Abstract
Acute liver failure (ALF) is a serious, often fatal condition. Up to half of pediatric ALF (PALF) patients do not survive without liver transplantation; however, early identification of those least likely to survive spontaneously remains difficult. Clinical experience suggests that recovery from ALF depends on the ability of the liver to regenerate. Based on this, we hypothesized that bio-markers of hepatic regeneration could have utility as predictors of recovery from PALF. In the studies reported here, we used comprehensive amino acid analysis to search for novel metabolomic markers of liver regeneration in mice subjected to partial hepatectomy. This analysis identified α-NH2-adipic acid and α-NH2-butyric acid as significantly increased in liver and plasma samples from mice subjected to partial hepatectomy compared to controls. Next, we tested whether serum levels of these markers were associated with clinical outcomes in PALF patients. This examination, performed on the initially collected serum samples from 40 randomly selected patients enrolled in the PALF Study Group, showed increased α-NH2-butyric-acid (Aab) and Aab:leucine (Aab:Leu) ratio in patients who survived without transplantation compared to those who were transplanted or died. These data indicate that Aab and the Aab:Leu ratio may predict clinical outcomes in PALF.
Keywords: liver, acute liver failure, biomarkers, animal, model
Introduction
Acute liver failure (ALF) is a severe, frequently fatal disorder in which previously healthy individuals present with significant acute liver injury and dysfunction characterized by varying degrees of hypertransaminasemia, coagulopathy, and encephalopathy (1;2). Clinical management of ALF is primarily supportive until recovery or death occurs or until orthotopic liver transplantation is performed. Approximately half of all adult (1) and pediatric (2) ALF patients do not recover without liver transplantation. However, early identification of those least likely to survive spontaneously remains extraordinarily challenging. As liver transplant list waiting times increase and suitable donor livers remain in short supply, timely recognition of ALF patients most likely to require transplantation in order to survive has become increasingly important (3) Currently, adult ALF patients are generally considered for liver transplantation based on King's College Hospital (KCH) Criteria (4). Such prognostication is based on etiology, age, plasma creatinine, and degrees of encephalopathy, acidosis, and coagulopathy. Various other predictive strategies have also been examined, including the Clichy criteria (5), serum phosphate (6;7), the MELD (Model for End-Stage Liver Disease) score (8), the APACHE II measurements (9;10), coagulation factors V and VII (11;12), histological evidence of liver regeneration on biopsy (13;14), hepatic volume on CT scan (15), serum α-fetoprotein (16–18), and serum actin-free Gc-globulin (19). Each of these models has significant limitations, and improved strategies for predicting outcomes in ALF continue to be sought (20). In addition, a pediatric ALF (PALF) risk-stratifying algorithm based on peak prothrombin time (PT), bilirubin, and ammonia has been reported (21). Nevertheless, the clinical value of KCH Criteria and each of these other models in predicting clinical outcomes in pediatric patients with ALF remains untested (22;23).
Spontaneous recovery from ALF depends, in part, on the ability of the liver to regenerate following acute injury. Furthermore, low rates of spontaneous recovery from ALF in adult patients with idiopathic and drug-induced liver failure is thought to result from a low capacity for such regeneration (17). Therefore, we hypothesized that the presence of serum bio-markers of liver regeneration detectable at the time of clinical presentation might distinguish PALF patients most likely to survive without liver transplantation from those whose native livers are not recovering. In the experiments reported here, we performed comprehensive amino acid analysis on liver and corresponding plasma samples from mice subjected to partial hepatectomy (24) to identify novel metabolomic bio-markers of liver regeneration. Next, we tested whether levels of these markers determined on archived samples of serum from a randomly selected subset of patients enrolled in the NIH-sponsored PALF Study Group were predictive of clinical outcome.
Materials and Methods
Animal Husbandry and Surgery
8–12 week old C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME), maintained on 12 h dark-light cycles and standard mouse chow, were subjected to two-thirds partial hepatectomy, allowed to recover, and then sacrificed for plasma and tissue harvest as previously described (25–27). Three to six animals were examined at each time point. These experiments were approved by the Animal Studies Committee of Washington University and conducted in accordance with institutional guidelines and the criteria outlined in the “Guide for Care and Use of Laboratory Animals” (NIH publication 86-23).
Patient Sample Analysis and Study Design
The NIH-sponsored PALF Study Group is collecting and maintaining a database of clinical information and a repository of serially collected serum samples on consenting children with PALF. At the time samples were drawn for this study 556 patients were enrolled in the study, including 312 who survived at least 21 days without liver transplantation and 244 who did not. For this case-control study, we obtained from the PALF study group an aliquot of the initially collected serum sample and corresponding clinical data from 20 randomly selected patients in each of 2 outcomes groups: (a) survival without transplantation, and (b) either transplantation or death. The number of samples analyzed was chosen based on a power calculation using plasma amino acid analysis data obtained during standard clinical management of 10 PALF patients cared for at St. Louis Children’s Hospital from 2002–2006, the results of which indicated that 20 patients in each outcomes group had at least 80% power to detect significant differences (p<0.05) between groups in levels of markers identified as associated with hepatic regeneration in the mouse model. Random sample selection was conducted by the PALF Study Group Data Coordinating Center, and serum amino acid analysis was performed without knowledge of outcome group. Patient demographic and clinical data, including age, gender, initial encephalopathy grade, etiology, and number of positive KCH Criteria at the time of presentation were compared between patient outcomes groups. These experiments were conducted in accordance with institutional guidelines and those set forth in the 1975 Declaration of Helsinki, and were approved by the Human Studies Institutional Review Board of Washington University.
Amino acid analysis
Amino acid analyses on mouse liver and plasma and human serum was performed by the St. Louis Children’s Hospital Metabolic Genetics Laboratory. Whole tissue lysates of mouse liver were prepared at a density of 100 mg/mL as previously described (25). Blood and tissue samples were deproteinated using 0.7% sulfosalicylic acid. Free amino acids were quantified by cation exchange chromatography using a LiCl step gradient and s-aminoethylcysteine as internal standard on a Beckman 7300 (Beckman-Coulter: Brea, CA). Amino acid-ninhydrin conjugates were identified by retention time and quantified using integrated peak areas. Imprecision of measurement was 5–7% across a concentration range of 4–650 µmol/L. The limit of detection (concentration distinguishable from zero) for each metabolite was 1 µmol/L.
Statistical Analysis
SigmaStat (SPSS, Chicago, IL), SAS (SAS Institute Inc., Cary, NC), and Stata (StataCorp LP, College Station TX) were used for all analyses. Data are reported as mean ± standard error. For evaluating the amino acid analysis data obtained on mouse liver and plasma, Student’s t test was used to compare detectable metabolite levels between un-operated, sham operated, and regenerating livers and also between corresponding plasma samples (with p<0.05 used as threshold for significance). In cases where a specific metabolite was un-detectable in all replicates from one group, i.e. where a test of normality failed, the nonparametric Wilcoxon Rank Sum test was used to compare these groups (p<0.05). For evaluating human serum, median and range are reported in addition to mean ± standard error and the Wilcoxon Rank Sum test was used to compare levels of metabolites between patients who survived without transplantation and those who either died or were transplanted (p<0.05). Pearson’s chi-square test for association (without continuity correction) or Fisher’s exact test was used to compare categorical patient demographic, clinical, and amino acid data between outcomes and to compare patient outcomes at α-NH2-butyric acid:leucine ratio cutoff points selected to maximize discrimination. Receiver-Operator-Characteristic (ROC) curves for α-NH2-butyric acid:leucine, PT, and bilirubin were generated and areas under the fitted curve compared using a nonparametric test.
Results
Novel Metabolomic Markers of Murine Liver Regeneration
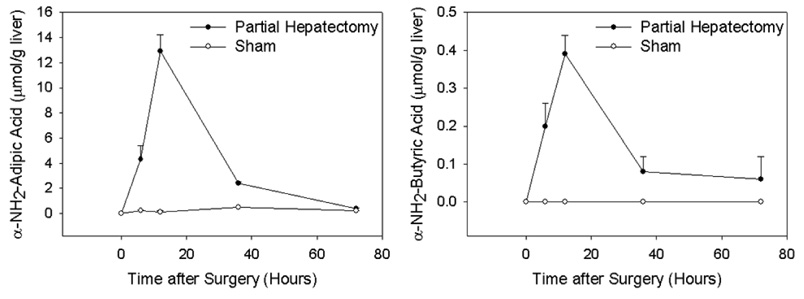
Results of amino acid analysis on liver tissue and plasma recovered from mice subjected to partial hepatectomy were compared to those from un-operated and sham-operated animals. This analysis identified two amino acids that were significantly increased in murine samples of both liver and plasma recovered 6 hours after partial hepatectomy (compared to controls, Table 1): α-NH2-adipic acid (Aaa) and α-NH2-butyric acid (Aab). Subsequent analysis of duplicate liver samples at serial times from 0–72 hours after partial hepatectomy or sham surgery verified the specificity of these changes and demonstrated the kinetics of appearance and disappearance of each of these metabolites in regenerating liver (Figure 1).
Table 1.
Amino Acids Higher in Both Regenerating Mouse Liver and Corresponding Plasma Recovered Six Hours after Partial Hepatectomy
| Amino Acid | Surgery | Liver (µmol/g) | p value | Plasma (µmol/l) | p value |
|---|---|---|---|---|---|
| α-NH2-adipic acid | None | 0.0±0.0 | 0.03b | 2.3±2.3 | 0.04a |
| Sham | 0.2±0.1 | 0.03a | 4.0±2.6 | 0.05a | |
| Partial Hepatectomy | 4.3±1.1 | - | 22.0±5.0 | - | |
| α-NH2-butyric acid | None | 0.0±0.0 | 0.006b | 1.0±1.0 | 0.02a |
| Sham | 0.0±0.0 | 0.03b | 0.0±0.0 | 0.02b | |
| Partial Hepatectomy | 0.2±0.1 | - | 12.3±2.6 | - |
Student’s t test versus Partial Hepatectomy
Wilcoxon Rank Sum test versus Partial Hepatectomy (see Materials and Methods)
Figure 1. Change of α-NH2-Adipic Acid and α-NH2-Butyric Acid in Regenerating Mouse Liver over Time.
The mean and range of 2–4 replicate determinations of α-NH2-adipic acid (left panel) and α-NH2-butyric acid (right panel) levels on liver extracts for each time point and surgical group is shown.
Metabolomic Markers of Liver Regeneration in PALF Patient Serum
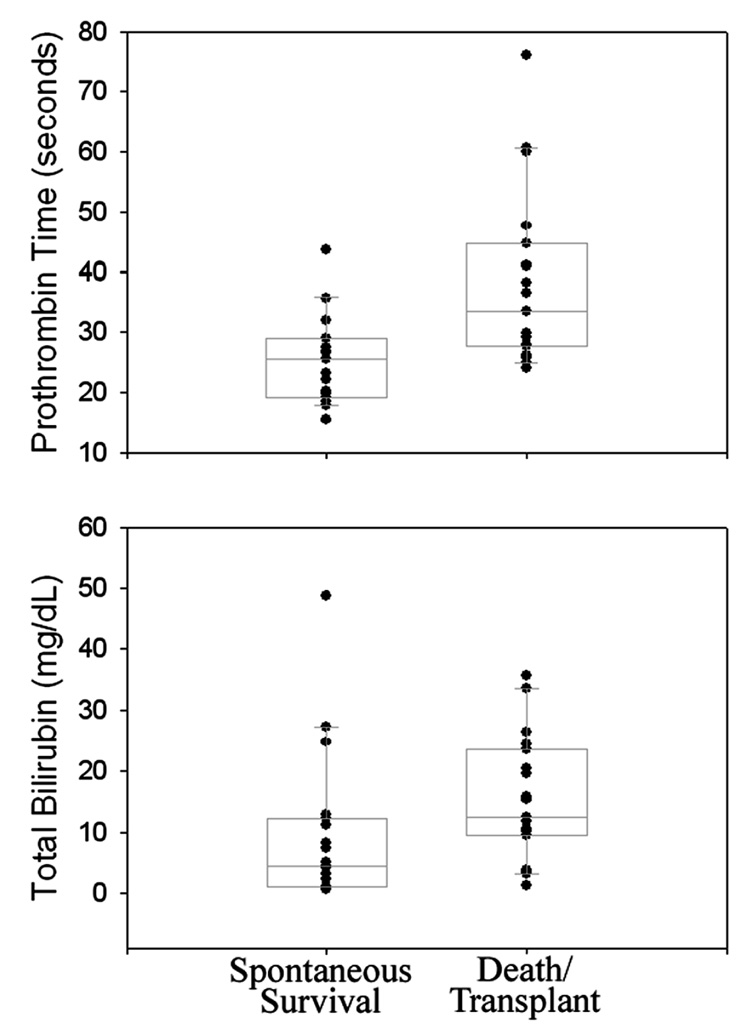
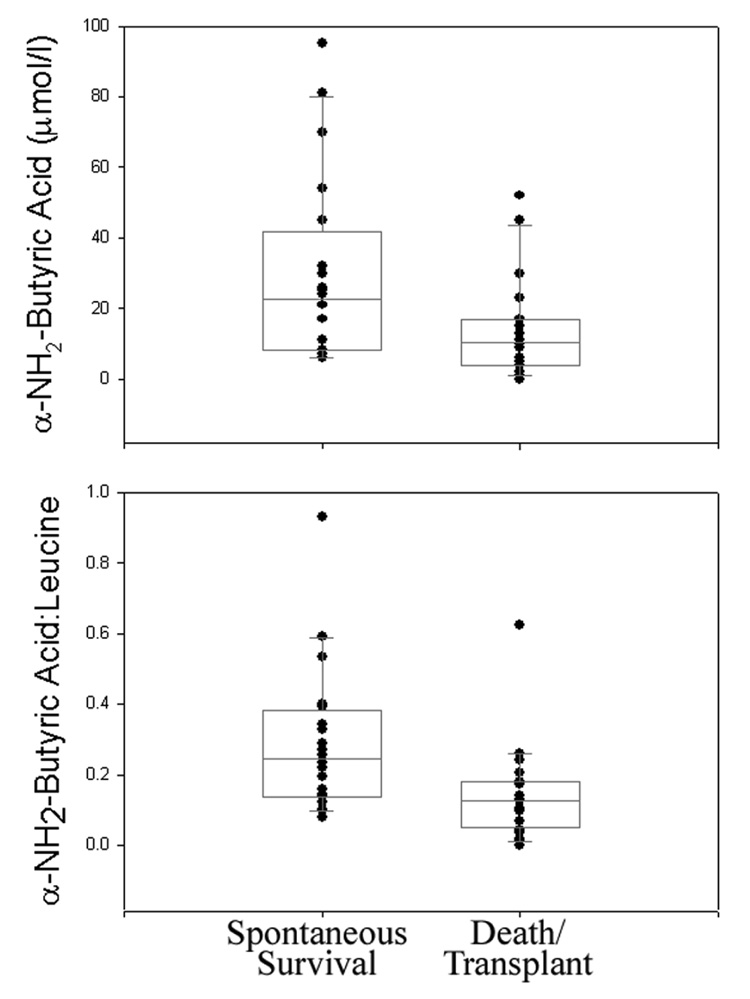
Levels of Aaa and Aab were determined on the initially collected serum sample from 20 randomly selected PALF patients in each of two outcomes groups (survival with native liver versus death/transplant). Demographic and clinical data such as age, gender, initial encephalopathy grade, etiology, and number of positive KCH Criteria at the time of presentation were comparable and did not distinguish between patients in the two groups (Table 2–Table 4). As previously reported (2), both PT and total bilirubin were significantly lower in serum samples from spontaneous survivors compared to those from patients in the death/transplant group (Figure 2 and Table 3). Comparison of serum metabolite levels between these groups showed no significant differences in Aaa levels (p=0.3), which were undetectable in the majority (37/40) of study patients. In contrast, levels of Aab were significantly greater in patients who survived without transplantation (29.2±5.9 µmol/l; median 22.5 µmol/l) compared to those who died or were transplanted (13.9±3.2 µmol/l, median 10.5 µmol/l, p=0.02, Figure 3). Based on these data, taken together with previously published studies proposing that the ratio of serum Aab to leucine (Aab:Leu) may be a specific indicator of alcoholic (28) and other forms (29) of liver injury, Aab:Leu was compared between outcomes groups. The results showed that this ratio was also significantly greater in PALF patients who survived with their native livers (0.29±0.05, median 0.25) compared to those who died or were transplanted (0.14±0.03, median 0.13, p=0.003, Figure 3). No significant difference in serum leucine levels between groups was observed (data not shown).
Table 2.
Summary of Demographic and Clinical Data for Study Patients
| Parameter | Survival with Native Liver (n=20) | Death/Transplant (n=20) | p value |
|---|---|---|---|
| Age | 0.80a | ||
| <2 years | 40% | 30% | |
| 2–10 years | 25% | 30% | |
| >10 years | 35% | 40% | |
| Gender (% female) | 65% | 40% | 0.11a |
| Initial Encephalopathy Graded | 0.58b | ||
| 0–1 | 80% | 65% | |
| 2–4 | 20% | 30% | |
| Diagnosis | 0.54b | ||
| Indeterminate | 45% | 70% | |
| Acetaminophen | 15% | 0% | |
| Other | 40% | 30% | |
| Application of King’s College Hospital Criteria on Enrollment | |||
| Acetaminophene | (n=3) | (n=0) | |
| 0–1 100% | 0–1 -- | ||
| 2–3 0% | 2–3 -- | ||
| 0.25c | |||
| Non-acetaminophen | (n=17) | (n=20) | |
| (# of positive indicators) | 0–1 88% | 0–1 70% | |
| 2 12% | 2 20% | ||
| 3 0% | 3 10% | ||
Pearson chi-square test for association
Pearson chi-square exact test
Fisher’s exact test
Data not available from 1 patient in ‘Death/Transplant’ group
pH>7.3 or any 1 of PT> 100s and Serum Cr > 300 µM with grade III or IV encephalopathy
Table 4.
Detailed Application of King’s College Hospital Criteria to Study Patients
| Survival with Native Liver | Death/Transplant | ||
|---|---|---|---|
| For acetaminophen patients | N=3* | N=0* | |
| 1) pH < 7.3 | 0/0 | - | |
| or | |||
| 2) Any 1 of PT >100 s and serum creatinine >300 umol/L with grade III or IV encephalopathy | 0/3 | - | |
| For nonacetaminophen patients | N=17* | N=20* | |
| 1) | PT >100 s | 0/16 | 0/20 |
| or | |||
| 2) | age <10 | 11/17 | 12/20 |
| Etiology: NANB, drug-induced hepatitis | 1/17 | 1/20 | |
| Jaundice >7days before coma onset | 0/17 | 3/20 | |
| PT>50 s | 0/16 | 2/20 | |
| Total bilirubin > 300 umol/L | 3/17 | 7/18 | |
| No positive indicator | 4 | 3 | |
| Any 1 of 5 indicators** | 11b | 11a | |
| Any 2 of 5 indicators** | 2 | 4c | |
| Any 3 of 5 indicators | 0 | 2 | |
For n/N, n represents the number of study patients positive for the indicator and N represents the number with information available for the indicator.
includes one patient with missing total bilirubin
includes one patient with missing PT
includes one patient with missing total bilirubin
Figure 2. Scatter and Box Plots of PT and Bilirubin in PALF Patient Serum Samples.
Results from PALF patients who spontaneously survived are compared to those from patients who died or were transplanted (10th, 25th, 50th, 75th, and 90th percentile are indicated).
Table 3.
Detailed Demographic and Clinical Data for Study Patients
| Survival with Native Live N=20 (%) | Death/Transplant N=20 (%) | p value | |
|---|---|---|---|
| Outcome | |||
| Death without transplant | 0 (0.0) | 4 (20.0) | |
| Death after transplant | 0 (0.0) | 2 (10.0) | |
| Transplant only | 0 (0.0) | 14 (70.0) | |
| Survival with native liver | 20 (100.0) | 0 (0.0) | |
| Demographics | |||
| Age (year) | |||
| Less than 2 years old | 8 (40.0) | 6 (30.0) | 0.80a |
| 2 to 10 years old | 5 (25.0) | 6 (30.0) | |
| More than 10 years old | 7 (35.0) | 8 (40.0) | |
| Median | 3.6 | 6.1 | 0.99b |
| Min, Max | 0.1, 16.8 | 0.1, 17.0 | |
| Sex | |||
| Male | 7 (35.0) | 12 (60.0) | 0.11a |
| Female | 13 (65.0) | 8 (40.0) | |
| King’s College Criteria parameters | Survival with Native Liver | Death/Transplant | |
| Days from hospitalization to study enrollment | |||
| Median | 1 | 4 | 0.008b |
| Min, Max | 0, 10 | 0, 18 | |
| Days from first symptom to study enrollment | |||
| # missing | 0 | 2 | |
| Median | 6 | 13 | 0.03b |
| Min, Max | 2, 159 | 2, 40 | |
| pH at study admission | |||
| # missing | 11 | 11 | |
| Median | 7.4 | 7.4 | 0.99c |
| Min, Max | 7.2, 7.5 | 7.2, 7.6 | |
| Prothrombin time at study admission (sec) | |||
| # missing | 1 | 0 | |
| Median | 25.9 | 36.3 | 0.004b |
| Min, Max | 15.4, 45.6 | 21.9, 76.0 | |
| Serum creatinine at study admission (mg/dl) | |||
| Median | 0.6 | 0.6 | 0.65b |
| Min, Max | 0.2, 2.5 | 0.1, 2.7 | |
| Total bilirubin at study admission (mg/dl) | |||
| # missing | 0 | 2 | |
| Median | 4.9 | 15.5 | 0.04b |
| Min, Max | 0.6, 48.7 | 1.2, 33.6 | |
| Potassium at study admission (mmol/L) | |||
| # missing | 0 | 2 | |
| Median | 3.8 | 3.8 | 0.56b |
| Min, Max | 2.6, 5.5 | 3.0, 7.8 | |
| Alpha-fetoprotein at study admission | |||
| # missing | 10 | 12 | |
| Median | 164 | 168 | 0.90c |
| Min, Max | 13.0, 228000.0 | 0.8, 11345.0 | |
| Encephalopathy grade at study admission | |||
| # missing | 0 | 1 | |
| 0 | 9 (45.0) | 7 (36.8) | 0.58d |
| I | 7 (35.0) | 6 (31.6) | |
| II | 4 (20.0) | 3 (15.8) | |
| III | 0 (0.0) | 2 (10.5) | |
| IV | 0 (0.0) | 1 (5.3) | |
| Initial diagnosis | 0.47d | ||
| Acetaminophen | 3 (15.0) | 0 (0.0) | |
| Hemophagocytic syndrome | 0 (0.0) | 1 (5.0) | |
| Veno-occlusive disease | 1 (5.0) | 0 (0.0) | |
| Wilson’s disease | 1 (5.0) | 1 (5.0) | |
| Metabolic, other | 2 (10.0) | 3 (15.0) | |
| Hepatitis A | 1 (5.0) | 0 (0.0) | |
| Autoimmune hepatitis | 2 (10.0) | 1 (5.0) | |
| Drug-induced hepatitis | 1 (5.0) | 1 (5.0) | |
| Mushroom toxicity | 1 (5.0) | 0 (0.0) | |
| Indeterminate | 8 (40.0) | 13 (65.0) | |
Pearson chi-square test for association
Wilcoxon rank sum test
Wilcoxon rank sum exact test
Fisher’s exact test
Figure 3. Scatter and Box Plots of α-NH2-Butyric Acid and the α-NH2-Butyric Acid:Leucine Ratio in PALF Patient Serum Samples.
Results from PALF patients who spontaneously survived are compared to those from patients who died or were transplanted (10th, 25th, 50th, 75th, and 90th percentile are indicated).
Prognostic Value of Aab:Leu, PT, and Bilirubin on PALF Outcomes
Analyses of Aab:Leu thresholds selected to maximize accuracy of prediction of spontaneous survival (for Aab:Leu ≥ threshold), death or transplant (for Aab:Leu < threshold), or both showed that 80% (12 of 15) of patients with an Aab:Leu ratio of at least 0.210 survived without transplantation and 68% (17/25) of patients with a ratio less than 0.210 either died or were transplanted (p=0.006, Table 5), for an overall diagnostic accuracy of 72.5% (29/40) at this threshold. This predictive accuracy is better than that of KCH criteria (4) with which only 55% (22/40) of clinical outcomes are correctly predicted when using a cutoff of 3 or more positive KCH indicators (Table 2 and Table 4). However, the predictive accuracy of KCH criteria in this analysis could be confounded, in part, by absence of any patients with PT > 100 seconds (Table 3) which may be the result of differences in PT determination (30) in North America (39/40 study patients including 19/20 spontaneous survivors) versus the United Kingdom (1/40 study patients).
Table 5.
Performance of Aab:Leu for Predicting PALF Outcomes at Various Thresholds
| Aab:Leu Threshold | Spontaneous Survival Predictive Valuea | Death/Transplant Predictive Valueb | Sensitivityc | Specificityd |
|---|---|---|---|---|
| 0.05 | 20/35 (57%) | 5/5 (100%) | 20/20 (100%) | 5/20 (25%) |
| 0.1 | 18/31 (58%) | 7/9 (78%) | 18/20 (90%) | 7/20 (35%) |
| 0.15 | 14/21 (67%) | 13/19 (68%) | 14/20 (70%) | 13/20 (65%) |
| 0.19 | 13/17 (76%) | 16/23 (70%) | 13/20 (65%) | 16/20 (80%) |
| 0.2 | 12/16 (75%) | 16/24 (67%) | 12/20 (60%) | 16/20 (80%) |
| 0.21 | 12/15 (80%) | 17/25 (68%) | 12/20 (60%) | 17/20 (85%) |
| 0.25 | 10/12 (83%) | 18/28 (64%) | 10/20 (50%) | 18/20 (90%) |
| 0.30 | 7/8 (88%) | 19/32 (59%) | 7/20 (35%) | 19/20 (95%) |
number of spontaneous survivors/number with Aab:Leu≥threshold
number dead or transplanted/number with Aab:Leu<threshold
number with Aab:Leu≥threshold/20 spontaneous survivors
number with Aab:Leu<threshold/20 dead or transplanted
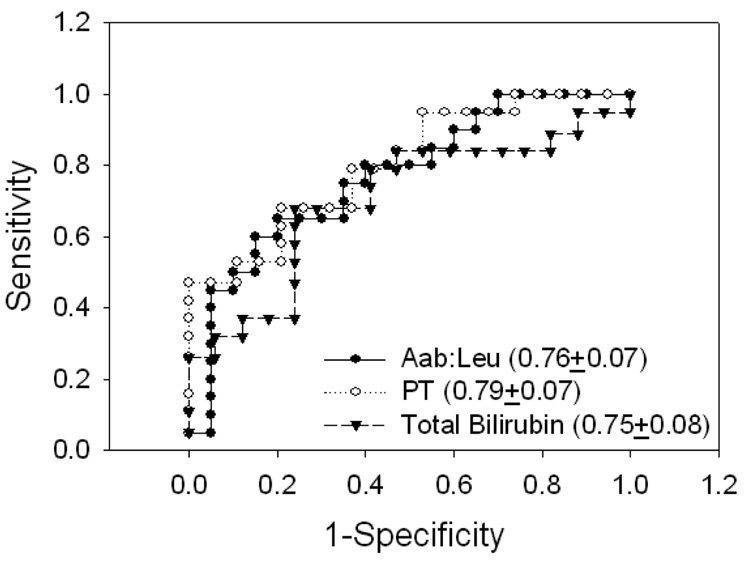
As noted above, PT and bilirubin were significantly lower in spontaneous survivors of PALF compared to those who died or underwent liver transplantation (Figure 2, Table 3 and (2)). Comparison of ROC analysis areas under the curve for Aab:Leu, PT, and bilirubin indicated no significant differences (Figure 4). Further analysis showed that combinations of Aab:Leu and PT thresholds that maximize the positive (PPV) and negative (NPV) predictive value of each individual parameter (Table 5 and Table 6) can result in overall improvement in outcomes prognostication compared to that provided by either parameter alone (Illustrated in Table 7).
Figure 4. ROC Analysis of α-NH2-Butyric Acid:Leucine (Aab:Leu), Prothrombin Time (PT), and Total Bilirubin on PALF Outcomes.
Areas under the fitted curve (AUC) and estimated standard error are shown.
Table 6.
Performance of PT for Predicting PALF Outcomes at Various Thresholds
| PT Threshold (sec) | Spontaneous Survival Predictive Valuea | Death/Transplant Predictive Valueb | Sensitivityc | Specificityd |
|---|---|---|---|---|
| 24 | 9/9 (100%) | 19/29 (66%) | 9/19 (47%) | 19/19 (100%) |
| 29 | 15/22 (68%) | 12/16 (75%) | 15/19 (79%) | 12/19 (63%) |
| 34 | 16/26 (62%) | 9/12 (75%) | 16/19 (84%) | 9/19 (47%) |
| 39 | 18/30 (60%) | 7/8 (88%) | 18/19 (95%) | 7/19 (37%) |
| 44 | 19/33 (58%) | 5/5 (100%) | 19/19 (100%) | 5/19 (26%) |
number of spontaneous survivors/number with PT≤threshold
number dead or transplanted/number with PT>threshold
number with PT<threshold/20 spontaneous survivors
number with Pt>threshold/20 dead or transplanted
Table 7.
Performance of Aab:Leu and PT for Predicting PALF Outcomes
| PALF Outcome | Threshold Criteria | Predictive Accuracy |
|---|---|---|
| Spontaneous Survival | Aab:Leu>0.25 | 10/12 (83%) |
| PT<24 sec | 9/9 (100%) | |
| Aab:Leu>0.25 OR PT<24 sec | 13/15 (87%) | |
| Death/Transplant | Aab:Leu<0.05 | 5/5 (100%) |
| PT>39 sec | 7/8 (88%) | |
| Aab:Leu<0.05 OR PT>39 sec | 11/12 (92%) |
Discussion
In the studies reported here, comprehensive amino acid analysis was used to identify Aaa and Aab as novel metabolomic markers of liver regeneration in the murine partial hepatectomy model. The mechanisms that account for increased hepatic and circulating levels of these amino acids are speculative and remain unknown. Aaa is a metabolic breakdown product of lysine (31), and it is intriguing that our analysis also showed increased lysine levels in regenerating mouse liver (data not shown). Aab is generated during catabolism of methionine and threonine (31). Together, these data suggest that precise regulation of lysine, methionine, or threonine metabolism may be important for normal hepatic regeneration.
Based on the hypothesis that recovery from ALF is dependent on regeneration of the injured liver, the metabolomic markers of liver regeneration identified in the mouse model were tested for their ability to predict clinical outcomes in PALF. The results showed that levels of Aab but not those of Aaa are significantly correlated with such outcomes. The explanation for the discordance between Aaa and Aab as markers of liver regeneration versus predictors of PALF outcomes is unknown, and may reflect differences in mouse versus human amino acid metabolism. Alternatively, these data raise the intriguing possibility that Aaa and Aab may correlate with distinct stages of the hepatic regenerative response, a consideration supported by data reported here showing more rapid and complete disappearance of Aaa than Aab from regenerating mouse liver 72 hours after partial hepatectomy (Figure 1). These observations also indicate that further investigation for additional (e.g. non-amino acid) metabolomic markers of hepatic regeneration may lead to the identification of other novel predictors of ALF outcomes.
Because the analysis of PALF serum reported here was performed on the initially-collected sample after enrollment into the Study Group, our data indicate that determination of serum Aab and the Aab:Leu ratio at the time of clinical presentation may be useful as an early predictor of outcome in PALF. The methodology used here for serum amino acid analysis can be completed in as little as several hours, and newer techniques utilizing mass spectrometry may permit similar analyses in less than an hour (32) thus allowing this test to be available for clinical decision making. Further investigations are warranted to verify the findings reported here, to characterize the temporal pattern of change in serum Aab:Leu in patients with ALF, and to prospectively evaluate the utility of this marker for predicting outcomes in pediatric and adult ALF in comparison to and in concert with other algorithms. For example, the data reported here show that thresholds of Aab:Leu and PT can be identified with which PALF outcomes can be predicted with greater reliability than is possible using either parameter alone (Table 5–Table 7). Future studies should address whether the predictive value of Aab and Aab:Leu varies with clinical or demographic variables. While plasma Aab:Leu has been reported to be elevated in alcoholic (28) and various metabolic forms (29) of liver injury, the utility of this marker for predicting outcomes in ALF caused by infectious, inflammatory, ischemic, and other toxic and metabolic causes of liver injury is unknown and should be determined. Finally, comparison of initial Aab:Leu levels between ALF patients who die prior to liver transplantation, those who are transplanted, and spontaneous survivors, and examination of levels in patients transplanted earlier with live donor-grafts versus those transplanted later with cadaveric grafts should be performed. Such analyses could lead to strategies for identification of those ALF patients who undergo liver transplantation but might have recovered spontaneously if given the opportunity. If the results of future studies confirm the observations reported here, serum amino acid analyses will become an important diagnostic tool with which to evaluate and direct clinical management of ALF patients.
Acknowledgements
These studies were supported in part by grants from NIH to DAR (DK068219) and to the PALF study group (5U01DK072146-03). YPT was supported by Institutional Training Grant T32-HD07409. The authors thank the PALF Study Group for its support of these studies including access to clinical registry data and biological samples. PALF study group member institutions (and principal and co-investigators) include: Baylor College of Medicine (Saul Karpen, MD-PhD), Birmingham Children’s Hospital, (UK, Dominic Dell Olio MD), Children’s Hospital of Pittsburgh (Robert Squires MD, Ben Schneider MD), Columbia Presbyterian (Steven Lobritto MD), Harvard Medical School (Maureen Jonas MD), Hospital for Sick Children (Toronto, Vick Ng MD), Indiana University Riley Hospital (Girish Subbarao MD), Johns Hopkins University (Kathleen Schwartz MD), King’s College Hospital (UK, Anil Dhawan MD), Mt. Sinai School of Medicine (Sukru Emre MD), Northwestern University (Estella Alonso MD), University of California, San Francisco (Philip Rosenthal MD), University of Cincinnati (John Bucuvalas MD, Nada Yazigi MD), University of Colorado (Michael Narkewicz MD), University of Michigan (M. James Lopez MD-PhD), University of Pennsylvania (Liz Rand MD), University of Texas Southwestern (Norberto Rodriguez Baez MD), University of Washington (Seattle, Karen Murray MD), Washington University (St. Louis, David Rudnick MD-PhD, Ross Shepherd MD). The authors are also grateful for support from National Institutes of Health (Patricia R. Robuck PhD-MPH, Director Clinical Trials Program, DDDN-NIDDK) and for assistance from members of the Data Coordinating Center (directed by Steven Belle PhD-MScHyg).
Reference List
- 1.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23(3):217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 2.Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tector AJ, Mangus RS, Chestovich P, Vianna R, Fridell JA, Milgrom ML, et al. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg. 2006;244(3):439–450. doi: 10.1097/01.sla.0000234896.18207.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97(2):439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 5.Bernuau J, Goudeau A, Poynard T, Dubois F, Lesage G, Yvonnet B, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986;6(4):648–651. doi: 10.1002/hep.1840060417. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology. 2002;36(3):659–665. doi: 10.1053/jhep.2002.35069. [DOI] [PubMed] [Google Scholar]
- 7.Macquillan GC, Seyam MS, Nightingale P, Neuberger JM, Murphy N. Blood lactate but not serum phosphate levels can predict patient outcome in fulminant hepatic failure. Liver Transpl. 2005;11(9):1073–1079. doi: 10.1002/lt.20427. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology. 2007;45(3):789–796. doi: 10.1002/hep.21503. [DOI] [PubMed] [Google Scholar]
- 9.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell I, Bihari D, Chang R, Wendon J, Williams R. Earlier identification of patients at risk from acetaminophen-induced acute liver failure. Crit Care Med. 1998;26(2):279–284. doi: 10.1097/00003246-199802000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Izumi S, Langley PG, Wendon J, Ellis AJ, Pernambuco RB, Hughes RD, et al. Coagulation factor V levels as a prognostic indicator in fulminant hepatic failure. Hepatology. 1996;23(6):1507–1511. doi: 10.1002/hep.510230630. [DOI] [PubMed] [Google Scholar]
- 12.Dymock IW, Tucker JS, Woolf IL, Poller L, Thomson JM. Coagulation studies as a prognostic index in acute liver failure. Br J Haematol. 1975;29(3):385–395. doi: 10.1111/j.1365-2141.1975.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 13.Scotto J, Opolon P, Eteve J, Vergoz D, Thomas M, Caroli J. Liver biopsy and prognosis in acute liver failure. Gut. 1973;14(12):927–933. doi: 10.1136/gut.14.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donaldson BW, Gopinath R, Wanless IR, Phillips MJ, Cameron R, Roberts EA, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology. 1993;18(6):1370–1376. [PubMed] [Google Scholar]
- 15.Shakil AO, Dvorchik I, Fung JJ, Rakela J. Liver transplantation for acute liver failure: outcome analysis. J Viral Hepat. 1997;4 Suppl 1:107–110. doi: 10.1111/j.1365-2893.1997.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 2005;41(1):26–31. doi: 10.1002/hep.20511. [DOI] [PubMed] [Google Scholar]
- 17.Blei AT. Selection for acute liver failure: have we got it right? Liver Transpl. 2005;11(11 Suppl 2):S30–S34. doi: 10.1002/lt.20595. [DOI] [PubMed] [Google Scholar]
- 18.Schiodt FV, Ostapowicz G, Murray N, Satyanarana R, Zaman A, Munoz S, et al. Alpha-fetoprotein and prognosis in acute liver failure. Liver Transpl. 2006;12(12):1776–1781. doi: 10.1002/lt.20886. [DOI] [PubMed] [Google Scholar]
- 19.Schiodt FV, Bangert K, Shakil AO, McCashland T, Murray N, Hay JE, et al. Predictive value of actin-free Gc-globulin in acute liver failure. Liver Transpl. 2007;13(9):1324–1329. doi: 10.1002/lt.21236. [DOI] [PubMed] [Google Scholar]
- 20.Macquillan G. Predicting outcome in acute liver failure: Are we there yet? Liver Transpl. 2007;13(9):1209–1211. doi: 10.1002/lt.21271. [DOI] [PubMed] [Google Scholar]
- 21.Liu E, MacKenzie T, Dobyns EL, Parikh CR, Karrer FM, Narkewicz MR, et al. Characterization of acute liver failure and development of a continuous risk of death staging system in children. J Hepatol. 2006;44(1):134–141. doi: 10.1016/j.jhep.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Dhawan A, Cheeseman P, Mieli-Vergani G. Approaches to acute liver failure in children. Pediatr Transplant. 2004;8(6):584–588. doi: 10.1111/j.1399-3046.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 23.Bucuvalas J, Yazigi N, Squires RH., Jr Acute liver failure in children. Clin Liver Dis. 2006;10(1):149–168. doi: 10.1016/j.cld.2005.10.006. vii. [DOI] [PubMed] [Google Scholar]
- 24.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 25.Rudnick DA, Perlmutter DH, Muglia LJ. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8885–8890. doi: 10.1073/pnas.151217998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 2004;40(6):1322–1332. doi: 10.1002/hep.20462. [DOI] [PubMed] [Google Scholar]
- 27.Liao Y, Shikapwashya ON, Shteyer E, Dieckgraefe BK, Hruz PW, Rudnick DA. Delayed hepatocellular mitotic progression and impaired liver regeneration in early growth response-1-deficient mice. J Biol Chem. 2004;279(41):43107–43116. doi: 10.1074/jbc.M407969200. [DOI] [PubMed] [Google Scholar]
- 28.Shaw S, Stimmel B, Lieber CS. Plasma alpha amino-n-butyric acid to leucine ratio: an empirical biochemical marker of alcoholism. Science. 1976;194(4269):1057–1058. doi: 10.1126/science.824734. [DOI] [PubMed] [Google Scholar]
- 29.Yudkoff M, Blazer-Yost B, Cohn R, Segal S. On the clinical significance of the plasma alpha-amino-n-butyric acid:leucine ratio. Am J Clin Nutr. 1979;32(2):282–285. doi: 10.1093/ajcn/32.2.282. [DOI] [PubMed] [Google Scholar]
- 30.Loeliger EA, Poller L, Samama M, Thomson JM, Van den Besselaar AM, Vermylen J, et al. Questions and answers on prothrombin time standardisation in oral anticoagulant control. Thromb Haemost. 1985;54(2):515–517. [PubMed] [Google Scholar]
- 31.Newsholme EA, Leech AR. Biochemistry for the Medical Sciences. New York: John Wiley & Sons; 1983. Amino Acid Metabolism; pp. 382–441. [Google Scholar]
- 32.Piraud M, Vianey-Saban C, Petritis K, Elfakir C, Steghens JP, Morla A, et al. ESI-MS/MS analysis of underivatised amino acids: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun Mass Spectrom. 2003;17(12):1297–1311. doi: 10.1002/rcm.1054. [DOI] [PubMed] [Google Scholar]