Abstract
Individuals with schizophrenia demonstrate behavioral and neurobiological deficits in episodic memory. However, recent work suggests that episodic memory deficits in schizophrenia may be mitigated through specific encoding strategies. The current study directly compared brain activity and memory performance associated with two different verbal encoding orientations in the same group of schizophrenia participants, in order to more fully characterize the role of strategy in memory processing in this population. Participants included 18 individuals with schizophrenia and 15 healthy comparison participants. Participants encoded words under two conditions during separate fMRI scanning runs. During Incidental encoding, participants were required to make abstract/concrete judgments for each word. During Intentional encoding, participants were instructed to memorize each word for a later memory test. Free recall and a recognition task (utilizing the Remember/Know paradigm) were performed outside of the scanner. Consistent with prior work, schizophrenia participants recognized more words encoded Incidentally than Intentionally, although free recall remained substantially impaired. Schizophrenia participants were also less likely to give Remember judgments for old words and more likely to give Guess judgments for both old and new words. When fMRI data were examined, we found that Incidental encoding was associated with substantially fewer between-group differences (Control > Schizophrenia) than Intentional encoding. Furthermore, schizophrenia participants exhibited intact activity during encoding of items that were subsequently retrieved. Our results suggest that use of an Incidental encoding strategy improved recognition memory among individuals with schizophrenia and resulted in a pattern of encoding-related brain activity that was more similar to that seen in control participants. However, we found that Incidental encoding did not improve free recall in schizophrenia participants and abnormal brain activity in some regions was observed, despite improvements in recognition memory.
Keywords: functional magnetic resonance imaging (fMRI), subsequent memory, incidental encoding
1. Introduction
Episodic memory (EM) deficits among individuals with schizophrenia are well-established (Aleman et al., 1999; Cirillo and Seidman, 2003). Such deficits have been linked to strategic memory failures (Koh, 1978; Brebion et al., 1997; Iddon et al., 1998) and may also be related to deficits in retrieval (Koh and Kayton, 1974; Calev, 1984). Many EM studies have used intentional encoding paradigms, in which participants are instructed to memorize words and are not oriented to use specific encoding strategies (Sengel and Lovallo, 1983; Gold et al., 1992; Brebion et al., 1997; Barch et al., 2002; Ragland et al., 2004; Weiss et al., 2004). However, individuals with schizophrenia show recognition memory improvements when oriented to use specific encoding strategies, such as making abstract/concrete judgments or living/non-living judgments for words (Hofer et al., 2003b; Ragland et al., 2003; Weiss et al., 2003; Bonner-Jackson et al., 2005).
Neuroimaging studies of memory in schizophrenia using intentional encoding paradigms consistently demonstrate abnormal encoding-related brain activity patterns during encoding (Hazlett et al., 2000; Ragland et al., 2001; Barch et al., 2002; Hofer et al., 2003a; Jessen et al., 2003; Achim and Lepage, 2005). However, conditions that encourage semantic encoding improve brain activity in individuals with schizophrenia in semantic processing areas (Bonner-Jackson et al., 2005; Ragland et al., 2005), reinforcing the notion that EM-related impairments in schizophrenia are related to difficulty in strategy generation and application.
Despite these findings, some questions about the ability of encoding strategies to improve EM in schizophrenia remain. For example, discrepancies across studies using supportive vs. unsupportive encoding may be due to population differences between studies (e.g., age, disease chronicity, etc.). A comparison of encoding conditions within the same participants is needed to rule out such confounds. Also, little is known about the patterns of brain activity that predict subsequent memory (SM) in schizophrenia and whether they are similar to those seen in controls.
The current study investigated activity associated with verbal encoding and subsequent memory during two different encoding paradigms in the same schizophrenia participants and healthy controls. We chose to compare the “standard” encoding approach often used in the literature (Intentional) with a more tightly controlled, supportive encoding task intended to promote memory (Incidental). Incidental encoding tasks that orient participants to process information “deeply” or semantically are well-known to significantly improve subsequent memory for those items (Craik and Lockhart, 1972; Craik and Tulving, 1975). Although there are inherent difficulties in comparing cognitive tasks that differ in terms of structure and task demands, we believe that this represents a more realistic and ecologically valid picture of everyday memory function. Furthermore, as Intentional encoding has been used in previous studies of EM in schizophrenia, this is a natural condition against which to compare strategic memory interventions.
We predicted that during Intentional encoding, schizophrenia participants would show abnormal patterns of activity in left prefrontal cortex (PFC; BA 45/47, 9/46) and medial temporal lobe (MTL; particularly hippocampus) as well as poorer SM performance (Barch et al., 2002; Hofer et al., 2003b; Jessen et al., 2003). Second, we predicted that during Incidental encoding, schizophrenia participants would: a) recruit typical deep encoding regions (left BA 45/47, left BA 9/46, left MTL), and b) show recognition rates equivalent to controls (Bonner-Jackson et al., 2005; Ragland et al., 2005). Third, we predicted more disparate memory performance and brain activity (particularly in left PFC and MTL regions) between groups for Intentional (relative to Incidental) encoding. Finally, we predicted that schizophrenia participants would show typical SM effects in regions such as BA 45/47 and hippocampus, with greater encoding-related activity for subsequently remembered, relative to forgotten, items. This prediction was based on previous research supporting the link between beneficial encoding conditions and improved memory-related brain activity among individuals with schizophrenia (Bonner-Jackson et al., 2005; Ragland et al., 2005).
2. Methods
2.1. Participants
Participants were 18 DSM-IV diagnosed individuals with schizophrenia and 15 healthy control participants who were recruited to participate in studies of brain structure and function at the Conte Center for the Neuroscience of Mental Disorders at Washington University. Exclusion criteria for all participants were: (a) meeting DSM-IV criteria for substance abuse or dependence within the past 3 months; (b) the presence of any clinically unstable or severe medical disorder; (c) head injury with documented neurological sequelae or loss of consciousness; or (d) meeting DSM-IV criteria for mental retardation (mild or greater in severity). Six participants (5 schizophrenia, 1 control) were excluded due to excessive movement during scanning, poor signal-to-noise ratios, or incomplete functional neuroimaging runs, and were not included in the sample described above. Demographic information is displayed in Table 1.
Table 1.
Demographic and Clinical Data
| Mean | Standard Deviation | ||||
|---|---|---|---|---|---|
| Characteristic | Control Participants | Participants with Schizophrenia | Control Participants | Participants with Schizophrenia | P-value for statistical test |
| Age (years) | 43.0 | 39.8 | 10.6 | 10.4 | P = 0.40 |
| Sex (% male) | 53.3 | 88.2 | P = 0.08 | ||
| Participant Education (years) | 15.2 | 12.0 | 2.5 | 2.0 | P = 0.001 |
| Parental education (years) | 12.2 | 12.9 | 1.8 | 1.8 | P = 0.34 |
| Handedness | 13R/2L | 15R/2L | |||
| Mean SAPS global item score | 0.8 | 0.6 | |||
| Mean SANS global item score | 1.0 | 0.7 | |||
| Poverty symptoms | 6.5 | 1.2 | |||
| Disorganization | 3.6 | 1.2 | |||
| Reality distortion | 3.9 | 1.7 | |||
| Atypical medications only (%) | 71 | ||||
| Combination typical/atypical (%) | 24 | ||||
| Anti-cholinergic medication (%) | 29 | ||||
Diagnostic information was collected using the Structured Clinical Interview for DSM-IV (SCID-IV; (Spitzer et al., 1990) and all available hospital records and corroborative family sources by a trained MSW-level research assistant, who regularly participated in ongoing training and reliability sessions for the SCID-IV as part of the Washington University Conte Center. Reliability among raters at these sessions was high (alpha values of 0.97, 0.91, and 0.94 for ratings of positive, negative, and disorganized symptoms, respectively). Written informed consent was obtained for all participants prior to research participation. All experimental procedures were approved by the Institutional Review Board (IRB) of Washington University and complied with these regulations.
2.2 Tasks and materials
The functional neuroimaging tasks included two encoding tasks, Incidental and Intentional, acquired in separate runs. During Incidental encoding, participants made abstract/concrete judgments for each word and pushed one of two buttons to indicate their response. During Intentional encoding, participants were instructed to memorize each word for a later memory test and to push a button after reading each word. Task order was counterbalanced across participants. Stimuli for each of the encoding tasks were 50 visually presented words, 3–10 letters in length, presented in 48 point Geneva font. Two word lists were constructed with words matched on length, frequency, and part of speech (Kucera and Francis, 1967). Each participant saw one word list for Incidental and one for Intentional, and the list used in each task condition was counterbalanced across participants.
Scanning runs lasted 5 minutes 20 seconds each. Stimuli were presented in an event-related design consisting of 50 task trials intermixed with 75 fixation trials. There were four fixation trials at the beginning (to allow MR signal to reach steady state) and end of each run. Each word was presented for 2 seconds followed by a 500 ms interstimulus interval. During fixation trials, a cross hair appeared continuously and participants fixated.
Following scanning, participants were given a free recall test (in order to assess unsupported memory retrieval), followed by a recognition memory test, in which 200 words were presented. One hundred of the words were “Old” (50 from each encoding task), and 100 were “New” (not previously presented). We used the Remember-Know paradigm (Tulving, 1985; Wheeler and Buckner, 2004), in order to identify words associated with recollection versus feelings of familiarity. Participants were required to push one of four keys for each word: Remember (recollection of having seen the word); Know (sense of familiarity without recollection); New (not previously seen); Guess (if unsure).
2.3. Scanning
All scanning was performed on the 1.5T Siemens VISION system. Functional images were collected using an asymmetric spin-echo echo-planar sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (T2*) (TR = 2500ms, TE = 50ms, FOV = 24cm, flip=90 degrees). During each functional run, 125 sets of axial images were acquired parallel to the anterior-posterior commissure plane (3.75×3.75mm in-plane resolution), allowing complete brain coverage at high signal-to-noise ratio (Conturo et al., 1996). Nineteen slices 7 mm thick were acquired in each image. Structural images were acquired using a coronal MP-RAGE 3D T1-weighted sequence (TR=9.7ms, TE=4ms, flip=10°; voxel size=1×1×1.2mm), and were used for between subject registration and anatomic localization.
2.4. Data analysis
2.4.1. Functional magnetic resonance imaging data (fMRI)
Functional neuroimaging data was analyzed using an in-house data analysis software package. fMRI preprocessing included (1) compensation for slice-dependent time shifts; (2) elimination of odd/even slice intensity differences due to interpolated acquisition; (3) realignment of all data acquired in each subject within and across runs to compensate for rigid body motion (Ojemann et al., 1997); (4) intensity normalization to a whole brain mode value of 1000; and (5) spatial smoothing with an 8-mm FWHM Gaussian kernel. The functional data were transformed into the stereotaxic atlas space of Talairach and Tournoux (Talairach and Tournoux, 1988) by computing a sequence ofaffine transforms (first frame EPI to T2-weighted TSE to MP-RAGE to atlas representative target) composed by matrix multiplication. All analyses were conducted on the basis of atlas-transformed data resampled to 3 mm cubic voxels.
For each participant, we estimated the magnitude of task-related activation by canvassing the brain on a voxel-by-voxel basis using a general linear model (GLM) and a boxcar task function convolved with a Boynton hemodynamic response function, with separate estimates for each encoding task. These contrasts then gave us regions of significant differences in brain activity. In order to assess the nature of these significant effects, we then performed ANOVAs and appropriate t-tests on these regions, with subjects treated as a random factor. For each participant, we created two sets of GLM contrasts. In the first set, we coded three trial types: 1) correct Incidental items (correct abstract/concrete response); 2) correct Intentional items (pushed the button when the item appeared); and 3) errors, collapsing across encoding tasks. In the second set, we coded each stimulus event within each encoding run as one the following categories, based on SM performance: 1) Remember (correct recognitions given a Remember response); 2) Know (correct recognitions given a Know response); 3) Guess; and 4) Miss (items incorrectly judged as New).
To identify regions of significant task-related activation, we used a multi-step approach outlined in Table 2. This approach involved the application of multiple statistical tests, with each test set at a relatively low statistical threshold. We have used this approach in a number of previous studies (e.g., Barch et al., 2001; Braver et al., 2003) and believe that it optimizes the trade-off between false positive protection (type 1 error) and sensitivity/power (type 2 error). A brain region was considered to be “significant” in an analysis when every voxel within that region was statistically significant (defined as P < 0.02) in each test required for a given effect (described in detail below). Voxels meeting these criteria had an α level of at least 0.0004 for the inference that they demonstrated all of the required patterns. It should be noted that this α level of 0.0004 is likely an overestimate of the true α level because of non-independence in the error terms of the statistical contrasts. This multi-step this approach does not change the significance level for any individual test (Nichols et al., 2005); however, it enhances the significance of the likelihood of all tests being significant simultaneously. In addition, a brain region was only considered to be “significant” in an analysis if it contained a cluster of 9 or more contiguous voxels. This cluster size requirement provides further protection against Type 1 error rates (Forman et al., 1995; McAvoy et al., 2001) and was chosen based on Monte Carlo simulations.
Table 2.
Conjunction Analyses Performed on Regional Brain Activity During Encoding in Participants With Schizophrenia and Healthy Controls
| Regional Brain Effects Required to be Present Simultaneouslya | ||
|---|---|---|
| Type of Conjunction Analysis | Number | Type |
| Within-group Analyses | ||
| Regions more responsive to Incidental than Intentional encoding | Two | Region was significantly more responsive to task than fixation for either Incidental or Intentional encoding (or both) |
| Region was significantly more responsive to Incidental than Intentional encoding | ||
| Regions more responsive to Intentional than Incidental encoding | Two | Region was significantly more responsive to task than fixation for either Incidental or Intentional encoding (or both) |
| Region was significantly more responsive to Intentional than Incidental encoding | ||
| Analyses comparing schizophrenia participants and control participants | ||
| Group differences in responses to Incidental encoding | Two | Region was significantly more responsive to task than fixation for Incidental encoding in either schizophrenia or control participants |
| Region was significantly more responsive to Incidental encoding in one group (control > schizophrenia, or schizophrenia > control) | ||
| Group differences in responses to Intentional encoding | Two | Region was significantly more responsive to task than fixation for Intentional encoding in either schizophrenia or control participants |
| Region was significantly more responsive to Intentional encoding in either group (control > schizophrenia, or schizophrenia > control) | ||
| Group differences in responses to Incidental vs. Intentional encoding | Two | Region was significantly more responsive to either Incidental or Intentional encoding in either group (control > schizophrenia, or schizophrenia > control) |
| Region showed a significant Group (control, schizophrenia) by Encoding Type (Incidental, Intentional) interaction | ||
| Group differences in SM activity | Two | Region was significantly more responsive during items that were subsequently remembered than subsequently missed (or vice versa), either schizophrenia or control participants |
| Region showed a significant Group (control, schizophrenia) by Subsequent Memory (Remember, Miss) interaction | ||
| Group overlap in SM activity | Two | Region showed a significant main effect of Subsequent Memory (Remember > Miss) |
| Region did not show a significant between-group difference in Subsequent Memory activity (between-group differences masked) | ||
Each effect was required to be significant at P = 0.02.
To examine within-group effects of Encoding Condition on encoding-related brain activity (for correct encoding trials only) within each group separately, we required voxels to show both: 1) significant activity greater than fixation in at least one encoding condition (Incidental > Fixation or Intentional > Fixation), and 2) a significant difference between conditions (Incidental > Intentional, or Intentional > Incidental). To identify significant between group differences in task-related activation during Intentional encoding, we required voxels to show: 1) significant task-related activation during Intentional encoding in either controls or participants with schizophrenia, using voxel-wise dependent sample t-tests; and 2) greater task-related activity for Intentional encoding in either group (control > schizophrenia, or schizophrenia > control), using voxel-wise independent samples t-tests. We performed an analogous set of contrasts for Incidental encoding.
We identified between-group differences in encoding activity associated with subsequently remembered items (“SM effect”) by requiring voxels to simultaneously show: 1) greater encoding activation during either subsequently remembered or subsequently missed items, in either group; and 2) a significant Group (control, schizophrenia) by Memory (Remember, Miss) interaction. The Remember vs. Miss contrast was chosen based on the large literature reporting robust SM activation using this technique (Fernandez et al., 1998; Wagner et al., 1998; Buckner et al., 2001; Otten and Rugg, 2001b; Fletcher et al., 2003). The statistical analyses of the fMRI signal were conducted individually for each voxel in the brain, which generated voxelwise statistical maps that were thresholded for significance using a cluster-size algorithm (Forman et al., 1995) that protects against an inflation of the false-positive rate with multiple comparisons. We used a cluster-size threshold of 9 contiguous voxels and a per-voxel alpha of at least 0.0004, corresponding to a corrected whole brain false positive rate of approximately 0.05. Some of the analyses presented below were conjunction analyses, in which we required multiple effects to be significant simultaneously. When two or more effects were required to be significant, a P-value threshold of 0.02 being required for each effect, and resulting in a combined significance of either 0.0004 (0.02*0.02) or 0.000008 (0.02*0.02*0.02) (Barch et al., 2001).
2.4.2. Behavioral data
Behavioral data was analyzed using appropriate t-tests and ANOVAs. Repeated measures ANOVAs were conducted on the memory performance and reaction time (RT) data with Encoding Condition (Incidental, Intentional) as the within-subjects variable and Group (control, schizophrenia) as the between-subjects variable.
3. Results
3.1. Behavioral data
Encoding and recognition data are presented in Table 3 and Figure 1. There was no accuracy measure for Intentional encoding. Controls performed significantly better than schizophrenia participants on the Incidental encoding task [t (30) = 2.98, P < 0.01]. The ANOVA revealed that RTs were longer for both groups during Incidental encoding [F (1,30) = 161.71, P < 0.001] and longer for participants with schizophrenia overall [F (1,30) = 6.22, P < 0.02], while the interaction was non-significant (F < 1).
Table 3.
Behavioral Data: Word Encoding and Retrieval
| Mean (SD) | |||
|---|---|---|---|
| Task | Measure | Control Participants | Participants with Schizophrenia |
| Word Encoding* (Incidental) | Accuracy | 0.82 (0.12)1 | 0.69 (0.13) |
| Reaction Time (ms) | 1090 (150)4 | 1250 (142)2,4 | |
| Word Encoding (Intentional) | Reaction Time (ms) | 719 (190) | 822 (202)2 |
| Word Recognition (New) | % Correct Rejections | 0.60 (0.25) | .43 (0.30) |
| Reaction Time (ms) | 1974 (433) | 2830 (1746) | |
| Word Recognition (Incidental) | Accuracy | 0.81 (0.18)3,4 | 0.70 (0.16)4 |
| Reaction Time (ms) | 1874 (465) | 2893 (1716) | |
| Word Recognition (Intentional) | Accuracy | 0.73 (0.16)3 | 0.57 (0.18) |
| Reaction Time (ms) | 1885 (385) | 2842 (1743) | |
| Memory Judgment | Word Type | ||
| Overall Accuracy | Old New |
0.77 (0.16)1 0.60 (0.25) |
0.60 (0.16) 0.43 (0.30) |
| Remember | Old New |
0.51 (0.23)1 0.13 (0.17) |
0.27 (0.21) 0.12 (0.17) |
| Know | Old New |
0.26 (0.16) 0.19 (0.16) |
0.33 (0.21) 0.17 (0.15) |
| Guess | Old New |
0.05 (0.06) 0.08 (0.08) |
0.19 (0.17)1 0.29 (0.25)1 |
| Memory Judgmen | Encoding Condition | ||
| Remember | Incidental Intentional |
0.55 (0.29)2 0.51 (0.21)1 |
0.32 (0.23) 0.26 (0.22) |
| Know | Incidental Intentional |
0.28 (0.21) 0.24 (0.12) |
0.37 (0.25) 0.29 (0.18) |
| Guess | Incidental Intentional |
0.03 (.04) 0.06 (0.08) |
0.15 (0.14)1 0.22 (0.20)1 |
| Free Recall | Encoding Condition | ||
| Overall | 7.2 (0.92)1 | 2.4 (0.87) | |
| Incidental Incidental |
5.2 (0.92) 9.1 (1.2)2 |
2.4 (0.87) 2.4 (1.1)2 |
|
Incidental encoding accuracy limited to trials on which a response was given; Incidental encoding RT data based on correct trials
P < 0.01
P < 0.02
P < 0.05
P < 0.001
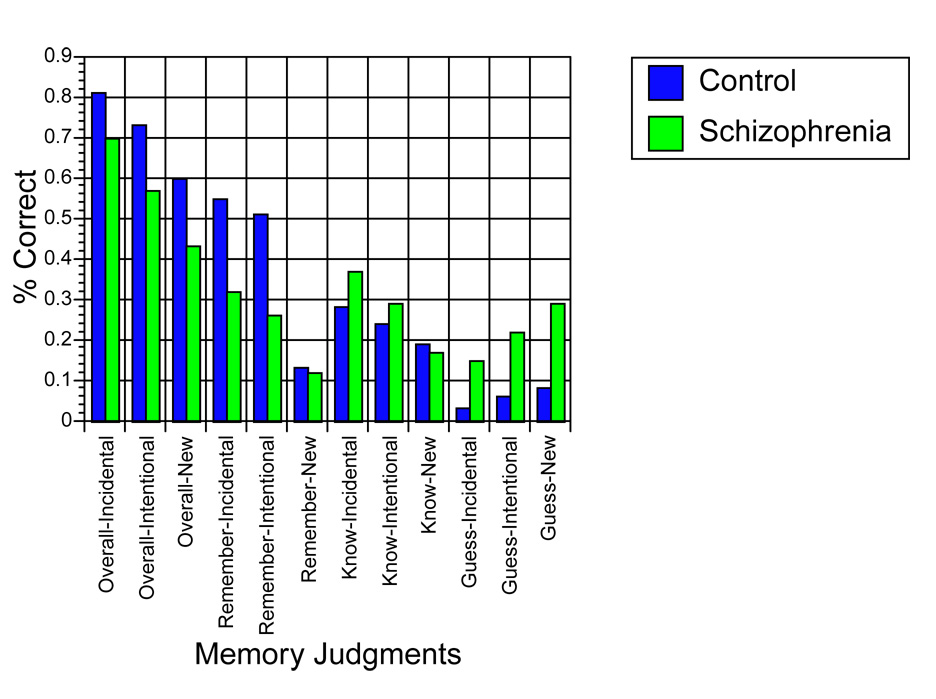
Figure 1.
Summary of word recognition data for control (blue bars) and schizophrenia (green bars) participants. Data are presented for overall accuracy, correct Remember judgments, correct Know judgments, and Guess judgments for Incidentally-encoded and Intentionally-encoded words, as well as New words.
3.1.1. Recognition accuracy
To assess the influence of encoding condition on SM performance, we conducted a 2 × 2 repeated measures ANOVA. This revealed that both groups recognized more items encoded Incidentally, as opposed to Intentionally [F(1,30) = 26.52, P < 0.001], and schizophrenia participants showed worse recognition overall [F(1,30) = 5.53, P < 0.05]. Although the Group × Encoding Condition interaction was non-significant (P > .24), we conducted separate group comparisons for each condition, given our a priori hypothesis of smaller group differences for Incidentally encoded items. Control participants showed significantly better memory performance than schizophrenia participants for Intentionally encoded words (P < .02; effect size = 0.93), with a trend-level group difference (control > schizophrenia) for Incidentally encoded words (P = 0.08; effect size = 0.64).
One possible concern was related to task order effects. Specifically, participants who performed the Incidental encoding task first could have used a similar deep encoding strategy during Intentional encoding, resulting in significantly better performance for Intentionally encoded words. Thus, in order to verify that encoding task order did not significantly influence memory success, we performed analyses comparing recognition rates for each task order (Incidental-Intentional vs. Intentional-Incidental). Results indicated that neither group performed significantly better following one task order relative to the other. In fact, we found evidence of numerically-greater memory performance for participants who saw Intentional encoding first, relative to those who saw Incidental encoding first, a finding which was in the opposite direction of what was expected. This strongly discounts the notion of carry-over effects and suggests that participants did not adopt the Incidental encoding strategy for use during Intentional encoding.
We then analyzed between-group differences in Remember judgments (for previously-seen words) as a function of encoding condition using a 2 × 2 repeated measures ANOVA. This revealed a trend toward more Remember judgments being given for Incidentally encoded (relative to Intentionally encoded) words [F (1,30) = 4.16, P = 0.05], as well as more Remember judgments given by controls than schizophrenia participants [F (1,30) = 8.80, P < 0.01]. The interaction was non-significant (P > 0.61). Parallel analyses were conducted for Know and Guess judgments for previously seen words as well. Results of the repeated measures ANOVA for Know judgments revealed that significantly more Know judgments were given for Incidentally encoded (relative to Intentionally encoded) words [F (1,30) = 9.37, P < 0.01], while the groups did not differ in their likelihood of making Know judgments (P > 0.32). The Group × Encoding Condition interaction was also non-significant (P > 0.43). Analysis of the data for Guess judgments revealed that significantly more Guess judgments were given following Intentional encoding (relative to Incidental encoding) [F (1,30) = 14.76, P < 0.005] and schizophrenia participants were significantly more likely to make Guess judgments to previously-seen words than control participants [F (1,30) = 9.51, P < 0.005]. The interaction showed trend-level significance [F (1,30) = 3.01, P = 0.093].
3.1.2. Free recall accuracy
The effect of encoding orientation on free recall accuracy was assessed using a 2 × 2 ANOVA, which revealed better overall recall following Intentional encoding [F(1,30) = 6.5, P < 0.02] and better recall by controls than schizophrenia participants [F(1,30) = 14.3, P = 0.001]. The significant interaction [F(1,30) = 6.1, P < 0.02] revealed that control participants recalled significantly more words following Intentional than Incidental encoding, whereas recall among schizophrenia participants did not differ following either condition.
Finally, in order to assess possible effects of anti-cholinergic medications on memory performance among schizophrenia participants, we compared the two subgroups (i.e., those who were taking anti-cholinergic medications at the time of study vs. those who were not) on all memory measures using independent samples t-tests. Results of all analyses were non-significant (all P’s > 0.26), leading us to believe that the subsample of schizophrenia participants who were taking anti-cholinergic medications were not differentially impaired relative to the remainder of the sample.
3.2. Neuroimaging data
3.2.1. Encoding orientation effects
The Incidental > Intentional contrast for controls (Table 4) revealed activity in regions such as left inferior frontal (BA 45) and right middle frontal gyrus (BA 6, 9). Control participants activated a number of temporal and occipital regions during Intentional > Incidental encoding (Table 4).
Table 4.
Regions of significant encoding-related activation: Incidental > Intentional
| Region of Interest | Brodmann Area(s) | X | Y | Z | ROI F-value for main effect of Encoding Condition | Effect size | |
|---|---|---|---|---|---|---|---|
| Control participants | |||||||
| Left inferior frontal gyrus | 45 | −44 | 16 | 21 | 21.49 | 1.42 | |
| Right middle frontal gyrus | 9 | 46 | 24 | 34 | 14.61 | 0.81 | |
| Left cerebellum | −42 | −54 | −32 | 14.47 | 0.89 | ||
| Right cerebellum | 7 | −56 | −31 | 13.31 | 1.27 | ||
| Medial superior frontal gyrus | 6 | 0 | 11 | 48 | 12.30 | 1.19 | |
| Right middle frontal gyrus | 6 | 32 | −3 | 48 | 10.43 | 0.72 | |
| Right inferior temporal gyrus | 20 | 42 | −12 | −30 | 9.87 | 1.37 | |
| Right precentral gyrus | 6 | 31 | −16 | 59 | 9.51 | 0.81 | |
| Left postcentral gyrus | 2 | −59 | −22 | 30 | 7.38 | 0.74 | |
| Left fusiform gyrus | 19 | −34 | −69 | −12 | 7.27 | 0.62 | |
| Left thalamus | −14 | −2 | 8 | 6.70 | 0.92 | ||
| Right insula | 31 | 18 | 9 | 6.68 | 1.12 | ||
| Right lingual gyrus | 18 | 26 | −70 | −10 | 6.63 | 0.64 | |
| Left cerebellum | −19 | −67 | −26 | 6.44 | 0.77 | ||
| Right cerebellum | 32 | −61 | −28 | 6.23 | 0.60 | ||
| Participants with schizophrenia | |||||||
| Right inferior frontal gyrus | 44 | 42 | 15 | 18 | 33.49 | 0.56 | |
| Right inferior frontal gyrus | 47 | 41 | 44 | −4 | 16.77 | 1.17 | |
| Right middle temporal gyrus | 21 | 61 | −50 | −1 | 15.94 | 1.26 | |
| Right middle occipital gyrus | 19 | 50 | −80 | −6 | 15.88 | 0.65 | |
| Left inferior frontal gyrus | 45 | −44 | 24 | 11 | 14.86 | 1.33 | |
| Left inferior parietal lobule | 40 | −41 | −46 | 53 | 14.45 | 0.83 | |
| Left precentral gyrus | 4 | −32 | −17 | 57 | 12.89 | 0.56 | |
| Left precentral gyrus | 6 | −51 | 2 | 33 | 11.99 | 0.63 | |
| Right thalamus | 11 | −17 | 5 | 10.77 | 0.74 | ||
| Left inferior parietal lobule | 40 | −32 | −56 | 40 | 10.51 | 0.91 | |
| Left medial frontal gyrus | 6 | −2 | 1 | 54 | 9.34 | 0.61 | |
| Left cerebellum | −30 | −80 | −23 | 9.09 | 0.37 | ||
| Right cerebellum | 30 | −68 | −26 | 8.69 | 0.54 | ||
| Right inferior temporal gyrus | 37 | 49 | −68 | 2 | 7.99 | 0.48 | |
| Right globus pallidus | 19 | −4 | 4 | 7.11 | 0.82 | ||
| Left superior temporal gyrus | 22 | −66 | −43 | 14 | 6.39 | 0.74 | |
Whole-brain corrected P-value for activations = 0.05
For participants with schizophrenia, the Incidental > Intentional contrast (Table 4) also revealed activity in typical verbal encoding areas (e.g., left BA 45), whereas Intentional > Incidental encoding was not associated with any regions of significant activity (Table 5).
Table 5.
Regions of significant encoding-related activation: Intentional > Incidenta
| Region of Interest | Brodmann Area(s) | X | Y | Z | ROI F-value for main effect of encoding condition | Effect size | |
|---|---|---|---|---|---|---|---|
| Control participants | |||||||
| Right superior temporal gyrus | 39 | 45 | −52 | 28 | 14.26 | 1.09 | |
| Right temporal lobe/Hippocampus | 39 | −30 | −7 | 12.07 | 1.29 | ||
| Right posterior cingulate | 30 | 2 | −46 | 17 | 10.25 | 0.79 | |
| Right paracentral lobe | 31 | 3 | −30 | 47 | 10.22 | 1.11 | |
| Left middle temporal gyrus | 21 | −57 | 0 | −9 | 10.13 | 0.93 | |
| Left middle temporal gyrus | 37 | −52 | −60 | 0 | 9.69 | 0.75 | |
| Left middle occipital gyrus | 19 | −39 | −70 | 13 | 8.16 | 0.77 | |
| Right cerebellum | 7 | −39 | −6 | 7.89 | 0.79 | ||
| Right cerebellum | 53 | −69 | −22 | 7.68 | 1.05 | ||
| Right precuneus | 19 | 28 | −75 | 36 | 6.96 | 0.67 | |
| Left superior temporal gyrus | 22 | −56 | −53 | 18 | 6.55 | 0.92 | |
| Participants with schizophrenia | |||||||
| None | |||||||
Whole-brain corrected P-value for activations = 0.05
Between-group comparisons for Intentional encoding revealed a large network of regions activated significantly more by controls (Figure 2), including right inferior frontal gyrus (BA 46, 47) and right parahippocampal gyrus (BA 30). Schizophrenia participants did not show significantly greater activity than controls during Intentional encoding.
Figure 2.
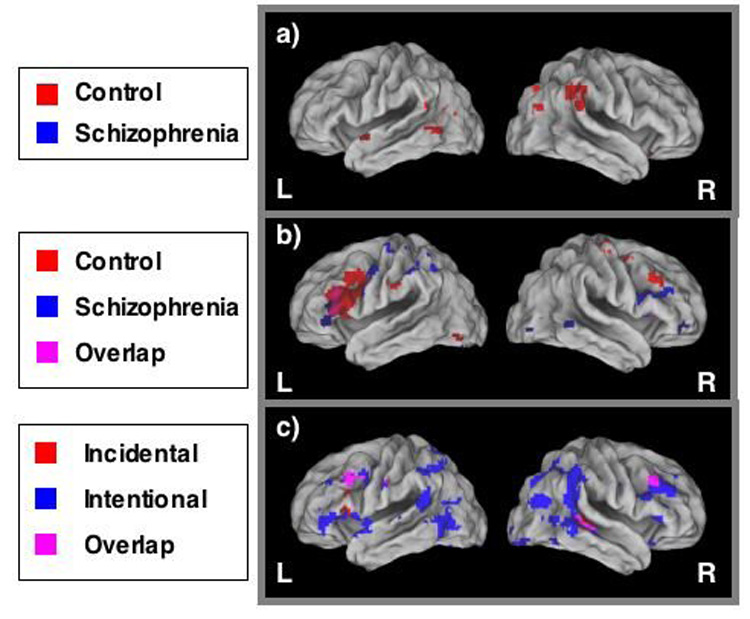
a) Regions of significant within group activity during Intentional (relative to Incidental) encoding; b) Regions of significant within-group activity during Incidental (relative to Intentional) encoding, including regions in which within-group activity overlapped between groups; c) Regions of significant between-group differences (Control > Schizophrenia) during Intentional and Incidental encoding, including regions in which between-group differences in both conditions overlapped.
Between-group comparisons for Incidental encoding revealed regions of significantly greater task-related activation among control participants compared with schizophrenia participants, including bilateral middle frontal gyrus (BA 9; Figure 2). However, the distribution and extent of between-group differences was markedly reduced, relative to Intentional encoding. The opposite contrast (schizophrenia > control) again failed to reveal any significant group differences.
Next, we performed a repeated measures ANOVA, with Group as the between subjects variable and Encoding Condition as the within subjects variable. Six regions showed a significant Group × Encoding Condition interaction (see Table 6). In 5 out of 6 regions, controls showed significantly greater activity than schizophrenia participants during Intentional encoding, with either no group differences in Incidental, or greater activity in schizophrenia participants than control participants in Incidental. Among the regions showing such an effect was left inferior frontal gyrus (see Figure 3). Only one region was activated more by controls than participants with schizophrenia during Incidental encoding with no differences in Intentional (BA 20; right inferior temporal gyrus).
Table 6.
Regions demonstrating significant Group x Encoding Condition interaction
| Region of Interest | Brodmann Area(s) | X | Y | Z | Intentional | Incidental |
|---|---|---|---|---|---|---|
| Right Inferior Temporal Gyrus | 20 | 47 | −11 | −28 | SCZ > CON* | CON > SCZ** |
| Right Insula | 45 | −2 | 8 | CON > SCZ* | SCZ > CON* | |
| Right Precuneus | 19 | 28 | −75 | 36 | CON > SCZ** | CON > SCZ* |
| Right Inferior Parietal Lobule | 40 | 54 | −45 | 40 | CON > SCZ** | CON = SCZ |
| Left Inferior Frontal Gyrus | 47 | −48 | 35 | −1 | CON > SCZ** | SCZ > CON* |
| Left Inferior Temporal Gyrus | 19 | −48 | −63 | −1 | CON > SCZ** | CON > SCZ* |
non-significant
P < 0.05
Figure 3.
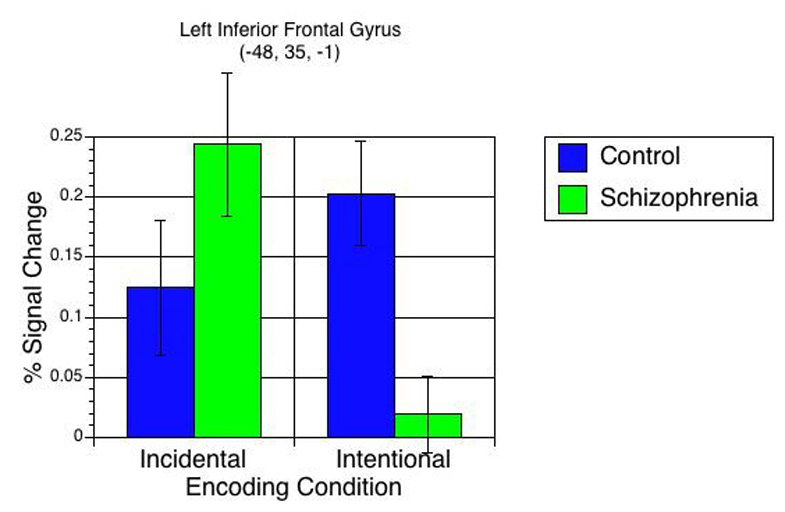
Group × Encoding Condition interaction for task-related activation in left inferior frontal gyrus in control (blue bars) and schizophrenia (green bars) participants. In this region, control participants demonstrated significantly greater task-related brain activity than schizophrenia participants during Intentional encoding, whereas schizophrenia participants demonstrated numerically-greater task-related brain activity than controls during Incidental encoding.
3.2.2. Subsequent memory effects
We next identified brain regions common to both groups showing significantly greater activity during encoding of subsequently remembered items than subsequently forgotten items by performing a repeated measures ANOVA, with Group (control, schizophrenia) as the between subjects variable and Memory Judgment (Remember, Miss) as the within subjects variable. We then masked the results of this ANOVA with the Group difference map (between-group differences for remembered vs. missed items) to exclude regions of between-group differences. This analysis revealed 8 regions (Table 7, Figure 4) that showed significant activity for subsequently remembered items in both groups.
Table 7.
Regions demonstrating a significant Main Effect of Memory Judgment (Remember > Miss
| Region of Interest | Brodmann Area(s) | X | Y | Z | Effect Size |
|---|---|---|---|---|---|
| Left postcentral gyrus | 40 | −39 | −34 | 57 | 0.50 |
| Right inferior frontal gyrus | 44 | 54 | 4 | 30 | 0.44 |
| Left superior parietal lobe | 7 | −30 | −66 | 47 | 0.43 |
| Left inferior frontal gyrus | 44 | −46 | 9 | 31 | 0.40 |
| Right cingulate gyrus | 32 | 16 | 9 | 35 | 0.37 |
| Right precental gyrus | 4 | 26 | −18 | 63 | 0.36 |
| Right parahippocampal gyrus | 36 | 33 | −24 | −27 | 0.35 |
| Right middle temporal gyrus | 21 | 62 | −44 | −6 | 0.35 |
Figure 4.
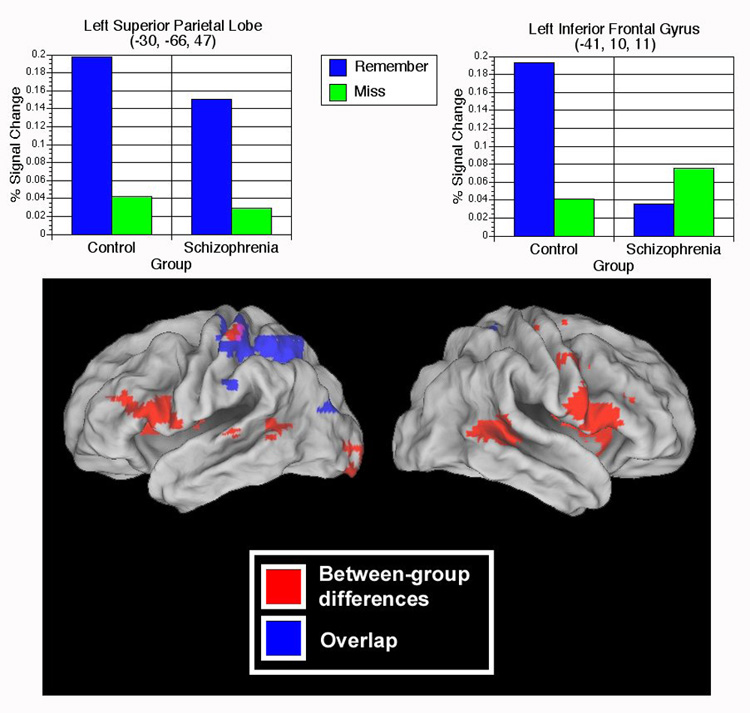
Upper panel: Graphs depicting average signal change related to subsequent memory (Remember > Miss) in two regions – left superior parietal lobe (-30, -66, 47) and left inferior frontal gyrus (-41, 10, 11). As can be seen, participants with schizophrenia show typical subsequent memory effects in the left superior parietal lobe region, but do not show such effects in left inferior frontal gyrus. Lower panel: Regions of significant overlap (blue) and regions of significant between-group differences (red) in subsequent memory activity (Remember > Miss). While the majority of between-group differences (largely Control > Schizophrenia) are confined to anterior/inferior regions, overlap between groups in subsequent memory activity occurs primarily in posterior/superior regions.
We then used the ANOVA described above to identify regions of significant group differences in SM effects (i.e., Group × Memory Judgment interactions). In the majority of the regions identified, controls showed significantly greater encoding-related activity for subsequently Remembered items (Table 8), including left inferior frontal (BA 44) and right middle frontal gyrus (BA 46). Participants with schizophrenia showed significantly greater activation during Remembered words in left precuneus (Figure 4).
Table 8.
Regions demonstrating a significant Group x Memory Judgment interactio
| Region of Interest | Brodmann Area(s) | X | Y | Z | Interaction |
|---|---|---|---|---|---|
| Right middle frontal gyrus | 46 | 45 | 35 | 22 | Con (R>M) |
| Right putamen | 29 | −2 | 9 | Con (R>M) | |
| Right middle temporal gyrus | 22 | 54 | −40 | 4 | Con (R>M) |
| Left superior temporal gyrus | 22 | −62 | −28 | 3 | Con (R>M) |
| Left inferior frontal gyrus | 44 | −41 | 10 | 11 | Con (R>M) |
| Left lingual gyrus | 17 | −14 | −98 | −7 | Con (R>M) |
| Left precentral gyrus | 6 | −62 | −1 | 21 | Con (R>M) |
| Left paracentral lobule | −11 | −13 | 46 | Scz (M>R) | |
| Left precuneus | 7 | −29 | −47 | 50 | Scz (R>M) |
The SM results could have been confounded by encoding condition, such that more Remember responses were produced following one encoding condition than the other. To rule out such a confound, we compared R > M activity in Incidental and Intentional encoding independently and confined our analyses to the previously identified regions of interest showing R > M effects. Our analyses revealed the same pattern of SM related brain activity as was previously found, regardless of encoding condition. It is also conceivable that task order (Incidental first vs. Intentional first) impacted brain activity patterns. Thus, we compared neuroimaging results from the two possible task orders (Incidental-Intentional vs. Intentional-Incidental). These analyses revealed no significant activation differences between the two task orders.
Discussion
In the current study, we investigated memory performance and encoding-related brain activity in response to two different encoding strategies in schizophrenia participants and healthy controls. To our knowledge, this is the first functional neuroimaging study to directly compare deep (supported) encoding and intentional (unsupported) encoding in the same group of schizophrenia participants and healthy controls. As predicted, schizophrenia participants showed memory improvements following Incidental encoding (relative to Intentional), in line with previous studies demonstrating memory benefits of encoding strategies (Ragland et al., 2003; Bonner-Jackson et al., 2005; Ragland et al., 2005). While controls recognized significantly more words than schizophrenia participants following Intentional encoding, the between-groups difference in recognition following Incidental encoding was non-significant and smaller in effect size. Our findings support hypotheses that faulty mnemonic processes during encoding underlie memory deficits in schizophrenia (Koh & Peterson, 1978; Brebion et al., 1997; Iddon et al., 1998). It is notable, however, that free recall performance in the schizophrenia group remained poor regardless of encoding orientation, which may highlight the need for supportive retrieval conditions in order to maximize memory performance in schizophrenia participants.
With regard to memory judgments, schizophrenia participants were significantly less likely to give Remember responses for Old words, although they made significantly more Remember judgments (35% vs. 28%) for previously seen words following Incidental encoding (relative to Intentional). For New words, however, schizophrenia participants made Guess responses nearly one-third (29%) of the time, compared to 5% for controls, suggesting an inability to effectively identify new words in the absence of retrieval strategies. Taken together, these results provide further evidence that recollection deficits are related to encoding deficits in schizophrenia, although retrieval impairments persist in the absence of supportive retrieval conditions.
The fMRI data revealed that during Intentional encoding, controls activated a wider network of brain regions than schizophrenia participants, including regions in bilateral PFC and right temporal lobe/hippocampus. Our results replicate previous findings of cortical underactivation during memory performance in schizophrenia (Ragland et al., 2001; Barch et al., 2002; Achim et al., 2005) and provide further evidence that both behavioral and neurobiological manifestations of memory deficits in schizophrenia may be tied to encoding failures. In contrast, Incidental encoding in schizophrenia participants was associated with increased activity in regions known to support EM function, consistent with previous work (Bonner-Jackson et al., 2005; Ragland et al., 2005). These regions included left (BA 45) and right inferior frontal gyrus (BA 44, 47), left inferior parietal lobule (BA 40), and cerebellum. We also detected fewer between-group differences in encoding-related brain activity during Incidental encoding, relative to Intentional encoding, suggesting that the provision of a semantic encoding strategy serves to “normalize” brain activity patterns to a certain degree among individuals with schizophrenia.
In addition, the Group × Encoding Condition interactions revealed an interesting pattern: controls largely showed greater activity than schizophrenia participants during Intentional encoding in areas commonly associated with EM processing (Buckner et al., 2001; Otten et al., 2001a). Among these were left inferior frontal gyrus (BA 47), left inferior temporal gyrus (BA 19), and right inferior parietal lobule (BA 40). These findings complement those from the between-group contrasts, reinforcing the notion that individuals with schizophrenia show the most pronounced activation deficits under unsupported encoding conditions. In contrast, the interaction also revealed that the groups were highly similar in terms of brain activation during Incidental encoding. This finding is most clearly typified by the activation patterns found in left inferior frontal gyrus (-48, 35, -1; Figure 3). Schizophrenia participants showed numerically (non-significantly) greater Incidental encoding-related activity than controls in left inferior frontal gyrus (BA 47), which is known to support EM function, whereas controls activated this region to a significantly greater degree than schizophrenia participants during Intentional encoding. Conversely, controls showed significantly greater Incidental encoding activity than schizophrenia participants in only one region (right inferior temporal gyrus; BA 20). Thus, it is clear that the Group × Encoding Type interaction was largely driven by the between-groups differences during Intentional encoding. These data suggest that when oriented to do so, individuals with schizophrenia recruit brain regions that control participants seem to utilize automatically. Our findings provide further evidence that the neural systems implicated in memory formation in healthy controls are also active in schizophrenia participants when memory strategies are provided.
Data from the SM analysis revealed that for both groups, encoding of subsequently remembered items was associated with activity in regions known to support successful encoding (Buckner et al., 2001; Otten et al., 2001b; Casasanto et al., 2002), including bilateral inferior frontal gyrus (BA 44), left superior parietal lobule (BA 7), and left postcentral gyrus (BA 40). To our knowledge, these results represent the first demonstration of functional overlap between subsequent memory regions in controls and schizophrenia participants, suggesting that individuals with schizophrenia and healthy controls recruit a set of similar brain regions during successful episodic encoding.
With regard to between-group differences in SM-related activity, we found that in the majority of regions controls showed more activity than schizophrenia participants during encoding of subsequently remembered items (compared to missed items). Left lingual gyrus (BA 17) and left inferior frontal gyrus (BA 44), for example, have both been implicated in subsequent memory studies in healthy controls (Otten et al., 2001a; Cansino et al., 2002) and are known to contribute to successful episodic encoding. Importantly, however, few of the regions showing between-group differences in SM activity (3/9) correspond to areas that have been previously identified as typical SM regions, perhaps suggesting that the majority of between-group differences identified here are related to other factors. In contrast, 5 of the 8 regions in which both groups demonstrated SM effects have been previously implicated in SM functions. For example, the groups showed overlap in SM activity in crucial prefrontal (bilateral inferior frontal gyrus) and medial temporal lobe regions (right parahippocampal gyrus) that have been linked to subsequent memory (Brewer et al., 1998; Otten et al., 2001a; Otten et al., 2001b). Furthermore, we found evidence for a clear functional dissociation between regions showing overlap and regions showing between group differences in SM activity (Figure 2). Between group differences (mostly control > schizophrenia) were largely confined to frontal areas, whereas the groups demonstrated significant overlap in posterior regions, such as left superior parietal lobe (BA 7),some of which have been postulated to provide a compensatory role in EM in schizophrenia (Heinze et al., 2006). These findings further support theories that some aspects of the EM system contributing to SM are intact in individuals with schizophrenia.
It is conceivable that the SM results were altered by the difficulty on the part of schizophrenia participants in accurately distinguishing between memory judgments, particularly Remember vs. Know. If schizophrenia participants were less accurate in identifying Remember responses, such that what they labeled as Remember responses were not associated with explicit recollection, this could have reduced estimates of SM-related activity. It is notable, however, that previous studies examining EM in individuals with schizophrenia have successfully utilized the Remember-Know paradigm (Huron et al., 1995; Danion et al., 1999), suggesting that individuals with schizophrenia, like controls, have the ability to make such distinctions. Future studies may profit by obtaining self-report measures regarding how schizophrenia participants completed this task, in order to assess understanding of the concept involved.
Finally, our predictions with regard to MTL activations were only somewhat fulfilled. As predicted, schizophrenia participants failed to activate MTL/hippocampal regions during Intentional encoding, though control participants did. However, neither group significantly activated MTL regions during Incidental encoding. The SM findings were also mixed with regard to MTL activity. Both groups showed significant activity in right parahippocampal gyrus during encoding of subsequently remembered words. We did not, however, detect between-group differences in other MTL regions, such as left hippocampus. Previous work has demonstrated that hippocampus is most active and engaged during relational binding of information (Dolan and Fletcher, 1997; Brown and Aggleton, 2001; Giovanello et al., 2004). Given the somewhat overlearned nature of word processing and the lack of an associative binding component in the orientation tasks, it is conceivable that MTL structures were not as crucial for the successful completion of these tasks as we had originally hypothesized.
One limitation of the current study is low memory performance in certain conditions, particularly among schizophrenia participants. Although this is problematic, schizophrenia participants made Remember judgments for old items (M = 27%) significantly more often than for new items (M = 12%), suggesting an ability to distinguish between old and new items to some degree. Another limitation stems from the non-significant Group × Encoding Condition interaction in the behavioral data. We cannot conclusively state that schizophrenia participants were selectively impaired following Intentional, relative to Incidental, encoding. However, given the overall pattern of behavior, a larger sample size could allow us to detect such an effect. Furthermore, the fMRI data did show a clear Group × Encoding Condition interaction: far more between-group differences were found during Intentional encoding, whereas the groups were quite similar during Incidental encoding. Thirdly, it can be argued that the difference in intentionality between encoding conditions significantly confounds the results, since intentional learning was emphasized in only one condition. Nevertheless, both groups performed better following unintentional encoding.
In summary, we demonstrated significant benefits of encoding orientation, both behaviorally and neurobiologically, on memory processing in individuals with schizophrenia, as well as evidence of additional memory and brain activation deficits not alleviated by provision of encoding strategies. We also identified SM effects among schizophrenia participants in a subset of brain regions known to support successful encoding in control participants, while in other regions schizophrenia participants failed to show normal SM effects. Future research should seek to further clarify the influence of strategies and the role of cortical structures in memory formation in schizophrenia.
Acknowledgments
The authors wish to thank Melissa Hanewinkel for assistance with participant recruitment and Kristen Haut for assistance in data collection, as well as four anonymous reviewers for their helpful comments and suggestions. This work was supported by NIMH grant MH071616 (Mapping Abnormal Neurodevelopment in Schizophrenia) as well as the Conte Center for the Neuroscience of Mental Disorders (MH071616).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. British Journal of Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder AV. Anterior cingulate cortex and response conflict: Effects of response modality and processing domain. Cerebral Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Barch DM, Csernansky J, Conturo T, Snyder AZ, Ollinger J. Working and long-term memory deficits in schizophrenia. Is there a common underlying prefrontal mechanism? Journal of Abnormal Psychology. 2002;111:478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut KM, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biological Psychiatry. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Brebion G, Amador X, Smith MJ, Gorman JM. Mechanisms underlying memory impairment in schizophrenia. Psychological Medicine. 1997;27:383–393. doi: 10.1017/s0033291796004448. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Glover GH, Gabrieli JDE. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler M, Sheridan M. Encoding processes during retrieval tasks. Journal of Cognitive Neuroscience. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Calev A. Recall and recognition in chronic nondemented schizophrenics: use of matched tasks. Journal of Abnormal Psychology. 1984;93:172–177. doi: 10.1037//0021-843x.93.2.172. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Casasanto DJ, Killgore WDS, Maldjian JA, Glosser G, Alsop DC, Cooke AM, Grossman M, Detre JA. Neural correlates of successful and unsuccessful verbal memory encoding. Brain and Language. 2002;80:287–295. doi: 10.1006/brln.2001.2584. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychology Review. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Conturo TE, McKinstry RC, Akbudak E, Snyder AZ, Yang T, Raichle ME. Sensitivity optimization and experimental design in functional magnetic resonance imaging. Society for Neuroscience Abstracts. 1996;26:7. [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Craik FIM, Tulving E. Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General. 1975;104:268–294. [Google Scholar]
- Danion JM, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Archives of General Psychiatry. 1999;56:639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weyerts H, Schrader-Bolsche M, Tendolkar I, Smid HGOM, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Heinze HJ. Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. Journal of Neuroscience. 1998;18:1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Stephenson CME, Carpenter TA, Donovan T, Bullmore ET. Regional brain activations predicting subsequent memory success: An event-related fMRI study of the influence of encoding tasks. Cortex. 2003;39:1009–1026. doi: 10.1016/s0010-9452(08)70875-x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. Journal of Abnormal Psychology. 1992;101:487–494. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Jeu LA, Nenadic I, Fleischman MB, Shihabuddin L, Haznedar MM, Harvey PD. Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophrenia Research. 2000;43:33–46. doi: 10.1016/s0920-9964(99)00178-4. [DOI] [PubMed] [Google Scholar]
- Heinze S, Sartory G, Muller BW, de Greiff A, Forsting M, Juptner M. Neural activation during successful and unsuccessful verbal learning in schizophrenia. Schizophrenia Research. 2006;83:121–130. doi: 10.1016/j.schres.2005.12.852. [DOI] [PubMed] [Google Scholar]
- Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW. Neural correlates of episodic encoding and recognition of words in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. American Journal of Psychiatry. 2003a;160:1802–1808. doi: 10.1176/appi.ajp.160.10.1802. [DOI] [PubMed] [Google Scholar]
- Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Kremser C, Felber S, Fleischhacker WW. An fMRI study of episodic encoding and recognition of words in patients with schizophrenia in remission. American Journal of Psychiatry. 2003b;160:911–918. doi: 10.1176/appi.ajp.160.5.911. [DOI] [PubMed] [Google Scholar]
- Huron C, Danion JM, Giacomoni F, Grange D, Robert P, Rizzo L. Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. American Journal of Psychiatry. 1995;152:1737–1742. doi: 10.1176/ajp.152.12.1737. [DOI] [PubMed] [Google Scholar]
- Iddon JL, McKenna PJ, Sahakian BJ, Robbins TW. Impaired generation and use of strategy in schizophrenia: evidence from visuospatial and verbal tasks. Psychological Medicine. 1998;28:1049–1062. doi: 10.1017/s0033291798006758. [DOI] [PubMed] [Google Scholar]
- Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Maier W, Schild HH, Heun R. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. American Journal of Psychiatry. 2003;160:1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- Koh SD. Remembering of verbal material by schizophrenic young adults. In: Schwartz S, editor. Language and Cognition in Schizophrenia. Hillsdale, NJ: Erlbaum; 1978. pp. 55–99. [Google Scholar]
- Koh SD, Kayton L. Memorization of "unrelated" word strings by young nonpsychotic schizophrenics. Journal of Abnormal Psychology. 1974;83:14–22. doi: 10.1037/h0036233. [DOI] [PubMed] [Google Scholar]
- Koh SD, Peterson RA. Encoding orientation and the remembering of schizophrenic young adults. Journal of Abnormal Psychology. 1978;87:303–313. [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational Analysis of Present-Day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ojemann J, Akbudak E, Snyder A, McKinstry R, Raichle M, Conturo T. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RNA, Rugg MD. Depth of processing effects on neural correlates of memory encoding. Brain. 2001a;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex. 2001b;11:1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: A PET cerebral blood flow study. American Journal of Psychiatry. 2001;158:1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Loughead J, Elliott M, Kohler C, Kanes S, Siegel SJ, Moelter ST, Gur RE. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. American Journal of Psychiatry. 2005;162:1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. American Journal of Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Moelter ST, McGrath C, Hill SK, Gur RE, Bilker WB, Siegel SJ, Gur RC. Levels-of-processing effect on word recognition in schizophrenia. Biological Psychiatry. 2003;54:1154–1161. doi: 10.1016/s0006-3223(03)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengel RA, Lovallo WR. Effects of cueing on immediate and recent memory in schizophrenics. Journal of Nervous and Mental Disease. 1983;171:426–430. doi: 10.1097/00005053-198307000-00006. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured clininical interview for DSM-III-R--patient edition (SCID-P, version 1.0) Washington, D.C.: American Psychiatric Press; 1990. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychologist. 1985;26:1–12. [Google Scholar]
- Wagner AD, Schacter D, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Schacter DL, Goff DC, Rauch SL, M, A N, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biological Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Zalesak M, DeWitt I, Goff DC, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biological Psychiatry. 2004;55:668–675. doi: 10.1016/j.biopsych.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]