fluor 594, fixed, permeabilized and stained with gp100 Abs HMB50 (GP, ΔRPT) or HMB45 (ΔPKD), and analyzed by laser scanning confocal microscopy. B, DM331 melanoma cells were incubated with leupeptin or chloroquine to inhibit endosomal proteases, and transfected with GP or ΔPKD gp100. Incubation in the presence of inhibitors was continued for 16 h, after which cells were fixed with paraformaldehyde and evaluated for epitope presentation to gp10044-59 specific murine CD4 T cells. C, Cell surface expression of class II MHC in DM331-GP, DM331-PKD or DM331-RPT after inhibitor treatment was analyzed by staining with L243 Ab and flow cytometry. Filled peak represents staining of untreated cells.
To investigate whether localization of gp100 in early or late endosomes correlated with epitope production in that compartment, we treated cells with leupeptin, which inhibits late endosomal serine and cysteine proteases, or chloroquine, to de-acidify these compartments (51, 56). Treatment with these agents did not affect the expression of MHC II or transfected gp100 molecules (Fig. 4C). DM331 also does not express the invariant chain (see Fig 7A below), and thus, leupeptin cannot alter epitope expression by blocking invariant chain degradation. As with wild-type gp100 (GP), chloroquine inhibited gp10044-59 production from the ΔPKD mutant, indicating that it also required intracellular processing in acidified endosomes (Fig. 4B). However, gp10044-59 presentation from GP gp100 was inhibited by leupeptin, while presentation of this epitope from the ΔPKD mutant was not. This difference might have been due to the large deletion in ΔPKD, which could render gp100 more susceptible to proteolytic degradation and thus less sensitive to inhibition. To further investigate this possibility we analyzed epitope presentation from a second gp100 mutant, ΔRPT, which has a deletion encompassing amino acids 314-424, but displays the same intracellular localization as GP gp100 (31). When expressed in DM331, ΔRPT colocalized with Lysotracker but not with internalized transferrin, indicating its distribution in late but not early endosomes (Fig. 4A). As with GP, presentation of gp10044-59 from ΔRPT was inhibited by both chloroquine and leupeptin (Fig. 4B). These results indicate that gp10044-59 can be generated in either early or late endosomes depending on the primary intracellular localization of the source protein.
Figure 7. AP2 depletion does not impair intracellular accumulation of new MHC II- peptide complexes in DM331 melanoma.
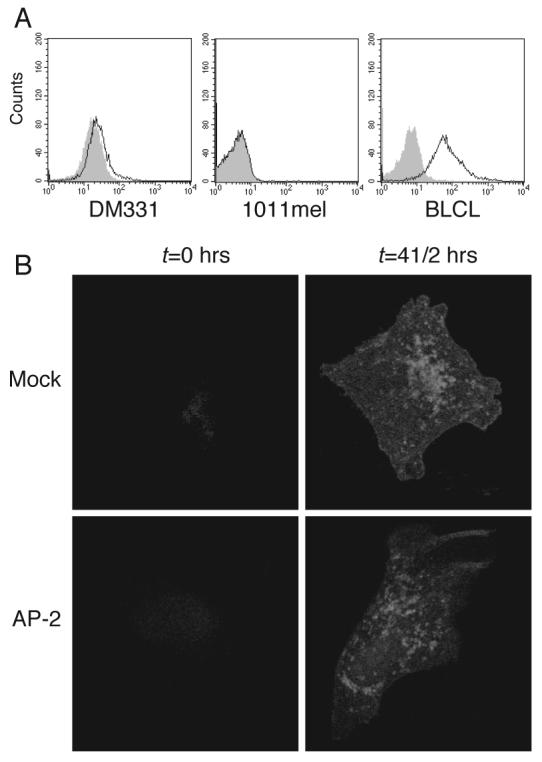
A, Expression of invariant chain by DM331 (left panel), 1011mel cells (middle panel) and BLCL (right panel) was evaluated by intracellular staining with LN2 Ab. Gray histogram represents isotype control. B, DM331-GPs cells transfected with control or AP2 specific siRNA were incubated with 3 μg/ml Brefeldin A for 12 h, washed and chased for 0 or 4½ h at 37°C, and then fixed, permeabilized, stained for mature MHC II molecules with L243 Ab and analyzed by confocal microscopy.
Wild-type gp100 is processed both in early and late endosomal compartments
Despite prevalent localization of GP gp100 to late endosomes, gp10044-59 presentation was only ∼ 55- 60% reduced after treatment with leupeptin. This, together with the demonstration that gp10044-59 is produced in early endosomes from ΔPKD gp100, led us to hypothesize that GP gp100 is also processed in this compartment. Primaquine prevents re-expression of internalized recycling MHC II molecules and inhibits presentation of epitopes produced in early endosomes (42). Treatment of ΔPKD expressing cells with primaquine inhibited gp10044-59 presentation to CD4 T cells to a similar extent as chloroquine (Fig. 5 upper left panel), while leupeptin had no effect either alone or in combination with primaquine. Primaquine did not reduce surface MHC II expression (not shown), HLA-A*0201 presentation of the Tyr369 epitope (Fig. 5 lower left panel), or presentation of exogenous gp10044-59 peptide pulsed on DM331 (Fig. 5 lower right panel). In keeping with the localization of the ΔPKD mutant protein and its insensitivity to leupeptin, this result suggests that the gp10044-59 derived from this molecule is presented by MHC II molecules recycling through early endosomes. In four independent experiments, primaquine treatment also reduced gp10044-59 presentation by DM331 cells expressing GP gp100 by 30-50% relative to chloroquine (Fig. 5 upper right panel and data not shown). The effects of leupeptin and primaquine on presentation by these cells were additive, and equivalent to treatment with chloroquine. These results suggest that gp10044-59 is also produced from wild-type gp100 in both late and early endosomes.
Figure 5. Gp10044-59 epitope can be produced from wild-type gp100 in early as well as late endosomes of DM331 melanoma.
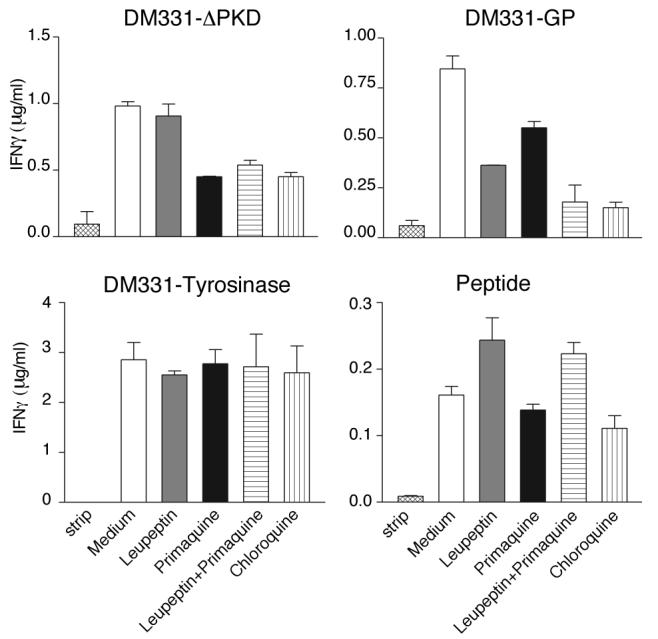
DM331 cells expressing ΔPKD (upper left panel), GP (upper right panel) or tyrosinase (lower left panel) were acid-stripped to remove existing peptide-MHC complexes and incubated for 16 h with the indicated inhibitors. Lower right panel, DM331 cells were incubated in the presence or absence of inhibitors together with 100 μg/ml gp10044-59 synthetic peptide. Following fixation with paraformaldehyde, cells were incubated with Tyr369- specific murine CD8 T cells (lower left panel) or gp10044-59 specific murine CD4 T cells (all other panels) and epitope presentation was evaluated based on IFN-γ secretion measured by ELISA. One experiment representative of four is shown.
The major source protein for gp10044-59 presented by HLA-DR*0401 is internalized from the plasma membrane by AP2
Gp100 might reach endosomal compartments for processing either directly from the Golgi or by transiting to the cell surface followed by internalization (16, 34, 57). The clathrin coat component AP2 has been implicated in endosomal targeting of newly synthesized proteins by endocytosis from the plasma membrane (58-61), and previous work was consistent with the possibility that gp100 was an AP2 cargo protein (33). To test this directly, we ablated AP2 expression in DM331-GPs using siRNA oligonucleotides directed to the μ2 subunit of the complex (59). In keeping with earlier work (58, 60), AP2 depletion led to a five fold increase in surface expression of LAMP-1, while the expression of mature MHC II molecules was unchanged (Fig. 6A). AP2 depletion also led to a significant increase in cell surface gp100, while having no effect on total cellular gp100 content (Fig 6A). By pre-labeling cell surface gp100 molecules with specific Ab, we also found that their subsequent internalization into endosomal compartments was substantially inhibited in AP2 depleted cells (Fig. 6B). In AP2-depleted cells, gp10044-59 presentation was substantially inhibited (Fig. 6C), suggesting that gp100 internalized from the plasma membrane is a major source of protein for epitope production.
Figure 6. Gp100 internalized from the plasma membrane by AP2 is the major source protein for gp10044-59-processing.
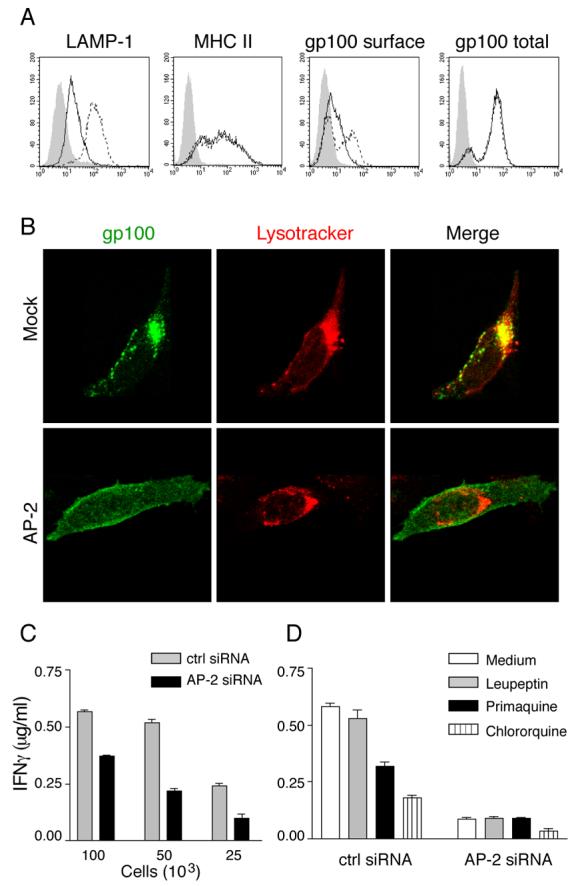
DM331-GPs cells were transfected with control or AP2-specific siRNAs, and cultured for 72 h (A-C) or 48 h (D). A, Cells were stained for cell surface expression of LAMP1 (LAMP-1 Ab), mature MHC II (L243 Ab) and gp100 (HMB50), and for total gp100 (HMB50) after permeabilization. Solid line, cells transfected with control siRNA, dotted line, cells transfected with AP2 siRNA, gray filled, isotype control. B, Cells were allowed to bind HMB50 for 30 min at 4°C, washed, and then incubated for 90 min at 37°C in the presence of Lysotracker- Alexa Fluor 594 (red). After washing and fixation, HMB50 Ab was detected with Alexa Fluor 488-conjugated second Ab (green). C, The indicated number of cells were incubated with gp10044-59-specific murine CD4 T cells and epitope presentation evaluated based on IFN-γ secretion measured by ELISA. D, Cells were stripped, washed and incubated in the presence of the indicated inhibitors for 16 h. Fixed cells were incubated with gp10044-59-specific murine CD4 T cells and epitope presentation evaluated based on IFN-γ secretion measured by ELISA.
Although AP2 deficiency did not alter the overall level of MHC II expression (Fig. 6A), it has been shown to direct internalization of newly synthesized MHC II molecules to endosomes by interaction with the invariant chain (Ii) (58). Thus, impaired gp10044-59 presentation in AP2 deficient cells could be due to limited availability of newly synthesized HLA-DR*0401 molecules in endosomes. However, DM331 melanoma fails to express significant amounts of Ii (Fig. 7A). To directly test whether AP2 knockdown decreased endosomal availability of newly synthesized HLA-DR*0401 molecules in DM331, we treated control and AP2-deficient cells with Brefeldin A for 12 h to trap newly synthesized MHC II complexes in the cis/medial-Golgi and clear late endosomal compartments of mature MHC II molecules (detected with L243 mAb) (Fig. 7B, left panel). When Brefeldin A was washed out and the cells incubated for 4.5 h, mature MHC II molecules were detected in endosomal compartments of both control and AP2 depleted cells (Fig. 7B, right panel). Thus, AP2 is not essential for endosomal trafficking of newly synthesized HLA-DR*0401 molecules and formation of new peptide-MHC II complexes in DM331 melanoma, presumably because of the absence of Ii. We conclude that the reduction of gp10044-59 epitope presentation after AP2 knockdown is a consequence of preventing access of cell surface gp100 to MHC II processing compartments.
Gp10044-59 epitope presentation is reduced in pigmented melanomas that contain melanosomes in addition to conventional endosomes
We next investigated whether the segregation of gp100 into melanosomes in pigmented cells reduced presentation of gp10044-59 relative to depigmented melanoma cells in which gp100 localizes to MHC II+ endosomes. For this evaluation, we used 1011mel and a second pigmented melanoma cell line, MNT1. As with DM331, neither of these cells expresses Ii (Fig 7A and data not shown). Both were transfected to express HLA-DR*0401 and sorted by flow cytometry to select cells expressing levels of surface MHC II molecules similar to DM331-GPs (Fig. 8A). Gp10044-59 presentation was markedly reduced in both 1011mel-DR4 and MNT1-DR4, requiring approximately 8 times as many cells as DM331-GPs to achieve T cell comparable activation (Fig. 8B). Although both of these cells expressed lower levels of HLA-A2 than DM331-GPs (Fig. 8C), 1011mel presented Tyr369 to specific CD8 T cells comparably to DM331-GPs, while MNT1 was about half as effective (Fig. 8D). Thus, the substantial differences in presentation of gp10044-59 were not a consequence of general differences in the ability to stimulate T cells.
Figure 8. Gp10044-59 epitope presentation is dramatically reduced in pigmented melanoma cells.
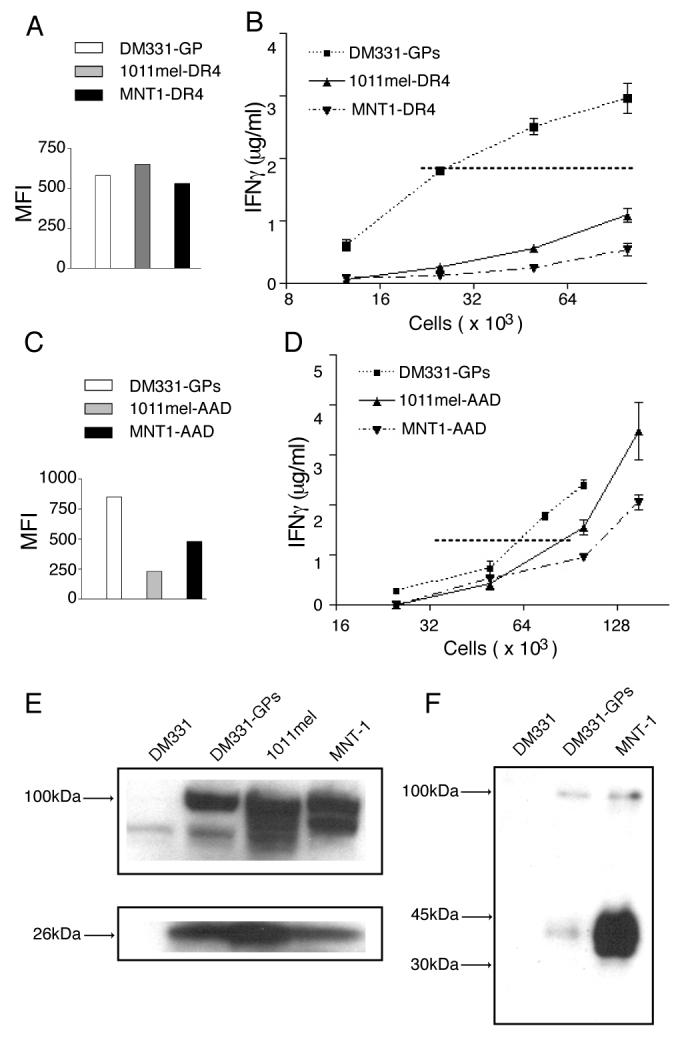
1011mel and MNT-1 pigmented melanoma cells were transfected with genes encoding DR*0401 α and β chains (A, C) or AAD (a chimeric molecules that has the HLA-A2 peptide binding site) (B, D), and incubated 48 h. A, DR*0401 transfectants were stained using the HLA-DR4-specific mAb NFLD.D10, and electronically- sorted to select cells expressing the same level of HLA-DR4 as DM331-GPs cells. Post- sort mean intensity fluorescence (MFI) of each cell line. B, Indicated numbers of sorted DR4*0401 transfectants were incubated with gp10044-59- specific murine CD4 T cells and epitope presentation measured by IFN-γ secretion. C, AAD transfectants were stained using the HLA-A2-specific mAb CR11-351. D, Indicated numbers of AAD transfectants were incubated with Tyr369- specific CD8 T cells and epitope presentation measured by IFN-γ secretion. E, The indicated melanoma cells were solubilized and 5 × 105 cell equivalents of each were separated by SDS-PAGE, followed by immunoblotting with Abs specific for the C-terminus of gp100 (αPep13h). F, Melanoma cells were lysed using M-PER, which allows recovery of gp100 from stage II melanosomes. SDS-PAGE and immunoblotting were performed using lumenal gp100- specific mAb HMB45.
Western blot analysis using the carboxyl-terminal specific Ab αPep13h showed that all three melanoma lines express similar levels of full-length gp100 and a 26kDa fragment that can be produced by proteolysis in either endosomes or early stage melanosomes (24, 29) (Fig. 8E). However, both MNT1 and 1011mel have much higher levels of the mature 34-38kDa gp100 fragment produced only in melanosomes and detected by mAb HMB45 (30), as compared with DM331-GPs (Fig. 8F and data not shown). While this gp100 fragment does not encompass the gp10044-59 peptide, its prevalence indicates that the relative paucity of gp10044-59 presentation is also not due to a lack of source protein expression. Instead, it suggests that this limitation is a consequence of source protein localization.
Gp10044-59 epitope presentation is completely abolished in AP2 depleted 1011mel- DR4 (Fig. 9A), demonstrating that gp100 internalized from plasma membrane is the only source protein for production of the gp10044-59 epitope in pigmented melanoma. To test whether gp10044-59 is produced in conventional early or late endosomes, despite the underrepresentation of gp100 localization in LAMP-1high compartments, we analyzed the effect of primaquine or leupeptin treatment on epitope presentation. 1011mel were transfected with DRB1*0401 and expression allowed to occur in the presence of inhibitors, then fixed and incubated with murine CD4 T cells specific for gp10044-59/DRB1*0401. Leupeptin had no effect on gp10044-59 presentation, while primaquine treatment led to a substantial reduction (Fig. 9B). These results indicate that, in pigmented melanomas, gp10044-59 is generated entirely within early endosomes/ stage I melanosomes and not in late endosomal compartments.
Figure 9. In pigmented melanoma, gp10044-59 epitope is produced in early but not late endosomes.
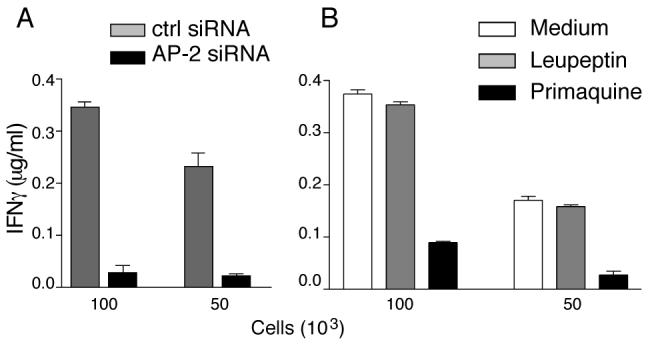
A, 1011mel pigmented melanoma were transfected DRB1*0401 α and β chains and control or AP2- specific siRNA. Seventy two h after transfection, the indicated number of cells were incubated with gp10044-59- specific murine CD4 T cells and epitope presentation evaluated based on IFN-γ secretion measured by ELISA. B, 1011mel pigmented melanoma were transfected with DRB1*0401 α and β chains. Twelve h after transfection, cells were incubated in the presence of the indicated inhibitor for 16 h. After fixation with paraformaldehyde, the indicated number of cells was incubated with gp10044-59- specific murine CD4 T cells and peptide presentation evaluated based on IFN-γ secretion measured by ELISA.
DISCUSSION
In the current work, we investigated the mechanism of presentation of an MHC II-restricted epitope from gp100/ Pmel17, a cellular protein with an endosomal/melanosomal localization. Using melanoma cells that have down-regulated components of the melanosomal pathway, but constitutively express HLA-DR*0401 molecules, we established that the majority of gp100 is sorted to LAMP-1hi/ MHC II+ conventional endosomes. In these cells, presentation of the gp10044-59 epitope required localization of gp100 source protein to endosomes and processing by acidic proteases. We also found that similar antigenic epitopes were produced in both early and late endosomes, despite differences in processing requirements. Epitope presentation depends in large part on gp100 trafficking to the plasma membrane and internalization mediated by the AP2 adaptor protein. We also established that the presentation of this epitope was dramatically reduced in pigmented melanomas that contain melanosomes in addition to conventional endosomes, consistent with the localization of gp100 to melanosomes and not to LAMP-1hi MHC II+ late endosomes. In these cells, gp10044-59 presentation still depends on protein internalization from the cell surface via AP2, but processing occurs entirely in early endosomes/ stage I melanosomes and not late endosomes. Collectively, our results emphasize that: processing of gp100 epitopes is directly tied to intracellular targeting of the source protein; melanosomes are relatively poor compartments for MHC II processing, despite their endosomal origin; and de-differentiation of melanoma cells, resulting in loss of melanosomes, augments presentation of epitopes from those MDP that continue to be expressed due to alterations in their endosomal targeting.
Previous studies demonstrated that endogenous cytoplasmic (49, 62) and transmembrane (50) proteins can be processed by the proteasome for presentation by MHC II molecules. The resulting peptides reach endosomal compartments through TAP (51) or LAMP-2 (63). However, high and sustained levels of cytosolic source protein may be required compared with class I-mediated recognition (64, 65). Here we show that inhibition of proteasomes by lactacystin had no effect on presentation of gp10044-59, although it stabilized gp100 fragments in both membrane and cytosol. This indicates that defective ribosomal products or poorly folded forms of the protein that were retrotranslocated into the cytosol are inefficient substrates for MHC II presentation, compared to full-length protein directly targeted to endosomes, even though the latter are fully folded and thus presumably more resistant to proteolytic degradation.
Using mutant forms of gp100 that localize to different compartments, we established that antigenically indistinguishable epitopes are produced in both early and late endosomes. Thus, gp10044-59 is among a limited number of epitopes for which presentation via recycling MHC II molecules in early endosomes has been demonstrated and the only one so far described to originate from an endogenous protein with an endosomal/ melanosomal localization; other endosomal recycling-dependent epitopes have been derived from exogenous (37, 40-42) or cytosolic (51) proteins. However, the involvement of AP2-mediated internalization of gp100 from the plasma membrane suggests that endocytosis is a common mechanism to deliver epitope source proteins, either endogenous or exogenous, to early endosomes for presentation by recycling MHC II molecules.
Other studies have demonstrated that distinct MHC II- restricted peptides are produced in early and late endosomal compartments. Epitopes produced in late endosomes require more substantial source protein denaturation, while those produced in early endosomes are produced by mild proteolysis, and may be destroyed in the harsher environment of late endosomes (37, 42). Here we demonstrated that gp10044-59 is generated by leupeptin-sensitive proteases in late endosomes, and by leupeptin-insensitive proteases in early endosomes. This may be facilitated by the fact that the protein itself is a normal resident of endosomes, and thus able to resist denaturation. On the other hand, the N-terminal region of gp100, which contains this epitope, seems to be dissociated and/or degraded during the maturation of melanosomes (35, 57), suggesting that it is accessible to endosomal proteases. Although the epitopes generated in these two compartments are antigenically indistinguishable, our data do not exclude the possibility that structurally different peptides containing the epitope are generated with greater or lesser efficiency in either compartment.
The inhibition of epitope presentation by siRNA-mediated depletion of AP2 suggests that the major forms of gp100 that serve as substrates for gp10044-59 epitope presentation were internalized from the plasma membrane. This observation supports and extends previous work demonstrating a correlation between gp100 cell surface expression and presentation of a DR*0701- restricted epitope, which led to the suggestion that gp100 accessed MHC II+ processing compartments by endocytosis from the plasma membrane (66). AP2 directs cargo internalization by recognition of tyrosine- or di-leucine-based sorting signals located in cytoplasmic domains (61, 67), and a di-leucine-based signal in the cytoplasmic domain of gp100, which is deleted in the natural silver mutation of mouse Pmel17, has been shown to be required for its efficient internalization and accumulation in melanosome precursors (34). These data suggest that AP2 facilitates internalization by interaction with the di-leucine-based motif. Interestingly, LePage et al (66) reported that deletion of the gp100 cytoplasmic domain reduced presentation of the DRB1*0701- restricted epitope, while deletion of the di-leucine motif did not, despite increased mutant protein localization to cell surface. However, that work employed gp100 transfectants to assess this issue, whereas we have used cell expressing the endogenous gp100 gene. Overexpression of gp100 minimizes the influence of the endocytosis signal on efficient localization into endosomes (31, 34), perhaps because of AP2-independent internalization or direct transport from the trans-Golgi network, and may explain the apparent discrepancy of our results and theirs. Our data do not exclude the possibility that a cohort of gp100 molecules traverses an alternative transport pathway from the trans-Golgi network directly to stage I melanosomes as has been proposed (33), but suggests that this is a minor pathway at best. Thus, our results establish that an endosomal protein reaches MHC II processing compartments by endocytosis mechanisms resembling those responsible for internalization of exogenous proteins from the plasma membrane.
Our results represent one of the first analyses of MHC II processing and presentation capabilities of melanosomes. Previous studies showed that melanosomes share a number of characteristics with conventional endosomes, including low luminal pH and the presence of lysosomal proteases and membrane proteins (18). In contrast, we found that MHC II molecules accumulated in conventional LAMP1hi late endosomes, and were largely excluded from stage II melanosomes. In accordance with previous reports (16), the majority of gp100 was localized in stage II melanosomes. In keeping with our results demonstrating that gp100 targeting to endosomes was the primary determinant of the presentation of gp10044-59 by class II molecules, these cells presented this epitope inefficiently, and the processing was confined to early endosomes. These data indicate that the MHC II molecules and MDP intersect at the early endosome/ stage I melanosome, but are segregated thereafter, presumably via specific sorting signals. It will be interesting to determine whether melanosomal targeting also diminishes the presentation of epitopes from other MDPs such as tyrosinase or Tyrp1, particularly if these epitopes require the more robust processing environment of late endosomal compartments. Conversely, it will also be interesting to determine whether mutations that interfere with transit of these proteins to melanosomes (68-71) enhance presentation of epitopes from these proteins.
An inverse correlation between expression of MHC II molecules and of MDPs has been previously reported (15). However, melanocytes and some melanomas express MHC II after induction with IFNγ (11-13). It remains to be determined whether IFNγ stimulation also alters the localization of MDP from melanosomes to late endosomes. Conversely, the limited ability of pigmented melanoma cells to present MHC II epitopes derived from MDPs is likely to make presentation of MDP-derived epitopes more reliant on uptake by professional APC. Thus, the contribution of epitope presentation directly by tumor or mediated by professional APC may vary among melanomas and may correlate with the degree of pigmentation.
During the process of malignant transformation and progression, melanocytes and/or early stage melanomas develop defects in melanosome biogenesis and pigment synthesis (20, 72). These may be due to alterations in mechanisms that segregate melanosomes from conventional endosomes (21, 22). Our results demonstrate that this de-differentiation results in the mistargeting of gp100 to conventional late endosomes. This shift results in a significantly higher presentation of gp10044-59. Thus, melanoma de-differentiation may be accompanied by increased efficiency of presentation of gp100- derived MHC II- restricted epitopes, leading to enhanced immune recognition.
ACKNOWLEDGEMENTS
The authors declare that they have no competing financial interests
Footnotes
This work was supported by USPHS grants AI20963 and AI33134 to VHE, AR041855 and EY015625 to MSM, and CIHR grant ROP-38369 to SD.
Abbreviations used in this paper: MDPs, melanocyte differentiation proteins; siRNA, small interfering RNA; ER, endoplasmic reticulum.
REFERENCES
- 1.Slingluff CL, Jr., Chianese-Bullock KA, Bullock TN, Grosh WW, Mullins DW, Nichols L, Olson W, Petroni G, Smolkin M, Engelhard VH. Immunity to melanoma antigens: from self-tolerance to immunotherapy. Adv. Immunol. 2006;90:243–295. doi: 10.1016/S0065-2776(06)90007-8. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A, Rosenberg SA, Robbins PF. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR- restricted tyrosinase epitopes. J. Exp. Med. 1996;183:1965–1971. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi H, Lu J, Celis E. Identification of helper T-cell epitopes that encompass or lie proximal to cytotoxic T-cell epitopes in the gp100 melanoma tumor antigen. Cancer Res. 2001;61:7577–7584. [PubMed] [Google Scholar]
- 5.Zarour HM, Storkus WJ, Brusic V, Williams E, Kirkwood JM. NY-ESO-1 encodes DRB1*0401-restricted epitopes recognized by melanoma- reactive CD4+ T cells. Cancer Res. 2000;60:4946–4952. [PubMed] [Google Scholar]
- 6.Touloukian CE, Leitner WW, Topalian SL, Li YF, Robbins PF, Rosenberg SA, Restifo NP. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. J. Immunol. 2000;164:3535–3542. doi: 10.4049/jimmunol.164.7.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapointe R, Royal RE, Reeves ME, Altomare I, Robbins PF, Hwu P. Retrovirally-transduced human dendritic cells can generate T cells recognizing multiple MHC class I and class II epitopes from the melanoma antigen gp100. J. Immunol. 2001;167:4758–4764. doi: 10.4049/jimmunol.167.8.4758. [DOI] [PubMed] [Google Scholar]
- 8.Robbins PF, el-Gamil M, Li YF, Zeng G, Dudley M, Rosenberg SA. Multiple HLA class II-restricted melanocyte differentiation antigens are recognized by tumor-infiltrating lymphocytes from a patient with melanoma. J. Immunol. 2002;169:6036–6047. doi: 10.4049/jimmunol.169.10.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kierstead LS, Ranieri E, Olson W, Brusic V, Sidney J, Sette A, Kasamon YL, Slingluff CL, Jr., Kirkwood JM, Storkus WJ. gp100/pmel17 and tyrosinase encode multiple epitopes recognized by Th1- type CD4+T cells. Br. J. Cancer. 2001;85:1738–1745. doi: 10.1054/bjoc.2001.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Topalian SL, Schwartzentruber DJ, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J. Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady MS, Eckels DD, Ree SY, Schultheiss KE, Lee JS. MHC class II-mediated antigen presentation by melanoma cells. J. Immunother. Emphasis. Tumor Immunol. 1996;19:387–397. doi: 10.1097/00002371-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Cerundolo V. T cells work together to fight cancer. Curr. Biol. 1999;9:R695–R697. doi: 10.1016/s0960-9822(99)80442-4. [DOI] [PubMed] [Google Scholar]
- 13.Zarour HM, Kirkwood JM, Kierstead LS, Herr W, Brusic V, Slingluff CL, Jr., Sidney J, Sette A, Storkus WJ. Melan-A/MART-1(51-73) represents an immunogenic HLA-DR4-restricted epitope recognized by melanoma-reactive CD4(+) T cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:400–405. doi: 10.1073/pnas.97.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Poole IC, Riker AI, Quevedo ME, Stennett LS, Wang E, Marincola W,FM, Kast WM, Robinson JK, Nickollof BJ. Interferon-gamma reduces melanosomal antigen expression and recognition of melanoma cells by cytotoxic T cells. Am. J. Pathol. 2002;160:521–528. doi: 10.1016/s0002-9440(10)64871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raposo G, Marks MS. The dark side of lysosome-related organelles: specialization of the endocytic pathway for melanosome biogenesis. Traffic. 2002;3:237–248. doi: 10.1034/j.1600-0854.2002.030401.x. [DOI] [PubMed] [Google Scholar]
- 16.Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 2001;152:809–824. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierre P, Denzin LK, Hammond C, Drake JR, Amigorena S, Cresswell P, Mellman I. HLA-DM is localized to conventional and unconventional MHC class II-containing endocytic compartments. Immunity. 1996;4:229–239. doi: 10.1016/s1074-7613(00)80431-8. [DOI] [PubMed] [Google Scholar]
- 18.Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J. Invest. Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- 19.Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J. Invest. Dermatol. 1999;3:24–28. doi: 10.1038/sj.jidsp.5640176. [DOI] [PubMed] [Google Scholar]
- 20.Sakai C, Ollmann M, Kobayashi T, Abdel-Malek Z, Muller J, Vieira WD, Imokawa G, Barsh GS, Hearing VJ. Modulation of murine melanocyte function in vitro by agouti signal protein. EMBO J. 1997;16:3544–3552. doi: 10.1093/emboj/16.12.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donatien PD, Diment SL, Boissy RE, Orlow SJ. Melanosomal and lysosomal alterations in murine melanocytes following transfection with the v-rasHa oncogene. Int. J. Cancer. 1996;66:557–563. doi: 10.1002/(SICI)1097-0215(19960516)66:4<557::AID-IJC22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Cohen SN. Tsg101. a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell. 1996;3:319–329. doi: 10.1016/s0092-8674(00)81111-3. [DOI] [PubMed] [Google Scholar]
- 23.Bouchard B, Fuller BB, Vijayasaradhi S, Houghton AN. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J. Exp. Med. 1989;169:2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berson JF, Harper DC, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Biol. Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raposo G, Marks MS. Melanosomes--dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theos AC, Truschel ST, Raposo G, Marks MS. The Silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Res. 2005;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cormier JN, Abati A, Fetsch P, Hijazi YM, Rosenberg SA, Marincola FM, Topalian SL. Comparative analysis of the in vivo expression of tyrosinase, MART-1/Melan-A, and gp100 in metastatic melanoma lesions: implications for immunotherapy. J. Immunother. 1998;21:27–31. doi: 10.1097/00002371-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Lapointe R, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2004;64:4056–4057. [PubMed] [Google Scholar]
- 29.Berson JF, Theos AC, Harper DC, Tenza D, Raposo G, Marks MS. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J. Cell Biol. 2003;161:521–533. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiamenti AM, Vella F, Bonetti F, Pea M, Ferrari S, Martignoni G, Benedetti A, Suzuki H. Anti-melanoma monoclonal antibody HMB-45 on enhanced chemiluminescence-western blotting recognizes a 30-35 kDa melanosome-associated sialated glycoprotein. Melanoma Res. 1996;6:291–298. doi: 10.1097/00008390-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoashi T, Muller J, Vieira WD, Rouzaud F, Kikuchi K, Tamaki K, Hearing VJ. The repeat domain of the melanosomal matrix protein PMEL17/GP100 is required for the formation of organellar fibers. J. Biol. Chem. 2006;281:21198–21208. doi: 10.1074/jbc.M601643200. [DOI] [PubMed] [Google Scholar]
- 33.Valencia JC, Watabe H, Chi A, Rouzaud F, Chen KG, Vieira WD, Takahashi K, Yamaguchi Y, Berens W, Nagashima K, Shabanowitz J, Hunt DF, Appella E, Hearing VJ. Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2: evidence for the polarized nature of melanocytes. J. Cell Sci. 2006;119:1080–1091. doi: 10.1242/jcs.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theos AC, Berson JF, Theos SC, Herman KE, Harper DC, Tenza D, Sviderskaya EV, Lamoreux ML, Bennett DC, Raposo G, Marks MS. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell. 2006;17:3598–3612. doi: 10.1091/mbc.E06-01-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper DC, Theos AC, Herman KE, Tenza D, Raposo G, Marks MS. Premelanosome amyloid-like fibrils are composed of only golgi-processed forms of Pmel17 that have been proteolytically processed in endosomes. J. Biol. Chem. 2008;283:2307–2322. doi: 10.1074/jbc.M708007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryant P, Ploegh H. Class II MHC peptide loading by the professionals. Curr. Opin. Immunol. 2004;16:96–102. doi: 10.1016/j.coi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Zhong G, Romagnoli P, Germain RN. Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J. Exp. Med. 1997;185:429–438. doi: 10.1084/jem.185.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 39.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 40.Lindner R, Unanue ER. Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO J. 1996;15:6910. [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin JP, Chu R, Harding CV. Early endosomes and a late endocytic compartment generate different peptide-class II MHC complexes via distinct processing mechanisms. J. Immunol. 1997;158:1523–1532. [PubMed] [Google Scholar]
- 42.Sinnathamby G, Eisenlohr LC. Presentation by recycling MHC class II molecules of an influenza hemagglutinin-derived epitope that is revealed in the early endosome by acidification. J. Immunol. 2003;170:3504–3513. doi: 10.4049/jimmunol.170.7.3504. [DOI] [PubMed] [Google Scholar]
- 43.Slingluff CL, Colella TA, Thompson L, Graham DD, Skipper JCA, Caldwell JA, Brinkerhoff L, Kittlesen DJ, Deacon DH, Oei C, Harthun NL, Huczko EL, Hunt DF, Darrow TL, Engelhard VH. Melanomas with concordant loss of multiple melanocytic differentiation proteins: immune escape that may be overcome by targeting unique or undefined antigens. Cancer Immunol. Immunother. 2000;48:661–672. doi: 10.1007/s002620050015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostankovitch M, Robila V, Engelhard VH. Regulated folding of tyrosinase in the endoplasmic reticulum demonstrates that misfolded full-length proteins are efficient substrates for class I processing and presentation. J. Immunol. 2005;174:2544–2551. doi: 10.4049/jimmunol.174.5.2544. [DOI] [PubMed] [Google Scholar]
- 45.Lampson LA, Levy R. Two populations of Ia-like molecules on a human B cell line. J. Immunol. 1980;125:293–299. [PubMed] [Google Scholar]
- 46.Drover S, Marshall WH, Kwok WW, Nepom GT, Karr RW. Amino acids in the peptide-binding groove influence an antibody-defined, disease-associated HLA-DR epitope. Scand. J Immunol. 1994;39:539–550. doi: 10.1111/j.1365-3083.1994.tb03411.x. [DOI] [PubMed] [Google Scholar]
- 47.Burkhart C, Britschgi M, Strasser I, Depta JP, von GS, Barnaba V, Pichler WJ. Non-covalent presentation of sulfamethoxazole to human CD4+ T cells is independent of distinct human leucocyte antigen-bound peptides. Clin. Exp. Allergy. 2002;32:1635–1643. doi: 10.1046/j.1365-2222.2002.01513.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee ZH, Hou L, Moellmann G, Kuklinska E, Antol K, Fraser M, Halaban R, Kwon BS. Characterization and subcellular localization of human Pmel 17/silver, a 110-kDa (pre)melanosomal membrane protein associated with 5,6,-dihydroxyindole-2-carboxylic acid (DHICA) converting activity. J. Invest. Dermatol. 1996;106:605–610. doi: 10.1111/1523-1747.ep12345163. [DOI] [PubMed] [Google Scholar]
- 49.Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J. Exp. Med. 2000;191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee P, Dani A, Bhatia S, Singh N, Rudensky AY, George A, Bal V, Mayor S, Rath S. Efficient presentation of both cytosolic and endogenous transmembrane protein antigens on MHC class II is dependent on cytoplasmic proteolysis. J. Immunol. 2001;167:2632–2641. doi: 10.4049/jimmunol.167.5.2632. [DOI] [PubMed] [Google Scholar]
- 51.Tewari M, Sinnathamby G, Rajagopal D, Eisenlohr L. A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nature Immunology. 2005;6:287–294. doi: 10.1038/ni1171. [DOI] [PubMed] [Google Scholar]
- 52.Mosse CA, Meadows L, Luckey CJ, Kittlesen DJ, Huczko EL, Slingluff CL, Jr., Shabanowitz J, Hunt DF, Engelhard VH. The class I antigen processing pathway for the membrane protein tyrosinase involves translation in the endoplasmic reticulum and processing in the cytosol. J. Exp. Med. 1998;187:37–48. doi: 10.1084/jem.187.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luckey CJ, King GM, Marto JA, Venketeswaran S, Maier BF, Crotzer VL, Colella TA, Shabanowitz J, Hunt DF, Engelhard VH. Proteasomes can either generate or destroy MHC class I epitopes: evidence for nonproteasomal epitope generation in the cytosol. J. Immunol. 1998;161:112–121. [PubMed] [Google Scholar]
- 54.Pinet VM, Long EO. Peptide loading onto recycling HLA-DR molecules occurs in early endosomes. Eur. J. Immunol. 1998;28:799–804. doi: 10.1002/(SICI)1521-4141(199803)28:03<799::AID-IMMU799>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 55.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J. Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 56.Fernandes DM, Vidard L, Rock KL. Characterization of MHC class II-presented peptides generated from an antigen targeted to different endocytic compartments. Eur. J. Immunol. 2000;30:2333–2343. doi: 10.1002/1521-4141(2000)30:8<2333::AID-IMMU2333>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 57.Yasumoto K, Watabe H, Valencia JC, Kushimoto T, Kobayashi T, Appella E, Hearing VJ. Epitope mapping of the melanosomal matrix protein gp100 (PMEL17): rapid processing in the endoplasmic reticulum and glycosylation in the early Golgi compartment. J. Biol. Chem. 2004;279:28330–28338. doi: 10.1074/jbc.M401269200. [DOI] [PubMed] [Google Scholar]
- 58.Dugast M, Toussaint H, Dousset C, Benaroch P. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J. Biol. Chem. 2005;280:19656–19664. doi: 10.1074/jbc.M501357200. [DOI] [PubMed] [Google Scholar]
- 59.McCormick PJ, Martina JA, Bonifacino JS. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc Natl Acad Sci U S A. 2005;102:7910–7915. doi: 10.1073/pnas.0502206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janvier K, Bonifacino JS. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol. Biol. Cell. 2005;16:4231–4242. doi: 10.1091/mbc.E05-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell. 2007;18:1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonifaz LC, Arzate S, Moreno J. Endogenous and exogenous forms of the same antigen are processed from different pools to bind MHC class II molecules in endocytic compartments. Eur. J. Immunol. 1999;29:119–131. doi: 10.1002/(SICI)1521-4141(199901)29:01<119::AID-IMMU119>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 63.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 64.Jaraquemada D, Marti M, Long EO. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II- restricted T cells. J. Exp. Med. 1990;172:947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malnati MS, Ceman S, Weston M, DeMars R, Long EO. Presentation of cytosolic antigen by HLA-DR requires a function encoded in the class II region of the MHC. J. Immunol. 1993;151:6751–6756. [PubMed] [Google Scholar]
- 66.Lepage S, Lapointe R. Melanosomal targeting sequences from gp100 are essential for MHC class II-restricted endogenous epitope presentation and mobilization to endosomal compartments. Cancer Res. 2006;66:2423–2432. doi: 10.1158/0008-5472.CAN-05-2516. [DOI] [PubMed] [Google Scholar]
- 67.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Bioch. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 68.Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, Bonifacino JS, Marks MS, Raposo G. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SR, Marks MS, Raposo G, Dell'Angelica EC. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Setty SR, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, Dell'Angelica EC, Raposo G, Marks MS. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Urabe K, Aroca P, Hearing VJ. From gene to protein: determination of melanin synthesis. Pigment Cell Res. 1993;6:186–192. doi: 10.1111/j.1600-0749.1993.tb00601.x. [DOI] [PubMed] [Google Scholar]


