Abstract
Background
Haptoglobin (Hp) genotype determines the efficiency of hemoglobin clearance after malaria-induced hemolysis and alters antioxidant and immune functions. The Hp2 allele is thought to have spread under strong selection pressure, but it is unclear whether this is due to protection from malaria or other diseases.
Methods
We monitored the incidence of febrile malaria and other childhood illnesses with regard to Hp genotype in a prospective cohort of 312 Kenyan children during 558.3 child-years of follow-up. We also conducted 7 cross-sectional surveys to determine the prevalence of Plasmodium falciparum parasitemia.
Results
The Hp2/2 genotype was associated with a 30% reduction in clinical malarial episodes (adjusted incidence rate ratio, 0.67; P = .008 for Hp2/2 vs. Hp1/1 and Hp2/1 combined). Protection increased with age; there was no protection in the first 2 years of life, 30% protection at ≥2 years of age, and 50% protection from 4-10 years of age. Children with the Hp1/1 genotype had a significantly lower rate of nonmalarial fever (P = .001).
Conclusions
Balancing selection pressures may have influenced the spread of the Hp gene. Our observations suggest that the Hp2 allele may have spread as a result of protection from malaria, and the Hp1 allele may be sustained by protection from other infections.
Keywords: Haptoglobin, malaria, infection, children
INTRODUCTION
Malaria is an important cause of childhood mortality [1] and has exerted selective pressure on the human genome. Haptoglobin (Hp) is an acute-phase plasma protein that binds rapidly and irreversibly with free hemoglobin (Hb) after the occurrence of malaria-induced hemolysis. Hp thus protects against Hb-iron-mediated oxidant damage [2] and modulates immune function and host response to inflammation. Binding of Hp-Hb complexes to CD163 macrophage receptors after hemolysis induces cytokine production [3]. Interestingly, Hp also alters leukocyte trafficking [4] and host response to bacterial lipopolysaccharides [5]. The functions of Hp are genotype dependent [6]. Two co-dominant alleles (Hp1 and Hp2) on chromosome 16q22 determine 3 phenotypes (the homodimer Hp1-1, the linear polymer Hp2-1, and the large circular polymer Hp2-2). The Hp2 allele is estimated to have arisen in India from an intragenic duplication of the Hp1 allele [7] and has since spread under strong selective pressure. Although the incidence and clinical expression of a number of diseases appear to be influenced by Hp type (reviewed in [6, 8]), it is unclear what selection pressures may have accounted for the spread of the Hp2 allele.
Clear functional differences exist between the Hp genotypes. Homozygosity for the Hp2 allele is associated with increased Hb-iron-induced oxidant damage [9], redox-active iron levels [10], and Hb-derived oxidation of lipids and proteins [11]. These differences are reflected in vivo. Serum vitamin C levels and serum ferroxidase levels in smokers are significantly reduced in individuals with the Hp2-2 phenotype (49.9 μmol/L and 52.9 U/L, respectively), but do not differ in individuals with the Hp1-1 (61.5 μmol/L and 63.4 U/L, respectively) or Hp2-1 (63.7 μmol/L and 65.2 U/L, respectively) phenotype [12, 13]. Oxidative damage to the erythrocyte membrane accelerates phagocytic removal of RBCs via aggregation of band 3 and deposition of immune complexes [14]. Interestingly, evidence suggests that this is a common mechanism for accelerated phagocytic removal of ring-parasitized erythrocytes in G6PD deficiency [15], sickle trait, and β-thalassaemia [16]. Moreover, Hp genotype appears to determine differences in immune function after hemolysis by altering the macrophage cytokine response [3] and modulating Th1 and Th2 balance [17].
Whether the spread of the Hp2 allele is due to protection from malaria or other diseases is controversial. Increased parasite densities were found in Hp knock-out mice [18], and in vitro Hp was toxic to malaria parasites (in order of toxicity, Hp1-1, followed by Hp2-2, followed by Hp2-1) [19]. On the basis of Hp phenotyping, a number of case-control and cross-sectional studies have suggested that Hp2 homozygosity has a protective effect and that there is increased susceptibility among individuals with the Hp1-1 phenotype, especially in relation to severe malaria and placental infection [20-24]. However, 2 additional studies did not find an association with Hp genotype [25, 26]. Previous studies have been cross-sectional or case-control in design. Here, we report on the effects of Hp genotype in a prospective cohort of children among whom we have measured the incidence of clinical malaria and other febrile diseases.
PARTICIPANTS, MATERIALS, AND METHODS
Study Design
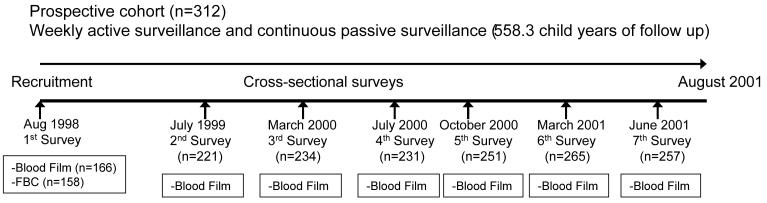
The study involved a cohort of 312 children (age, 0-10 years) living in the Ngerenya area of Kilifi District on the coast of Kenya and was conducted from August 1998 to August 2001. The study was nested within a larger study described in detail elsewhere [27, 28]. Children were mainly Giriama, a subgroup of the Mijikenda ethnic group. Figure 1 provides an overview of the study design. Children were recruited at the start of the study or at birth, if they were born into study households during the study period. The cohort was monitored by weekly active surveillance for clinical events, with a focus on malaria, and mothers could also bring their children to the dedicated research outpatient clinic at any time if the child was unwell. At each clinic visit, a clinician obtained a detailed clinical history, conducted an examination, and ascribed a primary and secondary diagnosis. During the study period, 7 cross-sectional surveys were conducted to assess the prevalence of Plasmodium falciparum parasitemia (figure 1). Children exited the study if informed consent was withdrawn or if they moved out of the study area for >2 months.
Figure 1.
Construction of a study of the incidence of Plasmodium falciparum malaria among children on the coast of Kenya.
Clinical Definitions
For the purposes of this study, clinical malaria was defined according to 2 alternative definitions. “Malaria definition 1” was indicated either by a measured fever (axillary temperature >37.5°C) at the time that the slides were prepared or by a clinical history of fever within the preceding 48 h, in conjunction with a slide positive for blood-stage asexual P. falciparum parasites at any density. “Malaria definition 2” was based on a measured fever in conjunction with the presence of P. falciparum parasites at any density (for children age <1 year) and at a density of >2,500 parasites/μL (for children age ≥1 year). Malaria definition 2, derived by means of multiple logistic regression methods, is associated with both a sensitivity and specificity of >80% for the diagnosis of clinical malaria in this area [28]. Asymptomatic malaria parasitemia was defined during cross-sectional surveys as a slide positive for P. falciparum in the absence of fever or other symptoms of clinical illness.
Nonmalarial fever was defined as a fever in a child whose blood slide was negative for P. falciparum and who had not received treatment with an antimalarial drug in the preceding 21 days: this definition included a range of nonmalarial febrile illnesses under other diagnostic categories. Upper respiratory tract infection was diagnosed in children whose principal symptoms were coryza or pharyngitis. Lower respiratory tract infection was diagnosed in children with cough and fast breathing and/or chest indrawing, in accordance with World Health Organisation clinical criteria [29]. Gastroenteritis was defined as diarrhoea (> 3 watery stools/day), with or without vomiting. Fever of unknown cause was a diagnosis of exclusion and was only assigned if no etiology could be found for the fever. Helminth infection was diagnosed in children who presented to the clinic with a history of passing worms of any species, and skin infection was diagnosed in children who presented with a variety of dermatological conditions, including scabies, boils, and impetigo.
Laboratory Procedures
Blood films were prepared with Giemsa stain and examined for malaria parasites according to standard methods. Parasite densities (i.e., the number of parasites per microliter of whole blood) were recorded as a ratio of parasites to WBCs or, for “heavier” infections, as a ratio of parasites to RBCs. Parasite densities were calculated from complete hematological assessments, if available; if not available, parasite densities were calculated on the assumption of a WBC count of 8 × 103 cells/ μL or a RBC count of 5 × 106 cells/ μL. Hp genotype was determined by allele-specific PCR, as described previously [30]. Hb types (HbA and HbS) were characterized by electrophoresis using cellulose acetate gels (Helena) and the common African 3.7-kb α-globin deletion was typed by PCR reaction as described elsewhere [31].
Statistical Analysis
Analyses were conducted using Stata software, version 8.0 (Stata Corp.). In all of our regression models, we accounted for the potential clustering of events within individual study children by using the sandwich estimator, which inflates CIs and adjusts P values upwards [32]. Variables that were not normally distributed were log-transformed before analysis. Multivariate regression models included the explanatory variables of age (as a continuous variable), sex, ethnic group, and season (defined in 3-month blocks); in addition, sickle cell trait and α+-thalassaemia genotypes were included in multivariate analyses of malaria or Hb levels.
We derived incidence rate ratios (IRRs) for malaria and other diseases using univariate and multivariate Poisson regression. Prior data suggested that the Hp2-2 phenotype has unique properties, compared with the Hp1-1 and Hp2-1 phenotypes [12, 30]. This study was designed to test the hypothesis that these functional differences would result in a reduced incidence of clinical malarial episodes in individuals with the Hp2/2 genotype. Thus, for malaria, the primary analysis compared individuals who had the Hp2/2 genotype with those who had the Hp2/1 or Hp1/1 genotype, and a secondary analysis treated each genotype separately. Because we did not have a prior hypothesis regarding the effect of Hp genotype on nonmalarial disease, each genotype was considered separately. Children were considered not to be at risk for malaria (and were omitted from both the numerator and denominator populations) for 21 days after receipt of treatment with an antimalarial drug. ORs for the prevalence of asymptomatic parasitemia over 7 cross-sectional surveys were derived by logistic regression analysis. Log-transformed parasite densities were analysed using linear regression models. The likelihood ratio test was used to assess for interactions between explanatory variables, as appropriate; no significant interactions were found. We investigated the possibility that age may have acted as an effect modifier in the association between malaria and Hp genotype by comparing models that included or excluded interaction terms between Hp genotype and age, using the Wald test. The duration of follow-up for study children was accounted for in all analyses.
Ethical permission for the study was granted by the Kenya Medical Research Institute National Ethical Review Committee, and procedures complied with the Helsinki Declaration of 1975 (revised in 1983). Written informed consent was provided by children's parents or guardians before recruitment.
RESULTS
Characteristics of Study Population
A total of 4437 clinic visits were made by 312 children during 558.3 child-years of follow-up, and 7 cross-sectional surveys of parasite prevalence were conducted during this time (figure 1). Of these children, 87 (27.9%) carried the Hp1/1 genotype, 146 (46.8%) carried the Hp2/1 genotype, and 79 (25.3%) carried the Hp2/2 genotype. Hp genotypes were in Hardy-Weinberg equilibrium, which predicts allele and genotype frequencies within a nonevolving population. No association was found between Hp genotype and either sickle cell trait or α+-thalassaemia genotype. Children with the Hp1/1 genotype were slightly younger than children with the Hp2/1 or Hp2/2 genotype (P = .08); the effect of age was accounted for in all multivariate analyses. Hb levels were measured in 158 children at the start of the study (table 1). Hb levels were lower in children with the Hp1/1 genotype than in others (P = .08, by log likelihood ratio testing), probably because of their younger age. Table 1 summarizes the characteristics of the study population according to Hp genotype.
Table 1.
Characteristics of study population, by haptoglobin genotype.
| Characteristic | Patients with haptoglobin 1-1 |
Patients with haptoglobin 2-1 |
Patients with haptoglobin 2-2 |
|---|---|---|---|
| Sample Size (n = 312) | 87 (27.9) | 146 (46.8) | 79 (25.3) |
| Age, mean months (95% CI) | 34.7 (28.7-40.7) | 44.6 (38.9-50.4) | 43.5 (36.3-50.7) |
| Male sex | 46 (52.9) | 78 (53.4) | 37 (46.8) |
| Ethnic Groupa | |||
| Giriama | 74 (86.0) | 120 (82.8) | 70 (88.6) |
| Chonyi | 7 (8.1) | 13 (9.0) | 7 (8.9) |
| Other Mijikenda | 5 (5.8) | 12 (8.3) | 2 (2.5) |
| Hemoglobin type (n = 296) | |||
| HbAA | 70 (86.4) | 125 (89.3) | 65 (86.7) |
| HbAS | 11 (13.6) | 15 (10.7) | 10 (13.3) |
| α+-Thalassaemia genotype (n = 294) | |||
| Normal (αα/αα) | 24 (28.6) | 43 (31.4) | 23 (31.5) |
| Heterozygote (−α/αα) | 44 (52.4) | 63 (46.0) | 38 (52.1) |
| Homozygote (−α/−α) | 16 (19.0) | 31 (22.6) | 12 (16.4) |
| Prevalence of asymptomatic parasitemiab |
|||
| No. of positive slide results/total no. of slides (%) |
110/440 (25.0) | 181/757 (23.9) | 85/428 (19.9) |
| OR (95% CI) | 1 | 0.95 (0.64-1.4) | 0.62 (0.37, 1.0)c |
| P | .79 | .065 | |
| Hemoglobin level, g/L (95% CI)d | 85.4 (79.9-90.9) | 93.2 (90.3-96.2) | 91.3 (85.8-96.8) |
NOTE. Data are no. (%) of children, unless otherwise indicated.
Two children were missing data on ethnic group.
These data were taken from 7 cross-sectional surveys combined during the study period. The adjusted ORs were calculated by multivariate logistic regression analysis that included the presence of malaria parasites on blood film as a dependent variable and the explanatory variables haptoglobin genotype, hemoglobin type, α+-thalassaemia type, age (as a continuous variable), sex, season, and ethnic group. Ninety-five percent CIs and P values were adjusted to take account of potential within-subject clustering of events using the sandwich estimator [32].
OR for haptoglobin 2-2 versus haptoglobin 1-1 and haptoglobin 2-1 combined, 0.64 (95% CI, 0.42-0.99, P = .043).
Hemoglobin levels were measured at cross-sectional survey (n = 158) in August 1998 (P = .08, by log likelihood ratio test, for the effect of haptoglobin genotype on hemoglobin level).
Hp genotype and malaria
In primary analysis, the incidence of clinical malaria was significantly lower in children with the Hp2/2 genotype, compared with children with the other genotypes; the degree of protection was ~30% for each of the 2 definitions of clinical malaria considered (for definition 1 [i.e., any density of parasitemia], the adjusted IRR was 0.72 [95% CI, 0.54-0.95; P = .02]; for definition 2 [parasite density of >2500 parasites/ μL for children aged ≥1 year ], the adjusted IRR was 0.67 [95% CI, 0.49-0.90; P = .008]) (table 2). A secondary analysis comparing the 3 genotypes also indicated a reduced incidence of clinical malaria for children with the Hp2/2 genotype (for definition 1, the adjusted IRR was 0.70 [95% CI, 0.50-0.96; P = .029]; for definition 2, the adjusted IRR was 0.70 [95% CI, 0.49-0.99; P = .049]). The incidence of malaria did not differ among children with the Hp1/1 versus those with the Hp2/1 genotype (for definition 1, the adjusted IRR was 0.96 [95% CI, 0.75-1.22; P = .72]; for definition 2 the adjusted IRR was 1.08 [95% CI, 0.82-1.42; P = .58]).
Table 2.
Incidence of clinical malaria, by haptoglobin (Hp) genotype.
| Clinical malaria definition |
Hp genotype | No. of episodes |
Incidence, no. of episodes per child-year of follow-up c |
IRR (95% CI) | P |
|---|---|---|---|---|---|
| Definition 1a | Hp1-1/Hp2-1 | 964 | 2.38 | 1 | |
| Hp2-2 | 280 | 1.83 | 0.72 (0.54-0.95) |
.02 | |
| Definition 2b | Hp1-1/Hp2-1 | 573 | 1.41 | 1 | |
| Hp2-2 | 153 | 0.99 | 0.67 (0.49-0.90) |
.008 |
NOTE. The analysis represents 233 children carrying the Hp1-1 and Hp2-1 genotypes during 405.2 child-years of follow-up and 79 children carrying the Hp2-2 genotype during 153.1 child-years of follow-up. Subjects were considered not to be at risk of malaria and were dropped from both numerator and denominator populations for 21 days after receiving treatment with an antimalarial drug. The adjusted incidence rate ratios (IRRs) were calculated using Poisson regression models that included each definition of malaria as a dependent variable and the explanatory variables of haptoglobin genotype, sickle cell, and α+-thalassaemia genotypes, age (as a continuous variable), sex, season (defined in 3-months blocks) and ethnic group. The 95% CIs and P values were adjusted to account for the potential clustering of events within individual study children by use of the sandwich estimator [32].
Defined as fever (axillary temperature, >37.5°C) or a history of fever in preceding 48 h in conjunction with a slide positive for blood-stage asexual Plasmodium falciparum at any density.
Defined as fever (axillary temperature, >37.5°C) in conjunction with a slide positive for blood-stage asexual P. falciparum at any parasite density for children aged <1 year and at a parasite density of >2,500 parasites/μL for children aged ≥ 1 year.
Crude incidence of malaria per person-year of observation
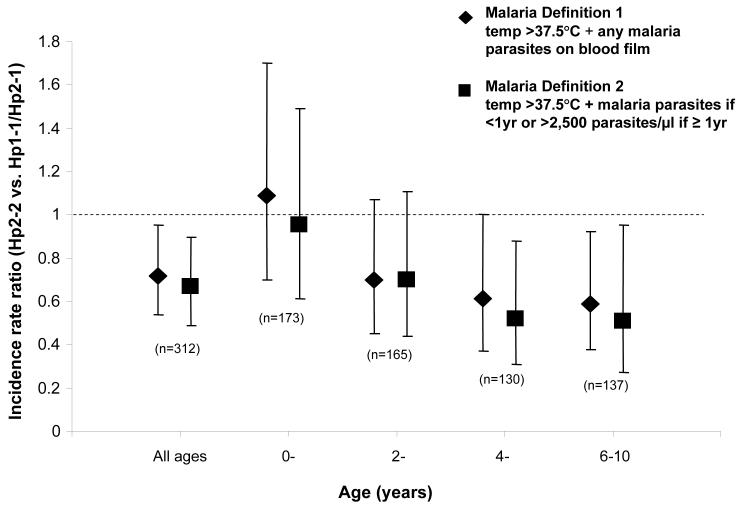
For all age groups combined, the Hp2/2 genotype was associated with ~30% protection against clinical malarial episodes. However, this rate of protection varied with age: there was no protection in the first 2 years of life, a 30% rate of protection at ≥ 2 years of age, and a ~50% rate of protection from 4-10 years (figure 2). Although a significant interaction was not found between age and protection over the full range of ages (Χ24, 4.26; P = .37), the data support the impression of acquired protection with age.
Figure 2.
Incidence rate ratios (IRRs) for clinical malaria in children with haptoglobin (Hp)2/2 versus the Hp1/1 and Hp2/1 genotypes combined, by age. Because this was a longitudinal cohort study, some children contributed data to >1 age stratum. Adjusted IRRs for malaria in children with Hp2/2, compared with baseline (individuals with Hp1-1 and those with Hp2-1) were calculated individually for each age stratum using a Poisson regression model, which included each definition of malaria as a dependent variable and the explanatory variables hemoglobin type (HbAA or HbAS), α+-thalassaemia genotype, season (defined as 3-month blocks), sex, ethnic group, and age (as a continuous variable). Ninety-five percent CIs and significance values were adjusted to take account of potential within-subject clustering of events using the sandwich estimator [32].
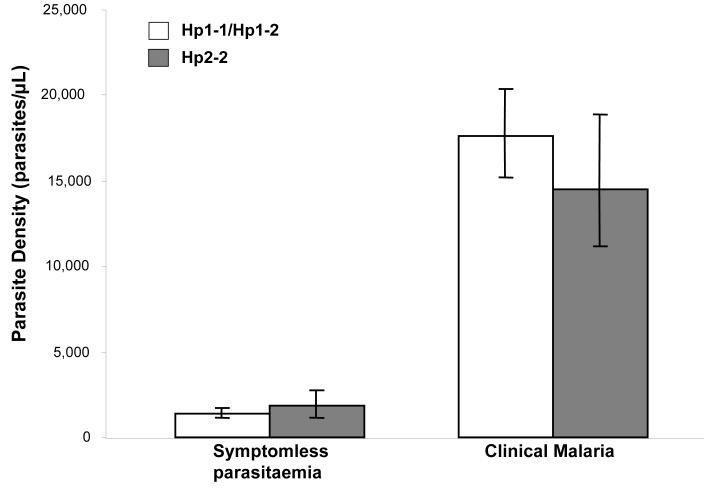
The prevalence of asymptomatic parasitemia during the 7 cross-sectional surveys was marginally lower in the Hp2/2 genotype group than in the other groups (adjusted OR for Hp2/2 vs. Hp1/1 and Hp2/1 combined, 0.64 [95% CI, 0.42-0.99; P = .043]; adjusted OR for heterogeneity between the 3 genotypes, 0.62 [95%CI, 0.37-1.0; P = .065] (table 1). Parasite prevalence did not differ between the Hp1/1 genotype group and the Hp2/1 genotype group (adjusted OR, 0.95; 95% CI, 0.64-1.4; P = .79). The overall mean parasite density did not differ by Hp genotype, either during febrile malaria episodes (P = 0.24) or during asymptomatic infection (as detected by 7 cross-sectional surveys; P = .82) (figure 3). However, in subgroup analysis, individuals with the Hp2/2 genotype aged ≥4 years had lower parasite densities during febrile malaria episodes, compared with children who carried the other genotypes (coefficient for Hp2/2 vs. Hp1/1 and Hp2/1 combined, −0.22; 95% CI −0.43 to −0.13; P = .038).
Figure 3.
Geometric mean parasite densities, by haptoglobin (Hp) genotype. The data on asymptomatic parasitemia derive from 7 cross-sectional surveys of malaria parasite prevalence in the study cohort. The data on Hp1-1 and Hp2-1 reflect 291 slides that tested positive for malaria parasites (of 1197 blood slides) among 233 children, and the data for Hp2-2 reflect 85 slides that tested positive for malaria parasites (of 428 blood slides) among 79 children. The data on clinical malaria derive from episodes of malaria (defined as an axillary temperature of >37.5°C in association with a slide positive for blood-stage asexual Plasmodium falciparum at any parasite density) detected during active and passive surveillance over 558.3 child-years of follow-up. The data on Hp1-1 and Hp2-1 reflects 964 episodes of malaria in 233 children, and data on Hp2-2 reflects 280 episodes of malaria among 79 children. Comparisons were made by linear regression with adjustment for the effects of age, season, sex, ethnic group, hemoglobin type (HbAA or HbAS) and α+-thalassaemia genotype. For asymptomatic parasitemia, the coefficient was 0.026 (95% CI, −0.20 to 0.25; P = .82), and for clinical malaria, the coefficient was −0.08 (95% CI, −0.22 to 0.06, P = .24). Bars 95% CIs.
Hp genotype and nonmalarial diseases
We did not have a prior hypothesis regarding the effect of Hp genotype on nonmalarial childhood diseases. However, we found that the Hp1/1 genotype was associated with a 30% reduction in the incidence of nonmalarial febrile episodes (adjusted IRR, 0.70; 95% CI, 0.56-0.86; P = .001) (table 3). The incidence of nonmalarial febrile episodes did not differ in children with the Hp2/1 genotype versus those with the Hp2/2 genotype (adjusted IRR, 0.98; 95% CI 0.79-1.21; P = .83). We did not find a significant association between Hp1/1 genotype and any particular primary nonmalarial clinical diagnosis. It is possible that the Hp1/1 genotype protects against a range of febrile illnesses, and our study may have been insufficiently powered to detect differences in individual diseases.
Table 3.
Incidence of nonmalarial childhood diseases, by haptoglobin (Hp) genotype
| Diagnosis, a Hp genotype | No. of episodes |
Incidence, no. of episodes per |
IRR (95% CI) | P |
|---|---|---|---|---|
|
| ||||
| All clinic visits b | ||||
| Hp1-1 | 1227 | 8.82 | 1 | |
| Hp2-1 | 2145 | 8.78 | 1.07 (0.92-1.25) | .38 |
| Hp2-2 | 1065 | 7.26 | 0.91 (0.75-1.10) | .32 |
| Nonmalarial fever (negative slide results)c | ||||
| Hp1-1 | 146 | 0.98 | 1 | |
| Hp2-1 | 319 | 1.24 | 1.44 (1.16-1.78) | .001 |
| Hp2-2 | 189 | 1.23 | 1.40 (1.11-1.78) | .005 |
| Upper respiratory tract infection | ||||
| Hp1-1 | 296 | 2.12 | 1 | |
| Hp2-1 | 592 | 2.42 | 1.23 (1.0-1.53) | .047 |
| Hp2-2 | 286 | 1.95 | 1.02 (0.80-1.30) | .86 |
| Lower respiratory tract infection | ||||
| Hp1-1 | 127 | 0.91 | 1 | |
| Hp2-1 | 213 | 0.87 | 1.10 (0.78-1.52) | .61 |
| Hp2-2 | 127 | 0.87 | 1.17 (0.82-1.67) | .39 |
| Gastroenteritis | ||||
| Hp1-1 | 114 | 0.82 | 1 | |
| Hp2-1 | 208 | 0.85 | 1.21 (0.92-1.60) | .18 |
| Hp2-2 | 111 | 0.76 | 1.18 (0.83-1.68) | .36 |
| Fever of unknown cause | ||||
| Hp1-1 | 63 | 0.45 | 1 | |
| Hp2-1 | 97 | 0.39 | 0.91 (0.63-1.31) | .62 |
| Hp2-2 | 59 | 0.40 | 0.95 (0.63-1.44) | .82 |
| Skin infection | ||||
| Hp1-1 | 165 | 1.18 | 1 | |
| Hp2-1 | 214 | 0.88 | 0.81 (0.60-1.10) | .17 |
| Hp2-2 | 120 | 0.82 | 0.81 (0.56-1.15) | .23 |
| Helminth infection | ||||
| Hp1-1 | 42 | 0.30 | 1 | |
| Hp2-1 | 64 | 0.26 | 0.88 (0.55-1.39) | .58 |
| Hp2-2 | 35 | 0.24 | 0.81 (0.48-1.36) | .42 |
NOTE. The analysis represents 87 children with the Hp1-1 genotype during 148.8 child-years of follow-up, 146 children with the Hp2-1 genotype during 256.4 child-years of follow-up, and 79 children with the Hp2-2 genotype during 153.1 child-years of follow-up. Incidence rate ratios (IRRs) were calculated by Poisson regression analysis that included each diagnosis separately as the dependent variable and the explanatory variables haptoglobin genotype, age (as a continuous variable), sex, season (defined as 3-month blocks), and ethnic group. The 95% CIs and P values were adjusted to take account of potential within-subject clustering of events using the sandwich estimator.
Diagnoses represent those recorded as either the primary or secondary diagnoses at each visit by the consulting clinician. See Participants, Materials and Methods for clinical definitions of the diseases.
All clinic visits also includes children presenting with malaria to clinic.
Nonmalarial fever was defined as a temperature >37.5°C in a child with a negative blood film result who had not been treated for malaria infection within the prior 21 days. Children with nonmalarial fever may also be represented under other nonmalarial diagnostic categories.
DISCUSSION
The association between Hp polymorphisms and malaria infection has been the subject of controversy and debate for >30 years. Some studies have suggested that the Hp2-2 phenotype is protective against malaria [20, 21, 23], and other studies have not found a clear association with Hp genotype [25, 26]. However, many of these studies were small, and all were of case-control or cross-sectional design. Here, we report a prospective cohort study that examined the association between Hp genotype and the incidence of clinical malaria and other childhood diseases. We found that the Hp2-2 genotype was associated with a 30% reduction in the incidence of clinical malaria (adjusted IRR for Hp2/2 vs. Hp1/1 and Hp2/1 combined, 0.67; 95% CI, 0.5-0.9; P = .008). Moreover, the degree of protection from malaria was not constant but increased with age, reaching a 50% rate of protection among children from 4-10 years of age. This suggests that, like the sickle cell trait [33], the Hp2-2 genotype may result in accelerated acquisition of immunity to malaria. Although our observations are in agreement with previous studies that have indicated a protective effect of Hp2-2 phenotype [20, 21, 23], we did not find a significant effect of the Hp2-1 genotype.
So how might the Hp2-2 genotype protect against malaria infection? We recently described a greater decrease in Hb level among children with the Hp2-2 genotype and speculated that this may have resulted from a failure to quench Hb- iron-mediated oxidant stress during malaria-induced hemolysis [30]. Studies suggest that reduced antioxidant protection may be specific to Hp2-2 and that there is little difference between the Hp2-1 and Hp1-1 phenotypes in this regard [12, 13]. Experimental evidence suggests that enhanced phagocytosis of ring-parasitized erythrocytes in sickle cell, G6PD deficiency, and β-thalassaemia is mediated by a mechanism essentially similar to the phagocytosis of oxidatively damaged RBCs [15, 16]. Increased reactive oxygen species (from the developing malaria parasite and from increased free Hb and iron levels in the Hp2-2 genotype) may similarly lead to membrane-bound hemichromes and iron, aggregation of band 3 proteins, and binding of autologous IgG and complement. It is possible, therefore, that enhanced immunity in the Hp2-2 genotype may be mediated by accelerated acquisition of antibodies to altered host antigens, such as the band 3 protein expressed on the ring-parasitized erythrocyte. Similar to other genetic polymorphisms associated with oxidant stress, such as G6PD deficiency and β-thalassaemia, Hp2-2 genotype (under conditions of oxidant stress) may protect against malaria at the expense of causing anaemia [30].
Differences in host response to inflammation may represent an alternative explanation for the protective effect of the Hp2-2 genotype. The cytokine response produced by macrophages in response to hemolysis differs by Hp genotype. By binding to macrophage CD163 receptors, Hp and Hb complexes mimic antibody binding and trigger cytokine secretion [3]. This scavenging of Hp/Hb complexes induces an antiinflammatory effect by strongly stimulating IL-10 secretion and heme oxygenase-1 synthesis [34]. Evidence suggests that the Hp2-2/Hb complex may promote a Th1 cytokine response by stimulating the release of less IL-10 and IL-6, compared with the Hp1-1/Hb complex [17]. Enhanced macrophage iron loading in individuals with the Hp2-2 phenotype after hemolysis [35] may also inhibit TNF-α, and IFN-γ production [36]. The significance of these possible effects remains to be elucidated. However, it is possible that, by modulating the inflammatory response during hemolysis, individuals who carry the Hp2-2 genotype may have an altered course of malaria infection.
Interestingly, we found that the Hp1-1 genotype was associated with significant protection from nonmalarial febrile illnesses. Mounting evidence suggests that Hp plays an important role in host defence against infection. Hp modulates leukocyte trafficking [4] and host response to bacterial lipopolysaccharides [5]. Hp (of the Hp1-1 type) is a ligand for the Mac-1 leukocyte integrin β2 (CD11b/CD18) receptor on monocytes, macrophages, and natural killer cells [4]. Moreover, Hp suppresses lipopolysaccharide-induced release of TNF-α, IL-10, and IL-12 in vitro and protects the host against the deleterious effect of lipopolysaccharides in vivo [5]. Furthermore, by binding to free Hb and reducing iron availability Hp exerts a bacteriostatic effect on many organisms such as Escherichia coli [37]. Lower levels of available iron in the Hp1-1 phenotype compared to the Hp2-2 type [10] may thus limit bacterial growth. In clinical studies, Hp2-2 is associated with increased mortality among patients with tuberculosis [38] and HIV infection [39]. It is likely that many of the nonmalarial febrile illnesses suffered would be due to viral infections, and a limitation of the study is lack of microbiology data. However, it seems unlikely that this effect can be explained by protection from any organism individually, but rather that Hp1-1 may protect from a range of different febrile processes.
Our results suggest that homozygosity for the Hp2 allele protects against clinical malaria and that homozygosity for Hp1 protects against nonmalarial febrile illnesses. Both P. falciparum malaria and bacterial infection are major causes of childhood death in sub-Saharan Africa [1, 40] and have exerted selective pressure on the human genome. Although most febrile illnesses are likely to have been due to viral infections, it is intriguing to speculate that balancing selection pressures on the Hp alleles from malaria and bacteraemia may have determined the spread and geographical distribution of the Hp gene. Establishment of the role of the Hp gene and mechanisms of protection may further our understanding of the pathophysiology of malaria and other childhood infections.
Acknowledgements
We thank the study participants and their families for taking part in the study. This paper is published with the permission of the Director of the Kenya Medical Research Institute.
Financial support. Wellcome Trust and the European Union via the BioMalpar Network 6 Initiative.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–7. doi: 10.1016/s1471-4922(01)02031-1. [DOI] [PubMed] [Google Scholar]
- 2.Gutteridge JMC. The antioxidant activity of haptoglobin towards hemoglobin-stimulated lipid peroxidation. Biochimica et Biophysica Acta. 1987;917:219–223. doi: 10.1016/0005-2760(87)90125-1. [DOI] [PubMed] [Google Scholar]
- 3.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the hemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 4.El Ghmati SM, Van Hoeyveld EM, Van Strijp JG, Ceuppens JL, Stevens EA. Identification of haptoglobin as an alternative ligand for CD11b/CD18. J Immunol. 1996;156:2542–52. [PubMed] [Google Scholar]
- 5.Arredouani MS, Kasran A, Vanoirbeek JA, Berger FG, Baumann H, Ceuppens JL. Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology. 2005;114:263–71. doi: 10.1111/j.1365-2567.2004.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:1589–600. [PubMed] [Google Scholar]
- 7.Maeda N, Yang F, Barnett DR, Bowman BH, Smithies O. Duplication within the haptoglobin Hp2 gene. Nature. 1984;309:131–5. doi: 10.1038/309131a0. [DOI] [PubMed] [Google Scholar]
- 8.McDermid JM, Prentice AM. Iron and infection: effects of host iron status and the iron-regulatory genes haptoglobin and NRAMP1 (SLC11A1) on host-pathogen interactions in tuberculosis and HIV. Clin Sci (Lond) 2006;110:503–24. doi: 10.1042/CS20050273. [DOI] [PubMed] [Google Scholar]
- 9.Melamed-Frank M, Lache O, Enav BI, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98:3693–8. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- 10.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96:435–41. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 11.Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry. 2004;43:3899–906. doi: 10.1021/bi0362626. [DOI] [PubMed] [Google Scholar]
- 12.Langlois MR, Delanghe JR, De Buyzere ML, Bernard DR, Ouyang J. Effect of haptoglobin on the metabolism of vitamin C. Am J Clin Nutr. 1997;66:606–10. doi: 10.1093/ajcn/66.3.606. [DOI] [PubMed] [Google Scholar]
- 13.Awadallah SM. Haptoglobin 2-2 phenotype is associated with decreased ferroxidase activity in smokers. Clin Chim Acta. 2003;334:71–6. doi: 10.1016/s0009-8981(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 14.Kay MM, Bosman GJ, Shapiro SS, Bendich A, Bassel PS. Oxidation as a possible mechanism of cellular aging: vitamin E deficiency causes premature aging and IgG binding to erythrocytes. Proc Natl Acad Sci U S A. 1986;83:2463–7. doi: 10.1073/pnas.83.8.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cappadoro M, Giribaldi G, O'Brien E, et al. Early Phagocytosis of Glucose-6-Phosphate Dehydrogenase (G6PD)-Deficient Erythrocytes Parasitized by Plasmodium falciparum May Explain Malaria Protection in G6PD Deficiency. Blood. 1998;92:2527–2534. [PubMed] [Google Scholar]
- 16.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–71. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 17.Guetta J, Strauss M, Levy NS, Fahoum L, Levy AP. Haptoglobin genotype modulates the balance of Th1/Th2 cytokines produced by macrophages exposed to free hemoglobin. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Hunt NH, Driussi C, Sai-Kiang L. Haptoglobin and malaria. Redox Rep. 2001;6:389–92. doi: 10.1179/135100001101536508. [DOI] [PubMed] [Google Scholar]
- 19.Imrie H, Ferguson DJ, Day KP. Human serum haptoglobin is toxic to Plasmodium falciparum in vitro. Mol Biochem Parasitol. 2004;133:93–8. doi: 10.1016/j.molbiopara.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Singh IP, Walter H, Bhasin MK, Bhardwaj V, Sudhakar K. Genetic markers and malaria. Observations in Gujarat, India. Hum Hered. 1986;36:31–6. doi: 10.1159/000153596. [DOI] [PubMed] [Google Scholar]
- 21.Elagib AA, Kider AO, Akerstrom B, Elbashir MI. Association of the haptoglobin phenotype (1-1) with falciparum malaria in Sudan. Trans R Soc Trop Med Hyg. 1998;92:309–11. doi: 10.1016/s0035-9203(98)91025-2. [DOI] [PubMed] [Google Scholar]
- 22.Bottini N, Ronchetti MP, Gloria-Bottini F, Fontana L. Malaria as a possible evolutionary cause of allergy. Allergy. 1999;54:188–9. doi: 10.1034/j.1398-9995.1999.00061.x. [DOI] [PubMed] [Google Scholar]
- 23.Quaye IK, Ekuban FA, Goka BQ, et al. Haptoglobin 1-1 is associated with susceptibility to severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:216–9. doi: 10.1016/s0035-9203(00)90281-5. [DOI] [PubMed] [Google Scholar]
- 24.Minang JT, Gyan BA, Anchang JK, Troye-Blomberg M, Perlmann H, Achidi EA. Haptoglobin phenotypes and malaria infection in pregnant women at delivery in western Cameroon. Acta Trop. 2004;90:107–14. doi: 10.1016/j.actatropica.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Aucan C, Walley AJ, Greenwood BM, Hill AV. Haptoglobin genotypes are not associated with resistance to severe malaria in The Gambia. Trans R Soc Trop Med Hyg. 2002;96:327–8. doi: 10.1016/s0035-9203(02)90114-8. [DOI] [PubMed] [Google Scholar]
- 26.Bienzle U, Eggelte TA, Adjei LA, et al. Limited influence of haptoglobin genotypes on severe malaria in Ghanaian children. Trop Med Int Health. 2005;10:668–71. doi: 10.1111/j.1365-3156.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- 27.Nyakeriga AM, Troye-Blomberg M, Dorfman JR, et al. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190:439–47. doi: 10.1086/422331. [DOI] [PubMed] [Google Scholar]
- 28.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–9. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . Acute respiratory infections in children: case management in small hospitals in developing countries: a manual for doctors and other senior health workers (WHO/ARI 90.5) Geneva: World Health Organisation; 1990. [Google Scholar]
- 30.Atkinson SH, Rockett K, Sirugo G, et al. Seasonal Childhood Anaemia in West Africa Is Associated with the Haptoglobin 2-2 Genotype. PLoS Med. 2006;3:e172. doi: 10.1371/journal.pmed.0030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong SS, Boehm CD, Higgs DR, Cutting GR. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood. 2000;95:360–2. [PubMed] [Google Scholar]
- 32.Armitage P, Berry G, Matthews J. Using STATA's robust cluster command as appropriate: statistical methods in medical research. 4th ed Oxford: Blackwell Scientific Publications; 2001. [Google Scholar]
- 33.Williams TN, Mwangi TW, Roberts DJ, et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–26. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 35.Langlois MR, Martin ME, Boelaert JR, et al. The haptoglobin 2-2 phenotype affects serum markers of iron status in healthy males. Clin Chem. 2000;46:1619–25. [PubMed] [Google Scholar]
- 36.Weiss G, Wachter H, Fuchs D. Linkage of cell-mediated immunity to iron metabolism. Immunol Today. 1995;16:495–500. doi: 10.1016/0167-5699(95)80034-4. [DOI] [PubMed] [Google Scholar]
- 37.Eaton JW, Brandt P, Mahoney JR. Haptoglobin: A Natural Bacteriostat. Science. 1982;215:691–692. doi: 10.1126/science.7036344. [DOI] [PubMed] [Google Scholar]
- 38.Kasvosve I, Gomo ZA, Mvundura E, et al. Haptoglobin polymorphism and mortality in patients with tuberculosis. Int J Tuberc Lung Dis. 2000;4:771–5. [PubMed] [Google Scholar]
- 39.Delanghe JR, Langlois MR, Boelaert JR, et al. Haptoglobin polymorphism, iron metabolism and mortality in HIV infection. AIDS. 1998;12:1027–1032. [PubMed] [Google Scholar]
- 40.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]